Introduction
Hepatocellular carcinoma (HCC) cells commonly
develop following liver cirrhosis and fibrosis (1). Liver fibrosis is considered a
premalignant disease, which occurs initially during pathological
changes of the liver (2,3). During chronic liver damage, hepatic
stellate cells (HSCs), the predominant type of hepatic
non-parenchymal cells, transdifferentiate into extracellular
matrix-producing myofibroblasts and are activated (4). The activated HSCs proliferate and
migrate towards the area of tissue remodelling, secreting
extracellular matrix (ECM) proteins and growth factors, and
providing an important microenvironment for hepatic regeneration
(5,6), which are vital for hepatocellular
function and the response of the liver to injury (7). Therefore, activated HSCs are the
predominant source of ECM proteins in liver fibrosis, on which HCC
commonly develops, and serves as an important mediator in the
inflammation-fibrosis-carcinoma axis and in tumor metastasis
(7,8).
There are apparent interactions between HCC cells
and HSCs during normal physiological processes and pathological
changes of the liver. The majority of investigations have focused
on the importance of HSCs in the development of HCC and revealed
that HSCs stimulate the invasion and migration of HCC cells,
promoting the tumorigenicity of HCC cells (9–11).
Previous studies have demonstrated that HCC cells secrete
cytokines, which promote the activation of HSCs (12–14).
The cross-talk between HCC cells and the surrounding
microenvironment is considered to be important in modulating the
biological behavior of the tumor. However, the molecular
mechanisms, which connect inflammation and cancer in the activation
of HSCs remain to be fully elucidated.
The present study investigated the cluster of
differentiation (CD)147 molecule, which is markedly expressed in
HCC cells. It is a key factor in the activation of HSCs and is an
important molecule during HCC cell-HSC cross-talk. The aim of the
present study was to investigate the mechanism underlying the
interaction between HCC cells and the surrounding microenvironment,
with a particular focus on the role of HCC cells in modulating the
biological activities of the HSCs.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) was
purchased from Hycylone (Logan, UT, USA) and fetal bovine serum
(FBS) was purchased from Sijiqing Biological Engineering Materials
(Hangzhou, China). TRIzol reagent and goat anti-mouse
secondary-antibodies conjugated with Alexa Fluor 594 (cat. no.
A-11005) or fluorescein isothiocyanate (FITC; cat. no. A16079) were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
ReverTra Ace-a-TM was purchased from Toyobo Co., Ltd, (Osaka,
Japan) and a mouse monoclonal antibody against α-smooth muscle
actin (α-SMA) (cat. no. ab7817) and CD147 (cat. no. ab78106) were
purchased from Abcam (Cambridge, UK). Goat anti-mouse secondary
antibodies, conjugated with horseradish peroxidase (cat. no.
BA1004), were purchased from Boster, Ltd. (Wuhan, China).
Cell culture and collection of
HCC-conditioned medium (CM)
The FHCC-98 human HCC cell line and the LX-2 human
HSC line (5×105 cells/100 mm culture dish) (Department
of Cell Biology, Fourth Military Medical University, Xi’an, China),
were cultured at 37°C in a humidified atmosphere, containing 5%
CO2 in DMEM, containing 5% FBS, 100 U/ml penicillin and
100 mg/ml streptomycin (HyClone Laboratories, Inc., Logan, UT,
USA).
For co-culture, the FHCC-98 and LX-2 cells
(5×105 cells/6-well plate) were mixed at a ratio of 1:1
and cultured in DMEM, containing 5% FBS, 100 U/ml penicillin and
100 mg/ml streptomycin.
The cells were grown until 70% confluent and were
subsequently incubated with fresh DMEM. Following incubation for 24
h, the HCC-CM was collected and centrifuged at 600 × g for 10 min
at room temperature to remove debris, filtered through a 0.2 mm
filter (EMD Millipore, Billerica, MA, USA) and stored at −20°C
until use.
Proliferation assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Aladdin Industrial, City of Industry, CA, USA) was performed
to detect the proliferation rate of the LX-2 cells. Briefly,
5×103 LX-2 cells were seeded into a 96-well plate
(Corning, Inc., Corning, NY, USA) for 24 h at 37°C and the cells
were then starved for an additional 24 h in DMEM supplemented with
0.5% FBS. The medium was replaced with DMEM containing 2% FBS and
different quantities of CM (10, 20, 30, 40, 50, 60 and 70%) or
CD147 (0.125, 0.25, 0.5 and 1.0 μg/ml) for 24 h. A total of
10 μl MTT solution (10 mg/ml) was added to each well and
incubated at 37°C for 4 hours. The media was then removed, 100
μl dimethyl sulfoxide (Shanghai Ziyi Reagent Factory,
Shanghai, China) was added to each well, and the plate was agitated
for 10 min, in order to dissolve the crystals that had formed. The
plate was then incubated at 37°C for 10 min, and absorbance was
measured at a wavelength of 490 nm using an enzyme-linked
immunosorbent assay detector (DG5031; Nanjing East China
Electronics Group Co., Ltd., Nanjing, China). Cell proliferation
was measured using an MTT assay based on the change in absorbance
at 490 nm using the formula: (A490experimental −
A490control) × 100% / A490control.
