Introduction
The effects of insulin are mediated by the
activation of a signaling pathway involving insulin receptor
subAstrate-1 (IRS-1) and phosphatidylinositol-3 kinase (PI3K)
(1,2). Previous studies have reported a
common polymorphism (rs1801278) in the IRS-1 gene, in which a
Gly/Arg substitution occurs at codon 972 (Arg972)
(3,4). The presence of Arg972
IRS-1 is associated with impaired insulin/IRS-1 signaling to
activate PI3K (3,4). In our previous study, an association
between Arg972 IRS-1 and increased risk and severity of
rheumatoid arthritis (RA), a chronic inflammatory disease with
progressive joint destruction, was identified (5,6).
However, the role of Arg972 IRS-1 in the pathogenesis of
RA remains to be elucidated.
RA is characterized by an imbalance in bone
remodeling and bone loss (7). It
is well established that osteoclasts are the principal type of cell
responsible for bone loss in RA (7). Tumor necrosis factor-α (TNF-α), an
inflammatory cytokine has been is elevated in the synovial fluid
and the synovium of patients with RA (8) and has been demonstrated to have a
central role in the pathogenesis of RA (9). Substantial in vitro and in
vivo evidence has suggested that TNF-α induces apoptosis in
osteoblasts (10–13). Insulin/IRS-1 signaling reportedly
activates the PI3K/Akt pathway, which is important in cell survival
against apoptotic stress (14).
Thus, in the present study, the effects of Arg972 IRS-1
and IRS-1 on TNF-α-induced apoptosis in normal and RA osteoblasts
were examined.
Materials and methods
Plasmids and reagents
A fragment of human genomic DNA containing the
entire coding sequence of IRS-1 was cloned and ligated into a pcDNA
3.1 expression vector and the Arg972 IRS-1 expression
vector (Invitrogen Life Technologies, Carlsbad, CA, USA) was
constructed, as previously described (4,15).
The cDNA construct containing the Arg972 substitution
was generated by site-directed mutagenesis using polymerase chain
reaction (PCR) with the wild-type IRS-1 as a template. The PCR
fragment containing the codon 972 variant of IRS-1 was digested
with BamHI and NheI restriction endonucleases and
inserted into pcDNA3-WT-IRS-1, which was previously digested with
the same enzymes. The presence of the substitution and the entire
sequence of the fragment inserted was confirmed by sequencing.
SuperFect transfection reagent was purchased from Qiagen (Valencia,
CA, USA). Anti-β-actin (cat. no. 8H10D10, 3700) antibody was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
IRS-1 (cat. no. sc-29376-V) short hairpin (sh)RNA lentiviral
particles, control shRNA lentiviral particles-A (cat. no.
sc-108080), selective PI3K inhibitor BKM120 (cat. no. sc-364437A)
and rabbit anti-human polyclonal IRS-1 (C-20; cat. no. sc-559;
1:1,000 dilution), mouse anti-human monoclonal Akt (5C10) (cat. no.
sc-81434; 1:1,000 dilution) and rabbit anti-human polyclonal
phosphorlyated (phospo)-Akt [Serine 473 (ser473) cat no. sc-101629;
1:1,000 dilution] antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The DeadEnd™
Fluorometric terminal deoxynucleotidyl transferase mediated
nick-end labeling (TUNEL) system was purchased from Promega
(Madison, WI, USA). Recombinant human TNF-α, G418, puromycin and
insulin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Life
Technologies (Beijing, China).
Cell culture
Adult human osteoblasts, isolated from normal
individuals (cat. no. 406-05a) and patients with RA (cat. no.
406RA-05a) were purchased from Cell Applications (San Diego, CA,
USA). The cells were grown in DMEM supplemented with 10% fetal
bovine serum and 1% penicillin/streptomycin (Sigma-Aldrich) at 37°C
in a humidified, 5% CO2 atmosphere. The normal and the
RA osteoblasts were genotyped by sequencing and found to be wild
type IRS-1 homozygotes.
Transfection and lentiviral
transduction
The IRS-1 and Arg972 IRS-1 expression
constructs were transfected into the osteoblast cells using
Superfect™ transfection reagent (Qiagen) according to the
manufacturer’s instructions. Pools of stable transductants were
generated via selection using G418 (600 μg/ml) according to
the manufacturer’s instructions. Lentiviral transduction was
performed and pools of stable transductants were generated via
selection with puromycin (4 μg/ml).
Western blot analysis
The osteoblasts were lysed in 250 μl 2X SDS
loading buffer containing 62.5 mm TrisHCl, (pH 6.8), 2% SDS, 25%
glycerol, 0.01% bromphenol blue, 5% 2-mercaptoethanol
(Sigma-Aldrich), and incubated at 95°C for 10 min. Equal quantities
of the proteins (100 μg) of each sample were separated by
8–15% SDS-polyacrylamide gel electophoresis and blotted onto a
polyvinylidene difluoride microporous membrane (Millipore,
Billerica, MA, USA). The membranes were incubated for 1 h with a
1:1,000 dilution of primary antibody, washed three times with
phosphate-buffered saline for 5 min and incubated with secondary
antibodies with horseradish peroxidase conjugate (1:5,000, 1 h. The
peroxidase was visualized using a GE Healthcare
electrochemiluminescence kit (Shanghai, China).
