Introduction
Cervical cancer remains one of the leading causes of
cancer-associated mortality in females, accounting for 14% of all
mortality due to gynecological cancer worldwide (1). The most common symptom of cervical
cancer is abnormal vaginal bleeding, however, in the majority of
cases there may be few clear manifestations until it progresses
into the advanced stage. The treatment of cervical cancer may
include surgery, chemotherapy, radiotherapy, immunotherapy and
vaccine therapy or a combination of these. Surgery, including local
excision is suitable for this type of cancer in the early stages.
Radiotherapy combined with cisplatin-based chemotherapy can
markedly improve the survival rate of patients with cervical cancer
at advanced stages (2), however,
these strategies may fail if tumors have metastasized. Furthermore,
chemotherapy resistance has created a major challenge for the
treatment of cervical cancer. Thus, the identification of new
strategies and agents for the treatment of cervical cancer is
urgently required (3).
Cancer targeting Gene-Viro-Therapy represents a
promising approach for cancer therapy, which is designed through
inserting an antitumor gene into an oncolytic viral vector that has
the ability to selectively replicate in tumor cells but not in
normal cells (4–6). More than 12 different oncolytic
viruses are currently undergoing phase I–III clinical trials
against various types of cancer (7). ZD55-TRAIL is an oncolytic adenovirus
(OA) previously constructed in the laboratory of Xinyuan Institute
of Medicine and Biotechnology at Zhejiang Sci-Tech University
(Hangzhou, China), with the antitumor gene tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) inserted into the
oncolytic vector ZD55 with E1B55-kDa deletion (8). Several in vitro and in
vivo experiments have demonstrated the antitumor effects of
ZD55-TRAIL in models of cervical, lung, breast, colorectal and
hepatocellular cancer (8–10). However, due to their genetic
heterogeneity, it is likely that certain cancer cells within a
tumor have different sensitivities to oncolytic viruses. Therefore,
it is necessary to reinforce the antineoplastic activity of the
virus to obtain the highest therapeutic intervention.
Suberoylanilide hydroxamic acid (SAHA) is a potent
inhibitor of class I and II histone deacetylases (HDACs). These
enzymes are responsible for deacetylation of histones and certain
other proteins, thus maintaining chromatin in a more relaxed state,
and thereby allowing transcription of different regulatory genes
that are involved in tumor development and growth (11–13).
In xenograft and in vitro models, certain HDAC inhibitors,
including SAHA have demonstrated antitumor activity against
cervical cancer (14–16). The present study investigated
whether SAHA is able to enhance the anticancer activity of
ZD55-TRAIL against cervical cancer, and the mechanism underlying
the synergistic therapeutic effects of ZD55-TRAIL and SAHA.
Materials and methods
Reagents
The OA ZD55-TRAIL was constructed as described
previously (8) and preserved in
the laboratory of Xinyuan Institute of Medicine and Biotechnology
at Zhejiang Sci-Tech University. The HDAC inhibitor SAHA was
purchased from Calbiochem (San Diego, CA, USA). All the compounds
used in the present study were dissolved in dimethyl sulfoxide
(DMSO) and diluted with culture medium (0.1% v/v DMSO). Caspase-8,
β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
antibodies were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Caspase-3, poly adenosine diphosphate
(ADP)-ribose (PARP), IκBα, p65 and p50 antibodies were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell lines and culture conditions
The human cervical cancer cell line HeLa was
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The HeLa-luc cell
line, a light producing cell line from HeLa cells stably
transfected with the luciferase gene, was purchased from Perkin
Elmer (Waltham, MA, USA). Cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with heat-inactivated fetal bovine serum (FBS;
Gibco-BRL) at 37°C in a humidified air atmosphere with 5%
CO2.
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was determined using an MTT assay.
Briefly, HeLa cells were plated in 96-well plates at a density of
1×104 in 100 μl culture medium. Cells were
treated with SAHA (0.1, 0.2, 0.4 or 0.8 μM) and ZD55-TRAIL
(2, 4, 8 or 16 MOI) independently or in combination for 48 h. After
48 h, MTT (Sigma-Aldrich, St. Louis, MO, USA) solution (10
μl, 5 mg/ml) was added to the cells, which were then
cultivated for a further 3 h. Absorbance was measured as 490 nm
using a fluorescence reader (Finstruments Multiskan EX; MTX LAB
Systems Inc., Männedorf, Switzerland).
Hoechst 33342 staining
Morphological characteristics of cell apoptosis were
detected using an Hoechst staining assay. HeLa cells were treated
with SAHA (0.4 μM), ZD55-TRAIL (8 MOI) or SAHA in
combination with ZD55-TRAIL for 24 h. Subsequently, 5 μl of
Hoechst 33342 (Sigma-Aldrich) was added to the cells for 30 min and
results were observed under an inverted fluorescence microscope
(IX71; Olympus, Tokyo, Japan). Untreated cells served as the
control.
