Introduction
Cytokinins are critical hormones for plant growth
and development. Historically, they were defined by their ability
to promote cell division when combined with auxin in tobacco tissue
culture (1). Further studies
demonstrated that cytokinins were involved in several processes in
plants (2). Chemically, cytokinins
are N6-substituted adenine derivatives (3). Cytokinins are divided into three
categories, according to their substituent, as follows: Isoprenoid
cytokinins, including isopentenyladenine, zeatin and zeatin
riboside; furfural derivatives, including kinetin and kinetin
riboside; and aromatic cytokinins, including ortho-topolin riboside
(oTR), ortho-methoxytopolin-riboside (MeoTR) and
N6-benzyladenosine (4).
Since the first aromatic cytokinin oTR was isolated
from mature leaves of poplar (5),
aromatic cytokinins have also been detected in oil palm (6) and microalgae (7). Previous studies have shown that
aromatic cytokinins exhibit toxicity against different types of
human cancer cells and their effectiveness in the treatment of
tumors was reported to be mediated through inhibition of cell
proliferation and/or differentiation (8–11).
N6-benzyladenosine was found to induce cell cycle arrest
and apoptosis in bladder carcinoma T24 cells (12). A previous study revealed that oTR
had a strong apoptosis-inducing effect on the human hepatoma
SMMC-7721 cell line (11). MeoTR
and oTR share an identical parental structure, except for the
substitution of the hydroxyl in benzene with a methoxy group.
However, the effect of MeoTR on human cancer cells has not yet been
studied.
Indole-3-acetic acid (IAA) is a naturally present
auxin and a vital regulator of plant cell division, elongation and
differentiation (13). Previous
studies have shown that IAA affected several different mammalian
cell functions; IAA, activated by horseradish peroxidase (HRP)
(14,15) or intense pulsed light (16,17),
was found to exhibit potent toxicity against cancer cells,
including G361 human melanoma cells (14) and PC-3 prostate cancer cells
(18). However, the effect of IAA
treatment alone against tested cancer cells did not produce
significant results (14,17,18).
Furthermore, to the best of our knowledge, a comparative and
systematic study of the effects of a combination treatment with
cytokinin and auxin on human cancer cells has yet to be
performed.
The aim of the present study was to investigate the
inhibitory effects of MeoTR and/or IAA in the human cervical cancer
HeLa cell line. We showed that treatment with MeoTR alone arrested
cell cycle progression, and accumulated in S phase, and induced
apoptosis via intrinsic and extrinsic caspase-dependent pathways
in vitro. These effects were enhanced by combination
treatment with IAA. Although IAA did not affect cell proliferation,
it induced significant cell accumulation in S phase, indicating
that its potentiating effect on MeoTR may be mediated by its
cytostatic rather than cytotoxic activity. MeoTR treatment blocked
the Akt pathway, and these effects were enhanced by IAA, although
IAA had no effect on the Akt pathway when used alone.
Materials and methods
Reagents
MeoTR was purchased from OlChemIm Ltd. (Olomouc,
Czech Republic) and IAA, Z-Val-Ala-Asp-fluo romethylketone
(Z-VAD-FMK), LY294002 and violet staining solution were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Insulin-like growth factor
I (IGF-I), an Akt activation factor, was purchased from PeproTech,
Inc. (Rocky Hill, CT, USA). The maximum final concentration of
dimethyl sulfoxide (DMSO) in the culture medium was <0.5%. XTT
and phenazine methosulfate (PMS) were purchased from Sangon Biotech
Co., Ltd (Shanghai, China). All additional solid reagents were
purchased from Sigma-Aldrich. The following primary antibodies:
Monoclonal anti-cleaved poly-adenosine diphos-phate-ribose
polymerase (PARP; cat. no. 5625), polyclonal anti-total PARP (cat.
no. 9542), monoclonal anti-cytochrome c (cat. no. 11940),
monoclonal anti-B cell lymphoma 2 (Bcl-2; cat. no. 2870),
monoclonal anti-survivin (cat. no. 2808), monoclonal
anti-phosphorylated (p)-pyruvate dehydrogenase kinase 1 (PDK1; cat.
no. 3438), monoclonal anti-p-Akt (Ser473; cat. no. 4060),
monoclonal anti-Akt (cat. no. 4685), monclonal anti-p-glycogen
synthase kinase 3β (GSK-3β; cat. no. 5558), monoclonal anti-GSK-3β
(cat. no. 12456) and monoclonal anti-β-actin (cat. no. 3700), were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
The following primary antibodies: Polyclonal anti-cyclin A (cat.
no. sc-751), poly-clonal anti-p-cyclin-dependent kinase 2 (CDK2)
(Thr160) (cat. no. sc-101656), polyclonal anti-CDK2 (cat. no.
sc-163), poly-clonal anti-p21 (cat. no sc-397) and polyclonal
anti-p27 (cat. no. sc-528), were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and primary antibodies,
polyclonal anti-caspase-3 (cat. no. 29010), polyclonal
anti-caspase-9 (cat. no. 22978) and polyclonal anti-caspase-8 (cat.
no. 22977) were purchased from Signalway Antibody (College Park,
MD, USA). Goat anti-mouse (#7072) and goat anti-rabbit (#7071)
secondary IgG antibodies were purchased from Cell Signaling
Technology, Inc.
Cell culture
Human cervical cancer HeLa cells (Cell Bank of the
Chinese Academy of Sciences, Shanghai, China) were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum, 100
μg/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C.
Cell viability assay
Vehicle (0.4% DMSO)-treated cells were considered
the control cells. Cell viability was determined using the XTT
assay. HeLa cells were seeded in 96-well plates at a density of
5.0×104 cells/well for 12 h prior to treatment. For the
dose-dependent assay, solutions containing various concentrations
of MeoTR (25, 50, 75 and 100 μM) and IAA (20, 50, 100, 500
and 1000 μM), independently, or MeoTR (25, 50, 75 and 100
μM) in combination with 1 mM IAA were added to each well in
100 μl RPMI-1640 medium and incubated for 48 h. For the time
course assay, solutions containing 50 μM MeoTR and 1 mM IAA,
alone or in combination, were added to each well in 100 μl
RPMI-1640 medium and incubated for 0, 12, 24 and 48 h. Following
treatment, 50 μl XTT solution (1 g/l XTT and 5 mg/l PMS in
RPMI-1640 medium) was added to each well for a further 4 h at 37°C.
Absorbance was read at 595 nm using a microplate reader (Thermo
Electron Corporation, Waltham, MA, USA). Three independent
experiments were performed from six replicates of each treatment
group. The percentage cell viability was calculated as follows:
[sample optical density (OD)/control OD] ×100%.
The coefficient of drug interaction (CDI) was used
to analyze the synergistically inhibitory effect of drug
combinations. CDI=AB/(AxB), where AB represents the cell viability
of the combination group and A or B represent the cell viabili-ties
of the single agent groups, as determined by measuring the
absorbance of each group. CDI<1 indicates that the drugs are
synergistic; CDI=1 indicates that the drugs are additive; CDI>1
indicates that the drugs are antagonistic; in addition, CDI <0.7
indicates that the drugs are significantly synergistic, as
previously described (19).
Crystal violet staining
HeLa cells were seeded in 6-well plates at a density
of 5.0×104 cells/well for 12 h. The cells were treated
with MeoTR (50 and 100 μM) and/or 1 mM IAA for a further 48
h. Cells were then fixed with 4% paraformaldehyde and stained with
crystal violet staining solution. The cell morphology was observed
using a Nikon Eclipse Ti-U Multi-port inverted microscope (Nikon
Corp., Tokyo, Japan).
