Introduction
Primary breast cancer (PBC) is one of the most
common malignancies amongst females, accounting for 23% of total
cancer diagnoses and 14% of all cancer-associated mortalities in
females worldwide (1). Significant
progress has been made in the treatment of primary tumors, and
multiple randomized trials have demonstrated the efficacy of
adjuvant chemotherapy and hormonal treatment in prolonging the
survival of patients with breast cancer (2–4).
Current treatment strategies include wide local excision and
radiotherapy or mastectomy, depending on the size of the tumor. In
addition, the majority of patients receive postoperative
radiotherapy (5). As for adjuvant
systemic therapy, endocrine-responsive tumors are treated with
tamoxifen or aromatase inhibitors with adjuvant chemotherapy, while
tumors that are endocrine non-responsive are treated with
chemotherapy (6). However, despite
significant progression in improving early detection and treatment
strategies, 30–50% of patients are at high risk of metastasis and
10–15% of patients develop distant metastases within 10 years of
initial diagnosis (7). The most
significant predictors of PBC disease recurrence and outcome,
include tumor size, histological grade, lymph node involvement,
expression of estrogen and/or progesterone receptors, human
epidermal growth factor receptor 2 expression and the presence of
circulating tumor cells (8,9).
Metastasis involves local tissue invasion by tumor
cells via cytoskeletal reorganization, migration of cells through
the tissue into the vascular or lymphatic system via lamellipodia
and establishment of secondary tumors at distant sites via the
activity of adhesion proteins (10). The management of metastasis
currently remains a major challenge for patients with PBC and there
has been a recent focus on targeting signaling pathways between the
primary tumor and disseminated metastases (11).
Rac, a member of the Rho family of GTPases, has been
shown to mediate multiple signaling pathways involved in
organization of the actin cytoskeleton, as well as invasion and
migration of tumor cells via p21-activated kinases (PAKs) (12–14).
PAKs have been reported to phosphorylate and activate LIM kinase,
which subsequently activates cofilin in order to regulate the
turnover of actin filaments (15).
Nischarin, a novel tumor suppressor, was initially
identified as an ~190 kDa cytosolic protein, which mapped to 3p21
(16,17). Nischarin was found to bind to the
α5 subunit of integrins, and inhibited Rac-mediated cell motility
and invasion in breast and colon epithelial cells (18–21).
Notably, IRAS, the human homolog of Nischarin, was described as an
imidazoline receptor (22) with
anti-apoptotic activity (23,24).
Nischarin mRNA expression levels have been reported to be
significantly higher in the brain and kidney compared with those in
the heart, liver, lung and skeletal muscle (19). Additionally, Nischarin expression
was recently revealed to be widely distributed in rat brain tissue,
particularly in the cerebral cortex and hippocampus, and is
hypothesized to exhibit a significant role in neuronal migration
(25).
The expression levels of Nischarin were previously
demonstrated to be significantly higher in normal breast tissue
compared with breast cancer tissue, and the loss of Nischarin
expression in breast cancer tissue is hypothesized to be due to a
loss of heterozygosity (26).
However, to the best of our knowledge, the role of Nischarin in
breast cancer metastasis has previously only been studied in
vitro (27) and the mechanisms
underlying Nischarin-mediated inhibition of metastasis remain to be
elucidated. In the present study, the expression of Nischarin
protein in PBC and adjacent normal tissues was evaluated. The
correlation between Nischarin expression levels and breast cancer
metastasis was also examined, in order to aid the elucidation of
the role of Nischarin in the occurrence, development and metastasis
of PBC.
Materials and methods
Reagents
The NISCH ELISA kit was purchased from USCN Life
Sciences (Wuhan, China). CHAPS, Tris buffer and urea were obtained
from Bio-Rad Laboratories, Inc. (Hercules, CA, USA) and the
bicinchoninic acid (BCA) protein quantification kit was purchased
from Shanghai Sangong Biotech Co., Ltd. (Shanghai, China).
