Introduction
Atopic dermatitis (AD) has a complex etiology
encompassing immunological responses, susceptibility genes,
environmental triggers and compromised skin-barrier function
(1). AD, also known as eczema, is
a chronic inflammatory skin disease, which can occur in children
and adults (2). AD is accompanied
by symptoms of pruritus, erythema, edema and xerosis (3). The mechanisms underlying these skin
diseases are also associated with genetic and environmental factors
of AD pathogenesis (4).
Immunoglobulin (Ig)E, which is important in the pathogenesis of
allergy, is produced by B-lymphocytes following exposure of
dendritic cells to a foreign antigen under the control of T-helper
lymphocytes (5). An association
between 70–85% of human AD and IgE hyper-production has been
demonstrated and the extent of IgE sensitization is directly
associated with the severity of the disease (6). When two or more molecules of IgE
bound to the surface of an immune cell are simultaneously linked to
its specific allergen, the immune cell immediately releases
preformed inflammatory mediators, including histamine, which
induces the immediate effects or early phase of an allergic
reaction (1). Following IgE
release, immune cells also synthesize newly formed inflammatory
molecules, including cytokines, such as interleukins (IL) (1,7,8). The
AD animal model, 2,4-dinitrochlorobenzene (DNCB)-induced contact
hypersensitivity of the skin, is commonly used for investigating
the pathogenesis of allergic contact dermatitis (9). DNCB, an allergen, can be internalized
by local antigen presenting cells, including dermal dendritic cells
and macrophages, processed and presented to T cells in the lymph
nodes for activation (5), and
cause AD like-symptoms, including eczema, erythema, scaling and
hemorrhaging in skin lesions (1,6,8,10,11).
Chamaecyparis obtusa (C. obtusa), a species
of cypress, is a tropical tree native to the central part of Japan
and the southern part of Korea. Essential oil extracted from C.
obtusa contains several types of terpenes, including
monoterpenes, sesquiterpenes and diterpenes. The crude extract of
C. obtuse reduces allergic reactions in an AD mouse model
and effectively suppresses the levels of serum IgE and
proinflammatory cytokines, as well as mast cell appearance under
the dermis and hypodermis (12).
C. obtusa, regarded as the most representative medicinal
plant, contains active terpene compounds, which may have
pharmacological effects (13).
Volatile organic compounds (VOC) have been found to demonstrate an
association with asthma and immune responses (14–16).