Flow cytometric analysis of apoptosis and
cell cycle
Cell apoptosis and cell cycle distribution were
analyzed using flow cytometry. HeLa cells were pre-treated with
MeoTR (50 and 100 μM) and/or 1 mM IAA for 48 h. Cell
apoptosis was assessed using a fluorescein isothiocyanate-Annexin V
apoptosis kit (BD Biosciences, San Jose, CA, USA) according to the
manufacturer’s instructions. The stained cells were then analyzed
using a FACSCalibur flow cytometer (BD Biosciences). For cell cycle
analysis, HeLa cells were harvested and washed twice with ice-cold
phosphate-buffered saline (PBS), resuspended in 1 ml 75% ethanol
(-20°C) and fixed at −20°C overnight. Fixed cells were washed with
PBS, treated with 50 μg/ml RNase A for 5 min at room
temperature and stained with propidium iodide (PI; 50 μg/ml)
in the dark at 4°C for 30 min. The stained cells were then assessed
using a FACSCalibur flow cytometer. Each assay was performed in
triplicate.
Western blotting
Following treatment with the indicated
concentrations of MeoTR and/or 1 mM IAA for 48 h, the cells were
lysed in RIPA lysis buffer. The concentration of total protein was
determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Waltham, MA, USA). For western blot analysis, 30
μg protein was resolved using 10% SDS-PAGE and transferred
to polyvinylidene difluoride membranes. Blots were blocked in
tris-buffered saline with Tween-20 (TBST), containing 0.01%
Tween-20 and 5% non-fat dry milk for 1 h, and incubated with
primary antibodies indicated above overnight at 4°C with agitation.
Membranes were then washed five times with TBST for 10 min each,
followed by incubation with the indicated secondary antibodies for
2 h at 4°C. Membranes were washed with TBST five times (10 min
each) and peroxidase activity was visualized using the enhanced
chemiluminescence method. Results were quantified through grey
correlation analysis of bands using Image J software (National
Institute of Health, Bethesda, MD, USA). Assays were performed in
triplicate.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean for three individual experiments. Statistical significance
was determined using the Student’s t-test. P<0.05 and P<0.01
were considered to indicate statistically significant differences
between values.
Results
IAA enhances the effect of MeoTR on the
inhibition of HeLa cell viability
The chemical structure of MeoTR is shown in Fig. 1A. The effect of MeoTR and/or IAA on
the survival of HeLa cells was assessed using an XTT assay. MeoTR
alone or in combination with IAA was found to significantly
decrease HeLa cell viability in dose- and time-dependent manners
compared with that of the vehicle-treated control or MeoTR-treated
cells, respectively (P<0.05) or P<0.01) (Fig. 1B, C and D), whereas IAA alone had
no cytotoxic effects (Fig. 1E and
F). MeoTR reduced HeLa cell viability to 54.1, 38.5, 27.1 and
21.0% of the control values at concentrations of 25, 50, 75 and 100
μM, respectively, and combination treatment with IAA further
reduced cell viability to 47.5, 32.9, 18.3 and 11.3% at these
concentrations, respectively (Fig. 1B
and D). The CDI indicated that IAA acted signifi-cantly
synergistically with MeoTR (Table
I). In addition, the potent growth inhibition of MeoTR alone
and in combination with IAA was further confirmed in the crystal
violet staining assay (Fig. 1G).
Additionally, the obvious decrease of cell numbers, microscopic
examination of cell morphology revealed certain major patterns of
shape change in response to treatment with MeoTR alone or in
combination with IAA. The treated cells were smaller, rounder and
deep-stained compared with those of the control group, as well as
detached from the culture well surface, indicating marked cell
damage (Fig. 1G).
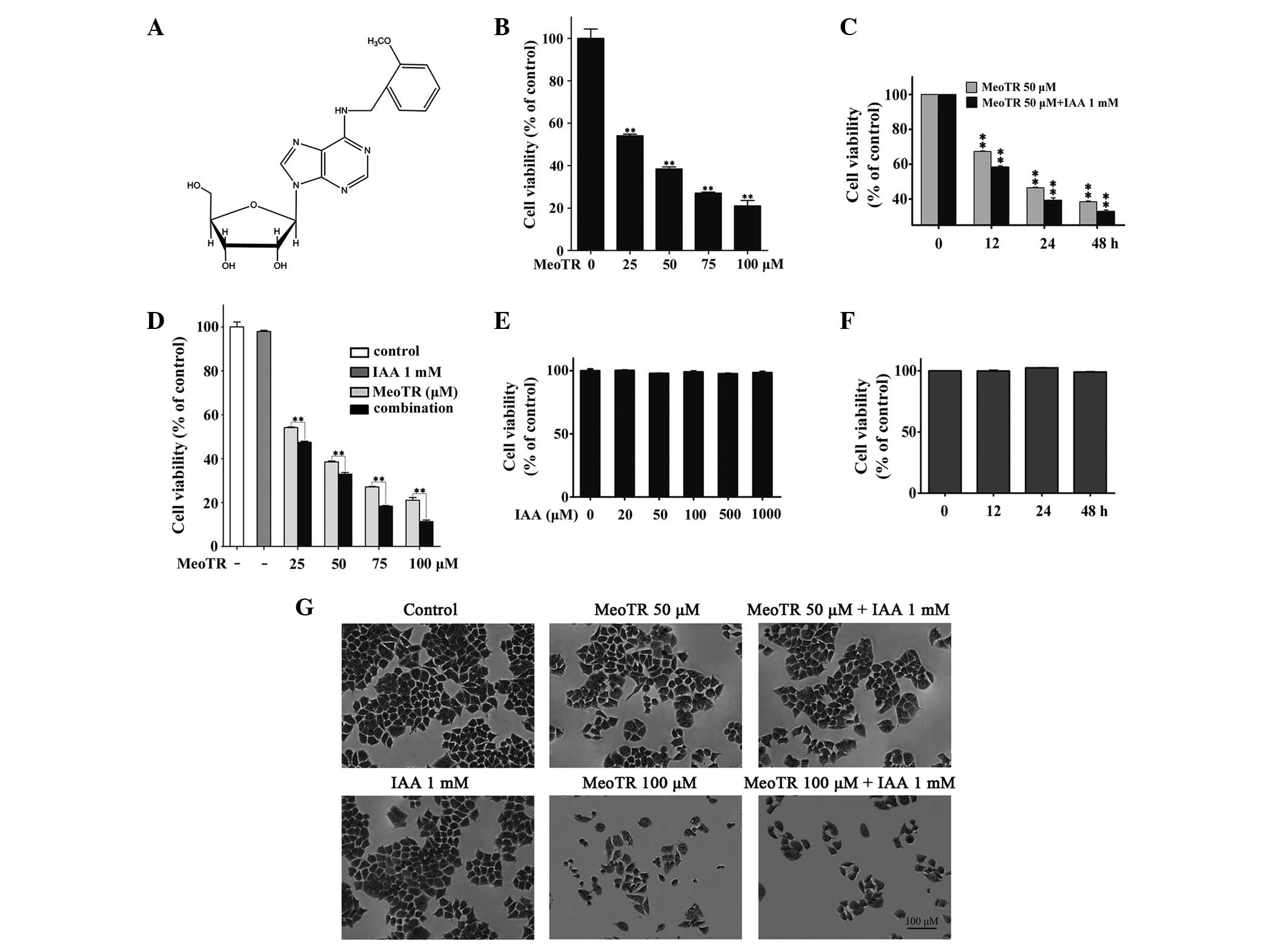 | Figure 1Cytotoxic effects of MeoTR and/or IAA
on HeLa human cervical cancer cells. (A) Structure of MeoTR
(molecular weight, 387.4). HeLa cells were cultured with different
concentration of MeoTR and/or IAA, and cell viability was measured
using the XTT assay. (B) Cell viability following treatment with
MeoTR (25, 50, 75 and 100 μM) for 48 h. (C) Cell viability
following treatment with 50 μM MeoTR and/or 1 mM IAA for
increasing incubation times (0, 12, 24 and 48 h). (D) Cell
viability following treatment with MeoTR alone or in combination
with 1 mM IAA for 48 h. (E) Cell viability following treatment with
IAA (20, 50, 100, 500 and 1000 μM) alone for 48 h. (F) Cell
viability following treatment with 1 mM IAA for increasing
incubation times (0, 12, 24 and 48 h). Values are expressed as the
mean ± standard error of the mean for three independent
experiments. *P<0.05 and **P<0.01 vs.