Detection of metalloproteinase (MMP)
secretion using gelatin zymography
Primary-cultured HSCs (5×103) at passage
two were seeded into a 96-well plate. At 70% confluence, the cells
were starved overnight in serum-free DMEM at 37°C prior to the
media being replaced with DMEM-supplemented 2% FBS and different
concentrations of CD147 (0.125, 0.25, 0.5 and 1.0 μg/ml).
Following incubation for 24 h, the cells were cultured in
serum-free DMEM for 24 h. The medium was harvested and the
expression levels of the MMPs were measured using gelatin
zymography, which was conducted as follows. Samples (40 μl)
to be tested were mixed with 2X Tris-glycine SDS sample buffer and
were rested for 10 min at room temperature. The gel, which
contained 0.1% Gelatin (Beijing YiRan Biological Technology Co.,
Ltd., Beijing, China), was ran with 1X Tris-glycine SDS running
buffer. The gels were then incubated at room temperature with
agitation in zymogram renaturing buffer for 30 min, then with
zymogram developing buffer for 30 min. The buffer was then
refreshed and it was incubated at 37°C overnight. The gels were
then stained with 0.5% (w/v) Coomassie Blue R-250(Beijing YiRan
Biological Technology Co., Ltd.) for 2 h, then were destained with
Coomassie R-250 destaining solution until areas of protease
activity appeared as clear bands. The Tris-glycine SDS
sample/running buffers, the zymogram renaturing/developing buffers
and Coomassie R-250 destaining solution were all prepared by the
Environment Related Gene Key Laboratory of Ministry of Education
(Xi’an, China). Densitometric analysis of the expression of MMP was
performed using a calibrated GS-670 densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
The LX-2 cells were seeded into a 6-well plate and
were treated with different concentrations of CD147 0.125, 0.25,
0.5 and 1.0 μg/ml). Following 24 h treatment, the medium was
removed and the cells were harvested using radioimmunoprecipitation
(RIPA) cell lysis buffer (Beyotime Institute of Biotechnology,
Nantong, China). For the tissue specimens, the liver tissues were
collected from rat models of hepatic fibrosis induced by carbon
tetrachloride (CCl4; The Third Chemical Reagent Factory,
Tianjin, China). The tissues were cut into small sections and the
cells were disrupted using a tissue homogenate method. Briefly, the
tissue sections were added to RIPA, placed on ice, and subsequently
homogenized using a pro200 Homogenizer (Pro Scientific, Inc.,
Oxford, CT, USA). The total protein was extracted from 50 mg tissue
samples and LX-2 cells in a 6-well plate using RIPA cell lysis
buffer, and the protein concentration was measured using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Western blotting was performed, according to a
standard method.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
After treatment with 40% CM or 0.125, 0.25, 0.5 and
1 μg/ml CD147, total RNA was extracted from the LX-2 cells
using TRIzol reagent, according to the manufacturer’s instructions
(Promega Corporation, Madison, WI, USA). cDNA was reverse
transcribed from 1 μg total RNA, using ReverTra Ace-α™ kit (Toyobo
Co., Ltd., Osaka, Japan). RT-qPCR analysis was performed using SYBR
Green PCR Master mix (Applied Biosystems, Foster City, CA, USA),
according to the manufacturer’s instructions, using a StepOnePlus™
Real-Time PCR system (Applied Biosystems). A total of 1.6 μl
template cDNA was used for amplification, and the PCR conditions
were set at: Initial denaturation at 95°C for 30 sec, 95°C for 5
sec and 58°C for 30 sec for 35 cycles, then 95°C for 15 sec, 60°C
for 1 min, 95°C for 15 sec and annealing and extension at 58°C for
30 sec. The gene expression levels of α-SMA, collagen I and TIMP
were measured and compared against the expression of β-actin. The
sequences of the oligonucleotides used are shown in Table I. The data were analyzed usign
StepOne v2.3 software (Life Technologies, Carlsbad, CA, USA).
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| α-SMA | | |
| Sense |
TTCGTTACTACTGCTGAGCGTGAGA | 200 |
| Antisense |
AAGGATGGCTGGAACAGGGTC |
| Collagen I | | |
| Sense |
AACATGACCAAAAACCAAAAGTG | 253 |
| Antisense |
CATTGTTTCCTGTGTCTTCTGG |
| TIMP-1 | | |
| Sense |
AGACCTACACTGTTGGCTG | 130 |
| Antisense |
GACTGGAAGCCCTTTTCAGAG |
| β-actin | | |
| Sense |
TGCTGTCCCTGTATGCCTCTG | 261 |
| Antisense |
TTGATGTCACGCACGATTTCC |
Immunofluorescence staining
The FHCC-98 and LX-2 cells (1×105
cells/6-well plate) were mixed and seeded into 6-well plates with
coverslips (Huarui Medical Instrument Co., Ltd., Taizhou, China).