IRS-1-associated PI3K activity assay
The IRS-1-associated PI3K activities were
determined, as previously described (16). Briefly, 700 μg total protein
was immunoprecipitated with anti-IRS-1 antibody (Santa Cruz
Biotechnology, Inc.). PI3K activity was measured in a reaction
mixture containing phosphatidylinositol (Sigma-Aldrich) and
[γ-32P]ATP (Sigma-Aldrich). After 5 min, the reaction was stopped
by the addition of HCl and chloroform:methanol and analyzed by
thin-layer chromatography. PI3K activity was detected by the
appearance of a specific radioactive spot corresponding to
32P-labeled phosphatidylinositol 3-phosphate
[(32P)PI-3-P] (17).
The PI3K activity was normalized against 106 cells. The
autoradiographic signals were quantified using the National
Institutes of Health Image J software, version 1.63 (National
Institutes of Health, Bethesda, MO, USA).
Measurement of apoptosis using a TUNEL
assay
The TUNEL assay was performed using the DeadEnd™
Fluorometric TUNEL system according to the manufacturer’s
instructions (Promega). The cells were treated with 20 ng/ml TNF-α
for 6 and 12 h in the presence of 10 nM insulin. Subsequently, 50
μl of TdT reaction mix was added to the cells on an area no
larger than 5 cm2 and slides were covered with plastic
coverslips to ensure even distribution of the mix. The slides were
incubated for 60 min at 37°C in a humidified chamber. Apoptotic
cells exhibit a marked nuclear green fluorescence, which can be
detected using a standard fluorescein filter. Cells stained with
4′,6-diamidino-2-phenylindole (Sigma-Aldrich) exhibit a marked blue
nuclear fluorescence. The slides were visualized using fluorescence
microscopy (IX83; Olympus, Beijing, China) and the relative
quantity of apoptotic cells were determined by counting the number
of TUNEL-positive cells in five randomly selected fields
(magnification, ×100) for each sample.
Statistical analysis
Statistical analyses were performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). All continuous variable
values are expressed as the mean ± standard deviation. Comparisons
of the means among multiple groups were performed with one-way
analysis of variance, followed by post hoc pairwise comparisons
using Tukey’s test. P<0.05 was considered to indicate a
statistically significant difference for the two-tailed
analysis.
Results
The normal and RA osteoblasts were stably
transfected with Arg972 IRS-1 and IRS-1. By contrast,
the cells were stably transduced with IRS-1-shRNA to knock down
IRS-1. As shown in Fig. 1,
compared with the controls, use of the anti-IRS-1 antibody revealed
that IRS-1 and Arg972 IRS-1 were overexpressed
>3.5-fold, while the level of endogenous IRS-1 was knocked down
by ~70% in the normal and the RA osteoblasts. Insulin stimulation
(10 nM) had no significant effects on the overexpression of
Arg972 IRS-1 and IRS-1 or on the knock down of IRS-1 in
the cells (data not shown).
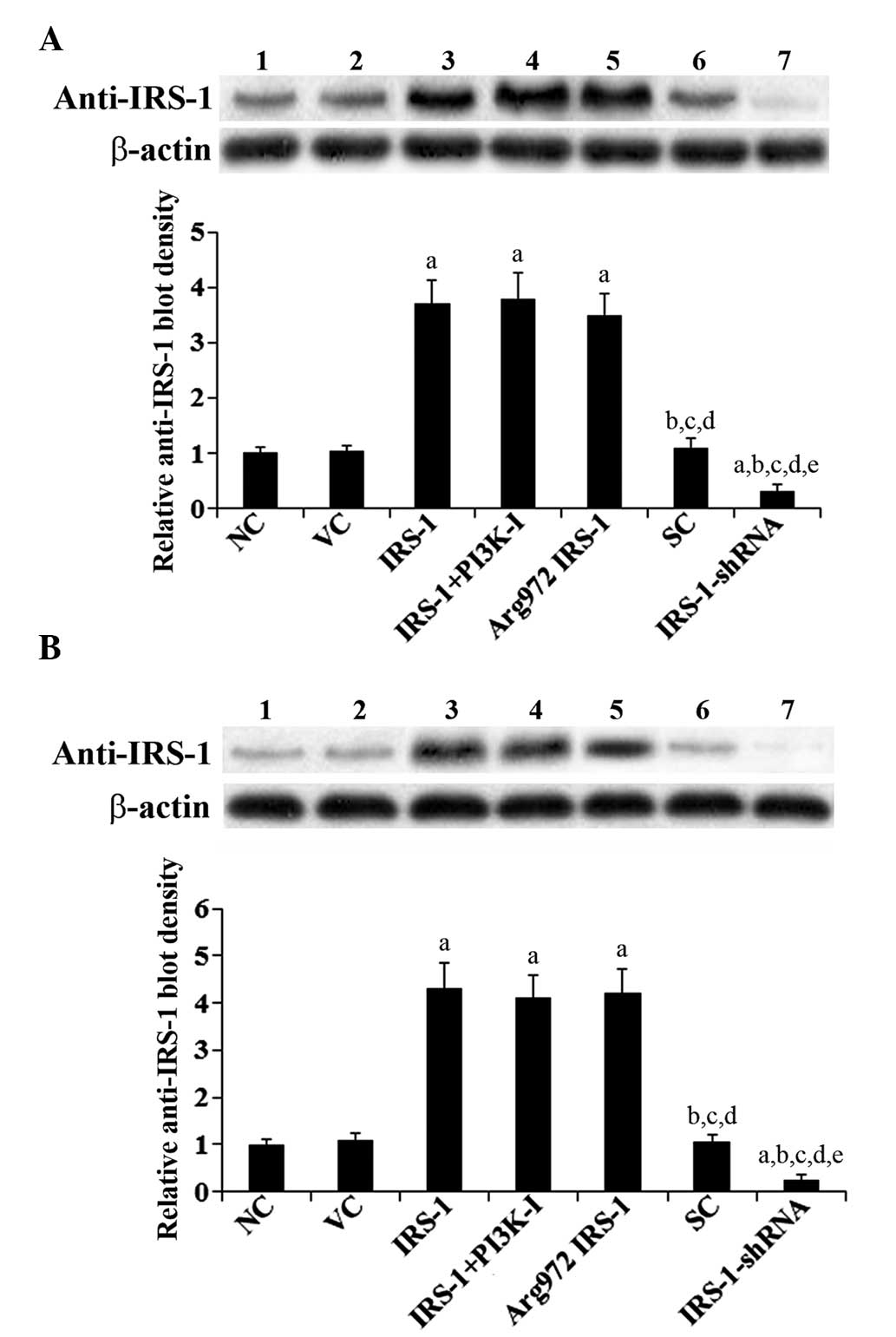 | Figure 1Stable overexpression of IRS-1 or
Arg972 IRS-1 or knock down of IRS-1 in normal human
osteoblasts and RA osteoblasts. Western blot analyses were
performed with an anti-IRS-1 antibody in (A) normal human
osteoblasts and (B) RA osteoblasts. Lane 1, control cells (NC);