vehicle-treated control cells or MeoTR-treated cells. (G) Changes
in cell morphology following incubation with MeoTR alone and in
combination with IAA was observed using crystal violet staining for
48 h. MeoTR, ortho-methoxytopolin-riboside; IAA, indole-3-acetic
acid. |
 | Table ISynergistically inhibitory effect of
MeoTR and IAA combination treatment. |
Table I
Synergistically inhibitory effect of
MeoTR and IAA combination treatment.
| MeoTR (μm)
|
|---|
| 25 | 50 | 75 | 100 |
|---|
| CDI | 0.896 | 0.874 | 0.692 | 0.551 |
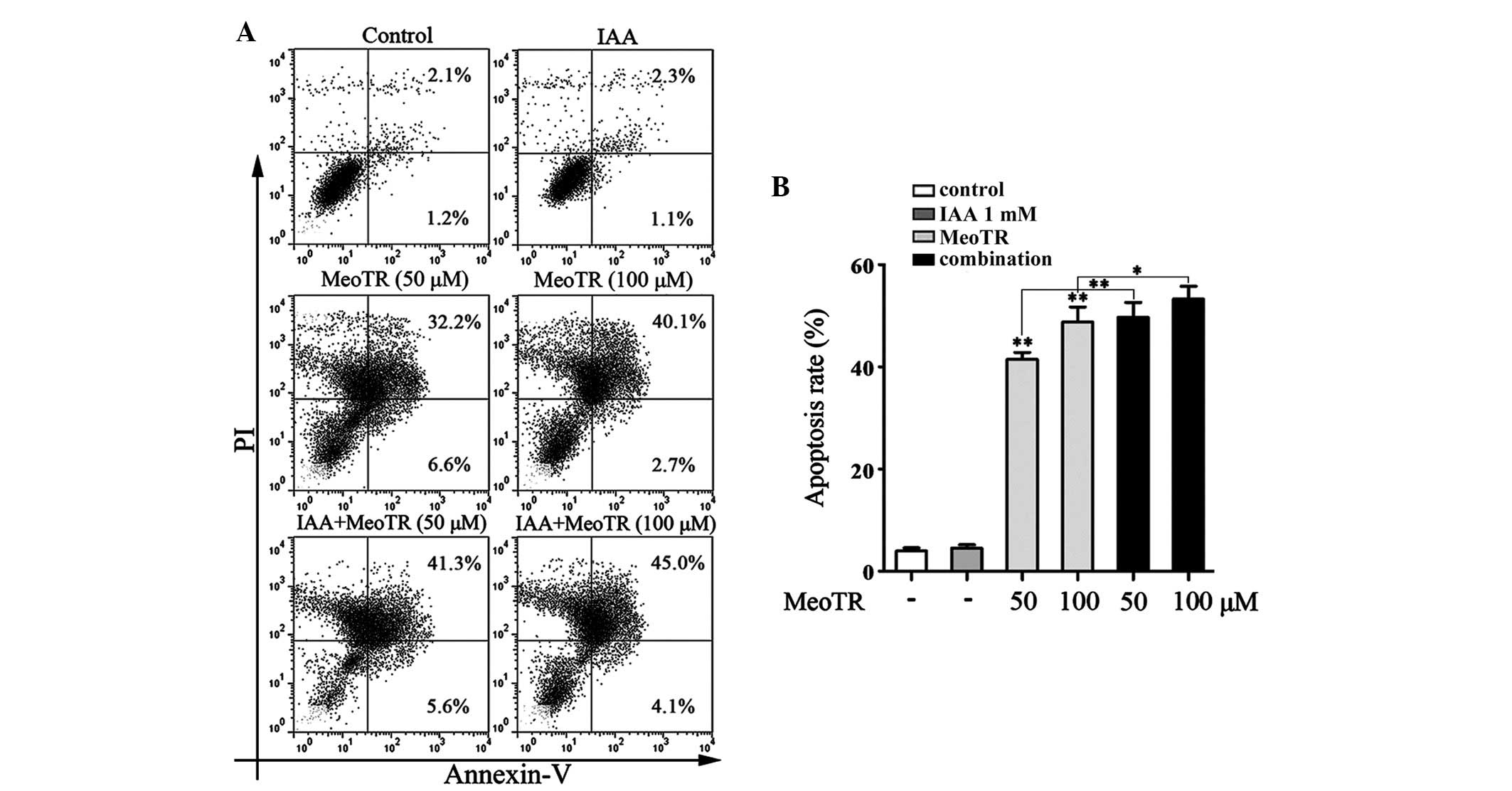
IAA enhances the effect of MeoTR on the
induction of HeLa cell apoptosis
In order to elucidate the mechanism of MeoTR-induced
cell death, the pro-apoptotic effects of MeoTR were measured using
flow cytometric analysis following Annexin V/PI staining. As shown
in Fig. 2, MeoTR induced cell
apoptosis in a dose-dependent manner and combined treatment with
IAA further enhanced this induction of apoptosis, while IAA alone
was unable to induce apoptosis. These findings indicated that
apoptosis may contribute to the anti-proliferative effect of MeoTR
in HeLa cells, the effect of which was enhanced in the presence of
IAA.
MeoTR induces apoptosis through intrinsic
and extrinsic caspase-dependent pathway and IAA potentiates this
effect
In order to investigate whether caspase-dependent
apoptotic pathways were activated in response to MeoTR, HeLa cells
were treated with different concentrations of MeoTR and/or 1 mM IAA
for 48 h and then lysed for western blot analysis. The release of
cytochrome c from mitochondria into the cytosol has an
important role in the pro-apoptotic mechanisms of the intrinsic
caspase-dependent pathways (20).
