Introduction
Osteosarcoma (OS) is the most common type of primary
malignant bone tumor, and predominantly occurs in infants (1). With the development of novel
chemotherapy protocols, surgical excision and radiological staging,
5-year survival rates and cure rates have increased to 60–80% in
patients with localized disease (2). However, problems with chemotherapy
remain, particularly the frequent development of drug resistance
(3). Therefore, there remains a
requirement for the development of alternative methods for the
treatment of OS. In recent years, the use of SDT has been widely
accepted as a promising therapeutic strategy to prevent the
development and recurrence of malignant diseases.
Sonodynamic therapy (SDT) is a non-invasive approach
for the treatment of diseases based on the synergistic effects of
low intensity ultrasound and sonosensitization (4–6). The
ultrasound energy can be focused on targeted tissues to induce
local cytotoxicity by activating sonosensitizers with minimal
damage to healthy tissues (7,8).
Recently, SDT has been widely used in the treatment of tumors and
has been demonstrated to mediate apoptosis in numerous experimental
systems in vitro or in vivo. SDT has been shown to
induce cell death by apoptosis in a number of human tumor cell
lines, such as liver, oral, leukemia, lung and colon cancer cell
lines (9).
Therefore, SDT has great advantage as a targeted
cancer therapy. The ultimate goal of anticancer therapy is to kill
cancer cells rapidly and effectively. In the present study, the
effects of SDT on the MG-63 human osteosarcoma cell line were
investigated using in vitro assays for proliferation and
apoptosis. The mechanisms of apoptosis were analyzed by measurement
of the protein expression of poly ADP-ribose polymerase (PARP),
cleaved PARP, procaspase-3, cleaved caspase-3 and cleaved
caspase-9.
Materials and methods
Cell culture
The MG-63 human osteosarcoma cell line was obtained
from the American Type Culture Collection (Rockville, MD, USA). The
cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM,
Life Technologies, Inc., Grand Island, NY, USA) supplemented with
10% fetal bovine serum (FBS, Hyclone, NY, USA), 100 U/ml penicillin
and 100 μg/ml streptomycin (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) at 3700b0C in a humidified
atmosphere of 5% CO2 in air. Experiments were conducted
in logarithmic growth phase cells.
Ultrasonic exposure system and SDT
treatment protocols
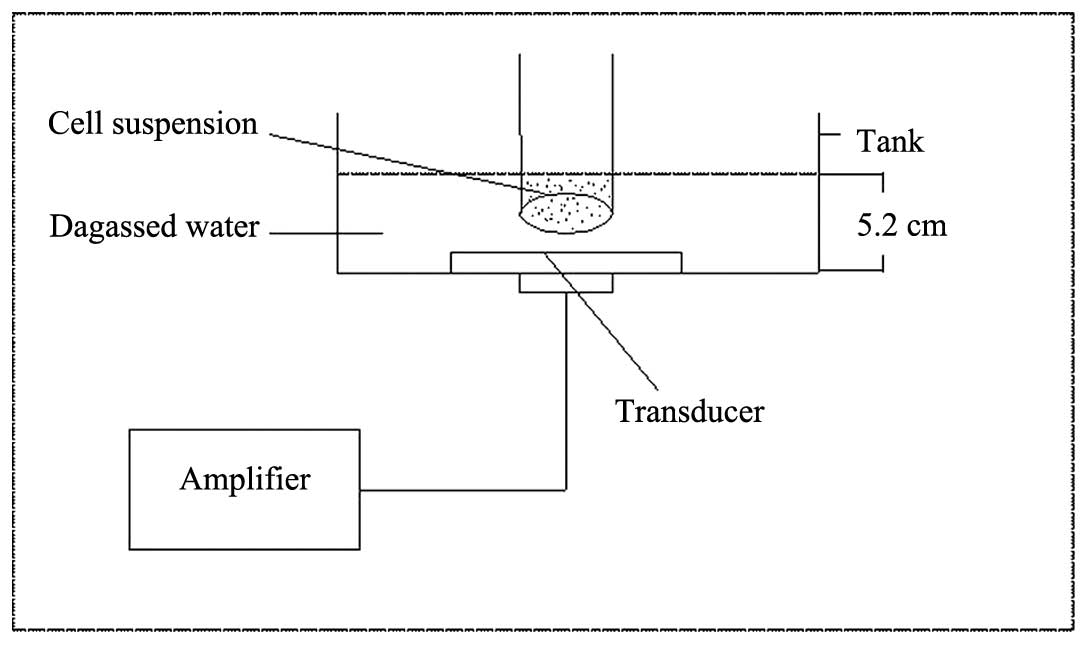
The ultrasonic exposure system (Fig. 1) used in our study was
multifunctional physiotherapy ultrasonic equipment (Tianshi
Technologies Ltd Co., Beijing, China). Using this device,
ultrasound at 1 MHz could be produced. The transducer (diameter,
2.5 cm; centre frequency, 1.0 MHz; duty factor, 10%; and repetition
frequency, 100 Hz) was placed upward at the bottom of a water tank.