Sample collection
A total of 60 primary cancer tissues and the
corresponding adjacent normal tissues were collected from patients
with breast cancer during modified radical mastectomy at the
Department of Breast Surgery of the Zhejiang Cancer Hospital
(Hangzhou, China) between February 2008 and February 2010. Tissues
were stored at −70°C prior to use. Seven tissue samples were
classified as grade I ductal carcinoma, 33 tissue samples were
grade II and 20 tissue samples were grade III. Pathological
examination indicated the presence of lymph node metastasis in 30
of the tissue samples, while 30 tissue samples were negative for
lymph node metastasis. Of the 30 samples with lymph node
metastasis, 21 tissue samples had <3 lymph node metastases,
while nine tissue samples had >3 lymph node metastases. All 60
tissue samples were identified as invasive ductal carcinoma in
post-operative pathological examinations. Cancer stages were graded
according to the AJCC Cancer Staging Manual (28,29)
and histological grade was determined according to the Nottingham
Combined Histological Grade (30).
None of the patients had received chemotherapy or
physical therapy prior to surgery. The study was reviewed and
approved by the Ethics Committee of Zhejiang Cancer Hospital
(Huangzhou, China). Written informed consent was obtained from all
patients involved in the study.
Protein extraction
Tissue samples were washed three times in normal
saline and residual water was removed with a filter. Tissue samples
were resuspended in 200 μl lysis buffer (4% CHAPS, 30 mM
Tris buffer and 8 M urea; pH 8.5) and sonicated (JY92-II DN; Ningbo
Scientz Biotechnology Co., Ltd, Zhejiang, China) on ice at 200 W
for a total of 150 sec, with an interval of 10 sec between 10 sec
bursts. The sonicated samples were centrifuged at 12,000 × g for 30
min at 4°C and the protein concentration of the supernatant was
determined.
ELISA
Nischarin expression levels were determined using a
NISCH ELISA kit (containing detection solution B, substrate
solution and stop solution; USCN Life Science, Inc., Wuhan, China)
according to the manufacturer’s instructions. Briefly, 100
μl standards (5, 2.5, 1.25, 0.625, 0.312 and 0.156 ng/ml) or
samples were incubated at 37°C for 2 h. The wells were washed three
times with 350 μl washing solution and were subsequently
incubated with 100 μl of freshly prepared detection solution
B for 30 min at 37°C. The wells were washed five times and then
incubated for 15–25 min in the dark with 90 μl substrate
solution at 37°C. The reaction was stopped by the addition of 50
μl stop solution and the absorbance (OD) was measured at 450
nm using a SpectraMAX M3 microplate reader (Molecular Devices,
Sunnyvale, CA, USA). Standard curves were constructed and the
following regression equation was calculated in order to determine
the concentration of the samples: Concentration=5(OD)-0.03,
R2=1.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation. Differences in mean age and Nischarin
concentrations between patients with and without lymph node
metastasis were analyzed by independent two-sample t-tests.
Differences in mean concentrations of Nischarin between cancer
tissues and adjacent noncancerous tissues were analyzed by paired
t-tests. Differences in the mean concentrations of Nischarin in
cancer tissues from various grades were analyzed by nonparametric
Kruskal Wallis test, due to the low number of grade I and III
cases. All statistical assessments were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed with SPSS 18.0 statistics
software (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
The average age of the 60 patients was 51.1±9.9
years. There was no significant difference in age between patients
with and without lymph node metastasis (50.9±9.6 vs. 51.3±10.4
years; P=0.898). All clinical characteristics of the patients
evaluated, including cancer stage and histological grade, are
summarized in Table I.
 | Table IClinical characteristics of patients
with primary breast cancer. |
Table I
Clinical characteristics of patients
with primary breast cancer.