To the best of our knowledge, there are no studies indicating that
exposure to the volatile organic compounds of C. obtusa
(VOCCo) can reduce allergic symptoms and, in particular, specific
IgE responses and immune cell infiltration. In addition, the
underlying mechanisms of the hypo-allergic effects of VOCCo have
not yet been elucidated. The aim of the current study was to
investigate whether VOCCo exposure is able to improve the symptoms
of AD and to determine whether it is amenable for use as a
pharmaceutical candidate.
Materials and methods
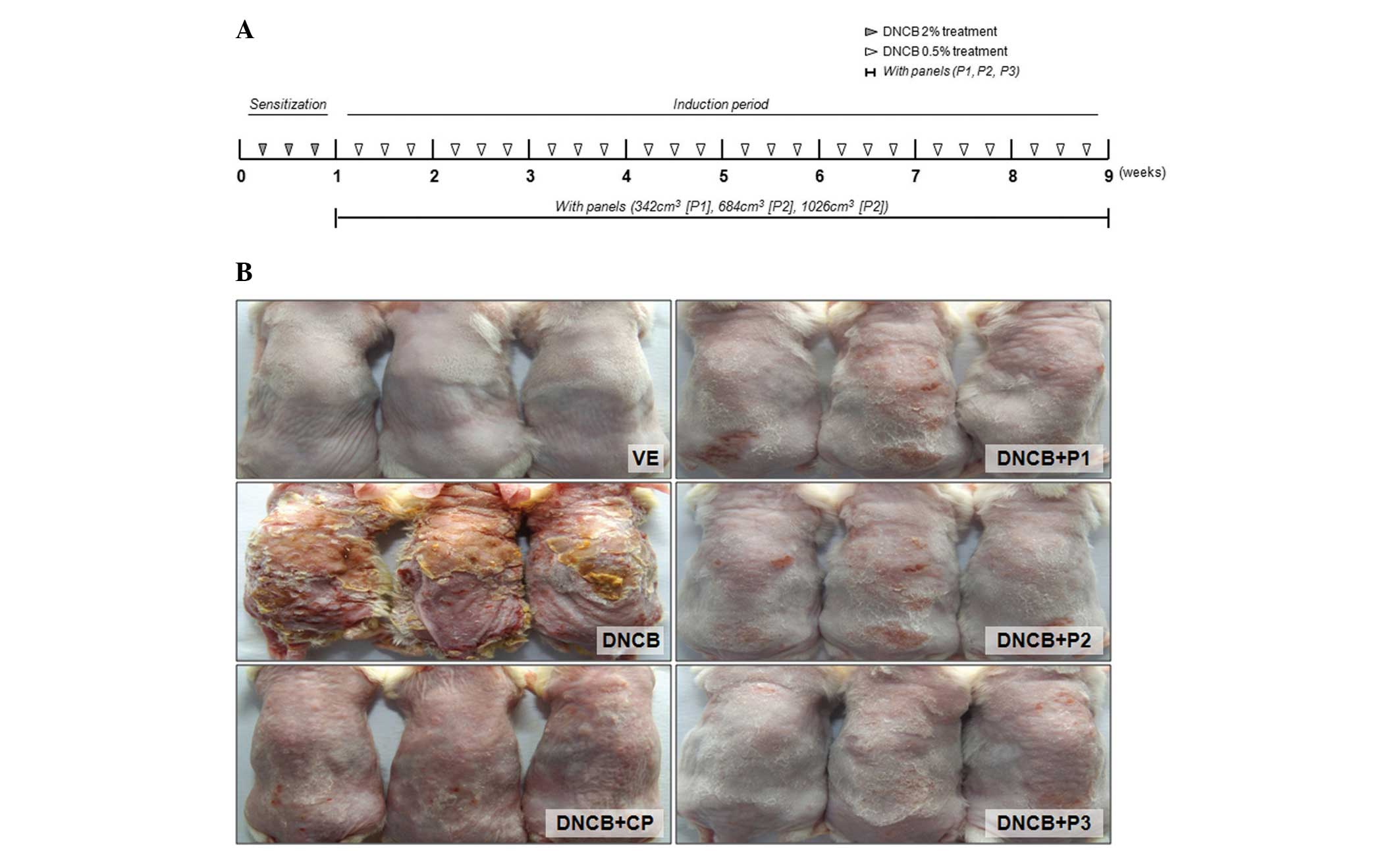
Animals and induction of an AD mouse
model
BALB/c mice (7 weeks old) were purchased from
Koatech (Pyeongtaek, Republic of Korea) and were housed in
polycarbonate cages with C. obtusa panel and corn cob
bedding and acclimated in an environmentally controlled room
(temperature, 23±2°C; relative humidity, 50±10%; frequent
ventilation and a 12:12-h light-dark cycle). The animal experiments
were approved by the Chungbuk National University Animal Care and
Use Committee (Cheongju, Korea) and all procedures were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals published by the National Institutes of Health (Bethesda,
MD, USA). DNCB (Sigma-Aldrich, St. Louis, MO, USA) dissolved in
acetone:olive oil (4:1) was used for the induction of dermatitis in
BALB/c mice. Briefly, hair was removed from a 2×4 cm region
spanning from the neck to the pelvis and the dorsal skin was
sensitized with 100 μl of 2% DNCB for 2 days during the
first week. Following the first sensitization, 100 μl of
0.5% DNCB was applied three times a week to the dorsal skin for an
additional 8 weeks. The VOCCo-untreated groups included the
vehicle-treated (VE) group, DNCB treatment only group (DNCB) and
the Clobetasol propionate ointment group (DNCB+CP). The
VOCCo-treated groups included the DNCB+P1 group (C. obtusa
panel, 342 cm3), DNCB+P2 group (684 cm3) and
the DNCB+P3 group (1,026 cm3). No substances were
applied to the skin surface on the final day of the experiment. The
mice were then sacrificed by ether inhalation and the skin, lymph
nodes and blood samples were collected for further analysis.
Level of serum IgE in an AD-like mouse
model
Blood samples were collected directly from the
inferior vena cava using a capillary tube at the end of the
experiment. Serum was obtained by centrifugation at 3,000 × g for
10 min at 4°C and stored at −70°C until use. Serum IgE levels were
measured using a mouse IgE ELISA Ready-Set-Go kit (eBiosciences,
San Diego, CA, USA) according to the manufacturer’s
instructions.