The tank was filled with degassed water and the inner surface was
padded with ultrasound-absorbing materials (Tianshi Technologies
Co., Ltd., Beijing, China) to minimize the wave reflection. The
spatial average and temporal average intensities (ISATA) was
measured with a stainless-steel ball radiometer (diameter, 0.32 cm;
Tianshi Technologies Co., Ltd.) over 30 sec. The output intensity
was 1 W/cm2 during exposure. MG-63 cells were seeded in
35-mm petri dishes and placed in the degassed water at a distance
of ~5.2 cm from the ultrasonic transducer. During exposure, the
temperature of the water was maintained at 3700b0C. During the
sonication procedure, the temperature of the solution inside the
petri dishes was measured prior to and following ultrasound
treatment with a digital thermometer, and no significant variation
of temperature was detected (±0.500b0C).
HMME was obtained from Yingfa Kangmei Ltd. Co.,
(Beijing, China). The cells were divided into the following four
groups: Sham group, which received no treatment; the HMME group,
which was treated with 20 μg/ml HMME alone; the ultrasound
group treated with 1 W/cm2 ultrasound alone and the
ultrasound + HMME (SDT) group, which was treated with 1
W/cm2 ultrasound plus 20 μg/ml HMME.
Cell viability assays
Cells were seeded into 35-mm petri dishes (Bogoo
Biotechnology Company, Shanghai, China) and incubated with
different concentrations of HMME (0, 5, 10, 20, 30, 40 and 50
μg/ml) for 3 h in the dark. They were then exposed to
ultrasound for 30 sec. After SDT (6 h), the cell survival rate was
determined by an MTT assay. The MTT assay provides a fast and
simple method to evaluate the viability of the cells in the SDT
(10). This assay was performed as
a regular procedure and the absorbance at 570 nm was recorded using
a microplate reader (BIOTEK ELx800, BioTek, San Diego, CA, USA)
against the reference value at 690 nm. Results were expressed as a
percentage of the control.
Intracellular localization of
hematoporphyrin monomethyl ether
MG-63 cells were incubated with 20 μg/ml HMME
for 4 h, then co-loaded with 10 nM Mito Tracker Green (MT-G,
Molecular Probes, Invitrogen Life Technologies, Carlsbad, CA, USA)
and 1 μg/ml Hoechst 33342 (Ho; Molecular Probes, Invitrogen
Life Technologies). After loading, cells were washed with
phosphate-buffered saline (PBS) and imaged using an inverted
confocal laser scanning microscope (TCS SP5Leica, Wetzlar,
Germany). In the multi-channel imaging, photomultiplier
sensitivities and offsets were set to a level at which bleed
through effects from one channel to another were negligible.
Cell apoptosis assays
Cell apoptosis was detected using the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (KeyGen, Nanjing, China). In brief, 1×105
treated cells were harvested following trypsinization and
centrifugation at 111.8 × g for 5 min. Cells were washed with PBS
and resuspended in 500 ml binding buffer, to which 5 ml Annexin
V-FITC was added. After a 5 min incubation, 5 ml PI was added,
followed by a 5 min incubation in the dark. Apoptotic cells were
analyzed by flow cytometry (FC500 system; Beckman Coulter,
Fullerton, CA, USA).
Western blot analysis
PARP, cleaved PARP, procaspase-3, cleaved caspase-3
and cleaved caspase-9 were measured by western blot analysis.
SDS-PAGE and immunoblotting were conducted according to set
standard procedures.
Briefly, cells were harvested and collected by
centrifugation at 111.8 × g for 5 min, then precooling lysate was
added (containing 0.25% sodium deoxycholate, 1% Triton X-100, 1%
Nonidet P-40, 5 mM EDTA, 10 μg/ml leupeptin, 10 μg/ml
aprotinin and 1 mM phenylmethylsulfonyl fluoride; all from
Sigma-Aldrich, St. Louis, MO, USA) on ice. The cells were
centrifuged at 111.8 x g for 5 min, and the supernatant was
collected, followed by the addition of 5X loading buffer
(containing 10% SDS, 5% β-mercaptoethanol, 15% glycerol, 0.01%
bromophenol blue and 200 mM Tris-HCl; pH 6.7) in boiling water for
5 min and separated on a 10–15% linear SDS polyacrylamide gel.
