Introduction
Gap junctions are membrane channel structures
between neighboring cells that mediate gap junction intercellular
communication (GJIC), which is an important pathway involved in
intercellular substance and signal transmission. GJIC occurs
throughout vascular tissues and is pivotal in the maintenance of
normal vascular function (1–3) and
the repair of vascular damage (4,5). The
basic structural unit of the gap junction is connexin. The major
types of connexins expressed in vascular tissue include Cx43, Cx40,
Cx37 and Cx45 (6), with Cx43
exhibiting the highest level of expression in this tissue (7,8). The
abnormal expression or dysfunction of Cx43 can affect the
proliferation, migration and differentiation of vascular smooth
muscle cells (VSMCs) (9–11). In addition, Cx43 participates in
the pathophysiological processes of vasoconstriction (12–14),
coronary artery spasm (15,16)
and atherosclerosis (17–19).
Insulin is an important hormone involved in
metabolic regulation and is required for the maintenance of normal
blood vessel function (20). In
insulin-resistant states, high levels of insulin in the blood are
closely associated with various cardiovascular diseases (21–23).
However, the involvement of insulin in the pathogenesis of
cardiovascular diseases through its effects on connexin and GJIC
remains unclear. In this study, by treating VSMCs with different
doses of insulin, the effects of insulin on GJIC, Cx43
phosphorylation, and Cx43 expression were explored under
physiological and pathological conditions (presence of 1 or 100
nmol/l insulin, respectively). Furthermore, the possible mechanisms
underlying these effects of insulin were investigated. The aim of
this study was to clarify the molecular mechanisms that contribute
to vascular dysfunction in conditions associated with insulin
resistance.
Materials and methods
Cell culture
All animal treatment procedures were performed in
accordance with the provisions of the Institutional Animal Ethics
Committee of China Medical University. The present study was
approved by the ethics committee of China Medical University
(Shenyang, China). Eight-week-old healthy Sprague-Dawley rats were
obtained from the Experimental Animal Center of China Medical
University (Shenyang, China). The rats were housed under specific
pathogen-free conditions (23±2°C, 60±5% humidity, 12:12 h
light:dark cycle) and allowed free access to food and water. The
rats were sacrificed by ether inhalation after 1 week and their
thoracic aortas were harvested under sterile conditions. As
described previously (24),
primary VSMCs were dissociated. Briefly, the thoracic aortas were
washed in pre-cooled phosphate-buffered saline (PBS), and the
adventitia and intima were removed. Subsequently, the reserved
media tunica was transferred to a culture flask, cut into ~1
mm3 sections, stuck to the bottom and the bubbles were
pressed out by tweezers. The flask was gently flipped upside down
and 5–6 ml Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand
Island, NY) containing 10% fetal bovine serum (FBS; Hyclone, Logan,
UT, USA) was added. The culture flask was cultured in an incubator
containing 5% CO2 and 95% air, at 37°C. After 4 h, the
flask was returned to an upright position and the tissue was
completely immersed in the culture medium for 5–7 days. The VMSCs
that had grown out from the edges of the tissue blocks were
digested using 0.25% trypsin (Beyotime Institute of Biotechnology,
Haimen, China), seeded in a new flask and cultured in DMEM
containing 10% FBS, 2 mmol glutamate (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China), 100 U/ml penicillin
(Beijing Solarbio Science & Technology Co., Ltd.) and 100
μg/ml streptomycin (Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C in a cell culture incubator with 5%
CO2. The purity of cultured VSMCs was determined using
immunocytochemical stanining with a monoclonal antibody specific
for α-actin.
Immunocytochemistry
Briefly, VMSCs were grown to subconfluence on
coverslips, fixed in 4% paraformaldehyde (Sinopharm Chemical
Reagent, Shanghai, China) and permeabilized with 0.5% Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd.). Following
treatment with 3% hydrogen peroxide (Sinopharm Chemical Reagent)
and 5% bovine serum albumin (Beijing Solarbio Science &
Technology Co., Ltd.), the cover-slips were incubated overnight at
4°C with a mouse monoclonal antibody targeting α-actin (1:300
dilution; cat. no. sc-58669; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The cover-slips were then incubated at 37°C for
40 min with a biotinylated goat anti-mouse immunoglobulin G
antibody (cat. no. A0286; Beyotime Institute of Biotechnology).
Immunostaining was detected using streptavidin-horseradish
peroxidase conjugate (Beyotime Institute of Biotechnology) and
visualized with 3, 3′-diaminobenzidine (Beyotime Institute of
Biotechnology). Finally, the sections were counterstained with
hematoxylin (Beijing Solarbio Science & Technology Co., Ltd.)
and observed under a light microscope (DP73; Olympus, Tokyo,
Japan).