Following co-culture for 1, 2 or 4 days, the cells were washed with
1X phosphate-buffered saline (PBS) and were fixed in cold acetone
(Nanjing Chemical Reagent Co., Ltd., Nanjing, China) for 10 min at
room temperature. The aspecific sites were blocked for 30 min using
2% bovine serum albumen (Shanghai Gaochuang Chemical Technology
Co., Ltd., Shanghai, China) in PBS. The coverslips were incubated
with mouse monoclonal primary antibodies against α-SMA (cat. no.
ab7817) and CD147 (cat. no. ab78106), diluted 1:500 with blocking
solution, overnight at 4°C. The control groups were only incubated
with one of α-SMA or CD147. Following three washes in PBS, the
cells were incubated with secondary antibodies conjugated to
fluorophores (FITC, BA1101; Cy3, BA1031; Invitrogen Life
Technologies) for 1 h at room temperature, following which,
4,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, USA)
was added to stain the nuclei. The coverslips were washed, as
above, and then mounted (PBS-glycerin; Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China) and observed using
fluorescence microscopy (BX53 microscope and DP72 charge-coupled
device camera; Olympus Corporation, Tokyo, Japan).
Rat models of hepatic fibrosis
The present study was approved by the ethics
committee of Xi’an Jiaotong University Laboratory Animal Center
(Xi’an, China). Specific pathogen-free male Sprague-Dawley rats
(n=10; weight, ~250 g) were supplied by the Experimental Animal
Centre of the Fourth Military Medical University (Xi’an, China) and
were maintained in a sterile room at 25°C with 40% humidity, a
natural light/dark cycle and ad libitum access to food. The
rats were randomly assigned into three groups: Control group, 4
weeks CCL4 treatment group and 8 weeks CCL4
treatment group. For the induction of liver fibrosis, the animals
were intraperitoneally injected with 2 ml CCl4/peanut
oil (Shandong Luhua Group Co., Ltd., Laiyang, China) solution (20%
v/v) twice a week for 4 or 8 weeks (CCl4 group). The
rats were administered 2 ml physiological saline (PS; 0.9% NaCl in
ddH2O), replacing the CCl4, in the PS group.
The rats were subsequently sacrificed by cervical dislocation
following 4 or 8 weeks of CCl4 induction and with
intraperitoneal anesthetization with 0.6 ml sodium pentobarbital
(2% w/v; Shanghai XiTang Biotechnology Co., Ltd., Shanghai,
China).
Detection of α-SMA and CD147 by
immunohistochemical staining
The liver tissues were fixed in 10% formalin (Xi’an
Fuli Chemical Plant, Xi’an, China) at room temperature and embedded
in paraffin (solid; melting point, 56–58°C) at 60°C Sections were
then cut (5 μm) from the tissues. Following
deparaffinization by an ascending gradient of alcohol (Xi’an
Chemical Reagent Factory, Xi’an, China) from 70% to 100% and
hydration by a descending gradient of alcohol from 100% to 70%, the
tissue sections were immunohistochemically stained, according to
the manufacturer’s instructions. Briefly, the tissue sections were
boiled for 20 min in citrate buffer solution for antigen retrieval
and incubated for 10 min with 30% H2O2 in an
80% methanol solution to inactivate the endogenous peroxidases.
Following washing three times with PBS, the liver tissue sections
were incubated overnight at 4°C with primary antibodies against
α-SMA (cat. no. ab7817) and CD147 (cat. no. ab78106), which were
diluted 1:100 in blocking buffer (PBS containing 5% bovine serum
albumin and 0.1% Triton X-100; Sigma-Aldrich). Following washing,
as above, the slides were incubated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (cat. no.
BA1004; 1:200) for 1 h at room temperature. Diaminobenzidine (DAB
Horseradish Peroxidase Chromogenic Reagent kit; Beyotime Institute
of Biotechnology) was added to the slides to visualize specific
antigen, and hematoxylin (Shanghai Yuanye Biotechnology Co., Ltd.,
Shanghai, China) was used to stain the nucleus. Finally, the
tissues sections were dehydrated, hyalinized sequentially and were
then mounted for visualization (BX53; Olympus Corporation).