lane 2, cells stably transfected with empty pcDNA3 vector (VC);
lane 3, cells stably transfected with IRS-1; lane 4, cells stably
transfected with IRS-1 plus pre-treatment with selective PI3K
inhibitor (50 μM BKM120) for 30 min; lane 5, cells stably
transfected with Arg972 IRS-1; lane 6, cells stably
transduced with scramble control shRNA (SC); lane 7, cells stably
transduced with IRS-1-shRNA. β-Actin blotting was used as a loading
control. The density of the anti-IRS-1 blot was normalized against
that of β-actin to obtain a relative blot density, which is
expressed as the fold change to the relative anti-IRS-1 blot
density of NC (designated as 1). aP<0.05, vs. NC or
VC; bP<0.05, vs. IRS-1; cP<0.05, vs.
IRS-1+PI3K-I; dP<0.05, vs. Arg972 IRS-1;
eP<0.05, vs. SC. IRS-1, insulin receptor substrate-1;
PI3K, phosphatidylinositol-3 kinase; RA, rheumatoid arthritis. |
Arg972 IRS-1 is reportedly associated
with impaired insulin/IRS-1 signaling to activate the PI3K/Akt
pathway (3,4), which is important in cell survival
against apoptotic stress (14).
The present study also examined the IRS-1-associated PI3K activity
and Akt activation/phosphorylation in osteoblasts. In the absence
of insulin, the overexpression of Arg972 IRS-1 and IRS-1
and the knock down of IRS-1 exhibited no significant effects on
IRS-1-associated PI3K activity, Akt activation/phosphorylation or
TNF-α-induced apoptosis in the osteoblasts. (figs. 2Figure 34). Different techniques used to stimulate
insulin were assessed, which revealed that treatment with 10 nM
insulin for 30 min had the most marked stimulatory effects on
IRS-1-associated PI3K activity in the osteoblasts (data not shown).
Thus, in all the subsequent experiments, osteoblasts were
pre-stimulated with 10 nM insulin for 30 min. As shown in Fig. 2, overexpression of IRS-1 increased
IRS-1-associated PI3K activity by ~3 fold in the normal and RA
osteoblasts, compared with the controls, which was eliminated by
pretreatment with 50 μM BKM120, a selective PI3K inhibitor,
for 30 min. By contrast, the overexpression of Arg972
IRS-1 and the knock down of IRS-1, decreased IRS-1-associated PI3K
activity by 40 and 60%, respectively in the normal and RA
osteoblasts. Similar trends were observed in the phosphorylation of
Akt at ser473, which is required for full activation of Akt by PI3K
(Fig. 3) (14).
 | Figure 2IRS-1-associated PI3K activity in
normal human osteoblasts and RA osteoblasts with overexpression of
IRS-1 or Arg972 IRS-1 or the knock down of IRS-1.
IRS-1-associated PI3K activity was measured in (A) normal human
osteoblasts and (B) RA osteoblasts stimulated by 10 nM of insulin
for 30 min. The IRS-1-associated PI3K activities inLane 1, control
cells (NC); lane 2, cells stably transfected with empty pcDNA3
vector (VC); lane 3, cells stably transfected with IRS-1; lane 4,
cells stably transfected with IRS-1 plus pre-treatment with
selective PI3K inhibitor (50 μM BKM120) for 30 min; lane 5,
cells stably transfected with Arg972 IRS-1; lane 6,
cells stably transduced with scramble control shRNA (SC); lane 7,
cells stably transduced with IRS-1-shRNA. Blot density is expressed
as fold changes to that of NC (designated as 1).
aP<0.05, vs. NC or VC; bP<0.05, vs.