| Characteristic | LN metastasis
(n=30) | No LN metastasis
(n=30) | Total (n=60) |
|---|
| Age (years)a | 50.9±9.6 | 51.3±10.4 | 51.1±9.9 |
| Gender,
femaleb | 30 (100) | 30 (100) | 60 (100) |
| Cancer
stageb |
| Ia | 0 (0) | 3 (10.0) | 3 (5.0) |
| Ib | 0 (0) | 0 (0.0) | 0 (0) |
| IIa | 1 (3.3) | 26 (86.7) | 27 (45.0) |
| IIb | 18 (60.0) | 1 (3.3) | 19 (31.7) |
| IIIa | 6 (20.0) | 0 (0) | 6 (10.0) |
| IIIb | 1 (3.3) | 0 (0) | 1 (1.7) |
| IIIc | 4 (13.3) | 0 (0) | 4 (6.7) |
| Histological
gradeb |
| I | 2 (6.7) | 5 (16.7) | 7 (11.7) |
| II | 21 (70.0) | 12 (40.0) | 33 (55.0) |
| III | 7 (23.3) | 13 (43.3) | 20 (33.3) |
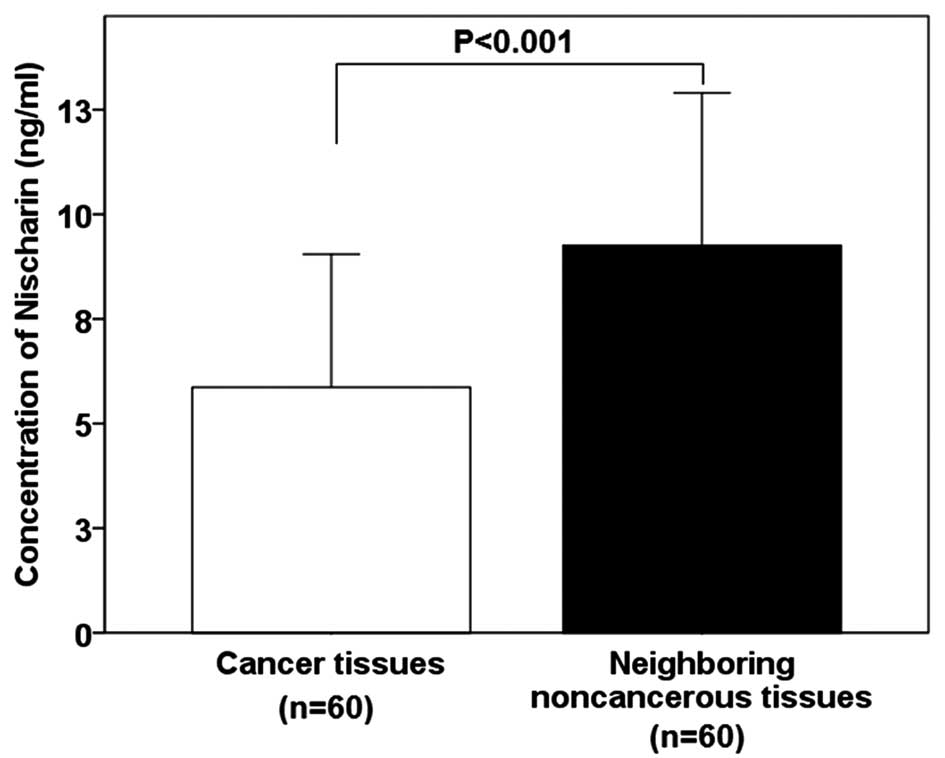
Nischarin concentration is lower in
breast cancer tissues
The mean protein concentration of Nischarin was
demonstrated to be significantly lower in breast cancer tissues
compared with that of the adjacent non-cancerous tissues (5.86±3.19
vs. 9.25±3.65 ng/ml; P<0.001; Fig.
1).