Experimental grouping and determination
of glucose uptake
VSMCs were inoculated into 24-well plates and
cultured in a cell culture incubator. After 24 h in culture, 1 or
100 nmol/l insulin was added to the culture medium, followed by
incubation for another 24 h. The glucose uptake of each group of
cells was measured as described by Pyla et al (25). Briefly, the cells were washed with
Krebs-Ringer-HEPES buffer containing 15 mmol/l HEPES, 105 mmol/l
NaCl, 5 mmol/l KCl, 1.4 mmol/l CaCl2, 1 mmol/l
KH2PO4, 1.4 mmol/l MgSO4 and 10
mmol/l NaHCO3, pH 7.4; followed by incubation in a 0.2
mmol/l 2-deoxy-D-glucose (2-DG) solution containing 1 μCi/ml
2-[3H] DG for 30 min. Subsequently, cells were washed
three times with pre-cooled phosphate-buffered saline and lysed
with 0.4 mol/l NaOH. After neutralization with HCl, aliquots of
cell lysate were used to determine glucose uptake with a liquid
scintillation spectrometer (PerkinElmer Wallac, Inc., Gaithersburg,
MD, USA).
Fluorescence recovery after
photobleaching (FRAP) assay
FRAP experiments were conducted as previously
described (26). After VSMCs grew
to 80% confluence, different concentrations of insulin (0, 1 or 100
nmol/l) were added to the culture medium. After 24 h, the culture
medium was discarded, and the cells were treated with
6-carboxyfluorescein diacetate (6-CFDA, Invitrogen Life
Technologies, Carlsbad, CA, USA) at a final concentration of 10
μmol/l for 20 min. 6-CFDA can easily pass through cell
membranes and decompose into 6-CF, which cannot pass through cell
membranes, but can spread through gap junctions (27). The excitation wavelength of 6-CF is
490 nm, and cells labeled with it can be observed as green under a
fluorescent microscope. After washing twice with D-Hank’s solution,
the cells were observed under a laser-scanning confocal microscope
(FV1000S-SIM/IX81; Olympus).
Three types of cells were selected for the FRAP
assay. The first type included cells that tightly connected with
neighboring cells and demonstrated GJIC. The fluorescence recovery
after photobleaching of these cells reflected the level of GJIC.
The second type included isolated cells that did not demonstrate
GJIC with neighboring cells and thus were used as a self-control in
this experiment. The third type included cells that were tightly
connected with neighboring cells and showed spontaneous
photobleaching. This type of cells did not require photobleaching
and thus were used for background correction. Photobleaching was
conducted with a laser power of 500 mW, duration of the
photobleaching pulse of 200 msec, a bleaching rate of 30–60%, a
bleaching intensity of 100%, and a scanning intensity of 20%. After
computer-assisted precise positioning, intracellular fluorescence
in selected cells was bleached with a laser beam. The bleached cell
was then monitored, and images were acquired (FV1000 confocal
operations software; Olympus) continuously every 10 sec for 5 min
for the detection of fluorescence recovery. The maximum
fluorescence recovery proportion and the fluorescence recovery rate
were used as indicators for the evaluation of GJIC in each group.
The fluorescence recovery rate after photobleaching was calculated
with the following formula:
[(It-I0)/(I-I0) × 100%]/t, where
It is the relative fluorescence intensity at the time
point t in the bleached cell, I0 is the relative
fluorescence intensity immediately after bleaching, I is the
relative fluorescence intensity prior to bleaching and t is the
recovery time. A third-party software program, Olympus Fluoview
V2.1C (Olympus), was used to automatically analyze the data.
Western blot analysis
Cells were lysed using radioim-munoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology), and
protein concentrations were quantified by the bicinchoninic acid
method (Beyotime Institute of Biotechnology). Equal quantities of
total proteins (40 μg) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrotransferred
onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% skimmed milk for 1 h and
incubated overnight at 4°C with the following primary anti bodies:
Rabbit polyclonal anti-Cx43 (cat. no. ab11370) or rabbit polyclonal
anti-p-Cx43 (cat. no. ab30559; Abcam, Cambridge, MA, USA) at
dilutions of 1:1,000 and 1:800, respectively. After washing with
PBS, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. sc-2054; Santa
Cruz Biotechnology, Inc.) at 1:5,000 dilutions for 1 h at 37°C.
Subsequently, the blotted protein bands were exposed to and
visualized with enhanced chemiluminescence reagents (Millipore).
Developed films were digitized by scanning, and the optical
densities were analyzed with Image J version 1.43b software
(National Institutes of Health, Bethesda, MD, USA). β-actin was
used as an internal control for the analysis of protein
expression.
Detection of H2O2
activity
Intracellular hydrogen peroxide levels were measured
using an Amplex Red Hydrogen Peroxide Assay kit (Invitrogen Life
Technologies), strictly following the instructions provided in the
user manual.
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparisons between groups were conducted using one-way
analysis of variance, and P<0.05 was considered to indicate a
statistically significant difference. GraphPad Prism 5.0 software
(GraphPad Software Inc., La Jolla, CA, USA) was used for graph
processing.