IRS-1; cP<0.05, vs. IRS-1+PI3K-I;
dP<0.05, vs. Arg972 IRS-1;
eP<0.05, vs. SC. IRS-1, insulin receptor substrate-1;
RA, rheumatoid arthritis; PI3K, phosphatidylinositol-3 kinase. |
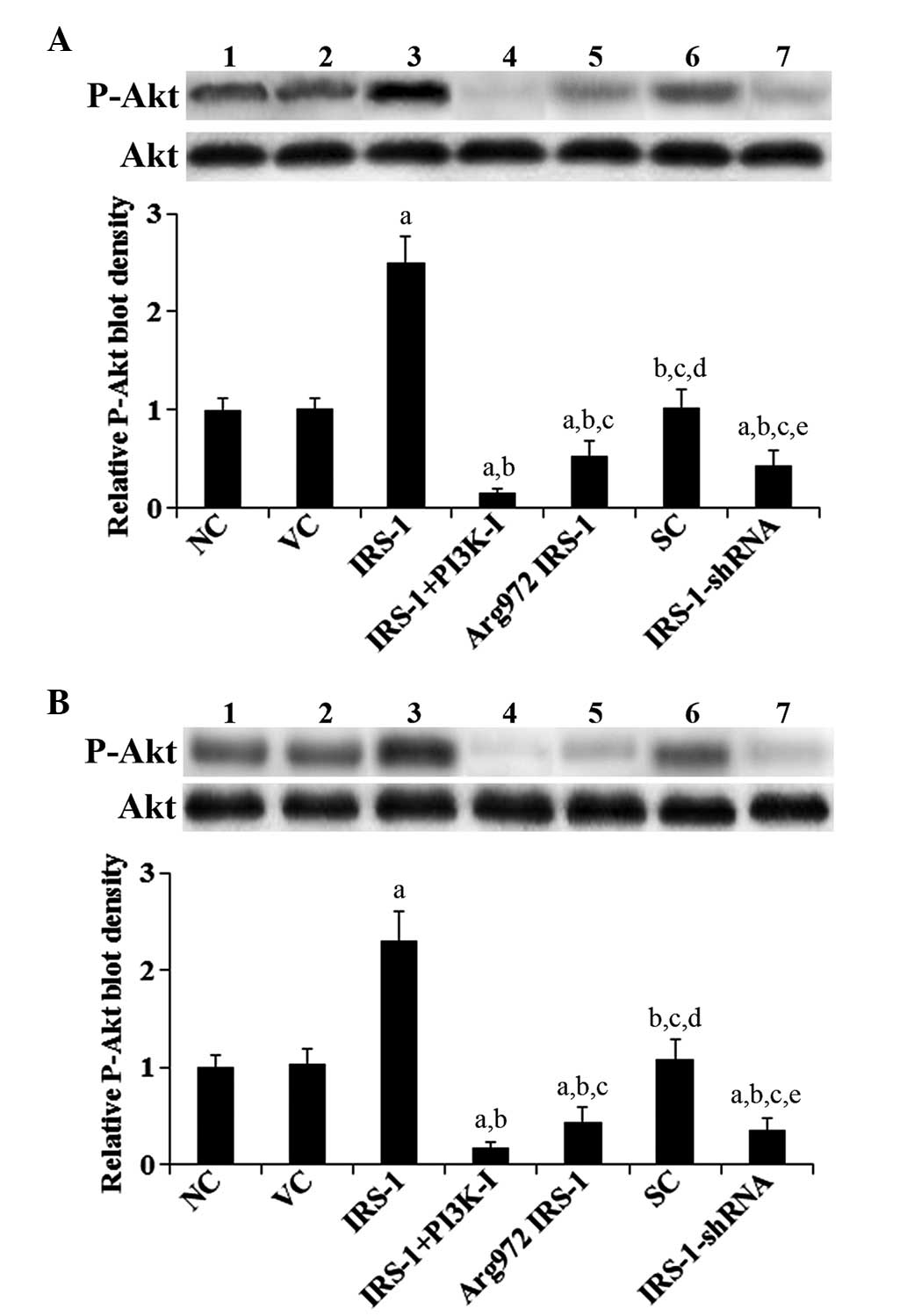 | Figure 3P-Akt level in normal human
osteoblasts and RA osteoblasts with overexpression of IRS-1 or
Arg972 IRS-1 or knock down of IRS-1. Western blot
analyses were performed to determine total Akt and P-Akt (at serine
473) levels in (A) normal human osteoblasts and (B) RA osteoblasts
stimulated by 10 nM insulin for 30 min. Lane 1, control cells (NC);
lane 2, cells stably transfected with empty pcDNA3 vector (VC);
lane 3, cells stably transfected with IRS-1; lane 4, cells stably
transfected with IRS-1 and pretreated with selective PI3K inhibitor
(50 μMBKM120) for 30 min (IRS-1+PI3K-I); lane 5, cells
stably transfected with Arg972 IRS-1; lane 6, cells
stably transduced with scramble control shRNA (SC); lane 7, cells
stably transduced with IRS-1-shRNA. The density of the P-Akt blot
was normalized against that of total Akt to obtain a relative blot
density, which is expressed as fold changes to the relative P-Akt
blot density of NC (designated as 1). aP<0.05 vs. NC
or VC; bP<0.05 vs. IRS-1; cP<0.05 vs.