Western blot analysis
HeLa cells were cultured and treated as mentioned
above. Following the indicated treatments, cells were washed twice
with phosphate-buffered saline (PBS) and lysed in RIPA buffer (0.5
M Tris-HCl pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40 and
10 mM EDTA). Proteins were separated by electrophoresis on 12% SDS
polyacrylamide gel, transferred onto a polyvinylidene fluoride
membrane, immunostained with the appropriate antibody and
visualized. The primary antibodies and dilutions used were as
follows: Rabbit monoclonal anti-caspase-8 (1:1,000), rabbit
monoclonal anti-caspase-3 (1:200), rabbit monoclonal anti-PARP
(1:200), rabbit monoclonal anti-IκBα (1:200), mouse monoclonal
anti-p65 (1:200), mouse monoclonal anti-p50 (1:200), rabbit
monoclonal anti-GAPDH (1:1,000) and rabbit monoclonal anti-β-actin
(1:1,000). The secondary antibodies used were IR Dye 700-conjugated
anti-rabbit (1:10,000) and IR Dye 800-conjugated anti-mouse
(1:5,000) immunoglobulin G antibodies (Lorne Laboratories, Ltd.,
Lower Early, UK).
Flow cytometric analysis
Apoptosis was determined using an annexin
V-fluorescein isothiocyanate apoptosis detection kit (BD
Pharmingen, San Diego, CA, USA) according to the manufacturer’s
instructions. Briefly, HeLa cells were treated with SAHA,
ZD55-TRAIL or SAHA in combination with ZD55-TRAIL for 24 h, and
then harvested by centrifugation at 300 × g for 5 min. Following
this, the cells were washed twice with 1X annexin V binding buffer,
resuspended in binding buffer and co-stained with annexin V and
propidium iodide (PI). The prepared cells were analyzed using a
FACScan flow cytometer and CELLQuest software (Becton Dickinson,
Franklin Lakes, NJ, USA).
To analyze the DNA distribution, cells were
harvested by trypsinization followed by washing twice with cold PBS
and then fixed in 70% ethanol at −20°C for at least 12 h.
Subsequently, cells were stained with phosphatidylinositol in the
dark at room temperature for 30 min and subsequently analyzed using
a FACScan flow cytometer (BD FACSAria I; Becton Dickinson). The
cell cycle profile was analyzed using FlowJo software (version
7.6.5; Treestar, Inc., Ashland, OR, USA).
Animal experiments
Female BALB/c nude mice (4-weeks old) were purchased
from the Shanghai Experimental Animal Center (Shanghai, China). The
study was approved by the Ethics Committee of the Women’s Hospital,
College of Medicine, Zhejiang University (Hanzhou, China). The
National Institutes of Health (Bethesda, MD, USA) guidelines on the
ethical use of animals were strictly followed in all the
experiments. For establishment of xenograft tumors, HeLa-luc cells
were subcutaneously injected into the right flank of each mouse at
a density of 5×106 cells in 100 μl DMEM. Once
tumors had reached 100–150 mm3 in size, mice were
divided randomly into three groups (n=6). The ZD55-TRAIL group was
treated with 1×109 plaque-forming units of ZD55-TRAIL
daily for 5 days by intratumoral injection and the combination
group was treated with the virus plus SAHA (25 mg/kg,
intraperitoneal injection). The control group received PBS. The
tumor burdens were monitored using the Xenogen IVIS Kinetic imaging
system (Caliper Life Sciences, Hopkinton, MA, USA).
Immunohistochemistry
At day 12, additional mice were humanely sacrificed
and tumors were excised. Following fixing in 4% paraformaldehyde
and embedding in paraffin, the tumors were cut into 4
μm-thick sections. Immunohistochemical staining with
anti-TRAIL primary antibody at 1:500 dilutions was conducted. For
expression detection, an avidin-biotin-peroxidase complex reagent
(Vector Laboratories, Burlingame, CA, USA) was used. Slides were
immersed in 3,3′-diaminobenzidine tetrahydrochloride solution
containing 0.006% hydrogen peroxide. Sections were counterstained
with hematoxylin and eosin (H&E).
Apoptosis was measured by TdT-mediated dUTP-biotin
nick end labeling (TUNEL) assay using an in situ Cell
Apoptosis Detection kit (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard
deviation and analyzed using SPSS 11.0 software (SPSS, Inc.,
Chicago, IL, USA). Data analysis was performed using the Student’s
t-test and P<0.05 was considered to indicate a statistically
significant difference.