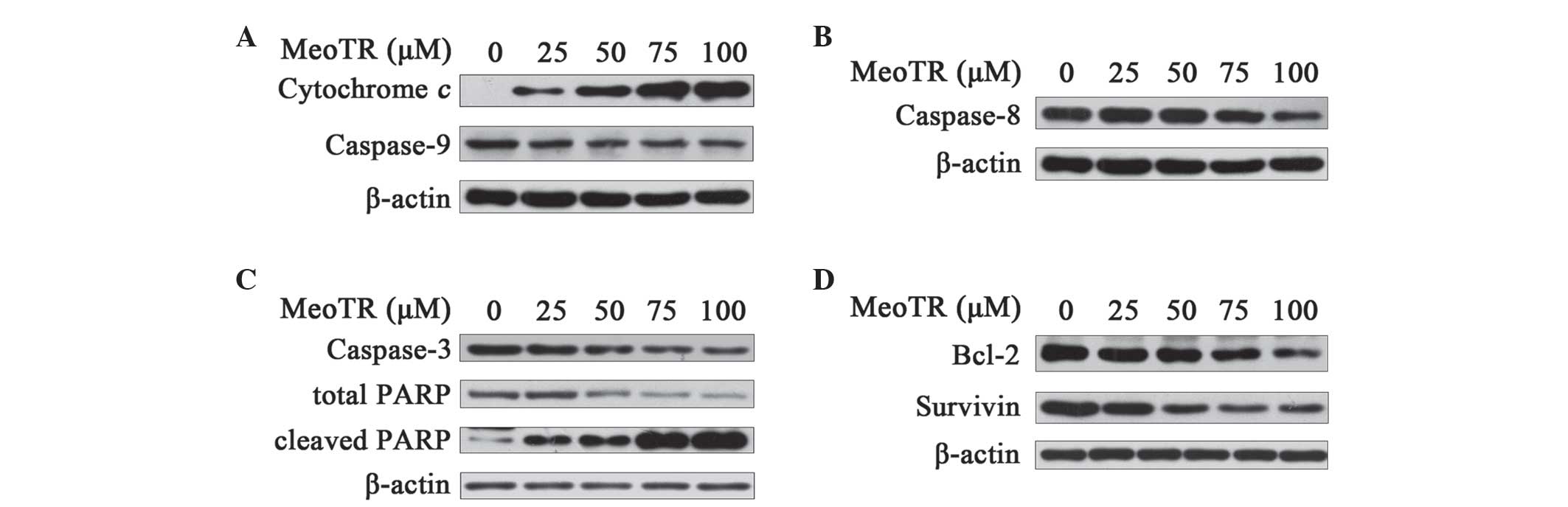
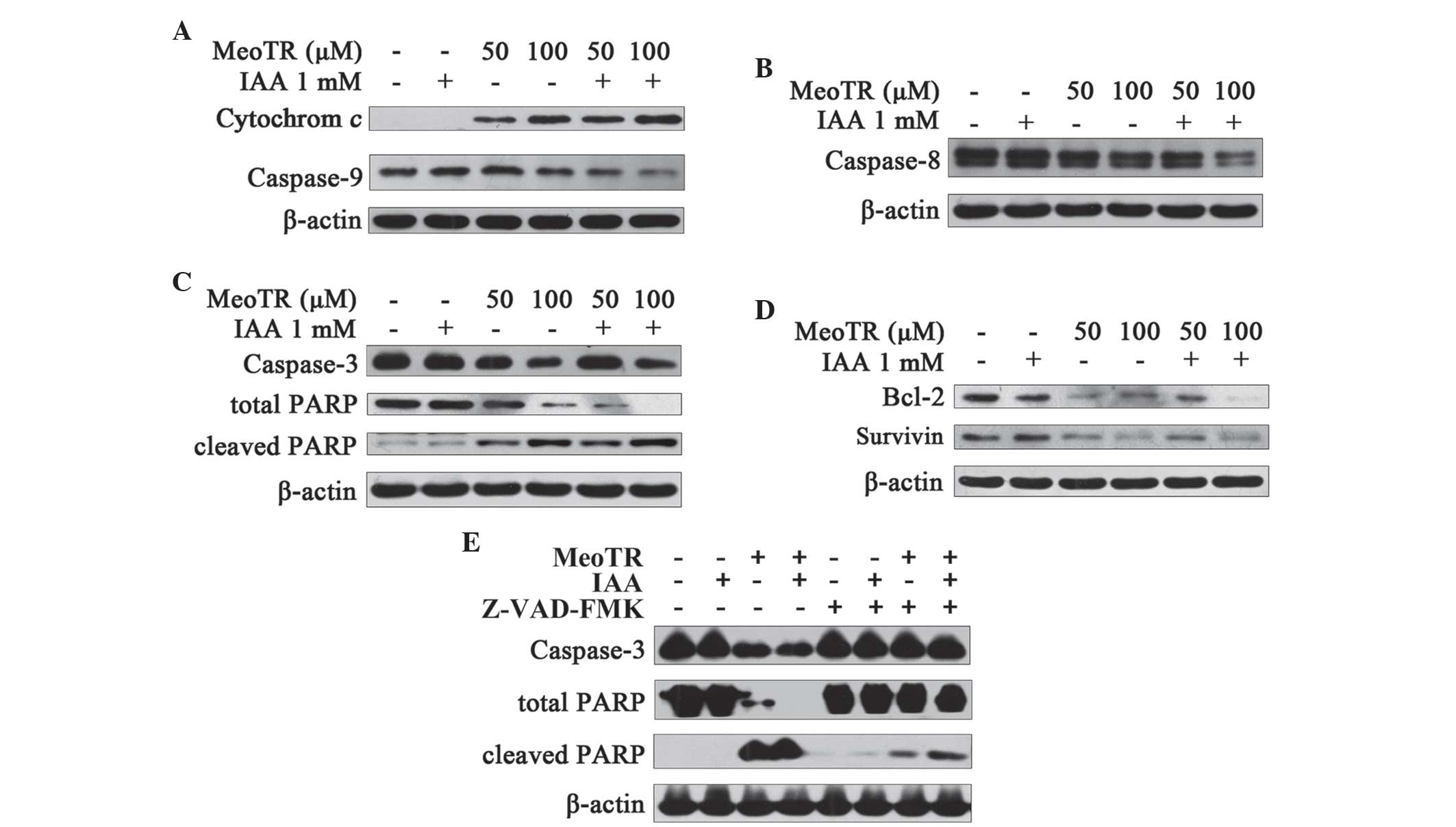
As shown in Fig. 3A, MeoTR
treatment increased the cytosolic levels of cytochrome c in a
dose-dependent manner, which led to the lysis of its downstream
protein zymogen caspase-9. However, no changes in cytochrome
c or caspase-9 were detected following pre-treatment with
IAA alone (Fig. 4A), whereas
combination treatment with MeoTR and IAA was more demonstrated a
more potent effect compared with that of MeoTR alone (Fig. 4A). Caspase-8 is the key initiator
caspase in the extrinsic caspase-dependent pathway and is activated
by auto-phosphorylation (21,22).
Treatment with MeoTR alone (Fig.
3B) or in combination with IAA (Fig. 4B) decreased the expression of the
caspase-8 zymogen in a dose-dependent manner; in addition, the
combination treatment was shown to accelerate the
auto-phosphorylation of caspase-8, whereas treatment with IAA alone
had no effect (Fig. 4B).
MeoTR induces the activation of
caspase-3, induces the cleavage of PARP and downregulates
anti-apoptotic proteins, and IAA potentiates this effect
PARP is the substrate of caspase-3, the executor of
apoptosis and a marker of apoptosis. As shown in Fig. 3C, decreased pro-caspase-3 and
increased cleaved PARP levels were observed in HeLa cells exposed
to different concentrations of MeoTR (25, 50, 75 and 100 μM)
in a dose-dependent manner, and this effect was enhanced when cells
were treated with MeoTR in combination with 1 mM IAA (Fig. 4C), whereas IAA alone did not affect
the cleavage of pro-caspase-3 and PARP (Fig. 4C).
In order to further confirm whether caspase cascade
activation is involved in the apoptotic mechanism of MeoTR in
combination with IAA, HeLa cells were pre-treated with 50 μM
Z-VAD-FMK, a pan-specific-caspase-inhibitor (23) for 1 h, and exposed to 50 μM
MeoTR and/or 1 mM IAA in the presence or absence of Z-VAD-FMK for a
further 48 h. The MeoTR and/or IAA-mediated activation of
caspase-3, decrease in total PARP and increase in cleaved PARP were
found to be partially, but not completely, antagonized in the
presence of Z-VAD-FMK; in addition, Z-VAD-FMK had no effect on
IAA-only treatment (Fig. 4E).