IRS-1+PI3K-I; dP<0.05 vs. Arg972 IRS-1;
eP<0.05 vs. SC. P-Akt, phosphorylated Akt; IRS-1,
insulin receptor substrate-1; RA, rheumatoid arthritis; PI3K,
phosphatidylinositol-3 kinase. |
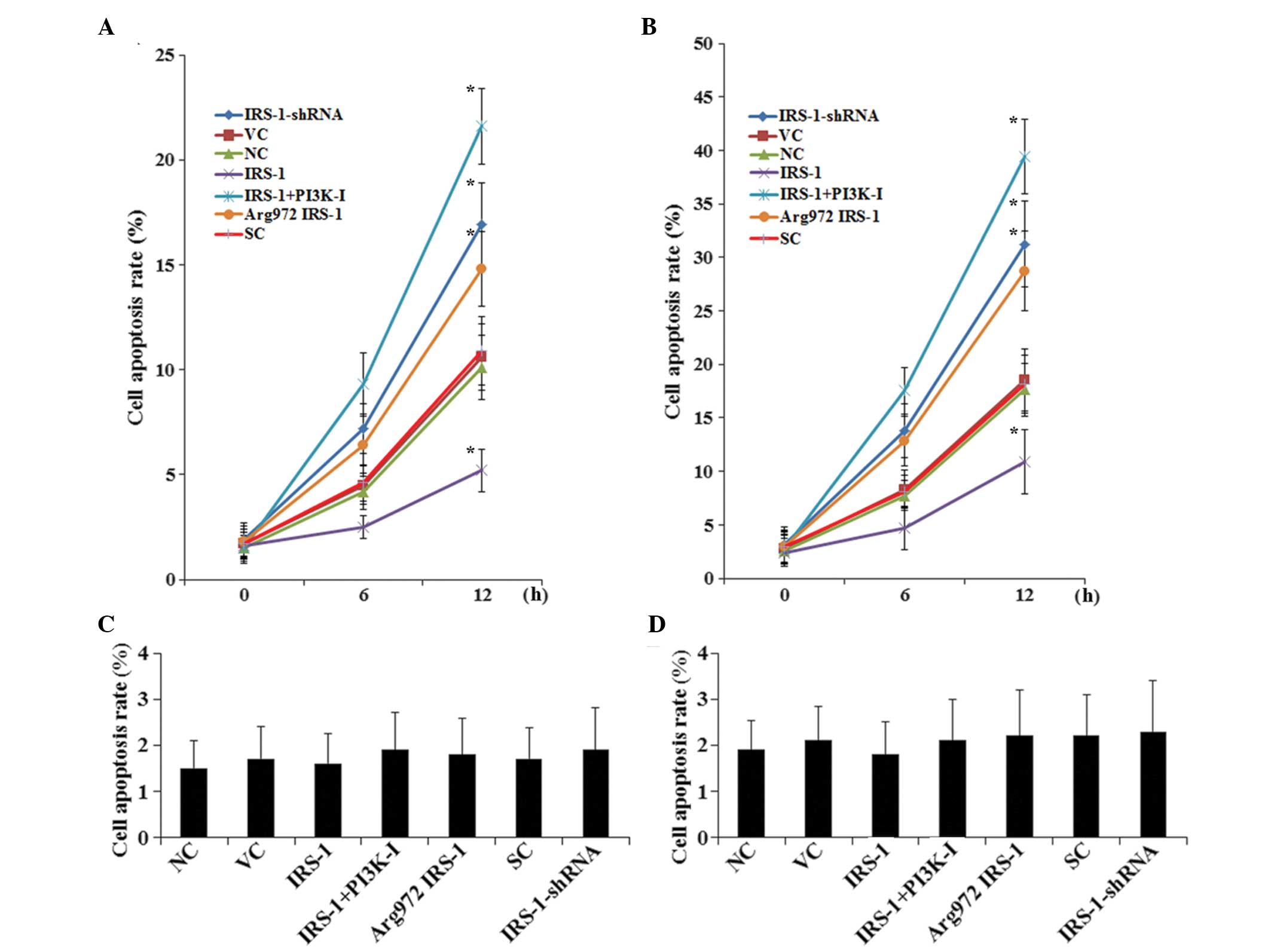 | Figure 4TNF-α-induced apoptosis in normal
human osteoblasts and RA osteoblasts with overexpression of IRS-1
or Arg972 IRS-1 or knock down of IRS-1. (A) Normal human
osteoblasts and (B) RA osteoblasts were stimulated by 10 nM insulin
for 30 min and then treated with 20 ng/ml TNF-α for 6 and 12 h
TUNEL assays were performed in the control cells (NC), cells stably
transfected with empty pcDNA3 vector (VC), cells stably transfected
with IRS-1, cells stably transfected with IRS-1 plus pre-treatment
with selective PI3K inhibitor BKM120 (50 μM) for 30 min
(IRS-1+PI3K-I), cells stably transfected with Arg972
IRS-1, cells stably transduced with scramble control shRNA (SC) and
cells stably transduced with IRS-1-shRNA. The rates of cell
apoptosis at 6 and 12 h are presented as the percentage of TUNEL
positive cells in the total cells. TUNEL assays were also performed
at 12 h in (C) normal human osteoblasts and (D) RA osteoblasts
stimulated by 10 nM insulin for 30 min, but without TNF-α
treatment. *P<0.05, compared with NC, VC or SC at 12
h. IRS-1, insulin receptor substrate-1; RA, rheumatoid arthritis;
PI3K, phosphatidylinositol-3 kinase; TNF-α, tumor necrosis
factor-α; TUNEL, terminal deoxynucleotidyl transferase mediated
nick-end labeling. |