Results
SAHA enhances ZD55-TRAIL-mediated growth
inhibition in HeLa cells
To assess whether the HDAC inhibitor SAHA enhances
ZD55-TRAIL-induced cell death in HeLa cells, an MTT assay was
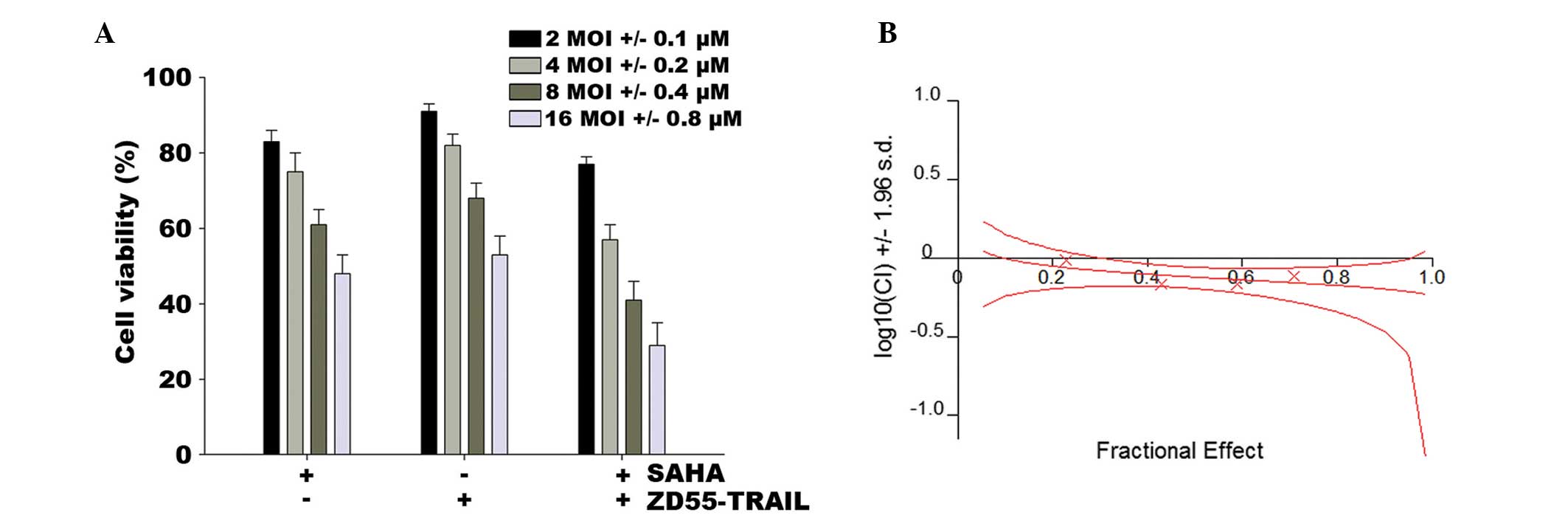
performed as mentioned in Materials and methods. Fig. 1A shows that the synergistic
combination of ZD55-TRAIL and SAHA induced cell death. HeLa cells
infected with ZD55-TRAIL at a MOI of 16 retained 53% cell
viability, whereas SAHA (0.8 μM) eliminated 51% of the
cells. However, when ZD55-TRAIL (16 MOI) and SAHA (0.8 μM)
were used together, 71% of the cells were killed. Combination index
(CI) analysis revealed that the CI for different concentrations of
the agents was 0.685–0.975, demonstrating that co-treatment of SAHA
and ZD55-TRAIL has a synergistic suppressive effect on HeLa cell
survival (Fig. 1B).