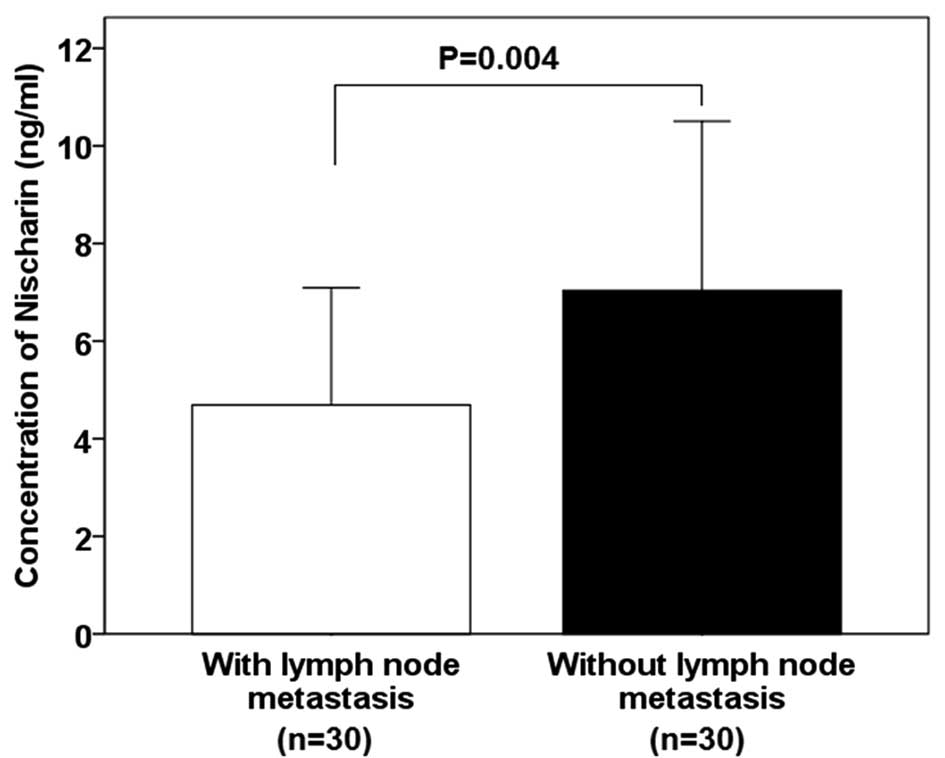
Nischarin concentration is lower in
patients with lymph node metastasis
The mean concentration of Nischarin protein was
found to be significantly lower in tissues from patients with lymph
node metastasis compared with those of patients without lymph node
metastasis (4.69±2.40 vs. 7.04±3.47 ng/ml; P=0.004; Fig. 2).
Nischarin concentration does not differ
between PBC grades
The expression of Nischarin protein in cancer
tissues from various grades of invasive ductal carcinoma were
evaluated, and no significant differences were detected between
each grade (grade I, 5.44±3.57; grade II, 6.42±3.85; grade III,
5.10±1.18 ng/ml; P=0.765; Fig.
3).
Discussion
In the present study, the expression levels of
Nischarin in breast cancer tissues were compared with those in
adjacent noncancerous tissues. Nischarin expression was also
compared between patients with and without lymph node metastasis,
and in patients with varying grades of breast cancer. The results
indicated that: i) Nischarin expression was significantly lower in
breast cancer tissues compared with that of normal tissues; ii)
Nischarin expression levels were significantly lower in patients
with lymph node metastasis compared with those of patients without
lymph node metastasis; and iii) there was no significant difference
in Nischarin expression levels between patients with grades I, II
or III breast cancer.
Integrins exhibit a critical role in multiple signal
transduction processes in order to regulate the cell cycle and cell
death (31,32). Upregulation of integrin α5β1
expression was demonstrated to inhibit tumor cell growth (33) and protect cells against mitogen
deprivation-induced apoptosis (34). Nischarin has been suggested to be
involved in the inhibition of tumor cell growth via upregulation of
the expression of the α5 subunit, reducing the phosphorylation of
focal adhesion protein tyrosine kinase and decreasing the Rac GTP
load (26). Low Nischarin
expression levels may therefore lead to increased tumor cell
proliferation and reduced cell apoptosis, resulting in
carcinogenesis.
The interaction of Nischarin with the α5 subunit of
integrins to regulate cell migration suggested that Nischarin may
have a role in mediating the metastasis of malignancies (26,27).
Concurrently, the overexpression of Nischarin was shown to result
in inhibition of cell migration of fibroblasts in vitro,
although this inhibition was not associated with cytotoxicity
(19). In addition, short
interfering RNA-mediated silencing of Nischarin expression was
observed to stimulate fibroblast migration (21). Overexpression of Nischarin in MCF-7
breast cancer cells also resulted in the inhibition of cell
migration, as indicated by a Transwell assay, although Nischarin
overexpression did not significantly influence cell adhesion
(35). Studies observing the
mechanisms underlying Nischarin-mediated inhibition of cell
migration demonstrated significantly higher rates of migration in
Rac-overexpressing cells compared with those of control cells, and
this migration was abrogated by the simultaneous overexpression of
Nischarin. Nischarin was also shown to directly interact with Rac
and PAK1, suggesting that Nischarin inhibited migration by
selectively interfering with the Rac-mediated signaling pathways,
which regulate cell migration via PAK (20,36).