Detection of α-SMA and CD147 in liver
tissues by western blotting
Tissue samples (50 mg) were lysed in non-ionic
detergent-containing buffer (RIPA lysis buffer). Following
centrifugation, the protein concentration was determined using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology). The
total protein (50 μg) from each sample was subjected to 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc.). The membrane was blocked using 5%
non-fat milk for 1 h at room temperature and was then incubated
with mouse monoclonal antibodies against α-SMA (cat. no. ab7817)
and CD147 (cat. no. ab78106) at 4°C overnight. Following washing
with Tris-buffered saline containing 0.1% Tween-20, the membrane
was incubated with horseradish peroxidase-conjugated goat
anti-mouse (cat. no. BA1004; 1:500) antibody for 1 h at room
temperature. Immunoreactive bands were visualized using an
enzyme-linked chemiluminescence detection kit (GE Healthcare,
Piscataway, NJ, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation of three independent samples. The results were analyzed
using Student’s t-test for independent samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of HSCs is induced by
HCC-CM
To determine the role of HCC-CM on the activation of
the LX-2 cells, the viability of the LX-2 cells was assessed using
an MTT assay following 24 h stimulation with CM. As shown in
Fig. 1A, treatment with HCC-CM at
concentrations between 10 and 70%, stimulated cell growth, however,
cell viability was increased more markedly by lower concentrations
of CM compared with higher concentrations. A significant increase
in proliferation rate (25.8%) was observed following the addition
of 40% CM.
 | Figure 1LX-2 cell activation is promoted by
CM. (A) LX-2 cells were incubated with different concentrations of
CM from FHCC-98 cells for 24 h and cell proliferation was measured
using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay. The proliferation rate was calculated as follows:
(A490 experimental − A490 control) × 100% /
A490 control. (B) Western blotting was used to detect
the expression of α-SMA in human LX-2 HSCs following treatment with
HCC-CM. β-actin was used as the internal standard for
normalization. (C) Relative expression of α-SMA was measured using
Quantity One density scanning software. (D) mRNA expression levels
of α-SMA, collagen I and TIMP-1 were determined by RT-qPCR. The
LX-2 cells were treated with 40% CM for 24 h and the cells were
harvested. The total RNA was extracted and was subjected to RT and
fluorescence qPCR. β-actin was used as an internal standard for
normalization. The data are expressed as the mean ± standard
deviation of three independent experiments (*P<0.05, compared
with the control group). CM, conditioned medium; HSC, hepatic
stellate cell; HCC, hepatocellular carcinoma; SMA, smooth muscle
actin; TIMP, tissue inhibitor of matrix metalloproteinase; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
The protein expression of α-SMA in human LX-2 HSCs
following treatment with HCC-CM was investigated by western
blotting. As shown in Fig. 1B,
when the LX-2 cells were treated with 20, 40 or 60% HCC-CM, the
expression of α-SMA increased compared with the untreated control
cells. The highest expression level of α-SMA was detected in the
LX-2 cells stimulated with 40% CM. The relative expression of α-SMA
in the LX-2 cells treated with HCC-CM is shown in Fig. 1C.
RT-qPCR was performed to determine whether HCC-CM
induced the activation of LX-2 cells. As shown in Fig. 1D, the expression levels of α-SMA,
collagen and TIMP were upregulated in the LX-2 cells following
treatment with 40% CM, suggesting that the LX-2 cells were
activated following stimulation with HCC-CM.
CD147 stimulates the activation of
HSCs
The present study used CD147 to estimate its role in
the activation of HSCs. LX-2 cell viability was detected using an
MTT assay 24 h after stimulation with CD147. As shown in Fig. 2A, CD147 at concentrations of 0.5
and 1 μg/ml promoted the proliferation of LX-2 cells in a
dose-dependent manner, as expected, with a maximum proliferation
rate of 32.5% following treatment with 1 μg/ml CD147.
RT-qPCR was used to detect the expression levels of
α-SMA, collagen I and TIMP in LX-2 cells following stimulation with
CD147 at different concentrations. As shown in Fig. 2B, these genes were upregulated in a
dose-dependent manner, suggesting that the LX-2 cells were
activated following treatment with CD147.
Gelatin zymography was used to analyze the secretion
of MMP-2 in primary HSCs following stimulation with CD147. The
secretion of MMP-2 in LX-2 cells treated with CD147 at different
concentrations increased compared with the control cells (Fig. 2C). The quantitative determination
of MMP-2 by scanning densitometry of the gelatin zymography
revealed a significant difference in the secretion of MMP-2 between
the stimulated and non-stimulated cells (Fig. 2D; P<0.05).