Results
Identification of VSMCs and the effect on
glucose uptake following different doses of insulin
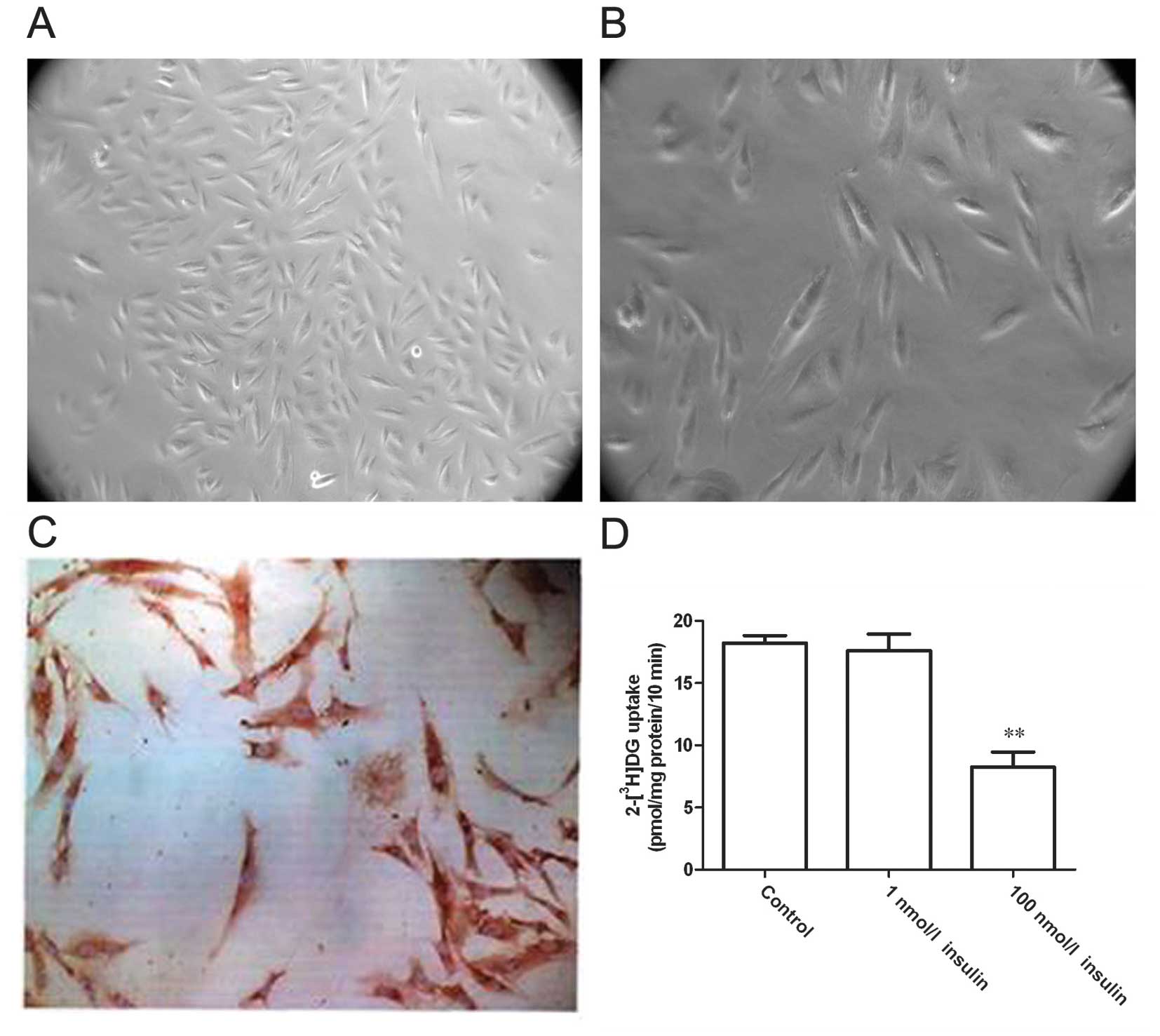
A tissue adherent cultivation approach was adopted
to separate VSMCs. After 5–7 days, smooth muscle cells grew out
from the edges of the tissue blocks. The cells were fusiform- or
spindle-shaped, relatively small in size with ovoid-shaped nuclei,
and the cytoplasm displayed strong light refraction (Fig. 1A and B). After culturing for 3–4
weeks, the cells formed monolayers and displayed a typical
‘peak-to-valley’ growth. Immunocytochemical staining for α-actin, a
specific marker of VSMCs, demonstrated that the majority of cells
were α-actin-positive with a large quantity of brown-yellowish
myofilaments in an arrangement parallel to the longitudinal axis of
the cell (Fig. 1C). These
observations indicated the successful acquisition of VSMCs. Then,
the cells were treated with different concentrations of insulin and
glucose uptake was assessed after 24 h (Fig. 1D). Compared with the control group,
the glucose uptake rate did not show significant changes in cells
treated with 1 nmol/l insulin (P>0.05), although this rate was
significantly decreased in cells treated with 100 nmol/l insulin
(P<0.01). These results indicate that high-dose insulin reduced
the sensitivity of VSMCs to insulin and led to a state of insulin
resistance.