SAHA enhances ZD55-TRAIL-induced
apoptosis in HeLa cells
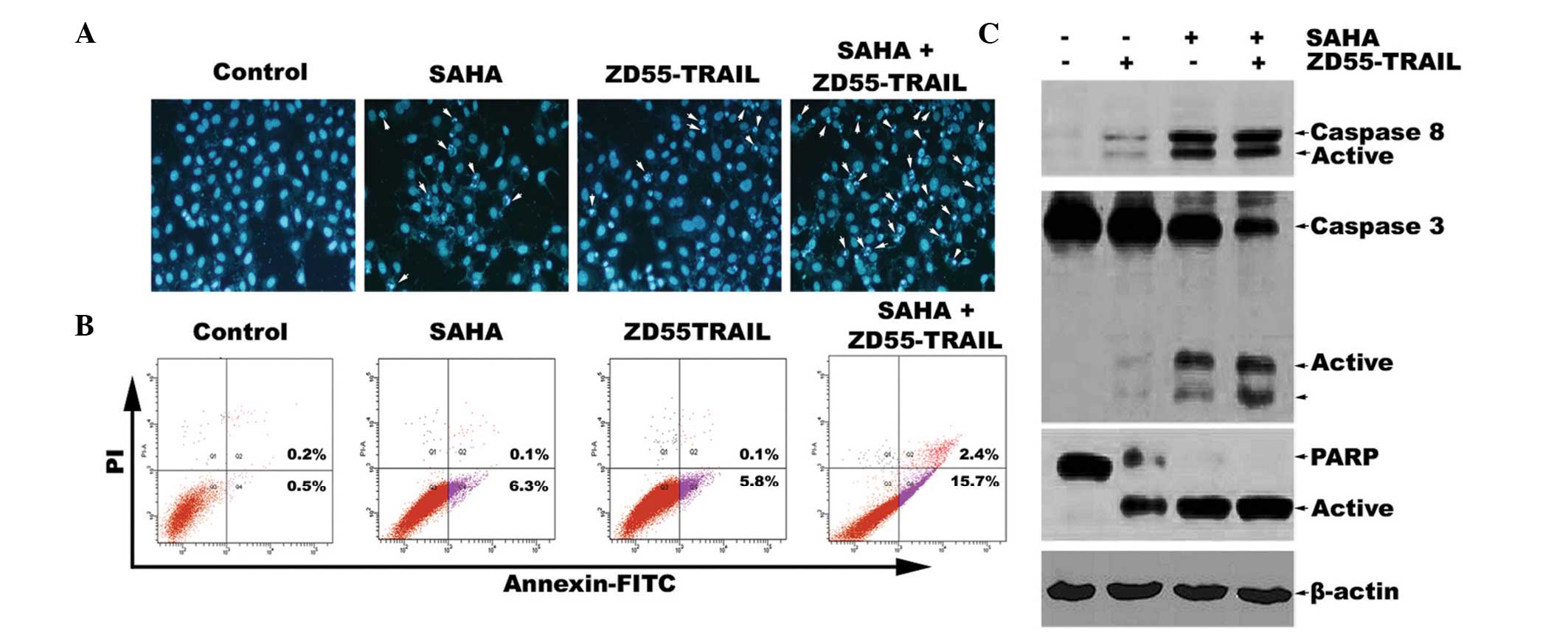
Subsequently, Hoechst 33342 staining was designed to
investigate the morphological alterations of HeLa cells treated
with a combination of SAHA and ZD55-TRAIL or SAHA and ZD55-TRAIL
alone. The results demonstrated that compared with treatment of
ZD55-TRAIL alone, co-treatment with SAHA led to marked apoptosis
characterized by chromatin condensation, nuclear fragmentation and
apoptotic bodies (Fig. 2A). To
quantify the effects of SAHA on TRAIL-induced apoptosis in HeLa
cells, Annexin-V-fluorescein isothiocyanate (FITC)/PI double
staining was used to analyze cell apoptosis (Fig. 2B). The results demonstrated that
the apoptotic rate of HeLa cells co-treated with ZD55-TRAIL and
SAHA was 15.7%, which is nearly three times more than that of
ZD55-TRAIL treatment alone (5.8%). Furthermore, western blot
analysis demonstrated that ZD55-TRAIL was able to induce activation
of caspase-8, caspase-3 and cleavage of PARP. The activation of
this caspase pathway was further increased by co-treatment of SAHA
and ZD55-TRAIL (Fig. 2C). Since
caspase-8, caspase-3 and PARP are the main apoptotic proteins
involved in the extrinsic apoptotic signaling pathway (17), our results suggested that SAHA
promotes ZD55-TRAIL-induced activation of the extrinsic apoptotic
signaling pathway, which may synergistically enhance
ZD55-TRAIL-induced HeLa cell apoptosis.