HCC cells induce the activation of
HSCs
The HCC cells and LX-2 cells were co-cultured, as
described above. The mixed cells were seeded into a 6-well plate
with coverslips. Following culture for 24, 48 or 96 h,
immunofluorescence staining was used to detect the expression
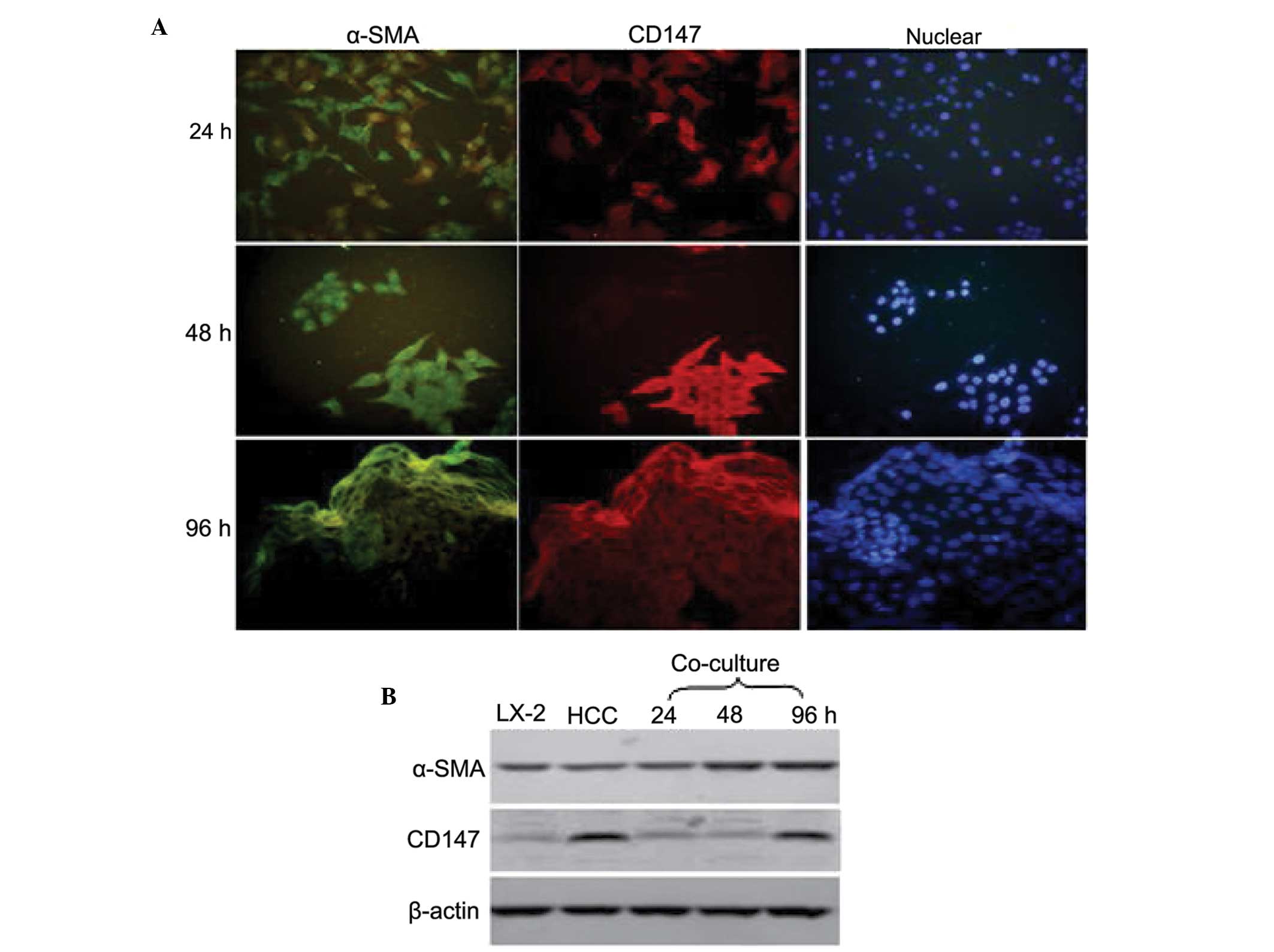
levels of α-SMA and CD147, as described above. As shown in Fig. 3A, following co-culture for 24 and
48 h, the HCC cells expressed CD147 (red fluorescence) and the LX-2
cells expressed α-SMA (green fluorescence). Following co-culture
for 96 h, the LX-2 cells expressed α-SMA and CD147, suggesting that
the LX-2 cells were activated and the shape changed to that of
fibroblasts.
To further investigate whether the expression of
CD147 in the LX-2 cells was stimulated by HCC cells, the expression
levels of α-SMA and CD147 were detected in HCC cells, LX-2 and
co-cultured cells by western blotting (Fig. 3B). The LX-2 cells expressed α-SMA
and CD147 on activation following co-culture with the HCC
cells.