Subsequently, the effects of IRS-1 and
Arg972 IRS-1 on TNF-α-induced apoptosis in osteoblasts
were examined. As shown in Fig. 4A and
B, osteoblasts treated with 20 ng/ml TNF-α for 12 h in the
presence of insulin (10 nM), exhibited significant differences in
apoptosis. Compared with the controls at 12 h, overexpression of
IRS-1 decreased cell apoptosis by ~5% in the normal osteoblasts and
by ~7% in the RA osteoblasts and this was eliminated completely by
pretreatment with 50 μM BKM120, a selective PI3K inhibitor,
for 30 min. By contrast, overexpression of Arg972 IRS-1
increased cell apoptosis by ~4.5% in the normal osteoblasts and by
~10.5% in the RA osteoblasts, and knock down of IRS-1 increased
cell apoptosis by ~6.5% in the normal osteoblasts and ~13% by in
the RA osteoblasts (Fig. 4A and
B). In the absence of TNF-α treatment, no significant
differences were obsserved in the apoptotic rate at 12 h in the
normal or the RA osteoblasts (Fig. 4C
and D). Representative fluorescent TUNEL assay images at 12 h
are shown in Fig. 5. DAPI staining
of the cell nuclei indicated a similar cell number/density in the
experiment groups (Fig. 5).
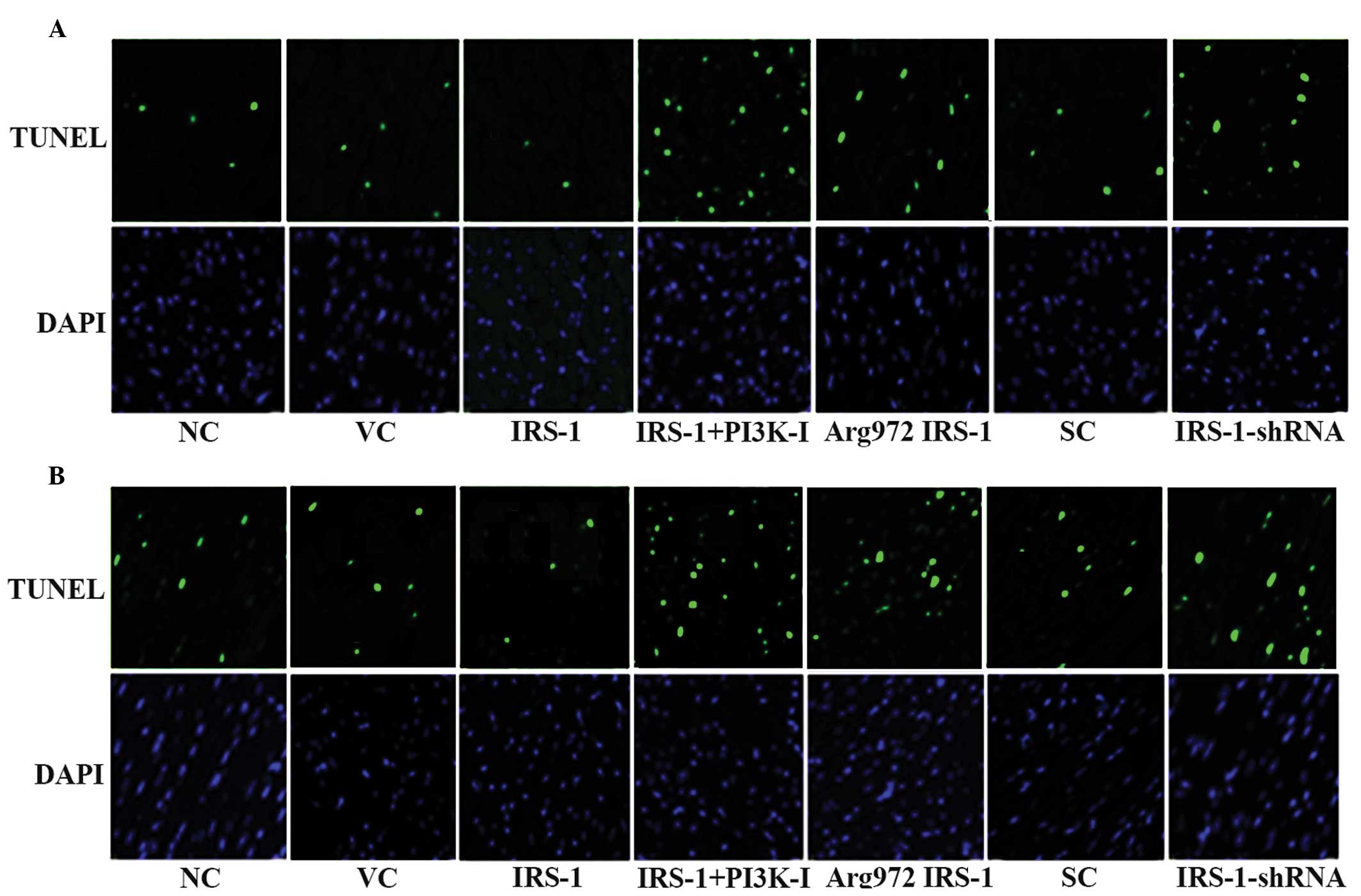 | Figure 5Fluorescent TUNEL assay in normal
human osteoblasts and RA osteoblasts with overexpression of IRS-1
or Arg972 IRS-1 or knock down of IRS-1. (A) Normal human
osteoblasts and (B) RA osteoblasts were stimulated by 10 nM insulin
for 30 min and then treated with 20 ng/ml TNF-α for 6 and 12 h.