These results indicated that MeoTR induced apoptosis in HeLa, which
was enhanced by IAA, the mechanism of which may be associated with
caspase activation.
Treatment of HeLa cells with increasing
concentrations of MeoTR significantly decreased the levels of the
anti-apoptotic proteins Bcl-2 and survivin (Fig. 3D), the effect of which was
potentiated by combination treatment with IAA (Fig. 4D). Treatment of HeLa cells with 1
mM IAA alone downregulated Bcl-2, whereas survivin expression
remained unchanged (Fig. 4D).
MeoTR blocks the Akt signaling pathway
and IAA potentiates this effect
In order to identify the signaling pathways involved
in MeoTR-mediated apoptosis in HeLa cells, the Akt signaling
pathway was investigated in HeLa cells following exposure to MeoTR
in the presence or absence of IAA. As shown in Fig. 5A and B, Akt phosphorylation was
inhibited in HeLa cells treated with MeoTR in a dose-dependent
manner, the inhibitory effect of which was enhanced by combination
treatment with IAA, whereas IAA alone had no effect on Akt
phosphorylation. GSK-3β is a tumor suppressor gene regulated by the
Akt signaling pathway, which is inactivated through phosphorylation
(24–26). MeoTR was found to strongly inhibit
the phosphorylation of GSK-3β in a dose-dependent manner, the
effect of which was enhanced by IAA (Fig. 5A and B), therefore protecting its
tumor suppressor function.
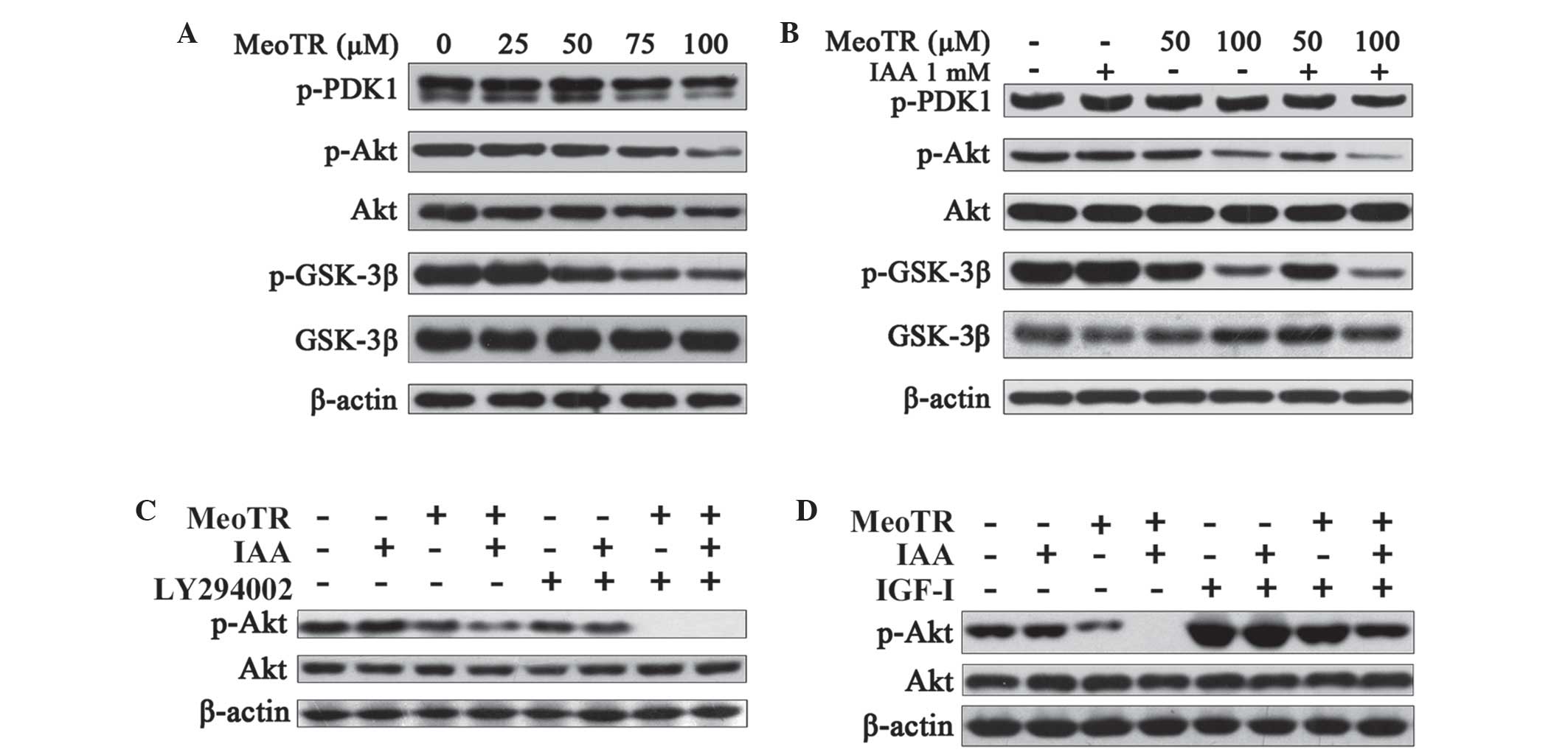 | Figure 5Effect of MeoTR and/or IAA on the Akt
signaling pathway in HeLa cells. Following treatment with MeoTR
(25, 50, 75 and 100 μM) alone or the combination of MeoTR
(50 and 100 μM) with IAA (1 mM) for 48 h, HeLa cells were
lysed for western blot analysis using specific antibodies. β-actin
was used as an internal protein loading control. Western blot
analysis of: (A) Effect of MeoTR on the Akt signaling pathway; (B)
effect of MeoTR alone or in combination with IAA on the Akt
signaling pathway; (C) effects of MeoTR and/or IAA on Akt
phosphorylation in the presence of a Akt inhibitor LY294002. HeLa
cells were pretreated with LY294002 (20 μM) for 30 min, and
exposed to MeoTR (50 μM) and/or IAA (1 mM) in the presence
or absence of LY294002 for a further 48 h; and (D) effects of MeoTR
and/or IAA on IGF-I stimulated phosphorylation of Akt. HeLa cells
were pretreated with MeoTR (50 μM) and/or IAA (1 mM) in
serum-free RPMI-1640 medium for 48 h, and stimulated with IGF-I (10
nM) for a further 30 min. MeoTR, ortho-methoxytopolin-riboside;
IAA, indole-3-acetic acid; PDK1, pyruvate dehydrogenase kinase 1;
GSK-3β, glycogen synthase kinase 3β; p-, phosphorylated; IGF-1,
insulin-like growth factor 1. |
In order to further confirm whether Akt inhibition
was involved in this mechanism of MeoTR-mediated apoptosis, HeLa
cells were pre-treated with 20 μM LY294002, an Akt inhibitor
(27) for 30 min and exposed to 50
μM MeoTR and/or 1 mM IAA in the presence or absence of
LY294002 for a further 48 h. As shown in Fig. 5C, pre-treatment with LY294002
significantly augmented the inhibitory effects of MeoTR on Akt
phosphorylation, alone and in combination with IAA. In addition,
following treatment with 50 μM MeoTR and/or 1 mM IAA in
serum-free RPMI-1640 medium for 48 h, HeLa cells were stimulated by
10 nM IGF-I, an Akt activation factor (28) for 30 min. MeoTR alone, as well as
combination with IAA, significantly inhibited the IGF-I-stimulated
Akt activation (Fig. 5D),
therefore further confirming the inhibitory effect of MeoTR on the
Akt pathway, the effect of which was enhanced by combination
treatment with IAA.