Histopathological alterations in an
AD-like mouse model
Dermis tissue was fixed by inflating the tissue with
10% formalin. The tissues were then embedded in paraffin, cut into
sections (5 μm) and stained with hematoxylin and eosin
(H&E). All tissue samples were examined and images were
captured and scored in a blinded manner under a light microscope
(BX51; Olympus, Tokyo, Japan). Images were captured on an Olympus
DP controller and manager under a microscope at (BX51; Olympus)
×100 magnification. For examination of the distribution of mast
cells beneath the dermis and hypo-dermis, the prepared tissues were
stained with toluidine blue and images were captured on an Olympus
DP controller and manager under a microscope (BX51; Olympus) at
×400 magnification. The number of mast cells in a 1 mm2
area was counted under a microscope at ×400 magnification.
RNA extraction and quantitative
polymerase chain reaction (PCR)
Total RNA was extracted from mouse skin using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. RNA concentrations were
measured using a microplate spectrophotometer (Epoch; BioTek
Instruments Inc., Winooski, VT, USA) at 260 nm. RNA quality was
evaluated by electrophoresis on 1% agarose gels. Total RNA (1
μg) was reverse transcribed into first-strand complementary
DNA (cDNA) using Moloney murine leukemia virus reverse
transcriptase (Invitrogen Life Technologies) and random primers
(9-mer; Takara Bio, Inc., Otsu, Shiga, Japan). Each cDNA sample (1
μl) was amplified with 10 μl of 2X SYBR®
Premix Ex Taq™ (Takara Bio, Inc.) and 10 pmol of each primer.
Amplification was performed using a 7300 Real-time PCR System
(Applied Biosystems, Foster City, CA, USA) with the following
parameters: Denaturation at 95°C for 5 min followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 45 sec. The following sequences of
oligonucleotide primers were used in the present study: IL-1β,
sense 5′-GAAATGCCACCTTTTGACAGTG-3′ and antisense
5′-CTGGATGCTCTCATCAGGACA-3′; IL-6, sense
5′-CTGCAAGAGACTTCCATCCAG-3′ and anti-sense
5′-AGTGGTATAGACAGGTCTGTTGG-3′; β-actin, sense
5′-TTCTACAATGAGCTGCGTGTG-3′ and antisense
5-ACCAGAGGCATACAGGGACA-3′. Relative expression levels in each
sample (normalized to that of β-actin) were determined using RQ
software (version 1.3; Applied Biosystems).
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed by one-way analysis of variance followed
by Tukey’s multiple comparison test. Statistical analyses were
performed using GraphPad Prism software (version 4.0; GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of VOCCo on skin lesions and serum
IgE levels in DNCB-treated mice
For investigation of the anti-allergic effects of
VOCCo in an AD-like mouse model, skin lesions were induced in
BALB/c mice using multiple topical applications of DNCB for 8 weeks
(Fig. 1). Marked inhibition of
AD-like skin lesions was observed in the VOCCo (P1, P2 and P3)
exposure groups. Treatment with DNCB resulted in induction of serum
IgE levels (up to 7-fold) compared with the VE group. The elevated
serum IgE level was decreased by clobetasol propionate (CP)
treatment (Fig. 2). Exposure to
VOCCo (P1, P2 and P3) resulted in a significant decrease in the
elevated IgE level and this anti-allergic effect on serum IgE level
was the highest in the P3 exposure group. The results indicated
that exposure to VOCCo can reduce IgE levels in the serum,
suggesting that VOCCo can ameliorate the hyper-allergic reaction in
an AD-like mouse model.