CD147 is expressed in rat models of
hepatic fibrosis induced by CCl4
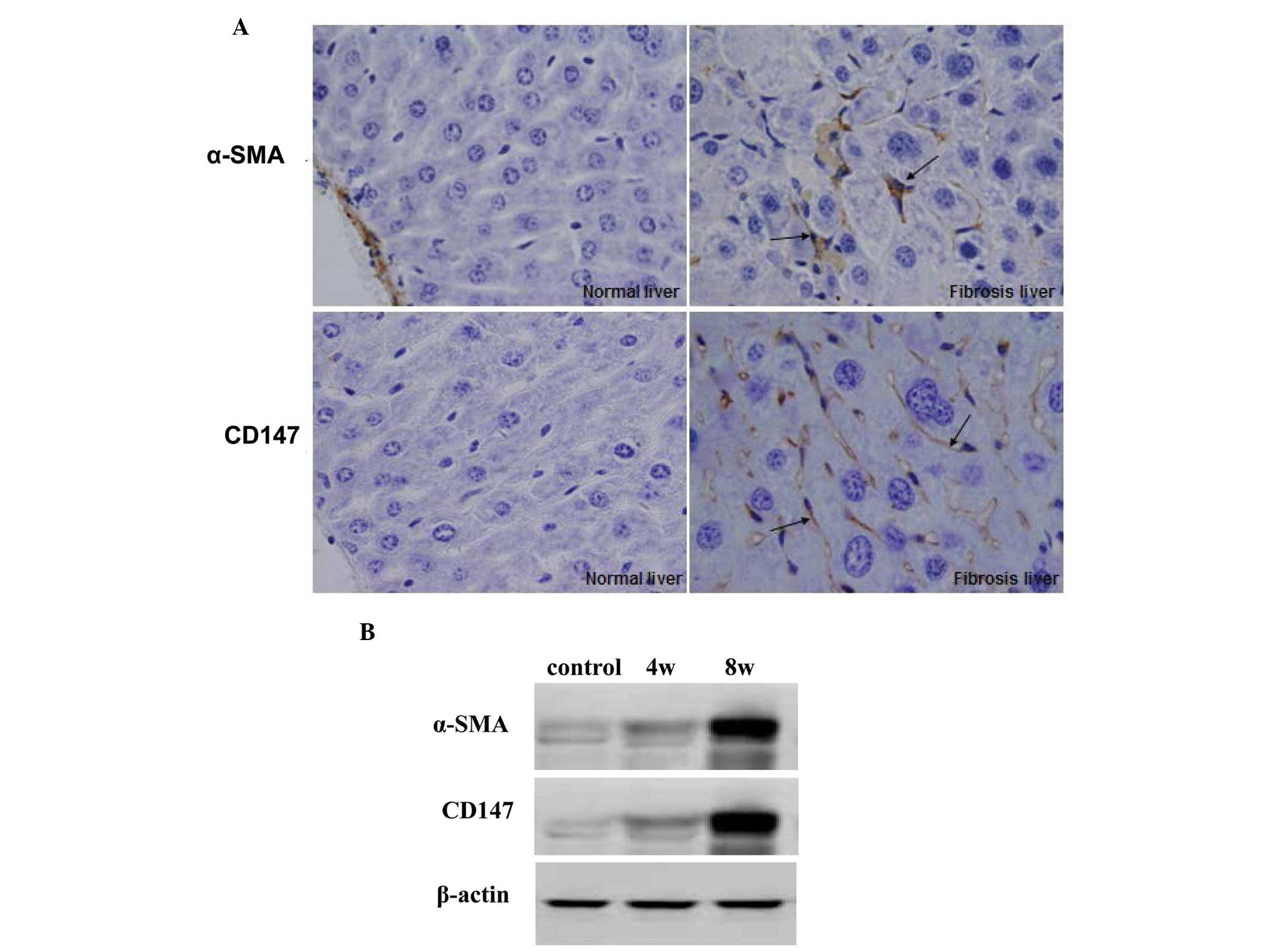
The present study investigated the expression of
CD147 in the liver of rat models of hepatic fibrosis, induced by
CCl4, to determine whether activated HSCs secreted CD147
Following treatment with CCl4 for 4 or 8 weeks, the
model rats were sacrificed and the liver tissues were used to
detect the expression levels of α-SMA and CD147 by
immunohistochemistry and western blotting. As shown in Fig. 4A, following treatment with
CCl4 for 8 weeks, significant increases in the
expression levels of α-SMA and CD147 were detected in the
CCl4-treated rat liver compared with the normal control
rats. Western blotting demonstrated identical results in the rat
hepatic tissues (Fig. 4B).
Discussion
Liver disease is characterized by excessive
deposition of ECM proteins (2,3). The
excess deposition of ECM proteins disrupts the normal architecture
of the liver, which alters the normal function and, ultimately,
leads to pathophysiological damage (3,15).
During the development of liver disease, HCC cells and HSCs secrete
cytokines (16) and subsequently
produce an inflammatory microenvironment and dynamic stroma in the
ECM (10), in which these two cell
types grow. Following liver injury, the HSCs undergo a complex
transformation or activation process, in which the cells change
from quiescent cells into myofibroblasts (6). This, in part, is characterized by the
appearance of the α-SMA cytoskeletal protein, therefore, the
expression of α-SMA has been considered as a useful marker of
activated HSCs (17). Activated
HSCs are also the primary cell type responsible for collagen
synthesis during liver disease (5).
There are reciprocal interactions between HCC cells
and HSCs during hepatocarcinogenesis. Numerous types of solid
malignant tumor arise on a background of inflamed and/or fibrotic
tissues, which are detected in >80% cases of HCC (18). The infiltration of α-SMA-positive
HSCs in the HCC stroma suggests that activated HSCs are important
in the occurrence and development of HCC in patients with cirrhosis
(19). Previous studies have
demonstrated that HSCs drive the progression of HCC (20,21).