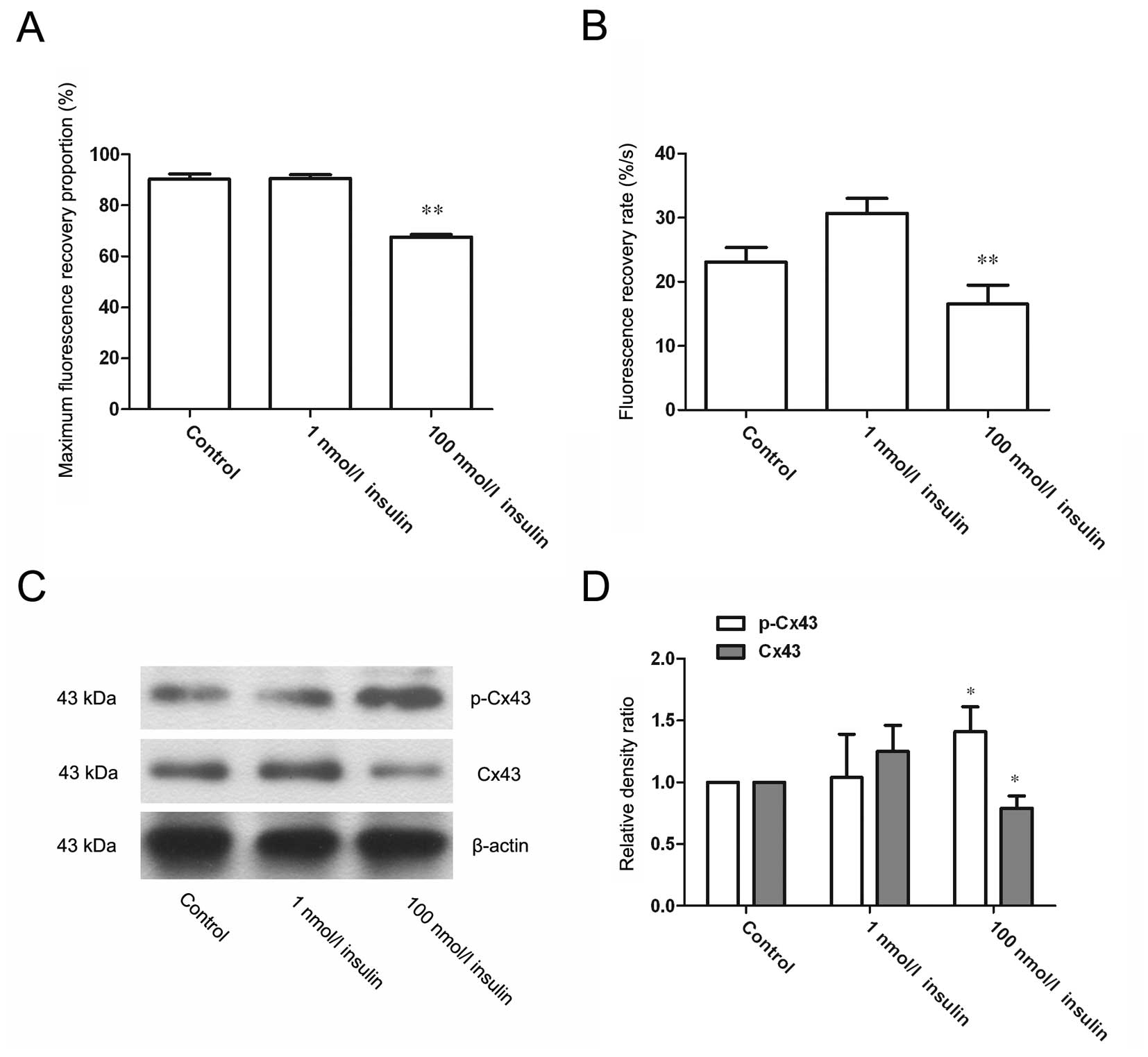
High-dose insulin suppresses GJIC and
induces the phosphorylation of Cx43 at s368
FRAP assays were performed to evaluate the effect of
insulin on GJIC in VSMCs (Fig. 2A and
B). The results showed that 1 nmol/l insulin did not induce
significant changes in the maximum fluorescence recovery proportion
or the fluorescence recovery rate of treated cells, while treatment
with 100 nmol/l insulin for 24 h significantly decreased these
readouts (P<0.01). These results suggested that high-dose
insulin could suppress the GJIC function of VSMCs. Furthermore,
western blotting showed that cells treated with 100 nmol/l insulin
demonstrated a significantly higher level of phosphorylated Cx43 at
s368 and a significantly decreased level of Cx43 (both P<0.05)
(Fig. 2C and D). This result
indicated that high-dose insulin promotes the phosphorylation of
Cx43 at s368 and downregulates the level of Cx43.