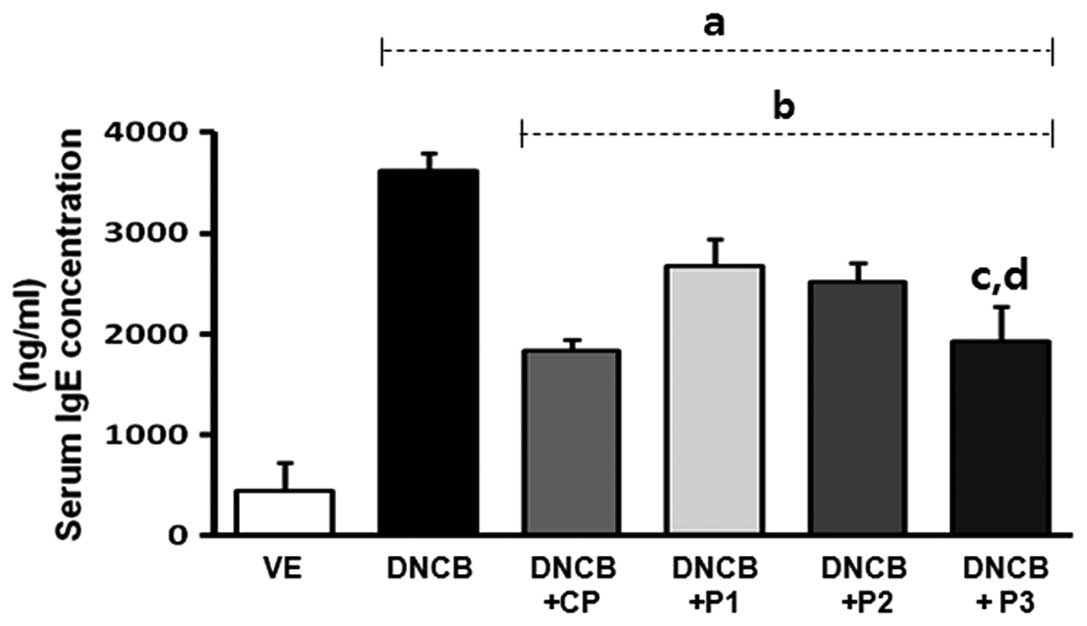 | Figure 2Effects of VOCCo on serum IgE levels
in DNCB-induced mice. The serum IgE levels in BALB/c mice were
measured at the end of the experiment (9 weeks later) using an
ELISA kit. VE, vehicle; DNCB, negative control; DNCB+CP, positive
control; DNCB+P1 (342 cm3), DNCB+P2 (684 cm3)
and DNCB+P3 (1,026 cm3) experimental groups. Values are
expressed as the mean ± standard deviation. aP<0.05
vs. VE; bP<0.05 vs. DNCB-treated group;
cP<0.05 vs. DNCB+P1; dP<0.05 vs.
DNCB+P2. VOCCo, volatile organic compounds of Chamaecyparis
obtusa; IgE, immunoglobulin E; DNCB, 2,4-dinitrochlorobenzene;
CP, clobetasol propionate. |
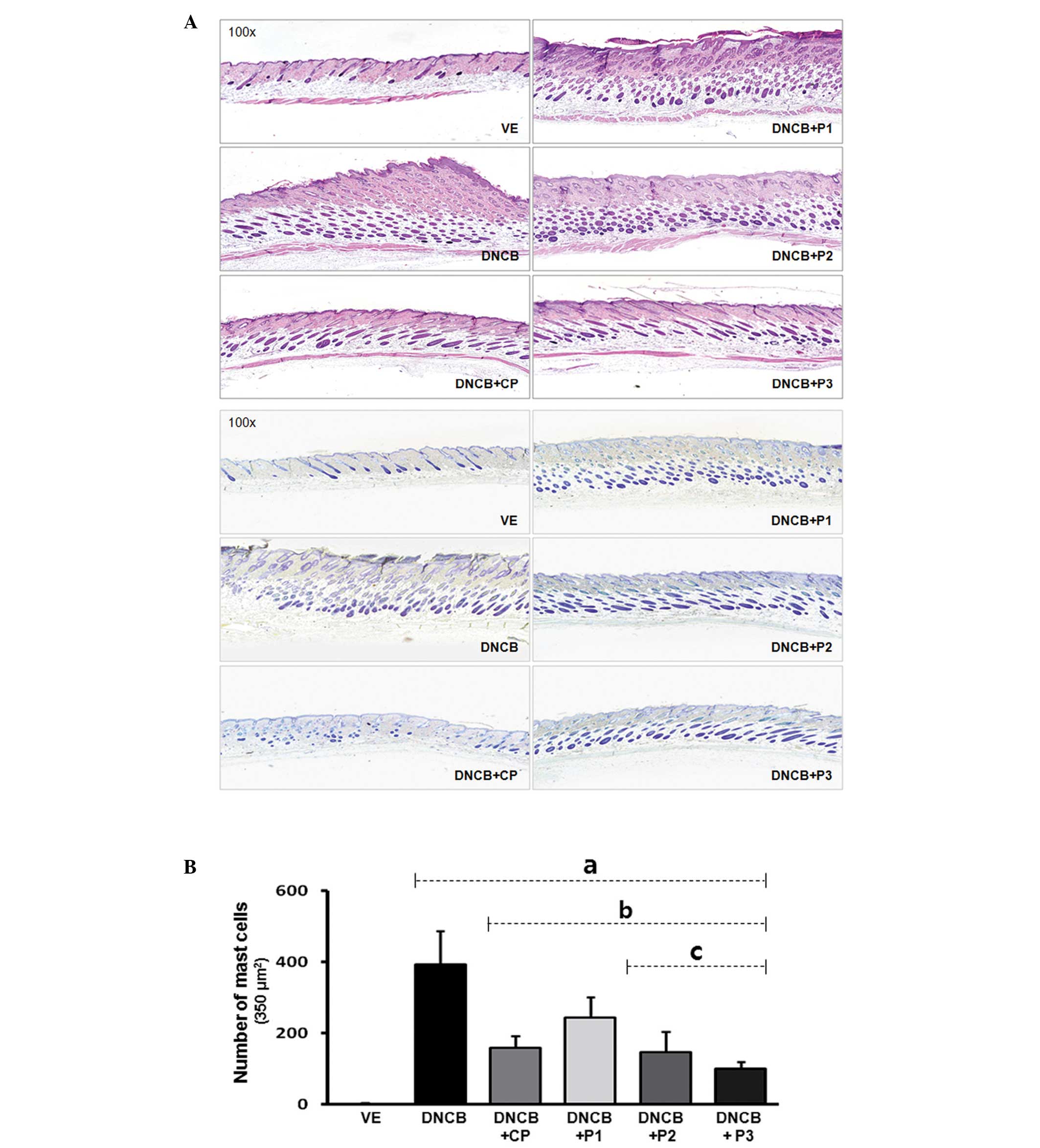
Histological findings in DNCB-treated
mice
For observation of skin thickness and immune cell
infiltration, DNCB-affected skin lesions were collected and stained
with H&E. As shown in Fig. 3,
DNCB induced an increase in skin thickness in DNCB-treated mice,
compared with the vehicle-treated group, and a decrease in
VOCCo-exposed mice. VOCCo (1,026 cm3) was expected to
have greater potential for recovery of AD-like skin compared with
the therapeutic control, CP. To determine the distribution of mast
cells beneath the dermis and hypodermis of AD-like mice, selected
tissues were stained with toluidine blue staining solution