Effects of SAHA and ZD55-TRAIL treatment
on the cell cycle
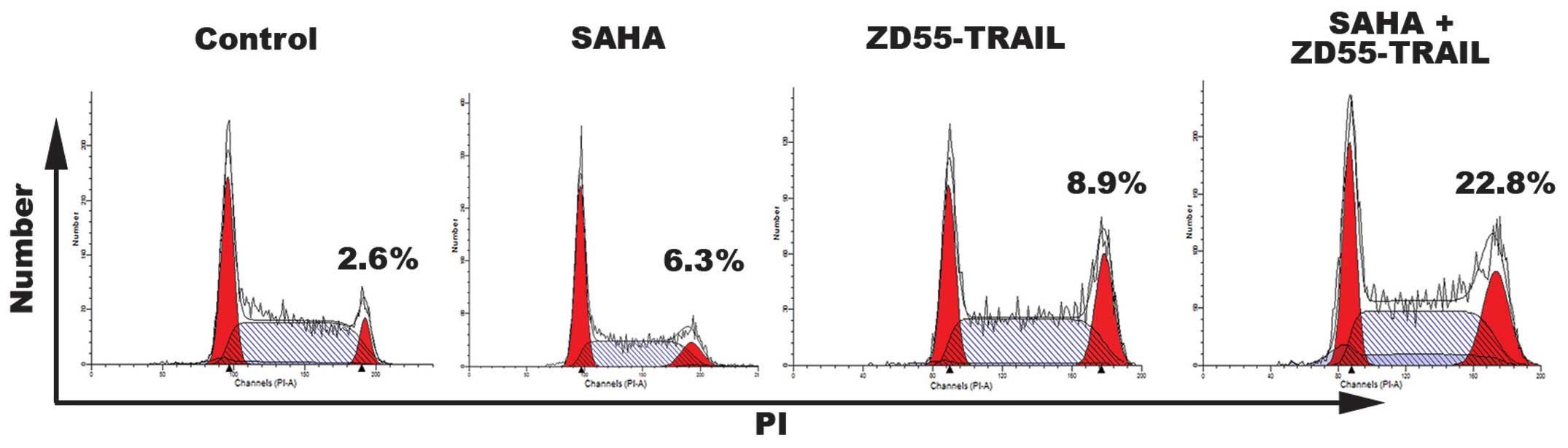
To determine whether the anti-proliferative effects
of ZD55-TRAIL and SAHA may also result from cell cycle arrest, cell
cycle analyses were performed on HeLa cells treated for 24 h. As
shown in Fig. 3, when used
individually, treatment with SAHA had only a partial effect on the
cell cycle. ZD55-TRAIL arrested a greater number of cells in the G2
phase (8.9%). However, treatment with a combination of ZD55-TRAIL
and SAHA caused 22.8% cells to enter the G2 cell cycle phase.
SAHA inhibits ZD55-TRAIL-induced
upregulation of IκBα, p50 and p65
To elucidate the mechanism of the enhanced
cytotoxicity of combined ZD55-TRAIL and SAHA, the protein
expression of IκBα, p65 and p50 was then determined by western
blotting. As shown in Fig. 4,
ZD55-TRAIL alone induced a partial increase in the levels of IκBα,
p65 and p50 protein, consistent with the hypothesis that activation
of the NF-κB pathway by ZD55-TRAIL leading to induced drug
resistance is responsible for the insensitivity of cancer cells to
this anticancer therapy agent (18–21).
By contrast, SAHA inhibited expression of IκBα, p65 and p50.
Notably, ZD55-TRAIL-induced upregulation of IκBα, p65 and p50 was
significantly inhibited by SAHA.
SAHA enhances ZD55-TRAIL-mediated
cervical tumor growth suppression in vivo
To determine whether SAHA plus ZD55-TRAIL treatment
enhances tumor growth suppression, in vivo experiments were
performed using a cervical tumor xenograft model established by
HeLa-luc cells. Compared with mice treated with PBS or ZD55-TRAIL,
mice treated with SAHA plus ZD55-TRAIL demonstrated significant
growth suppression (P=0.026 vs. ZD55-TRAIL; Fig. 5A). The average luciferase activity
in the mice receiving combination therapy was ~4.6×107
photons/sec (p/s) at the end of the experiment. Whereas, the
average luciferase activity of mice injected with ZD55-TRAIL or PBS
was 3×108 p/s and 1.5×109 p/s, respectively
(Fig. 5B). Furthermore, analysis
of tumors by immunohistochemical staining using anti-TRAIL antibody
revealed that there was a strong expression of TRAIL in xenografts
treated with ZD55-TRAIL and that the highest expression of TRAIL
was observed in tumor sections that received combined injections of
SAHA with ZD55-TRAIL (Fig. 5C). To
investigate the possible mechanisms underlying tumor growth
inhibition induced by ZD55-TRAIL combined with SAHA, a TUNEL assay
was used to verify whether the action of the combination therapy
was due to their proapoptotic effect. As shown in Fig. 5C, ZD55-TRAIL plus SAHA caused
profound cell death in the tumor mass via apoptosis. These results
demonstrated that the treatment of cervical tumors with ZD55-TRAIL
plus SAHA enhances growth suppression in parallel to enhanced
apoptosis, a finding consistent with our in vitro
results.
Discussion
Monotherapy of cancer often has limited success, as
the tumor cells usually develop resistance to the agents used and
tumors are usually genetically diverse (7,22).