IAA enhances the inhibitory effect of
MeoTR on HeLa cells through arresting cell cycle progression and
accumulating in S-phase
In order to investigate the potential synergistic
inhibitory mechanism of MeoTR and IAA on HeLa cells, HeLa cells
were treated with MeoTR (50 and 100 μM) and/or 1 mM IAA for
48 h and examined using flow cytometric analysis. MeoTR and IAA
induced a marked accumulation of cells in S-phase, as indicated by
the increased proportion of cells in S-phase, which was further
enhanced by combination treatment with IAA. In addition, the effect
of MeoTR alone or in combination with IAA cell cycle progression
occurred in a dose-dependent manner (Fig. 6A and B).
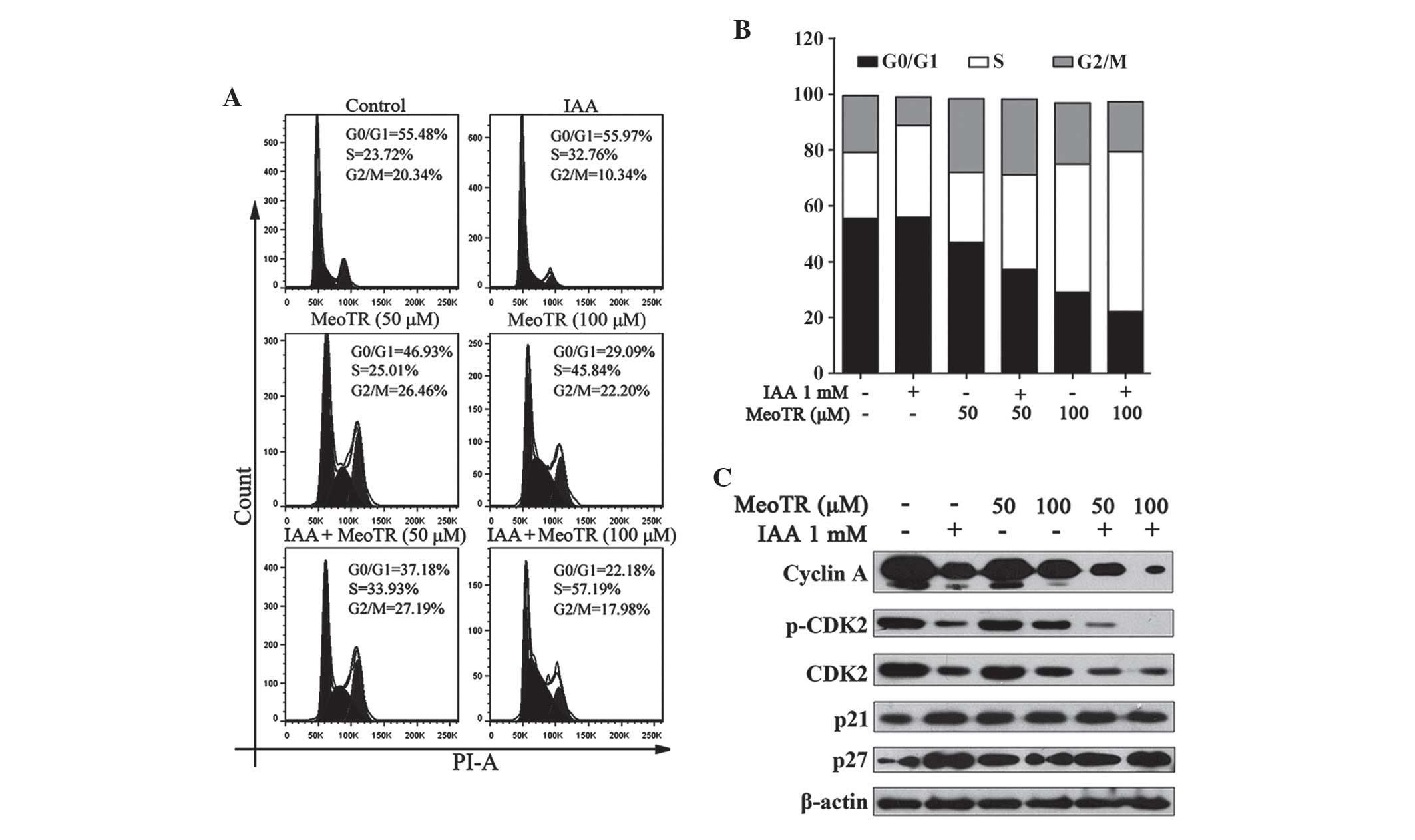 | Figure 6Effects of MeoTR and/or IAA on cell
cycle progression arrest. (A and B) Effect of MeoTR (50 and 100
μM) and/or 1 mM IAA on cell cycle progression arrest, as
determined using flow cytometric analysis with PI staining. The two
peaks represents cells in G1 (the first) and
G2/M (the second) phase, and the area between them
represents the S phase. (C) Effect of IAA and/or MeoTR on the
expression of cyclin A, CDK2, p-CDK2, p21 and p27 in HeLa cells.
Cells were pre-treated with MeoTR (50 and 100 μM) and/or IAA
(1 mM) for 48 h, and or lysed for western blot analysis using
specific antibodies. β-actin was used as an internal protein
loading control. MeoTR, ortho-methoxytopolin-riboside; IAA,
indole-3-acetic acid; CDK2, cyclin dependent kinase 2; p-,
phosphorylated; PI, propidium iodide. |
The complex of cyclin A and CDK2 has a key role in
the progression of cells from S-phase into G2/M-phase, and
threonine phosphorylation of CDKs has been reported to enhance the
binding of cyclins (29,30). As shown in Fig. 6C, treatment of HeLa cells with IAA
or MeoTR, independently, attenuated the expression of cyclin A as
well as that of total and p-CDK2; in addition, the combination
treatment resulted in a more potent inhibitory effect. IAA and/or
MeoTR treatment also resulted in a significant increase in p21 and
p27 expression levels (Fig. 6C),
which were previously reported to inhibit the formation of
CDK-cyclin complexes in order to negatively regulate the cell
division cycle (31). Overall,
these results indicated that IAA synergistically enhanced
MeoTR-induced anti-proliferative and apoptotic effects.
Discussion
MeoTR is a member of the aromatic cytokinin family.
The results of the present study showed that MeoTR had a marked
cytotoxic effect on HeLa cells (Fig.
1B), while combination treatment with IAA enhanced the effect
of MeoTR (Fig. 1D). The effect of
the combination treatment was further clarified through the
calculation of CDI (Table I).
Apoptosis is a physiological process that is
critical for animal development and tissue homeostasis and is
characterized by distinct morphological as well as biochemical
features (32,33). In addition, two caspase-dependent
pathways have been well characterized (34), which are important for cell death
induced by apoptosis (35). The
intrinsic caspase-dependent pathway is mediated by the release of
cytochrome c from mitochondria into the cytosol (20), where it promotes the interaction of
apoptosis protein-activating factor 1 (APAF-1) and pro-caspase-9,
leading to the formation of the apoptosome, which in turn results
in the activation of the initiator caspase-9 (21–36).