following formalin fixation (Fig.
3A). The number of mast cells was counted under a light
microscope (BX51; Olympus) and expressed as the number of cells per
square millimeter of dorsal skin (Fig.
3A). Infiltration of mast cells in DNCB-treated mice increased
upon treatment with DNCB only, while infiltration was significantly
decreased upon exposure in all VOCCo exposure groups (Fig. 3B). These findings suggest that
VOCCo can inhibit the progression of AD-like skin lesions in the
DNCB-induced AD mouse model.
Effect of VOCCo on the expression of
proinflammatory cytokines in AD-induced mice
To determine the effect of VOCCo on the
proinflammatory cytokines, IL-1β and IL-6, the transcriptional
expression of these cytokines in DNCB-induced AD was evaluated. As
shown in Fig. 4, the expression of
IL-1β and IL-6 cytokines of the skin were induced by DNCB
treatment. Elevated expression of dermal IL-1β and IL-6 mRNA was
attenuated by CP treatment in AD-induced mice. Expression of IL-6
mRNA demonstrated a decrease in all VOCCo groups (P1, P2 and P3)
compared with the DNCB only treatment group. In addition, dermal
IL-1β mRNA expression decreased. These results suggest that VOCCo
can inhibit proinflammatory gene expression in the skin, which may
be important in the suppression of allergic reactions in an AD
mouse model.