Therefore, it is important to identify combinations of two or more
therapeutic agents, acting through different mechanisms with
synergistic effects without increasing adverse effects. Preclinical
studies revealed that the efficacy of oncolytic viruses can be
improved by combination with chemotherapeutic agents (23–25).
ONYX-015, a E1B-55 kDa gene-deleted OA, in combination with
chemotherapy has been assessed in phase I–II trials for patients
with advanced cancer (26,27). The present study investigated the
possibility of combining ZD55-TRAIL with SAHA, an HDAC inhibitor
that may be a novel therapeutic agent for patients with cervical
squamous cell carcinoma (14–16,28,29),
in order to reinforce their antitumor activities. The present study
demonstrated that subclinically achievable doses of SAHA (30), namely, 0.1–0.8 μM,
significantly and synergistically increased ZD55-TRAIL oncotoxicity
in vitro. SAHA also has been demonstrated to reinforce the
cytotoxicity of the virus in vivo.
TRAIL is a promising anticancer agent due to its
selective induction of apoptosis in various types of cancer cells,
without toxic effects on normal cells (17,31).
ZD55-TRAIL has been demonstrated to exert strong cytopathic effects
on various cancer cell lines via expression of the TRAIL protein,
thereby inducing enhanced apoptosis (8–10).
However, TRAIL resistance has been observed in ~50% of assessed
cancer cells, including cervical cancer cells (32). Susceptibility to TRAIL-induced
apoptosis could be regulated at multiple levels in the apoptotic
signaling cascade. Previous studies have suggested that activation
of the NF-κB pathway contributes to resistance of cancer cells to
TRAIL-induced apoptosis (33,34).
It was reported that SAHA enhanced the cytotoxic and apoptotic
effects of TRAIL and upregulated the expression of TRAIL death
receptors (35–37). In the present study, the expression
of IκBα and the p50 and p65 subunits of NF-κB was upregulated in
HeLa cells infected with ZD55-TRAIL. By contrast, HeLa cells
treated with SAHA demonstrated a significant inhibition of IκBα,
p50 and p65 expression. Notably, addition of SAHA abrogated
upregulation of IκBα, p50 and p65, which may contribute to enhanced
cell death induced by combination therapy. These results are
consistent with previous observations that artesunate, an effective
and safe anti-malarial drug, effectively enhances TRAIL-mediated
cytotoxicity in human cervical carcinoma cells by suppressing
pro-survival proteins and the transcriptional activity of NF-κB
(32).
Apoptosis of cancer cells in response to TRAIL is
initiated by binding of the ligand to death receptors (DR4 and
DR5), followed by recruitment of caspase-8 via Fas-associated death
domain protein and activation of caspase-3 leading to PARP cleavage
(17). By contrast, it has been
demonstrated that extrinsic and intrinsic apoptotic pathways were
activated by treatment with SAHA, as evidenced by processing of
initiator caspase-8 and caspase-9 (38,39).
In our experiments activation of caspase-8 was observed in HeLa
cells treated with SAHA. In addition, there were increased levels
of caspase-8, caspase-3 and PARP cleavage in HeLa cells treated
with ZD55-TRAIL/SAHA combination compared with that of cells
treated with ZD55-TRAIL or SAHA alone. This suggested that the
enhanced activation of the extrinsic apoptotic pathway also
accounts for the observed enhanced therapeutic effects of combined
ZD55-TRAIL and SAHA.
In clinical settings, the therapeutic approach to
advanced cervical cancer is chemotherapy, however, severe adverse
effects and resistance are major problems. OAs provide a new
platform to treat cervical cancer as biotherapeutic agents that
lack cross-resistance with chemotherapy (40–42).
The present study demonstrated for the first time, to the best of
our knowledge, that SAHA sensitizes human cervical cancer cells to
ZD55-TRAIL-induced cell death in vitro and in vivo.
This synergistic effect is associated with inhibition of the NF-κB
signaling pathway and enhanced activation of the extrinsic
apoptosis pathway. These results suggest that ZD55-TRAIL, used in
combination with the HDAC inhibitor SAHA, represents a promising
novel targeted approach for treating cervical cancer.
Acknowledgments
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. Y2090455), the
Foundation of Zhejiang Municipal Commission of Population and
Family Planning (grant no. 2009-74) and the Foundation of Health
and Family Planning Commission of Zhejiang Province (grant no.