Notably, Nischarin selectively inhibited migration of MCF-7 cells
induced by PAK, but not migration induced by MEK kinase 1, a Rac
effector in the c-Jun N-terminal kinase pathway, or migration
induced by MEK1, which is an effector in the
Ras-Raf-MEK-extracellular-signal-regulated kinase pathway (20). A study also indicated that
Nischarin was able to regulate Rac1 signaling pathways independent
of PAK1 (37).
Further studies aiming to elucidate the mechanisms
underlying Nischarin-mediated regulation of cell migration and
invasion identified a direct association between Nischarin and LIM
kinase (LIMK), which is a downstream effector of PAK and is known
to have a significant role in cell motility, cell invasion and the
G2/M checkpoint of the cell cycle (38–40).
LIMK has been reported to regulate the phosphorylation and
dephosphorylation of cofilin, which is an important determinant of
actin-based cell motility (41,42).
Direct binding of Nischarin with LIMK has been shown to inhibit
LIMK activity, cofilin phosphorylation and LIMK-mediated invasion
of MCF-7 breast cancer cells (43). Nischarin has also recently been
shown to directly associate with tumor suppressor LKB1 in breast
cancer cells. The suppression of Nischarin and LKB1 in these cells
resulted in increased phosphorylation of PAK1 and LIMK1, and
upregulation of Cyclin D1 and CDK4 expression, resulting in
enhanced cell migration and tumor growth (27).
MicroRNAs (miRs) are small noncoding endogenous RNAs
that negatively regulate gene expression at the transcriptional or
translational level by binding to the 3′-untranslated region of
their target mRNAs (44). The
expression of miR23b and miR27b, which are highly expressed in
breast cancer cells, was shown to be inversely correlated with
Nischarin expression levels. Furthermore, Nischarin was shown to
negatively regulate the expression of miR23b/27b via the inhibition
of NFκB phosphorylation (45).
Further investigation into the Nischarin signaling pathways is
required in order to elucidate the mechanisms underlying
Nischarin-mediated inhibition of tumor cell migration and
metastasis in PBC and other types of cancer.
Nischarin has also been demonstrated to be
significantly downregulated in human breast cancer tissues compared
with normal tissues in patients with breast cancer from the USA,
and the overexpression of Nischarin in MDA-MB-231 breast cancer
cells significantly inhibited metastasis, suggesting that Nischarin
may function as a tumor suppressor (27). Nischarin expression was also
associated with more advanced tumor grades and a decrease in
survival (27). In the present
study ELISA analysis revealed significantly lower expression levels
of Nischarin in breast cancer tissues compared with adjacent normal
tissues in patients with PBC. Additionally, Nischarin expression
was found to be significantly lower in patients with lymph node
metastasis compared with that of patients without lymph node
metastasis, suggesting that Nischarin expression levels may be a
reliable indicator for the prediction of the invasiveness and
metastatic potential of breast cancer. In the present study, no
significant correlation was observed between tumor grade and
Nischarin expression levels. The results indicated the
reproducibility, high sensitivity, specificity and ease of use of
the Nischarin ELISA assay, suggesting that it may be efficiently
used in clinical practice.