Then, the proteins were transferred onto nitrocellulose filter
membranes (Millipore, Billerica, MA, USA). Membranes were incubated
at room temperature for 2 h and probed at 400b0C overnight with
mouse monoclonal PARP (cat. no. sc-56197), mouse monoclonal cleaved
PARP (cat. no. sc-56196), mouse monoclonal procaspase-3 (cat. no.
sc-7272), rabbit monoclonal cleaved caspase-3 (cat. no.
sc-22171-R), mouse monoclonal cleaved caspase-9 (cat. no. sc-70505)
as primary antibodies all purchased from Santa Cruz Biotechnology,
Inc. (San Diego, CA, USA). Membranes were probed with a goat
anti-rabbit antibody conjugated with secondary antibody (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
for 1 h and visualized by enhanced chemiluminescence (Tianshi
Technologies Co., Ltd.).
Statistical analysis
Statistical analysis was conducted using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). All values are
expressed as the mean ± standard error of the mean. Differences
between the treated cells and control were assessed with one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
HMME-SDT inhibits the proliferation of
MG-63 cells
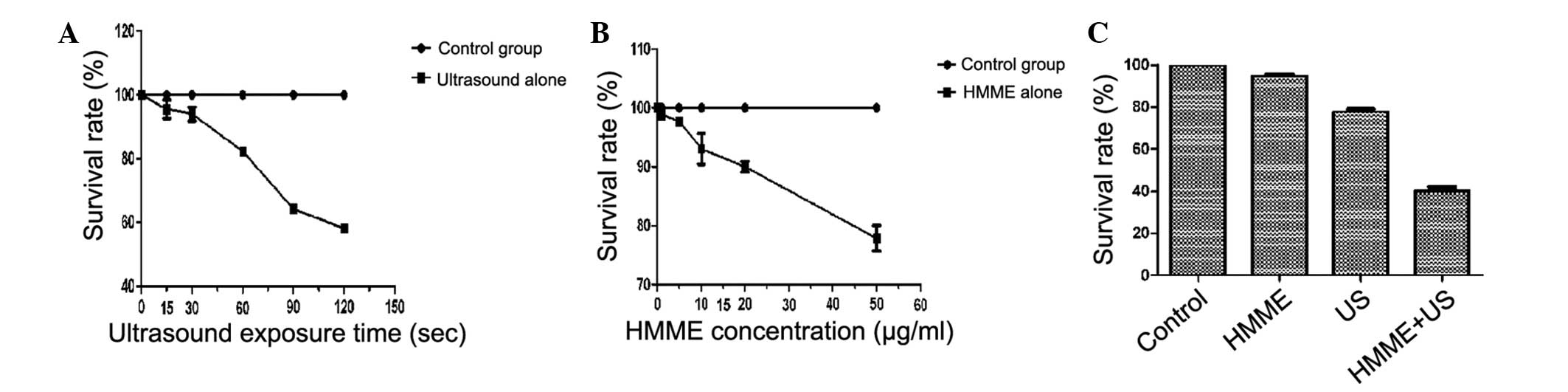
Cell viability was detected by an MTT assay. The
survival rate of MG-63 cells decreased with HMME concentration and
ultrasonic irradiation time increased. As shown in Fig. 2A, the survival rate was
significantly decreased with the increase in ultrasound exposure
time. As shown in Fig. 2B, the
survival rate was significantly decreased with increasing
concentration of HMME.
As shown in Fig.
2C, cell survival in 20 μg/ml HMME alone was 94.70±0.58%
compared with control, showing no obvious cytotoxic effect on MG-63
cells. When cells were treated with 1.0 W/cm2
ultrasound, the survival rate of cells was 77.68±0.89%. However,
when cells were treated with ultrasound in the presence of 20
μg/ml HMME, cell survival decreased to 40.18±1.35%
(P<0.01), demonstrating a synergistic enhancement of
sonochemically induced cytotoxicity.