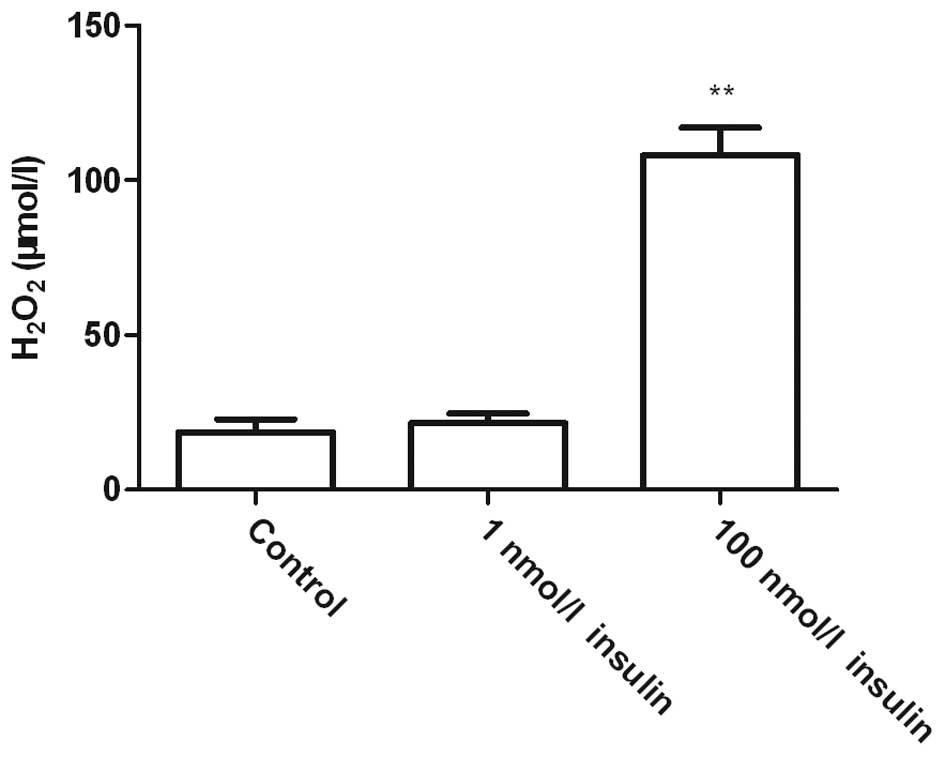
High-dose insulin induces
H2O2 release from VSMCs
H2O2 activity was measured to
explore the mechanisms underlying the effect of insulin on GJIC
(Fig. 3). Treatment with 1 nmol/l
insulin did not exert a significant impact on cellular
H2O2 activity, while treatment with 100
nmol/l insulin for 24 h significantly enhanced the cellular
H2O2 level (P<0.01), indicating that the
effect of high-dose insulin on GJIC may be correlated with the
release of H2O2.
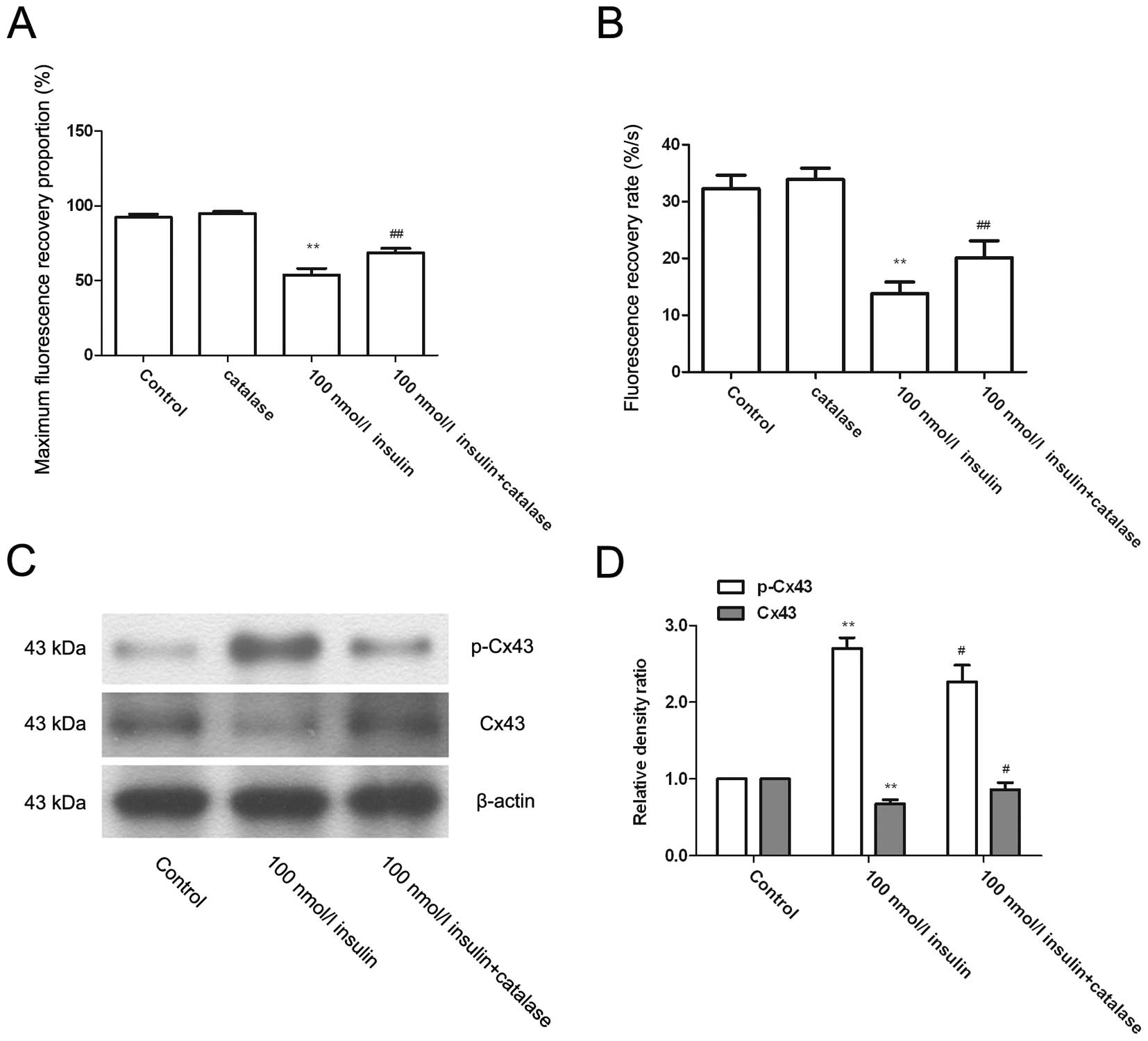
High-dose insulin promotes the
phosphorylation of Cx43 and inhibits GJIC via the release of
H2O2
VSMCs were pre-treated with 2,000 U/ml catalase 30
min prior to the insulin treatment, and then the GJIC level was
measured (Fig. 4A and B). Compared
with cells treated with only high-dose insulin, the maximum
fluorescence recovery proportion and the fluorescence recovery rate
were significantly increased in cells pretreated with catalase
(P<0.01). Western blotting further revealed that compared with
cells treated with only high-dose insulin, catalase-pretreated
cells showed a significantly decreased phosphorylation level of
Cx43 at s368 and an elevated Cx43 expression level (Fig. 4C and D; P<0.05).
Discussion
It has been shown that insulin affects the
proliferation, migration, phenotypic transformation and cellular
contraction of VSMCs, and is involved in the occurrence and
development of cardiovascular disease (28–30).
GJIC is an important pathway contributing to intercellular
substance and signal transmission and thus is crucial in the
maintenance of normal vascular function. However, the effect of
insulin on intracellular GJIC remains unclear. In this study, using
different concentrations of insulin to treat in
vitro-cultured VSMCs, it was observed that high-dose insulin
suppressed cellular GJIC function, promoted the phosphorylation of
Cx43 at s368 and downregulated the expression of Cx43. In addition,
high-dose insulin treatment enhanced H2O2
release. Furthermore, catalase pretreatment significantly restored
GJIC function, which was accompanied by decreased phosphorylation
of Cx43 at s368 and increased Cx43 expression. These results
indicate that high-dose insulin can regulate GJIC in VSMCs through
the mediation of oxidative stress.
Insulin can promote glucose uptake and utilization
in tissues and cells, and thus serves as an essential hormone in
glucose metabolism. In the clinic, insulin resistance refers to the
physiological condition in which reduced glucose uptake and
utilization efficiencies lead to a compensatory increase in insulin
secretion to maintain glucose hemostasis in the body (31). Hyperinsulinemia is often used as