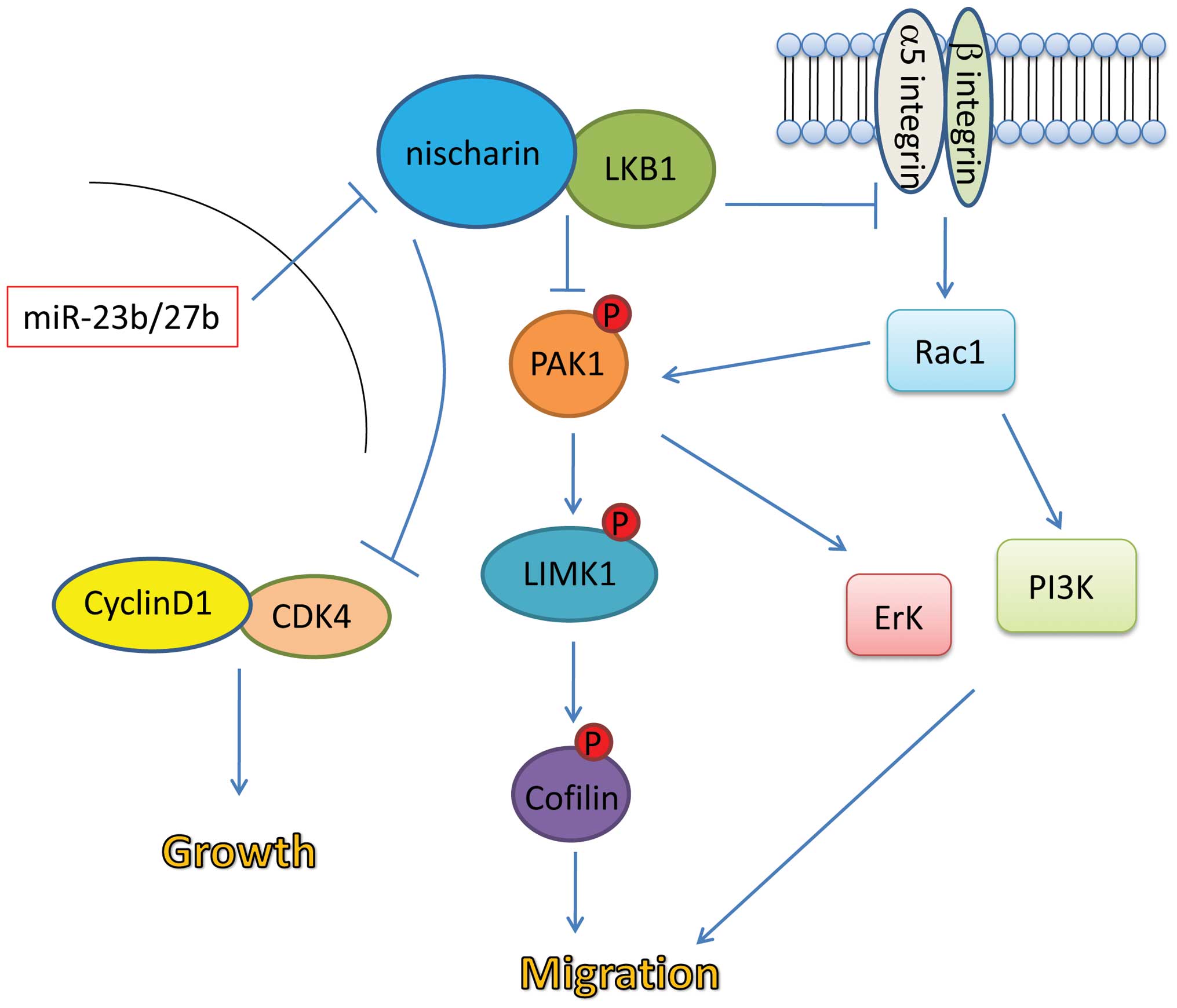
Based on the data available from previous studies as
well as the results of the present study, a potential model of the
role of Nischarin in cell migration and tumor growth was suggested
(Fig. 4). Binding of Nischarin to
LKB1 may inhibit integrin-mediated activation of the Rac1 pathway,
which promotes cell migration. The Nischarin-LKB1 interaction may
also inhibit phosphorylation and activation of the
PAK1-LIMK1-cofilin pathway, which promotes cell migration. Finally,
it is possible that the Nischarin-LKB1 interaction may inhibit cell
cycle progression via inhibition of the Cyclin D1/CDK4 complex.
High expression levels of miR23b/27b in breast cancer cells may
inhibit the interaction between Nischarin and LKB1, abrogating the
tumor suppressor effects of Nischarin.