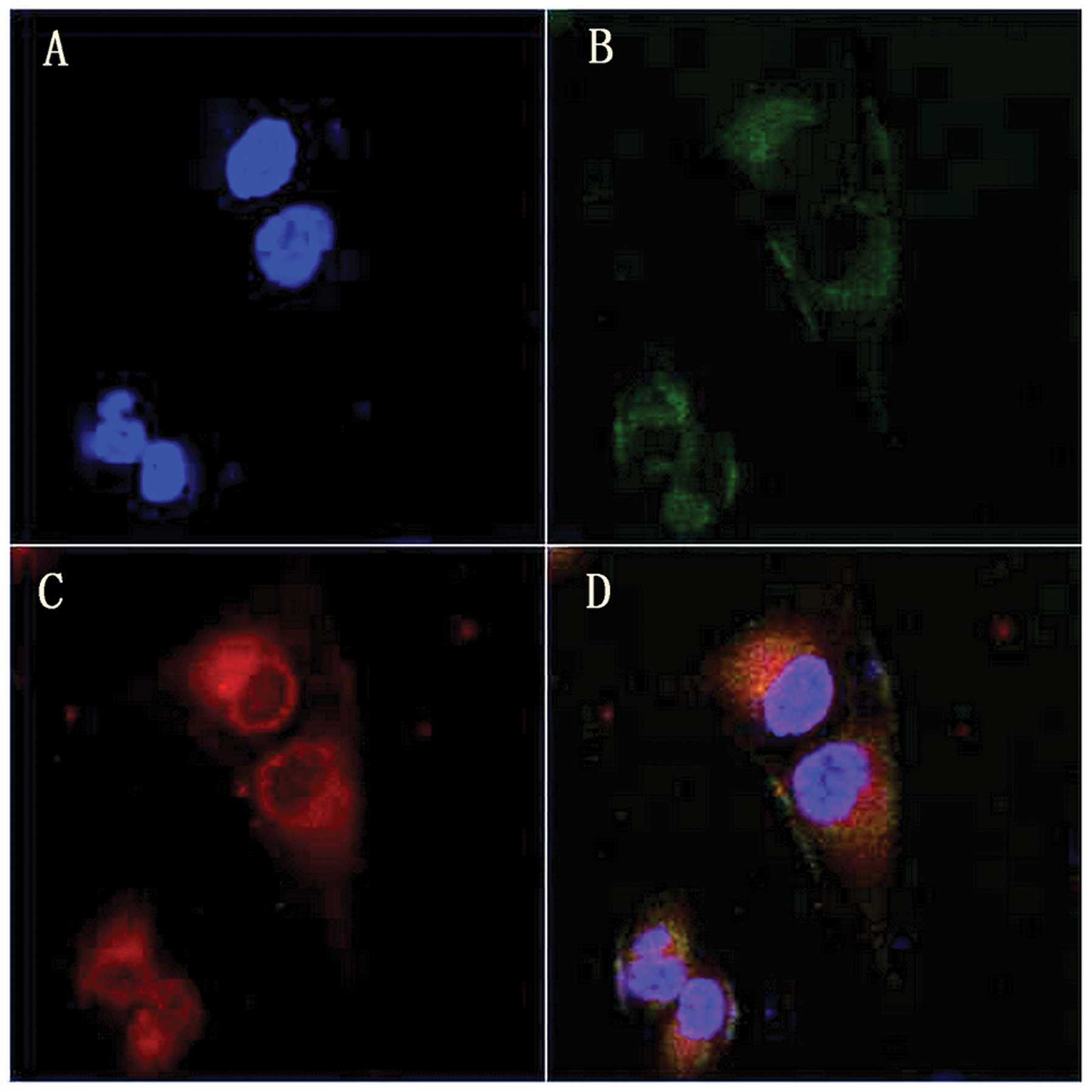
Intracellular localization of HMME
After 4 h incubation with HMME, cells were co-loaded
with the mitochondria probe MT-G (green) and nuclear probe Ho
(blue). As shown in Fig. 3, the
HMME fluorescence (red) corresponded well with MT-G green
fluorescence but without any Ho blue fluorescence overlap,
indicating that HMME predominantly accumulated in the mitochondria
of MG-63 cells.