one of the diagnostic criteria for insulin resistance. Due to the
limitations of human research, the majority of studies have treated
cells with high-dose insulin for studies of the pathogenesis of
insulin resistance (32,33). In this study, insulin doses of 1
and 100 nmol/l in VSMCs were used to evaluate its effects on
vascular states under physiological and pathological conditions.
The results demonstrated that insulin at a physiological
concentration did not significantly affect glucose uptake, while
high-dose insulin significantly reduced the glucose uptake rate.
The findings are consistent with the conclusions drawn in previous
studies (34).
Vascular tissue displays abundant GJIC, which is
extensively involved in various physiological functions of blood
vessels and is closely associated with the occurrence and
development of various diseases (35). By treating VSMCs with different
concentrations of insulin, it was observed that high-dose insulin
could suppress GJIC in VSMCs. Therefore, abnormal GJIC function may
contribute to the pathogenesis of insulin resistance. Among all
connexins present in vascular tissue, Cx43 shows the highest
protein expression level, and multiple studies have shown that
abnormal phosphorylation of Cx43 at s368 can downregulate Cx43
expression and inhibit GJIC function (36,37).
In this study, it was observed that high-dose insulin induced
abnormal phosphorylation of Cx43 at s368 and downregulated Cx43
expression, indicating that the high-dose insulin-induced reduction
of GJIC function may be associated with the abnormal
phosphorylation of Cx43 at s368.
Currently, it is hypothesized that high-dose insulin
induces insulin resistance, type 2 diabetes (38), atherosclerosis (39), hypertension (40) and obesity (41) primarily through the induction of
oxidative stress responses (42,43).
H2O2 is a stable component of reactive oxygen
species in VSMCs and is also a second messenger for various
stimulators in smooth muscle cells (44). The current study demonstrated that
high-dose insulin could induce a release of
H2O2, which is consistent with the
conclusions drawn from previous studies (45). In addition, the inhibition of GJIC
function by H2O2 through the induction of
Cx43 phosphorylation has been verified in various types of cells
(46,47). In this study, catalase pretreatment
reversed the high-dose insulin-induced inhibition of GJIC,
decreased the phosphorylation level of Cx43 at s368, and
upregulated Cx43 expression. These findings further confirmed that
high-dose insulin could promote Cx43 phosphorylation through the
induction of H2O2 release, thus suppressing
GJIC function.