In conclusion, the results of the present study
revealed that Nischarin expression was significantly lower in
breast cancer tissues compared with adjacent normal tissues in
Chinese patients with PBC. To the best of our knowledge, the
present study was the first to demonstrate that Nischarin
expression levels were significantly lower in patients with lymph
node metastasis compared with patients with no lymph node
metastasis. A major limitation of the present study was that the
mechanisms underlying the role of Nischarin in the inhibition of
metastasis were not investigated. It may also be important to
investigate the role of Nischarin in different types of cancer.
Further studies are required to verify the role of Nischarin as a
prognostic marker for breast cancer metastasis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez EA, Romond EH, Suman VJ, et al:
Trastuzumab plus adjuvant chemotherapy for human epidermal growth
factor receptor 2-positive breast cancer: planned joint analysis of
overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol.
32:3744–3752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albain KS, Barlow WE, Ravdin PM, et al:
Breast Cancer Intergroup of North America: Adjuvant chemotherapy
and timing of tamoxifen in postmenopausal patients with
endocrine-responsive, node-positive breast cancer: a phase 3,
open-label, randomised controlled trial. Lancet. 374:2055–2063
|
|
5
|
Clarke M, Collins R, Darby S, et al: Early
Breast Cancer Trialists’ Collaborative Group: Effects of
radiotherapy and of differences in the extent of surgery for early
breast cancer on local recurrence and 15-year survival: an overview
of the randomised trials. Lancet. 366:2087–2106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aebi S, Davidson T and Gruber G: Primary
breast cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 22(Suppl 6): vi12–vi24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tazhibi M, Fayaz M and Mokarian F:
Detection of prognostic factors in metastatic breast cancer. J Res
Med Sci. 18:283–290. 2013.PubMed/NCBI
|
|
8
|
Cadoo KA, Fornier MN and Morris PG:
Biological subtypes of breast cancer: current concepts and
implications for recurrence patterns. Q J Nucl Med Mol Imaging.
57:312–321. 2013.PubMed/NCBI
|
|
9
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Whale A, Hashim FN, Fram S, Jones GE and
Wells CM: Signalling to cancer cell invasion through PAK family
kinases. Front Biosci (Landmark Ed). 16:849–864. 2011. View Article : Google Scholar
|
|
11
|
Redig AJ and McAllister SS: Breast cancer
as a systemic disease: a view of metastasis. J Intern Med.
274:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kjoller L and Hall A: Signaling to Rho
GTPases. Exp Cell Res. 253:166–179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagrodia S and Cerione RA: Pak to the
future. Trends Cell Biol. 9:350–355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szczepanowska J: Involvement of
Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim
Pol. 56:225–234. 2009.PubMed/NCBI
|
|
15
|
Edwards DC, Sanders LC, Bokoch GM and Gill
GN: Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase
signalling to actin cytoskeletal dynamics. Nat Cell Biol.
1:253–259. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinez A, Walker RA, Shaw JA, Dearing
SJ, Maher ER and Latif F: Chromosome 3p allele loss in early
invasive breast cancer: detailed mapping and association with
clinicopathological features. Mol Pathol. 54:300–306. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Killary AM, Wolf ME, Giambernardi TA and
Naylor SL: Definition of a tumor suppressor locus within human
chromosome 3p21–p22. Proc Natl Acad Sci USA. 89:10877–10881. 1992.
View Article : Google Scholar
|
|
18
|
Ma H, Li W and Wu N: Advances in new
Nischarin protein. Chinese Pharmacological Bulletin. 26:42010.In
Chinese.
|
|
19
|
Alahari SK, Lee JW and Juliano RL:
Nischarin, a novel protein that interacts with the integrin alpha5
subunit and inhibits cell migration. J Cell Biol. 151:1141–1154.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alahari SK: Nischarin inhibits Rac induced
migration and invasion of epithelial cells by affecting signaling
cascades involving PAK. Exp Cell Res. 288:415–424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alahari SK and Nasrallah H: A membrane
proximal region of the integrin alpha5 subunit is important for its
interaction with nischarin. Biochem J. 377:449–457. 2004.