In the extrinsic caspase-dependent pathway, caspase-8 is the key
initiator caspase (21,22). The activation of the extrinsic
pathway is divided into two steps as follows: First, cell surface
death receptors engage with their specific ligands, including CD95,
tumor necrosis factor (TNF) receptor or TNF-related
apoptosis-inducing ligand receptor, which results in the assembly
of the death-inducing signal complex (DISC), allowing caspase-8 to
reach a sufficient local concentration of zymogen and enzymatic
activity; and secondly, the authophosphorylation and activation of
caspase-8 triggers its separation from DISC, which enables it to
access other substrates (37,38).
Once the initiator caspases are activated by either the intrinsic
or extrinsic signaling pathway, they trigger the activation of the
effector caspases-3/7 and the cleavage of PARP, leading to the
rapid and irreversible morphological and biochemical changes of
apoptosis (39).
In the present study, MeoTR treatment was found to
increase the content of cytochrome c in the cytosol of HeLa
cells, indicating the release of cytochrome c from
mitochondria into the cytosol, and caspase-9 was activated
(Fig. 3A). In addition, the
activation of the initiator caspase-8 was detected (Fig. 3B). These results suggested that
MeoTR-induced apoptosis in HeLa cells was mediated by the intrinsic
and extrinsic caspase-dependent pathways. In cells exposed to
combination treatment, the initiator proteins of the two pathways,
caspase-9 and caspase-8, were activated and the degree of
activation was increased (Fig. 4A and
B); however, IAA alone had no effect on the activation of these
proteins or apoptosis (Fig. 4A,B,
2D). These data further illustrate
that the enhancement effect of IAA on MeoTR was not a simple
superposition of cytotoxicity.
The intrinsic and extrinsic pathways are subject to
negative regulation by numerous anti-apoptotic proteins, including
Bcl-2 (40) and survivin (41,42).
In the present study, when used independently, MeoTR downregulated
these anti-apoptotic proteins, whereas IAA had no observable
effect, with the exception of Bcl-2, which was negatively regulated
by IAA (Fig. 3D and 4D). Combination treatment with MeoTR and
IAA exhibited a more potent effect on reducing the expression of
these anti-apoptotic proteins compared with that of each agent
alone (Fig. 4D). Therefore,
downregulation of the expression of Bcl-2 may be one of the
mechanisms underlying the potentiating effect of IAA on
MeoTR-induced apoptosis.
The Akt pathway has a fundamental role in cell
survival and proliferation, and is a potentially effective target
for the development of novel drugs for the treatment of malignant
cancers (43–45). GSK-3β, a pro-apoptotic protein
(24,25), acts downstream of the Akt pathway
and upstream of caspase-8, and it is inactivated through
phosphorylation at serine 9 mediated by the Akt pathway (26). As shown in Fig. 5A in the present study, MeoTR alone
attenuated the phosphorylation of Akt in a dose-dependent manner in
HeLa cells. In addition, MeoTR and IAA combination treatment
exhibited a more potent inhibitory effect on the phosphorylation of
Akt compared with that of MeoTR alone (Fig. 5B), although IAA alone had no effect
(Fig. 5B). The inhibition of Akt
phosphorylation by MeoTR alone and in combination with IAA was
further confirmed through the employment of specific Akt signal
inhibitor LY294002 (Fig. 5C) and
the Akt activation factor IGF-I (Fig.
5D). MeoTR inhibited the phosphorylation of GSK-3β (Fig. 5A), thereby protecting its
pro-apoptotic effect, and IAA potentiated the inhibitory effect of
MeoTR on GSK-3β phosphorylation or the promotion of
dephosphorylation (Fig. 5B). In
addition, the MeoTR-mediated inhibition of PDK1, an upstream
regulator of the Akt pathway (43), was comparably weak when used
independently (Fig. 5A) or in
combination with IAA (Fig. 5B),
indicating that MeoTR may directly interfere with Akt
phosphorylation and block this cell survival-promoting pathway. In
summary, the regulation of the Akt pathway and GSK-3β activity by
MeoTR and/or IAA may by the mechanism by which MeoTR mediated the
suppression of survival and proliferation as well as the induction
of apoptosis, and therefore contributed to the enhanced
cytotoxicity of combination treatment.
Previous studies have shown that IAA activation by
horseradish peroxidase or UVB may be used to investigate its
effects on cancer cells, while IAA alone has a weak cytotoxic
effect on the cell lines assessed (14–17,46,47).
The results of the present study were in agreement with those of
previous studies and showed that IAA had no effect on HeLa cell
viability and apoptosis induction. However, the results of the
present study demonstrated that IAA as well as MeoTR induced
significant accumulation of cells in S-phase, inhibited the
expression of S-phase-associated cyclin A, CDK2 and p-CDK as well
as increased the expression of cell cycle-inhibitory proteins p21
and p27, with enhanced effects under combination treatment. These
results suggested that IAA alone may induce significant delay in
progression through S-phase (Fig.
6A–C), which may therefore be the mechanism of IAA enhancement
on MeoTR-induced growth arrest and apoptosis induction. In plant
tissue culture, cytokinin and auxin are often used in combination
to control cell division and differentiation; however, their
combined effect on animal/human cancer cells in culture has not yet
been reported. The results of the present study confirmed that
MeoTR and IAA combination treatment exhibited a more potent effect
on HeLa cell viability and apoptosis compared with that of MeoTR
treatment alone.
In conclusion, the present results indicated that
MeoTR exerted a marked anti-tumor effect on human cervical cancer
HeLa cells through the induction of apoptosis and blockage of the
Akt pathway, the effect of which was enhanced when treated in
combination with IAA, while IAA alone had no cytotoxic effects. IAA
therefore exhibited a synergistic effect on MeoTR-induced growth
inhibition and apoptosis in HeLa cells through cell cycle
progression arrest and accumulation in S-phase, coupled with the
negative regulation of the Bcl-2. To the best of our knowledge, the
present study was the first to demonstrated that cytokinin and
auxin combination treatment had a synergistic antitumor effect
in vitro, suggesting the potential effectiveness of
combining two plant hormone-like substances for the future
treatment of malignant cervical cancer. However, further studies,
including pre-clinical studies and clinical trials, are required in
order to support this proposed therapeutic strategy.
Acknowledgments
The present study was supported, in part, by a grant
from the National Natural Science Foundation of China (No.
31030045).
References
|
1
|
Skoog F and Miller CO: Chemical regulation
of growth and organ formation in plant tissues cultured in vitro.
Symp Soc Exp Biol. 11:118–130. 1957.PubMed/NCBI
|
|
2
|
Dolezal K, Popa I, Hauserova E, et al:
Preparation, biological activity and endogenous occurrence of
N6-benzyladenosines. Bioorg Med Chem. 15:3737–3747. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strnad M: The aromatic cytokinins. Physiol
Plantarum. 101:674–688. 1997. View Article : Google Scholar
|
|
4
|
Barciszewski J, Massino F and Clark BF:
Kinetin-a multiactive molecule. Int J Biol Macromol. 40:182–192.
2007. View Article : Google Scholar
|
|
5
|
Horgan R, Hewett EW, Horgan JM, Purse J
and Wareing PF: A new cytokinin from Populus × robusta.
Phytochemistry. 14:1005–1008. 1975. View Article : Google Scholar
|
|
6
|
Jones LH, Martinkova H, Strnad M and Hanke
DE: Occurrence of aromatic cytokinins in oil palm (Elaeis
guineensis Jacq). J Plant Growth Regul. 15:39–49. 1996. View Article : Google Scholar
|
|
7
|
Ördög V, Stirk WA, Van Staden J, Novák O
and Strnad M: Endogenous cytokinins in three genera of microalgae
from the chlorophyta1. J Phycol. 40:88–95. 2004. View Article : Google Scholar
|
|
8
|
Cheong J, Goh D, Yong JW, Tan SN and Ong
ES: Inhibitory effect of kinetin riboside in human heptamoa, HepG2.