In conclusion, through the activation of oxidative
stress responses, high-dose insulin treatment induced Cx43
phosphor-ylation and downregulated Cx43 expression, thus
suppressing GJIC function. This process may contribute to the
pathogenesis of insulin resistance, although the detailed
mechanisms require further investigation in in-depth studies.
Acknowledgments
This study was supported by a grant from the
National Basic Research Program of China (973 Program, no.
2012CB518606).
References
|
1
|
Chadjichristos CE, Morel S, Derouette JP,
et al: Targeting connexin 43 prevents platelet-derived growth
factor-BB-induced phenotypic change in porcine coronary artery
smooth muscle cells. Circ Res. 102:653–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoguchi T, Yu HY, Imamura M, et al:
Altered gap junction activity in cardiovascular tissues of
diabetes. Med Electron Microsc. 34:86–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Figueroa XF, Isakson BE and Duling BR:
Connexins: gaps in our knowledge of vascular function. Physiology
(Bethesda). 19:277–284. 2004. View Article : Google Scholar
|
|
4
|
Coutinho P, Qiu C, Frank S, Tamber K and
Becker D: Dynamic changes in connexin expression correlate with key
events in the wound healing process. Cell Biol Int. 27:525–541.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao Y, Regan CP, Manabe I, et al: Smooth
muscle-targeted knockout of connexin43 enhances neointimal
formation in response to vascular injury. Arterioscler Thromb Vasc
Biol. 27:1037–1042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnstone S, Isakson B and Locke D:
Biological and biophysical properties of vascular connexin
channels. Int Rev Cell Mol Biol. 278:69–118. 2009.PubMed/NCBI
|
|
7
|
Evans WH and Martin PE: Gap junctions:
structure and function (Review). Mol Membr Biol. 19:121–136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haefliger JA, Nicod P and Meda P:
Contribution of connexins to the function of the vascular wall.
Cardiovasc Res. 62:345–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joshi CN, Martin DN, Shaver P, Madamanchi
C, Muller-Borer BJ and Tulis DA: Control of vascular smooth muscle
cell growth by connexin 43. Front Physiol. 3:2202012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnstone SR, Kroncke BM, Straub AC, et
al: MAPK phosphorylation of connexin 43 promotes binding of cyclin
E and smooth muscle cell proliferation. Circ Res. 111:201–211.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia G, Cheng G, Gangahar DM and Agrawal
DK: Involvement of connexin 43 in angiotensin II-induced migration
and proliferation of saphenous vein smooth muscle cells via the
MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 44:882–890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Earley S, Resta TC and Walker BR:
Disruption of smooth muscle gap junctions attenuates myogenic
vasoconstriction of mesenteric resistance arteries. Am J Physiol
Heart Circ Physiol. 287:H2677–H2686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rocha ML, Kihara AH, Davel AP, Britto LR,
Rossoni LV and Bendhack LM: Blood pressure variability increases
connexin expression in the vascular smooth muscle of rats.
Cardiovasc Res. 80:123–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slovut DP, Mehta SH, Dorrance AM, Brosius
FC, Watts SW and Webb RC: Increased vascular sensitivity and
connexin43 expression after sympathetic denervation. Cardiovasc
Res. 62:388–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong T, Wang H and Wang Y: Effects of gap
junctional blockers on cerebral vasospasm after subarachnoid
hemorrhage in rabbits. Neurol Res. 31:238–244. 2009. View Article : Google Scholar
|
|
16
|
Wang H, Hong T, Wang H and Wang Y: Altered
expression of connexin43 and its possible role in
endothelin-1-induced contraction in rabbit basilar artery. Neurol
Res. 31:67–73. 2009. View Article : Google Scholar
|
|
17
|
Wei JM, Wang X, Gong H, Shi YJ and Zou Y:
Ginkgo suppresses atherosclerosis through downregulating the
expression of connexin 43 in rabbits. Arch Med Sci. 9:340–346.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwak BR, Mulhaupt F, Veillard N, Gros DB
and Mach F: Altered pattern of vascular connexin expression in
atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 22:225–230.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwak BR, Veillard N, Pelli G, et al:
Reduced connexin43 expression inhibits atherosclerotic lesion
formation in low-density lipoprotein receptor-deficient mice.
Circulation. 107:1033–1039. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Breen DM and Giacca A: Effects of insulin
on the vasculature. Curr Vasc Pharmacol. 9:321–332. 2011.
View Article : Google Scholar
|
|
21
|
Anfossi G, Russo I, Doronzo G and Trovati
M: Contribution of insulin resistance to vascular dysfunction. Arch
Physiol Biochem. 115:199–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muniyappa R, Montagnani M, Koh KK and Quon
MJ: Cardiovascular actions of insulin. Endocr Rev. 28:463–491.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muniyappa R and Quon MJ: Insulin action
and insulin resistance in vascular endothelium. Curr Opin Clin Nutr
Metab Care. 10:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo HM, Hung CF, Tseng YL, Chen BH, Jian JS
and Wu WB: Lycopene binds PDGF-BB and inhibits PDGF-BB-induced
intracellular signaling transduction pathway in rat smooth muscle
cells. Biochem Pharmacol. 74:54–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pyla R, Poulose N, Jun JY and Segar L:
Expression of conventional and novel glucose transporters, GLUT1,
-9, -10 and -12, in vascular smooth muscle cells. Am J Physiol Cell
Physiol. 304:C574–C589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen J, Wang LH, Zheng LR, Zhu JH and Hu
SJ: Lovastatin inhibits gap junctional communication in cultured
aortic smooth muscle cells. J Cardiovasc Pharmacol Ther.