2012RCB013).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mountzios G, Soultati A, Pectasides D,
Pectasides E, Dimopoulos MA and Papadimitriou CA: Developments in
the systemic treatment of metastatic cervical cancer. Cancer Treat
Rev. 39:430–443. 2013. View Article : Google Scholar
|
|
4
|
Hermiston TW and Kuhn I: Armed therapeutic
viruses: strategies and challenges to arming oncolytic viruses with
therapeutic genes. Cancer Gene Ther. 9:1022–1035. 2002. View Article : Google Scholar
|
|
5
|
Zhang ZL, Zou WG, Luo CX, et al: An armed
oncolytic adenovirus system, ZD55-gene, demonstrating potent
antitumoral efficacy. Cell Res. 13:481–489. 2003. View Article : Google Scholar
|
|
6
|
Dong F, Wang L, Davis JJ, et al:
Eliminating established tumor in nu/nu nude mice by a tumor
necrosis factor-α-related apoptosis inducing ligand-armed oncolytic
adenovirus. Clin Cancer Res. 12:5224–5230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Bonifati S, Hristov G, et al:
Synergistic combination of valproic acid and oncolytic parvovirus
H-1PV as a potential therapy against cervical and pancreatic
carcinomas. EMBO Mol Med. 5:1537–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pei Z, Chu L, Zou W, et al: An oncolytic
adenoviral vector of Smac increases antitumor activity of TRAIL
against HCC in human cells and in mice. Hepatology. 39:1371–1381.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Gu J, Zhao L, et al: Complete
elimination of colorectal tumor xenograft by combined manganese
superoxide dismutase with tumor necrosis factor-related
apoptosis-inducing ligand gene virotherapy. Cancer Res.
66:4291–4298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SB, Tan Y, Lei W, et al: Complete
eradication of xenograft hepatoma by oncolytic adenovirus ZD55
harboring TRAIL-IETD-Smac gene with broad antitumor effect. Hum
Gene Ther. 23:992–1002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan J, Cang S, Ma Y, Petrillo RL and Liu
D: Novel histone deacetylase inhibitors in clinical trials as
anti-cancer agents. J Hematol Oncol. 3:52010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bots M and Johnstone RW: Rational
combinations using HDAC inhibitors. Clin Cancer Res. 15:3970–3977.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadnagy A, Beaulieu R and Balicki D:
Histone tail modifications and noncanonical functions of histones:
perspectives in cancer epigenetics. Mol Cancer Ther. 7:740–748.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han BR, You BR and Park WH: Valproic acid
inhibits the growth of HeLa cervical cancer cells via
caspase-dependent apoptosis. Oncol Rep. 30:2999–3005.
2013.PubMed/NCBI
|
|
15
|
Takai N, Kira N, Ishii T, Nishida M, Nasu
K and Narahara H: Novel chemotherapy using histone deacetylase
inhibitors in cervical cancer. Asian Pac J Cancer Prev. 12:575–580.
2011.PubMed/NCBI
|
|
16
|
Feng D, Wu J, Tian Y, et al: Targeting of
histone deacetylases to reactivate tumour suppressor genes and its
therapeutic potential in a human cervical cancer xenograft model.
PLoS One. 8:e806572013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhari BR, Murphy RF and Agrawal DK:
Following the TRAIL to apoptosis. Immunol Res. 35:249–262. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thaci B, Ulasov IV, Wainwright DA and
Lesniak MS: The challenge for gene therapy: innate immune response
to adenoviruses. Oncotarget. 2:113–121. 2011.PubMed/NCBI
|
|
19
|
Tamanini A, Nicolis E, Bonizzato A,
Bezzerri V, Melotti P, Assael BM and Cabrini G: Interaction of
adenovirus type 5 fiber with the coxsackievirus and adenovirus
receptor activates inflammatory response in human respiratory
cells. J Virol. 80:11241–11254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verdino P, Witherden DA, Havran WL and
Wilson IA: The molecular interaction of CAR and JAML recruits the
central cell signal transducer PI3K. Science. 329:1210–1214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong Y, Zhu W, Huang X, You L, Han X, Yang
C and Qian W: PI3K inhibitor LY294002 inhibits activation of the
Akt/mTOR pathway induced by an oncolytic adenovirus expressing
TRAIL and sensitizes multiple myeloma cells to the oncolytic virus.
Oncol Rep. 31:1581–1588. 2014.PubMed/NCBI
|
|
22
|
Kaiser J: Combining targeted drugs to stop
resistant tumors. Science. 331:1542–1545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alonso MM, Gomez-Manzano C, Jiang H, et
al: Combination of the oncolytic adenovirus ICOVIR-5 with
chemotherapy provides enhanced anti-glioma effect in vivo. Cancer
Gene Ther. 14:756–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Young BA, Spencer JF, Ying B, Tollefson
AE, Toth K and Wold WS: The role of cyclophosphamide in enhancing
antitumor efficacy of an adenovirus oncolytic vector in
subcutaneous Syrian hamster tumors. Cancer Gene Ther. 20:521–530.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng HT, Li L, Liu H, Wang Y, Li GC and
Qian WB: Homoharringtonine acts synergistically with SG235-TRAIL, a
conditionally replicating adenovirus, in human leukemia cell lines.