Mol Biosyst. 5:91–98. 2009. View
Article : Google Scholar
|
|
9
|
Ishii Y, Hori Y, Sakai S and Honma Y:
Control of differentiation and apoptosis of human myeloid leukemia
cells by cytokinins and cytokinin nucleosides, plant
redifferentiation-inducing hormones. Cell Growth Differ. 13:19–26.
2002.PubMed/NCBI
|
|
10
|
Ishii Y, Sakai S and Honma Y:
Cytokinin-induced differentiation of human myeloid leukemia HL-60
cells is associated with the formation of nucleotides, but not with
incorporation into DNA or RNA. Biochim Biophys Acta. 1643:11–24.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Sun C, Wang ZH and Guo GQ:
Mechanism of apoptotosis induced by orthotopolin riboside in human
hepatoma cell line SMMC-7721. Food Chem Toxicol. 50:1962–1968.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castiglioni S, Casati S, Ottria R,
Ciuffreda P and Maier JA: N6-isopentenyladenosine and its analogue
N6-benzyladenosine induce cell cycle arrest and apoptosis in
bladder carcinoma T24 cells. Anticancer Agents Med Chem.
13:672–678. 2013. View Article : Google Scholar
|
|
13
|
Goldsmith MH: Cellular signaling: new
insights into the action of the plant growth hormone auxin. Proc
Natl Acad Sci USA. 90:11442–11445. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DS, Jeon SE and Park KC: Oxidation of
indole-3-acetic acid by horseradish peroxidase induces apoptosis in
G361 human melanoma cells. Cell Signal. 16:81–88. 2004. View Article : Google Scholar
|
|
15
|
Kim DS, Kim SY, Jeong YM, et al:
Indole-3-acetic acid/horseradish peroxidase-induced apoptosis
involves cell surface CD95 (Fas/APO-1) expression. Biol Pharm Bull.
29:1625–1629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkes LK and Wardman P: Enhancing the
efficacy of photodynamic cancer therapy by radicals from plant
auxin (indole-3-acetic acid). Cancer Res. 63:776–779.
2003.PubMed/NCBI
|
|
17
|
Kim DS, Kim SY, Jeong YM, et al:
Light-activated indole-3-acetic acid induces apoptosis in g361
human melanoma cells. Biol Pharm Bull. 29:2404–2409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SY, Ryu JS, Li H, et al: UVB-activated
indole-3-acetic acid induces apoptosis of pc-3 prostate cancer
cells. Anticancer Res. 30:4607–4612. 2010.PubMed/NCBI
|
|
19
|
Wang D, Wang Z, Tian B, Li X, Li S and
Tian Y: Two hour exposure to sodium butyrate sensitizes bladder
cancer to anticancer drugs. Int J Urol. 15:435–441. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vander Heiden MG, Chandel NS, Williamson
EK, Schumacker PT and Thompson CB: Bcl-xL regulates the membrane
potential and volume homeostasis of mitochondria. Cell. 91:627–637.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alnemri ES, Livingston DJ, Nicholson DW,
et al: Human ICE/CED-3 protease nomenclature. Cell. 87:1711996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu TZ, Cheng JT, Yiin SJ, et al:
Isoobtusilactone A induces both caspase-dependent and -independent
apoptosis in Hep G2 cells. Food Chem Toxicol. 46:321–327. 2008.
View Article : Google Scholar
|
|
24
|
Pap M and Cooper GM: Role of glycogen
synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell
survival pathway. J Biol Chem. 273:19929–19932. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pap M and Cooper GM: Role of translation
initiation factor 2B in control of cell survival by the
phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta
signaling pathway. Mol Cell Biol. 22:578–586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CF, Chen CL, Chiang CW, Jan MS, Huang
WC and Lin YS: GSK-3beta acts downstream of PP2A and the PI
3-kinase-Akt pathway and upstream of caspase-2 in ceramide-induced
mitochondrial apoptosis. J Cell Sci. 120:2935–2943. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bondar VM, Sweeney-Gotsch B, Andreeff M,
Mills GB and McConkey DJ: Inhibition of the phosphatidylinositol
3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma
cells in vitro and in vivo. Mol Cancer Ther. 1:989–997.
2002.PubMed/NCBI
|
|
28
|
Zheng WH and Quirion R: Insulin-like
growth factor-1 (IGF-1) induces the activation/phosphorylation of
Akt kinase and cAMP response element-binding protein (CREB) by
activating different signaling pathways in PC12 cells. BMC
Neurosci. 7:512006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Stephens PA, Middleton FK and
Curtin NJ: Targeting the S and G2 checkpoint to treat cancer. Drug
Discov Today. 17:194–202. 2012. View Article : Google Scholar
|
|
30
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon MK, Mitrea DM, Ou L and Kriwacki RW:
Cell cycle regulation by the intrinsically disordered proteins p21
and p27. Biochem Soc Trans. 40:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarraf CE and Bowen ID: Proportions of
mitotic and apoptotic cells in a range of untreated experimental
tumours. Cell and tissue kinetics. 21:45–49. 1988.PubMed/NCBI
|
|
33
|
Wyllie AH: Apoptosis (the 1992 Frank Rose
Memorial Lecture). Br J Cancer. 67:205–208. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Green DR: Apoptotic pathways: paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scoltock AB and Cidlowski JA: Activation
of intrinsic and extrinsic pathways in apoptotic signaling during
UV-C-induced death of Jurkat cells: the role of caspase inhibition.
Exp Cell Res. 297:212–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boatright KM, Renatus M, Scott FL, et al:
A unified model for apical caspase activation. Mol Cell.
11:529–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kruyt FA and Schuringa JJ: Apoptosis and
cancer stem cells: Implications for apoptosis targeted therapy.
Biochem Pharmacol. 80:423–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hockenbery DM, Oltvai ZN, Yin XM, Milliman
CL and Korsmeyer SJ: Bcl-2 functions in an antioxidant pathway to
prevent apoptosis. Cell. 75:241–251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim E, Matsuse M, Saenko V, et al:
Imatinib enhances docetaxel-induced apoptosis through inhibition of
nuclear factor-kappaB activation in anaplastic thyroid carcinoma
cells. Thyroid. 22:717–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee R and Collins T: Nuclear factor-kappaB
and cell survival: IAPs call for support. Circ Res. 88:262–264.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: a hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lawlor MA and Alessi DR: PKB/Akt: a key
mediator of cell proliferation, survival and insulin responses? J
Cell Sci. 114:2903–2910. 2001.PubMed/NCBI
|
|
45
|
Martelli AM, Tazzari PL, Tabellini G, et
al: A new selective AKT pharmacological inhibitor reduces
resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic
acid and ionizing radiation of human leukemia cells. Leukemia.
17:1794–1805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim SY, Ryu JS, Li H, et al: UVB-activated
indole-3-acetic acid induces apoptosis of PC-3 prostate cancer
cells. Anticancer Res. 30:4607–4612. 2010.PubMed/NCBI
|
|
47
|
Folkes LK and Wardman P: Oxidative
activation of indole-3-acetic acids to cytotoxic species-a
potential new role for plant auxins in cancer therapy. Biochem
Pharmacol. 61:129–136. 2001. View Article : Google Scholar : PubMed/NCBI
|