The role of HCC cells on the activation of HSCs has also been
investigated (22,23), however, few studies have
investigated the molecular mechanism underlying the reciprocal
interactions between HCC cells and HSCs. The present study
demonstrated that HCC increases the activation of HSCs, and that
CD147 is a key molecule involved in the cross-talk between HCC
cells and HSCs.
CD147, also termed EMMPRIN or basigin, is a
transmembrane protein, which is important in the metastasis and
progression of cancer via inducing the production of MMPs (24). Since this protein exhibits marked
expression levels in several types of carcinoma, HAb18G/CD147 acts
as a cancer-associated biomarker for the detection of cancer
(25), and is an effective target
molecule for its treatment (26).
CD147 is also expressed highly in HCC and promotes metastasis and
progression (27), however, the
function of CD147 in the activation of HSCs remains to be
elucidated.
In the present study, the LX-2 human HSC line,
induced by tumor-CM from human HCC cells, exhibited phenotypic
characteristics, including increased cell proliferation, secretion
of MMP-2 and gene expression levels of α-SMA, collagen I and
TIMP-1, which are also induced by CD147. The results suggested that
the CD147 molecule, secreted by the tumor cells, was involved in
the activation of HSCs. It has been reported that the recruitment
and activation of rat HSCs are under the control of tumor cells
(28), and that HCC cell stimulate
the growth, migration and expression of pro-angiogenic genes in
human HSCs (13), indicating a
different manner of tumor-induced activation from the classic
fibrosis type activation.
There was increased expression of CD147 in the
membranes of HCC cells, and also in the LX-2 cells following
co-culture with the HCC cells, with a change in shape of the LX-2
to that of fibroblasts, suggesting that HCC cells triggered the
epithelial-mesenchymal transformation of the HSCs via the secretion
of CD147, which led to further activation of LX-2 cells and the
secretion of CD147. These results demonstrated that HCC cells
stimulated the activation of HSCs and the expression of CD147 in
the LX-2 cells. The increased expression of CD147 triggers the
epithelial-mesenchymal transformation of HCC cells, leading to a
more aggressive and invasive phenotype (9,10).
The present study further detected the expression of CD147 in liver
tissues from rat models of hepatic fibrosis induced by
CCl4. Treatment with CCl4 for 8 weeks led to
marked expression of CD147 in the liver tissue. Combined with the
in vitro results, it was suggested that CD147 is a key
molecule involved in the cross-talk between HCC cells and HSCs.
A previous study demonstrated that HCC cells
stimulate the growth, migration and expression of pro-angiogenic
genes in human HSCs (13). Another
investigation revealed that the activation of cultured rat HSCs is
induced by tumoral hepatocytes and fetal bovine serum (12). The present study demonstrated that
HCC cells secreted CD147, promoted the activation of HSCs and
induced the expression of associated genes. These results are
consistent with a previous study, which suggested the same function
of HCC cells during the activation and transformation of HSCs
(14).
Previous studies have demonstrated that activated
HSCs promote the development of HCC (9,10,20,21),
and that HSC cross-talk in the liver results in a permissive
inflammatory microenvironment, which drives the progression of HCC
(7).
In conclusion, although sevceral proteins and growth
factors contribute to HCC cell-HSC interaction, the present study
demonstrated that CD147 contributed to this cross-talk and also
affected the tissue microenvironment. This affected the biological
properties of the HCC cells and HSCs, possibly inducing a different
clinical outcome. These findings emphasize the requirement for
novel therapies targeting different tissue microenvironment
components.
Acknowledgments
This study was supported by a grant from the Science
and Technology Research Projects of Shaanxi Province, China (no.
2010-KII-G2).
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HSC
|
hepatic stellate cell
|
|
CM
|
conditioned medium
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
α-SMA
|
α-smooth muscle actin
|
|
TIMP-1
|
tissue inhibitor of
metalloproteinase-1
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
ECM
|
extracellular matrix
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Mikula M, Proell V, Fischer AM and
Mikulits W: Activated hepatic stellate cells induce tumor
progression of neoplastic hepatocytes in a TGF-beta dependent
fashion. J Cell Physiol. 209:560–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guyot C, Lepreux S, Combe C, et al:
Hepatic fibrosis and cirrhosis: The (myo)fibroblastic cell
subpopulations involved. Int J Biochem Cell Biol. 38:135–151.