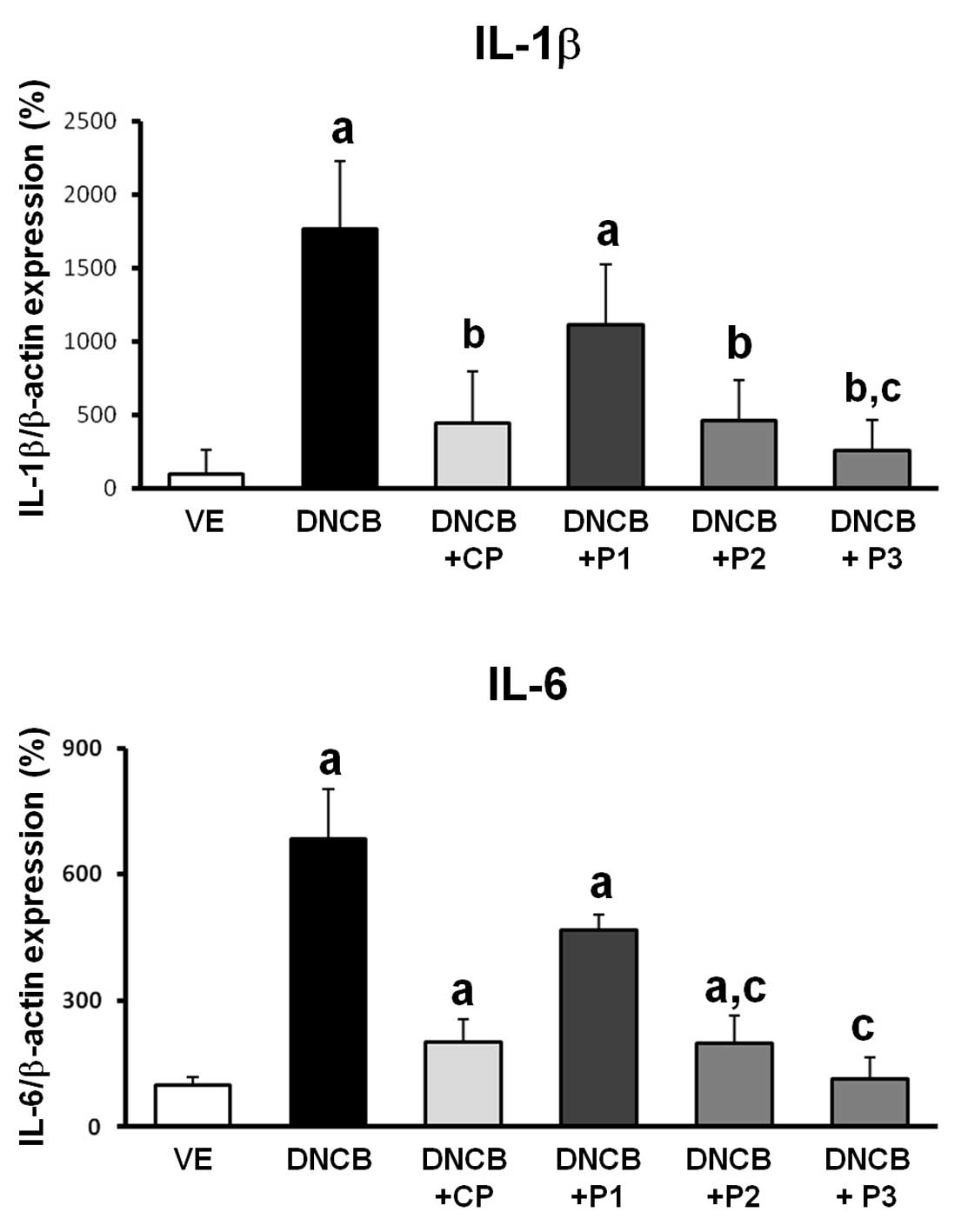 | Figure 4Proinflammatory cytokine mRNA
expression in dorsal skin lesions of DNCB-treated mice. Isolated
mRNA was analyzed by reverse transcription quantitative polymerase
chain reaction for IL-1β and IL-6. Results were internally
confirmed by the comparative CT method against β-actin as the
standard gene. VE, vehicle; DNCB, negative control; DNCB+CP,
positive control; DNCB+P1, DNCB+P2 and DNCB+P3 (2, 5 and 7%),
experimental groups. Values are presented as the mean ± standard
deviation. aP<0.05 vs. VE; bP<0.05 vs.
DNCB-treated group; cP<0.05 vs. DNCB+P1;
dP<0.05 vs. DNCB+P2. DNCB, 2,4-dinitrochlorobenzene;
CP, clobetasol propionate; VE, vehicle; IL, interleukin. |
Discussion
Medications for AD comprise of oral or topical
agents, including emollients, corticosteroids, calcineurin
inhibitors and immunosuppressants (17). Numerous natural products are
currently being investigated in order to determine whether they can
be used for the treatment of AD (1). Corticosteroids may be used for the
management of severe flares and rashes that cover a large part of
the body (17,18). Several corticosteroid ointments are
also available for the control of AD-symptoms and are considered to
have greater safety but a poorer efficacy compared with oral
corticosteroids for the treatment of AD (17,18).
However, long term use of oral and topical corticosteroids is
associated with a significant number of side effects and drug
tolerance in the endocrine system of the body (11,18).