15:296–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao R, Hu MF, Lou JT and Lei YL: Effects
of H pylori infection on gap-junctional intercellular communication
and proliferation of gastric epithelial cells in vitro. World J
Gastroenterol. 13:5497–5500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Wang Y, Wang X, et al: Insulin
promotes vascular smooth muscle cell proliferation via
microRNA-208-mediated downregulation of p21. J Hypertens.
29:1560–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CC, Gurevich I and Draznin B: Insulin
affects vascular smooth muscle cell phenotype and migration via
distinct signaling pathways. Diabetes. 52:2562–2569. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salt IP: Examining the role of insulin in
the regulation of cardiovascular health. Future Cardiol. 9:39–52.
2013. View Article : Google Scholar
|
|
31
|
Reaven GM: The insulin resistance
syndrome: Definition and dietary approaches to treatment. Annu Rev
Nutr. 25:391–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang WY, Lee JJ, Kim Y, et al: Effect of
eriodictyol on glucose uptake and insulin resistance in vitro. J
Agric Food Chem. 60:7652–7658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu P, Zhang Y, Shi D, Chen Y and Deng J:
Cardamonin ameliorates insulin resistance induced by high insulin
and high glucose through the mTOR and signal pathway. Planta Med.
79:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Hitomi H, Hosomi N, et al:
Mechanical stretch augments insulin-induced vascular smooth muscle
cell proliferation by insulin-like growth factor-1 receptor. Exp
Cell Res. 317:2420–2428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Figueroa XF and Duling BR: Gap junctions
in the control of vascular function. Antioxid Redox Signal.
11:251–266. 2009. View Article : Google Scholar
|
|
36
|
Lim MC, Maubach G and Zhuo L: TGF-beta1
down-regulates connexin 43 expression and gap junction
intercellular communication in rat hepatic stellate cells. Eur J
Cell Biol. 88:719–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Richards TS, Dunn CA, Carter WG, Usui ML,
Olerud JE and Lampe PD: Protein kinase C spatially and temporally
regulates gap junctional communication during human wound repair
via phosphorylation of connexin43 on serine368. J Cell Biol.
167:555–562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weyer C, Funahashi T, Tanaka S, et al:
Hypoadiponectinemia in obesity and type 2 diabetes: Close
association with insulin resistance and hyperinsulinemia. J Clin
Endocrinol Metab. 86:1930–1935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fujiwara T, Saitoh S, Takagi S, et al:
Development and progression of atherosclerotic disease in relation
to insulin resistance and hyperinsulinemia. Hypertens Res.
28:665–670. 2005. View Article : Google Scholar
|
|
40
|
DeFronzo RA and Ferrannini E: Insulin
resistance. A multifaceted syndrome responsible for NIDDM, obesity,
hypertension, dyslipidemia, and atherosclerotic cardiovascular
disease. Diabetes Care. 14:173–194. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lewis GF, Uffelman KD, Szeto LW and
Steiner G: Effects of acute hyperinsulinemia on VLDL triglyceride
and VLDL apoB production in normal weight and obese individuals.
Diabetes. 42:833–842. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Henriksen EJ, Diamond-Stanic MK and
Marchionne EM: Oxidative stress and the etiology of insulin
resistance and type 2 diabetes. Free Radic Biol Med. 51:993–999.
2011. View Article : Google Scholar :
|
|
43
|
Tiganis T: Reactive oxygen species and
insulin resistance: the good, the bad and the ugly. Trends
Pharmacol Sci. 32:82–89. 2011. View Article : Google Scholar
|
|
44
|
Cowan DB, Jones M, Garcia LM, et al:
Hypoxia and stretch regulate intercellular communication in
vascular smooth muscle cells through reactive oxygen species
formation. Arterioscler Thromb Vasc Biol. 23:1754–1760. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang M, Yang Y, Zhang S and Kahn AM:
Insulin-stimulated hydrogen peroxide increases guanylate cyclase
activity in vascular smooth muscle. Hypertension. 42:569–573. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hwang JW, Park JS, Jo EH, et al: Chinese
cabbage extracts and sulforaphane can protect
H2O2 -induced inhibition of gap junctional
intercellular communication through the inactivation of ERK1/2 and
p38 MAP kinases. J Agric Food Chem. 53:8205–8210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee KW, Hur HJ, Lee HJ and Lee CY:
Antiproliferative effects of dietary phenolic substances and
hydrogen peroxide. J Agric Food Chem. 53:1990–1995. 2005.
View Article : Google Scholar : PubMed/NCBI
|