Acta Pharmacol Sin. 30:1529–1536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galanis E, Okuno SH, Nascimento AG, et al:
Phase I-II trial of ONYX-015 in combination with MAP chemotherapy
in patients with advanced sarcomas. Gene Ther. 12:437–445. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Opyrchal M, Aderca I and Galanis E: Phase
I clinical trial of locoregional administration of the oncolytic
adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin
and cisplatin chemotherapy in patients with advanced sarcomas.
Methods Mol Biol. 542:705–717. 2009.
|
|
28
|
Jiang Y, Wang Y, Su Z, Yang L, Guo W, Liu
W and Zuo J: Synergistic induction of apoptosis in HeLa cells by
the proteasome inhibitor bortezomib and histone deacetylase
inhibitor SAHA. Mol Med Rep. 3:613–619. 2010.
|
|
29
|
Tambunan US, Bakri R, Prasetia T,
Parikesit AA and Kerami D: Molecular dynamics simulation of complex
Histones Deacetylase (HDAC) Class II Homo Sapiens with
suberoylanilide hydroxamic acid (SAHA) and its derivatives as
inhibitors of cervical cancer. Bioinformation. 9:696–700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kelly WK, O’Connor OA, Krug LM, et al:
Phase I study of an oral histone deacetylase inhibitor,
suberoylanilide hydroxamic acid, in patients with advanced cancer.
J Clin Oncol. 23:3923–3931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thanaketpaisarn O, Waiwut P, Sakurai H and
Saiki I: Artesunate enhances TRAIL-induced apoptosis in human
cervical carcinoma cells through inhibition of the NF-κB and
PI3K/Akt signaling pathways. Int J Oncol. 39:279–285.
2011.PubMed/NCBI
|
|
33
|
Williams AC, Smartt H, H-Zadeh AM,
Macfarlane M, Paraskeva C and Collard TJ: Insulin-like growth
factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced
apoptosis of human colorectal carcinoma cells through inhibition of
NF-kappaB. Cell Death Differ. 14:137–145. 2007. View Article : Google Scholar
|
|
34
|
Ricci MS, Kim SH, Ogi K, et al: Reduction
of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes
resistant human cancer cells to TRAIL-induced death. Cancer Cell.
12:66–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fandy TE, Shankar S, Ross DD, Sausville E
and Srivastava RK: Interactive effects of HDAC inhibitors and TRAIL
on apoptosis are associated with changes in mitochondrial functions
and expressions of cell cycle regulatory genes in multiple myeloma.
Neoplasia. 7:646–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lauricella M, Ciraolo A, Carlisi D, Vento
R and Tesoriere G: SAHA/TRAIL combination induces detachment and
anoikis of MDA-MB231 and MCF-7 breast cancer cells. Biochimie.
94:287–299. 2012. View Article : Google Scholar
|
|
37
|
Cao H, Cheng Y, You L, Qian J and Qian W:
Homoharringtonine and SAHA synergistically enhance apoptosis in
human acute myeloid leukemia cells through upregulation of TRAIL
and death receptors. Mol Med Rep. 7:1838–1844. 2013.PubMed/NCBI
|
|
38
|
Al-Yacoub N, Fecker LF, Möbs M, Plötz M,
Braun FK, Sterry W and Eberle J: Apoptosis induction by SAHA in
cutaneous T-cell lymphoma cells is related to downregulation of
c-FLIP and enhanced TRAIL signaling. J Invest Dermatol.
132:2263–2274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen S, Zhao Y, Gou WF, Zhao S, Takano Y
and Zheng HC: The anti-tumor effects and molecular mechanisms of
suberoylanilide hydroxamic acid (SAHA) on the aggressive phenotypes
of ovarian carcinoma cells. PLoS One. 8:e797812013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanerva A, Lavilla-Alonso S, Raki M, et
al: Systemic therapy for cervical cancer with potentially
regulatable oncolytic adenoviruses. PLoS One. 3:e29172008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang W, Sima N, Kong D, et al: Selective
targeting of HPV-16 E6/E7 in cervical cancer cells with a potent
oncolytic adenovirus and its enhanced effect with radiotherapy in
vitro and vivo. Cancer Lett. 291:67–75. 2010. View Article : Google Scholar
|
|
42
|
Wang H, Song X, Zhang H, et al:
Potentiation of tumor radiotherapy by a radiation-inducible
oncolytic and oncoapoptotic adenovirus in cervical cancer
xenografts. Int J Cancer. 130:443–453. 2012. View Article : Google Scholar
|