Fluorescent TUNEL assays were performed in control cells (NC),
cells stably transfected with empty pcDNA3 vector (VC), cells
stably transfected with IRS-1, cells stably transfected with IRS-1
plus pre-treatment with selective PI3K inhibitor BKM120 (50
μM) for 30 min (IRS-1+PI3K-I), cells stably transfected with
Arg972 IRS-1, cells stably transduced with scramble
control shRNA (SC) and cells stably transduced with IRS-1-shRNA.
Apoptotic cells exhibited marked nuclear green fluorescence, which
was detected using a standard fluorescein filter. All cells stained
with DAPI exhibited marked blue nuclear fluorescence. TUNEL,
terminal deoxynucleotidyl transferase mediated nick-end labeling;
DAPI, 4′,6-diamidino-2-phenylindole; IRS-1, insulin receptor
substrate-1; RA, rheumatoid arthritis PI3K, phosphatidylinositol-3
kinase; TNF-α, tumor necrosis factor-α. |
Discussion
Arg972 IRS-1 has been previously reported
an independent risk factor for RA and a case-control study
demonstrated it is significantly associated with the severity of RA
(5). In the present study, a
mechanistic explanation for this was obtained by revealing that
Arg972 IRS-1 enhanced TNF-α-induced apoptosis in normal
and RA osteoblasts.
The presence of Arg972 IRS-1 is
associated with impaired insulin/IRS-1 signaling to activate the
PI3K/Akt pathway (3,4). The results of the present study were
in agreement with this, which demonstrated that, in the presence of
insulin, stable overexpression of Arg972 IRS-1 and knock
down of IRS-1 significantly decreased IRS-1-associated PI3K
activity and Akt activation/phosphorylation (ser473) in
osteoblasts. By contrast, the stable overexpression of IRS-1
significantly increased IRS-1-associated PI3K activity and Akt
activation/phosphorylation (ser473), which was completely
eliminated by a selective PI3K inhibitor. For insulin stimulation,
the cells were pretreated with 10 nM insulin, which had been used
in a previous study to stimulate osteoblast-linage cells (18).
The present study demonstrated that treatment with
20 ng/ml TNF-α for 12 h induced significant apoptosis in the normal
and RA osteoblasts, concordant with a previous study, which
observed peaks in the activities of caspase-3, caspase-8 and
caspase-9 in human bone marrow stromal cells after 12 h of
treatment with 20 ng/ml TNF-α (19). In agreement with their inhibitory
effects on the PI3K/Akt pathway, which is important in promoting
cell survival against apoptotic stress (14), overexpression of Arg972
IRS-1 and knock down of IRS-1 in the present study significantly
enhanced TNF-α-induced apoptosis in the normal and RA osteoblasts.
This was corroborated by the finding that the overexpression of
IRS-1 significantly increased IRS-1-associated PI3K activity/Akt
phosphorylation and reduced TNF-α-induced apoptosis in the
osteoblasts. The overexpression of Arg972 IRS-1 and
knock down of IRS-1 in the RA osteoblasts exhibited a more
pronounced inhibitory effect on TNF-α-induced apoptosis compared
with the normal osteoblasts. This suggested that, Arg972
IRS-1, or the impairment of insulin/IRS-1 signaling, was important
in the pathogenesis of RA and that other signaling pathways,
besides insulin/IRS-1 signaling, are involved in the pathogenesis
of RA.
The normal and the RA osteoblasts used in the
present study were genotyped and found to be wildtype IRS-1
homozygotes. Thus, the overexpression of Arg972 IRS-1 in
the osteoblasts resulted in the expression of a mixture of
Arg972 IRS-1 and wild type IRS-1. This resembles an
Arg972 IRS-1 heterozygote, which is the major source of
Arg972 IRS-1 carriers and the frequency of
Arg972 IRS-1 heterozygote, wild-type IRS-1 homozygote
and Arg972 IRS-1 homozygote are 12.5, 87 and 0.6%,
respectively, in Exome Sequencing Project cohort populations
(http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=rs1801278).
By demonstrating that the overexpression of Arg972 IRS-1
and IRS-1 enhanced and inhibited TNF-α-induced apoptosis in normal
and RA osteoblasts, respectively, the results of the present study
suggested that Arg972 IRS-1 carriers may develop RA more
easily and that insulin/IRS-1 signaling is important in the
pathogenesis of RA. It also suggested that insulin/IRS-1 signaling
may be a new target for treating RA.
TNF-α has significant effects on other cell types in
the synovial membrane, including synoviocytes, macrophages,
chondrocytes and osteoblasts (20). Thus, it may be useful to examine
how Arg972 IRS-1 and IRS-1 affect the regulatory effects
of TNF-α on cell types other than osteoblasts in future studies. In
addition, inflammatory cytokines, including interleukin-1β and
interleukin-6 have also been found to have important roles in the
pathogenesis of RA (9). Therefore,
identifying whether insulin/IRS-1 signaling can regulate the
effects of interleukins on cells involved in the pathogenesis of RA
may be of interest.