View Article : Google Scholar
|
|
22
|
Ivanov TR, Jones JC, Dontenwill M,
Bousquet P and Piletz JE: Characterization of a partial cDNA clone
detected by imidazoline receptor-selective antisera. J Auton Nerv
Syst. 72:98–110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dontenwill M, Pascal G, Piletz JE, et al:
IRAS, the human homologue of Nischarin, prolongs survival of
transfected PC12 cells. Cell Death Differ. 10:933–935. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dontenwill M, Piletz JE, Chen M, et al:
IRAS is an anti-apoptotic protein. Ann N Y Acad Sci. 1009:400–412.
2003. View Article : Google Scholar
|
|
25
|
Ding Y, Zhang R, Zhang K, et al: Nischarin
is differentially expressed in rat brain and regulates neuronal
migration. PLoS One. 8:e545632013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baranwal S, Wang Y, Rathinam R, et al:
Molecular characterization of the tumor-suppressive function of
nischarin in breast cancer. J Natl Cancer Inst. 103:1513–1528.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain P, Baranwal S, Dong S, Struckhoff AP,
Worthylake RA and Alahari SK: Integrin-binding protein nischarin
interacts with tumor suppressor liver kinase B1 (LKB1) to regulate
cell migration of breast epithelial cells. J Biol Chem.
288:15495–15509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edge S, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th edition. Springer; New York, NY:
2010
|
|
29
|
Singletary SE, Allred C, Ashley P, et al:
Revision of the American Joint Committee on Cancer staging system
for breast cancer. J Clin Oncol. 20:3628–3636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harris L, Fritsche H, Mennel R, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Assoian RK: Anchorage-dependent cell cycle
progression. J Cell Biol. 136:1–4. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frisch SM and Ruoslahti E: Integrins and
anoikis. Curr Opin Cell Biol. 9:701–706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varner JA, Emerson DA and Juliano RL:
Integrin alpha 5 beta 1 expression negatively regulates cell
growth: reversal by attachment to fibronectin. Mol Biol Cell.
6:725–740. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Vuori K, Reed JC and Ruoslahti E:
The alpha 5 beta 1 integrin supports survival of cells on
fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci
USA. 92:6161–6165. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu JJ, Lei HT, Hou and YM: A new
evaluation method for tumor cell migration process. Chinese
Pharmacological Bulletin. 1:128–131. 2010.
|
|
36
|
Alahari SK, Reddig PJ and Juliano RL: The
integrin-binding protein Nischarin regulates cell migration by
inhibiting PAK. EMBO J. 23:2777–2788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reddig PJ, Xu D and Juliano RL: Regulation
of p21-activated kinase-independent Rac1 signal transduction by
nischarin. J Biol Chem. 280:30994–31002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davila M, Frost AR, Grizzle WE and
Chakrabarti R: LIM kinase 1 is essential for the invasive growth of
prostate epithelial cells: implications in prostate cancer. J Biol
Chem. 278:36868–36875. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshioka K, Foletta V, Bernard O and Itoh
K: A role for LIM kinase in cancer invasion. Proc Natl Acad Sci
USA. 100:7247–7252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bagheri-Yarmand R, Mazumdar A, Sahin AA
and Kumar R: LIM kinase 1 increases tumor metastasis of human
breast cancer cells via regulation of the urokinase-type
plasminogen activator system. Int J Cancer. 118:2703–2710. 2006.
View Article : Google Scholar
|
|
41
|
Nishita M, Tomizawa C, Yamamoto M, Horita
Y, Ohashi K and Mizuno K: Spatial and temporal regulation of
cofilin activity by LIM kinase and Slingshot is critical for
directional cell migration. J Cell Biol. 171:349–359. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soosairajah J, Maiti S, Wiggan O, et al:
Interplay between components of a novel LIM kinase-slingshot
phosphatase complex regulates cofilin. EMBO J. 24:473–486. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding Y, Milosavljevic T and Alahari SK:
Nischarin inhibits LIM kinase to regulate cofilin phosphorylation
and cell invasion. Mol Cell Biol. 28:3742–3756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jackson RJ and Standart N: How do
microRNAs regulate gene expression? Sci STKE. 2007:re12007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|