To avoid these side effects, certain medications are prescribed for
a short course in an effort to calm the rash. Use of compounds from
plants has previously been reported as an alternative method for
anti-AD treatment and this type of treatment is expected to prevent
the onset of allergic diseases and to ameliorate allergic symptoms
(19,20). The existence of Th1/Th2 subsets in
Th lymphocytes provides a framework for understanding normal and
pathological immune responses in allergic responses. Th1 and Th2
types of reactions can mutually regulate several immune signaling
cascades. Therefore, balancing the Th1/Th2 types of reactions may
be fundamental for the treatment of AD (21). Herbal therapy has become
increasingly popular in Asia and Europe as a result of its
successful use over extended time periods. A wide variety of
phenolic substances derived from plants have been reported to
retain marked antioxidant and anti-inflammatory activities, which
contribute to their chemopreventive potential (22). In addition, the VOC of plants have
been reported to exhibit different antioxidant activities, which
may support or directly act as an effective antioxidant (23). In previous studies, C.
obtusa oil has been demonstrated to possess antimicrobial and
antifungal activities (24,25)
and its use has resulted in the improvement of the skin condition
of eczematous lesions of AD caused by the mite antigen. A previous
study reported that treatment with C. obtusa oil resulted in
attenuation of the symptoms of AD in patients with dry scaly skin
lesions by modulation of interferon-γ and ILs (12).
The current study investigated the effects of VOCCo
in a DNCB-induced AD mouse model. The induced serum IgE level was
significantly decreased by application of VOCCo in DNCB-induced AD
mice. In addition, this treatment resulted in the recovery of
histological features. Exposure to VOCCo was demonstrated to result
in successful elimination of the AD symptoms of skin lesions and
mast cell infiltration beneath the hypodermis was also effectively
inhibited. Cytokine mRNAs (IL-1β and IL-6) from skin lesions of
DNCB-treated mice were significantly inhibited, suggesting that
VOCCo may contribute to suppression of the stimulation of T
cell-mediated cytokines in DNCB-induced mice.
Over the last half century, topical corticosteroids
have been the primary choice for the treatment of AD. However,
conditions, including skin atrophy, striae and perioral dermatitis,
in sensitive areas (face or skin folds) have prevented the
long-term use of corticosteroids for the treatment of AD (26). Previously, new topical calcineurin
inhibitors, including tacrolimus ointment and pimecrolimus cream,
have been used for monotherapy treatment of AD when conventional
treatments, including corticosteroids are unsuccessful, however,
the medicine was ineffective for the treatment of AD. Numerous
patients stop seeking help from conventional physicians and turn to
alternative medical approaches (27); these can include natural products.
Although the mechanisms of herbal remedies have not yet been fully
elucidated, a number of remedies may have scientific merit and
clinical benefit for patients (12,13,19,27,28).
In conclusion, the present study demonstrated the
anti-allergic effects of VOCCo in an AD mouse model. Based on the
results of the present study, it appears that VOCCo may be a
potential therapeutic compound for the treatment of AD, and control
of the levels of serum IgE and T cell-derived cytokines, IL-1β and
IL-6 in skin lesions of an AD mouse model.
Acknowledgments
This study was supported by the Korea Forest
Research Institute (grant no. KFRI-2013-P1) and the National
Research Foundation of Korea grant of Korean government (MEST)
(grant no. 2013–010514).
References
|
1
|
Abramovits W: Atopic dermatitis. J Am Acad
Dermatol. 53(Suppl 1): S86–S93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung DY, Boguniewicz M, Howell MD, Nomura
I and Hamid QA: New insights into atopic dermatitis. J Clin Invest.
113:651–657. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu N, Dairiki K, Ogawa S and Kaneko
T: Dietary whey protein hydrolysate suppresses development of
atopic dermatitis-like skin lesions induced by mite antigen in
NC/Nga mice. Allergol Int. 55:185–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang JS, Lee K, Han SB, et al: Induction
of atopic eczema/dermatitis syndrome-like skin lesions by repeated
topical application of a crude extract of Dermatophagoides
pteronyssinus in NC/Nga mice. Int Immunopharmacol. 6:1616–1622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grabbe S and Schwarz T: Immunoregulatory
mechanisms involved in elicitation of allergic contact
hypersensitivity. Immunol Today. 19:37–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bardana EJ Jr: Immunoglobulin E-(IgE) and
non-IgE-mediated reactions in the pathogenesis of atopic
eczema/dermatitis syndrome (AEDS). Allergy. 78:25–29. 2004.