In conclusion, the present study provided the first
evidence, to the best of our knowledge, that under insulin
stimulation, Arg972 IRS-1 and IRS-1 enhanced and
inhibited TNF-α-induced apoptosis in normal and RA osteoblasts,
respectively, by a PI3K-dependent mechanism. These findings suggest
an important role for insulin/IRS-1 signaling in the pathogenesis
of RA.
References
|
1
|
Zeng G, Nystrom FH, Ravichandran LV, Cong
LN, Kirby M, Mostowski H and Quon MJ: Roles for insulin receptor,
PI3-kinase, and Akt in insulin signaling pathways related to
production of nitric oxide in human vascular endothelial cells.
Circulation. 101:1539–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuboki K, Jiang ZY, Takahara N, et al:
Regulation of endothelial constitutive nitric oxide synthase gene
expression in endo-thelial cells and in vivo: a specific vascular
action of insulin. Circulation. 101:676–681. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fallucca F, Dalfrà MG, Sciullo E, et al:
Polymorphisms of insulin receptor substrate 1 and beta3-adrenergic
receptor genes in gestational diabetes and normal pregnancy.
Metabolism. 55:1451–1456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang C, Lin Z, Zhou Y, et al:
Arg(972) insulin receptor substrate-1 is associated with
elevated plasma endothelin-1 level in hypertensives. J Hypertens.
30:1751–1757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H, Liu S, Long M, Peng L, Deng H and
You Y: Arg972 Insulin receptor substrate-1 polymorphism
and risk and severity of rheumatoid arthritis. J Int Rheum Dis. In
press.
|
|
6
|
Nanke Y, Kotake S, Akama H and Kamatani N:
Alkaline phosphatase in rheumatoid arthritis patients: possible
contribution of bone-type ALP to the raised activities of ALP in
rheumatoid arthritis patients. Clin Rheumatol. 21:198–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neve A, Corrado A and Cantatore FP:
Osteoblast physiology in normal and pathological conditions. Cell
Tissue Res. 343:289–302. 2011. View Article : Google Scholar
|
|
8
|
Fütterer A, Mink K, Luz A, Kosco-Vilbois
MH and Pfeffer K: The lymphotoxin beta receptor controls
organogenesis and affinity maturation in peripheral lymphoid
tissues. Immunity. 9:59–70. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McInnes IB and Liew FY: Cytokine networks:
Towards new therapies for rheumatoid arthritis. Nat Clin Prac
Rheumatol. 1:31–39. 2005. View Article : Google Scholar
|
|
10
|
Kitajima I, Soejima Y, Takasaki I, Beppu
H, Tokioka T and Maruyama I: Ceramide-induced nuclear translocation
of NFkappa B is a potential mediator of the apoptotic response to
TNF-alpha in murine clonal osteoblasts. Bone. 19:263–270. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hill PA, Tumber A and Meikle MC: Multiple
extracellular signals promote osteoblast survival and apoptosis.
Endocrinology. 138:3849–3858. 1997.PubMed/NCBI
|
|
12
|
Jilka RL, Weinstein RS, Bellido T, Parfitt
AM and Manolagas SC: Osteoblast programmed cell death (apoptosis):
Modulation by growth factors and cytokines. J Bone Miner Res.
13:793–802. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hock JM, Krishnan V, Onyia JE, Bidwell JP,
Milas J and Stanislaus D: Osteoblast apoptosis and bone turnover. J
Bone Miner Res. 16:975–984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Monti S, Juszczynski P, et al: SYK
inhibition modulates distinct PI3K/AKT- dependent survival pathways
and cholesterol biosynthesis in diffuse large B cell lymphomas.
Cancer Cell. 23:826–838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porzio O, Federici M, Hribal ML, et al:
The Gly972-->Arg amino acid polymorphism in IRS-1
impairs insulin secretion in pancreatic beta cells. J Clin Invest.
104:357–364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagoshi T, Matsui T, Aoyama T, et al: PI3K
rescues the detrimental effects of chronic Akt activation in the
heart during ischemia/reperfusion injury. J Clin Invest.
115:2128–2138. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsui T, Li L, del Monte F, Fukui Y,
Franke TF, Hajjar RJ and Rosenzweig A: Adenoviral gene transfer of
activated PI 3-kinase and Akt inhibits apoptosis of hypoxic
cardiomyocytes in vitro. Circulation. 100:2373–2379. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Zhang X, Wang W and Liu J: Insulin
stimulates osteoblast proliferation and differentiation through ERK
and PI3K in MG-63 cells. Cell Biochem Funct. 28:334–341. 2010.
View Article : Google Scholar
|
|
19
|
Byun CH, Koh JM, Kim DK, Park SI, Lee KU
and Kim GS: α-lipoic acid inhibits TNF-α-induced apoptosis in human
bone marrow stromal cells. J Bone Miner Res. 20:1125–1135. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma X and Xu S: TNF inhibitor therapy for
rheumatoid arthritis (Review). Biomed Rep. 1:177–184. 2013.
|



