2006.
|
|
5
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novo E, di Bonzo LV, Cannito S, et al:
Hepatic myofibroblasts: a heterogeneous population of
multifunctional cells in liver fibrogenesis. Int J Biochem Cell
Biol. 41:2089–2093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coulouarn C, Corlu A, Glaise D, et al:
Hepatocyte-stellate cell cross-talk in the liver engenders a
permissive inflammatory microenvironment that drives progression in
hepatocellular carcinoma. Cancer Res. 72:2533–2542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang BB, Cheng JY, Gao HH, et al: Hepatic
stellate cells in inflammation-fibrosis-carcinoma axis. Anat Rec
(Hoboken). 293:1492–1496. 2010. View
Article : Google Scholar
|
|
9
|
Zhao W, Zhang L, Yin Z, et al: Activated
hepatic stellate cells promote hepatocellular carcinoma development
in immunocompetent mice. Int J Cancer. 129:2651–2661. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amann T, Bataille F, Spruss T, et al:
Activated hepatic stellate cells promote tumorigenicity of
hepatocellular carcinoma. Cancer Sci. 100:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung JO, Gwak GY, Lim YS, et al: Role of
hepatic stellate cells in the angiogenesis of hepatoma. Korean J
Gastroenterol. 42:142–148. 2003.In Korean. PubMed/NCBI
|
|
12
|
Xia Y, Chen R, Song Z, et al: Gene
expression profiles during activation of cultured rat hepatic
stellate cells by tumoral hepatocytes and fetal bovine serum. J
Cancer Res Clin Oncol. 136:309–321. 2010. View Article : Google Scholar
|
|
13
|
Sancho-Bru P, Juez E, Moreno M, et al:
Hepatocarcinoma cells stimulate the growth, migration and
expression of pro-angiogenic genes in human hepatic stellate cells.
Liver Int. 30:31–41. 2010. View Article : Google Scholar
|
|
14
|
Xia YH, Song ZJ, Chen RX, et al: Analysis
of differential gene expression in rat hepatic stellate cells
activated by culture or hepatocellular carcinoma cell induction.
Zhonghua Zhong Liu Za Zhi. 31:164–169. 2009.PubMed/NCBI
|
|
15
|
Nagoshi S: Liver diseases. Nihon Rinsho.
72:726–729. 2014.In Japanese. PubMed/NCBI
|
|
16
|
Tahashi Y, Matsuzaki K, Date M, et al:
Differential regulation of TGF-beta signal in hepatic stellate
cells between acute and chronic liver injury. Hepatology. 35:49–61.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Zohar R and McCulloch CA: Multiple
roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell
Res. 312:205–214. 2006. View Article : Google Scholar
|
|
18
|
Ooi LL, Bay BH, Ng RT, et al: An animal
model for the study of hepatic stellate cell and hepatocellular
carcinoma interaction. Ann Acad Med Singapore. 28:95–98.
1999.PubMed/NCBI
|
|
19
|
Kurogi M, Nakashima O, Miyaaki H, et al:
Clinicopathological study of scirrhous hepatocellular carcinoma. J
Gastroenterol Hepatol. 21:1470–1477. 2006.PubMed/NCBI
|
|
20
|
Zhao W, Zhang L, Xu Y, et al: Hepatic
stellate cells promote tumor progression by enhancement of
immunosuppressive cells in an orthotopic liver tumor mouse model.
Lab Invest. 94:182–191. 2014. View Article : Google Scholar
|
|
21
|
Han S, Han L, Yao Y, et al: Activated
hepatic stellate cells promote hepatocellular carcinoma cell
migration and invasion via the activation of FAK-MMP9 signaling.
Oncol Rep. 31:641–648. 2014.
|
|
22
|
Nevzorova YA, Hu W, Cubero FJ, et al:
Overexpression of c-myc in hepatocytes promotes activation of
hepatic stellate cells and facilitates the onset of liver fibrosis.
Biochim Biophys Acta. 1832:1765–1775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang DW, Zhao YX, Wei D, et al: H
Ab18G/CD147 promotes activation of hepatic stellate cells and is a
target for antibody therapy of liver fibrosis. J Hepatol.
57:1283–1291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muramatsu T and Miyauchi T: Basigin
(CD147): a multifunctional transmembrane protein involved in
reproduction, neural function, inflammation and tumor invasion.
Histol Histopathol. 18:981–987. 2003.PubMed/NCBI
|
|
25
|
Weidle UH, Scheuer W, Eggle D, et al:
Cancer-related issues of CD147. Cancer Genomics Proteomics.
7:157–169. 2010.PubMed/NCBI
|
|
26
|
Hao JL, Cozzi PJ, Khatri A, et al: C
D147/EMMPRIN and CD44 are potential therapeutic targets for
metastatic prostate cancer. Curr Cancer Drug Targets. 10:287–306.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu S, Li Y, Zhang Y, et al: Expression
and clinical implications of H Ab18G/CD147 in hepatocellular
carcinoma. Hepatol Res. 45:97–106. 2015. View Article : Google Scholar
|
|
28
|
Faouzi S, Lepreux S, Bedin C, et al:
Activation of cultured rat hepatic stellate cells by tumoral
hepatocytes. Lab Invest. 79:485–493. 1999.PubMed/NCBI
|