View Article : Google Scholar
|
|
7
|
Lee SO, Lou W, Nadiminty N, Lin X and Gao
AC: Requirement for NF-(kappa)B in interleukin-4-induced androgen
receptor activation in prostate cancer cells. Prostate. 64:160–167.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georas SN, Guo J, De Fanis U and Casolaro
V: T-helper cell type-2 regulation in allergic disease. Eur Respir
J. 26:1119–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garrigue JL, Nicolas JF, Fraginals R,
Benezra C, Bour H and Schmitt D: Optimization of the mouse ear
swelling test for in vivo and in vitro studies of weak contact
sensitizers. Contact Dermatitis. 30:231–237. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong JH, Shin D, Han SY, et al: Blockade
of airway inflammation by kaempferol via disturbing Tyk-STAT
signaling in airway epithelial cells and in asthmatic mice. Evid
Based Complement Alternat Med. 2013:2507252013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dharmage SC, Lowe AJ, Matheson MC, Burgess
JA, Allen KJ and Abramson MJ: Atopic dermatitis and the atopic
march revisited. Allergy. 69:17–27. 2014. View Article : Google Scholar
|
|
12
|
Joo SS, Yoo YM, Ko SH, et al: Effects of
essential oil from Chamaecypris obtusa on the development of atopic
dermatitis-like skin lesions and the suppression of Th cytokines. J
Dermatol Sci. 60:122–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park D, Jeon JH, Kwon SC, et al:
Antioxidative activities of white rose flower extract and
pharmaceutical advantages of its hexane fraction via free radical
scavenging effects. Biochem Cell Biol. 87:943–952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lehmann I, Rehwagen M, Diez U, et al:
Enhanced in vivo IgE production and T cell polarization toward the
type 2 phenotype in association with indoor exposure to VOC:
results of the LARS study. Int J Hyg Environ Health. 204:211–221.
2001. View Article : Google Scholar
|
|
15
|
Diez U, Kroessner T, Rehwagen M, et al:
Effects of indoor painting and smoking on airway symptoms in atopy
risk children in the first year of life results of the LARS-study.
(Leipzig allergy high-risk children study). Int J Hyg Environ
Health. 203:23–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wieslander G, Norbäck D, Björnsson E,
Janson C and Boman G: Asthma and the indoor environment: the
significance of emission of formaldehyde and volatile organic
compounds from newly painted indoor surfaces. Int Arch Occup
Environ Health. 69:115–124. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boguniewicz M and Leung DY: Atopic
dermatitis: a disease of altered skin barrier and immune
dysregulation. Immunol Rev. 242:233–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Bruin-Weller MS and Bruijnzeel-Koomen
CA: Topical immunomodulators, such as tacrolimus and pimecrolimus,
in the treatment of atopic dermatitis. Ned Tijdschr Geneeskd.
149:1096–1100. 2005.In Dutch. PubMed/NCBI
|
|
19
|
Kawai M, Hirano T, Higa S, et al:
Flavonoids and related compounds as anti-allergic substances.
Allergol Int. 56:113–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan HY, Zhang AL, Chen D, Xue CC and Lenon
GB: Chinese herbal medicine for atopic dermatitis: a systematic
review. J Am Acad Dermatol. 69:295–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heinzel FP, Sadick MD, Holaday BJ, Coffman
RL and Locksley RM: Reciprocal expression of interferon gamma or
interleukin 4 during the resolution or progression of murine
leishmaniasis. Evidence for expansion of distinct helper T cell
subsets. J Exp Med. 169:59–72. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Surh YJ, Na HK, Lee JY and Keum YS:
Molecular mechanisms underlying anti-tumor promoting activities of
heat-processed Panax ginseng CA Meyer. J Korean Med Sci.
16:S38–S41. 2001. View Article : Google Scholar
|
|
23
|
Jang HW, Ka MH and Lee KG: Antioxidant
activity and characterization of volatile extracts of Capsicum
annuum L. and Allium spp. Flavour Fragr J. 23:178–184. 2008.
View Article : Google Scholar
|
|
24
|
Hong EJ, Na KJ, Choi IG, Choi KC and Jeung
EB: Antibacterial and antifungal effects of essential oils from
coniferous trees. Biol Pharm Bull. 27:863–866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HO, Baek SH and Han DM: Antimicrobial
effects of Chamaecypris obutusa essential oil. Korean J Appl
Microbiol Biotechnol. 29:253–257. 2001.
|
|
26
|
Thaçi D: Long term management of childhood
atopic dermatitis with calcineurin inhibitors. Hautarzt.
54:418–423. 2003.In German.
|
|
27
|
Vender RB: Alternative treatments for
atopic dermatitis: a selected review. Skin Therapy Lett. 7:1–5.
2002.PubMed/NCBI
|
|
28
|
Wang W, Zhou Q, Liu L and Zou K:
Anti-allergic activity of emodin on IgE-mediated activation in
RBL-2H3 cells. Pharmacol Rep. 64:1216–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|