HMME-SDT induces apoptosis in MG-63
cells
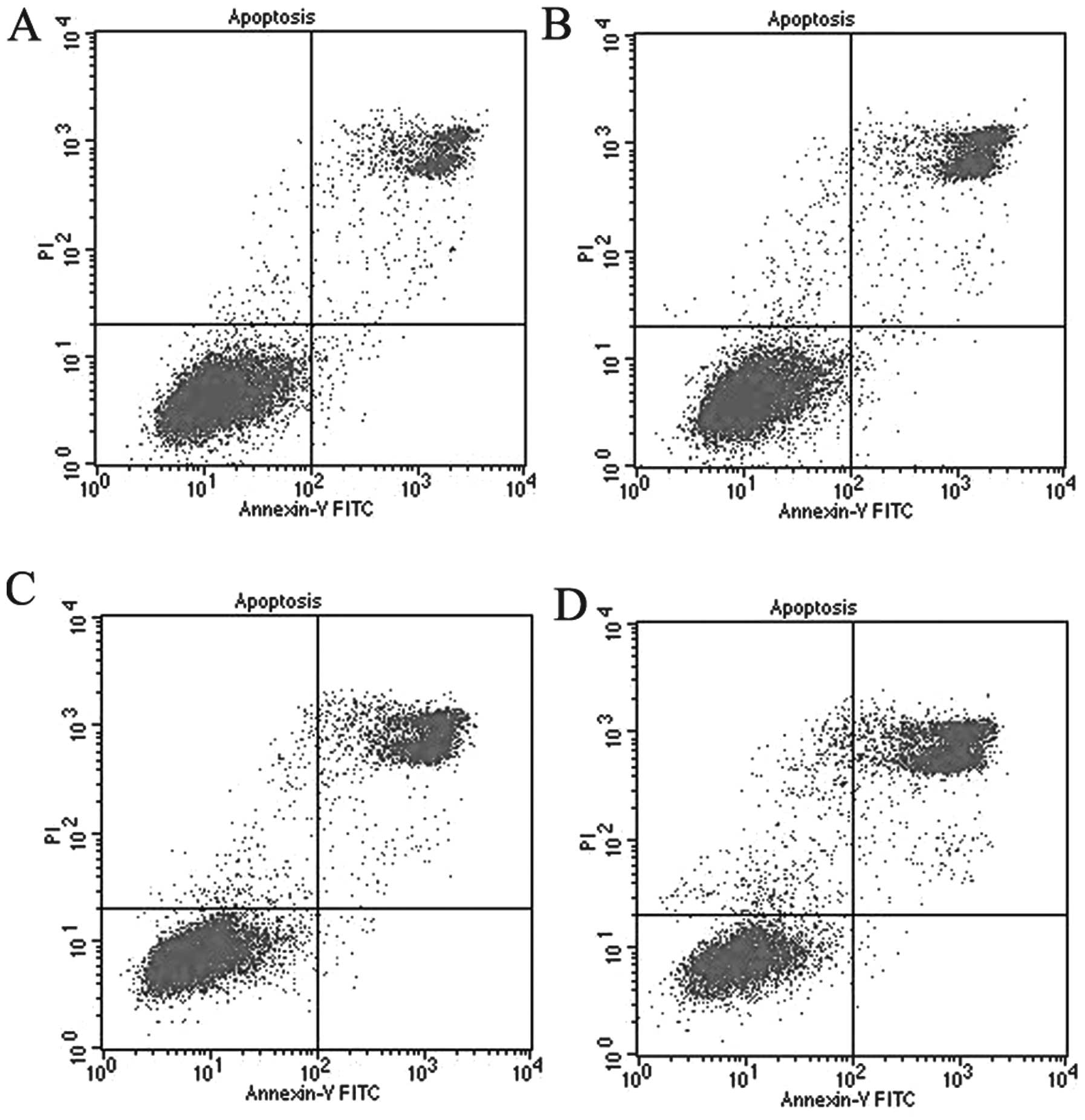
Flow cytometry with Annexin V (Annexin V-FITC) and
PI staining has been regarded as an important way to distinguish
early apoptosis from late apoptosis or necrosis (11). In the present study, flow cytometry
showed that HMME-SDT significantly increased the apoptosis of the
MG-63 cell population. Fig. 4
shows the MG-63 cells 12 h after sham treatment, 11.49% of the
cells had undergone apoptosis. Following treatment with HMME alone,
19.02% of the MG-63 cells had undergone apoptosis while following
ultrasound treatment alone 23.58% had undergone apoptosis. However,
in the MG-63 cells treated with HMME-SDT, there was a significant
increase in the percentage of cells that had undergone apoptosis to
43.56% at the intensity of 1.0 W/cm2 for 30 sec. These
results showed that the sonodynamic action of HMME could
effectively induce the apoptosis of OS cells.
Protein expression detected by western
blot analysis
Increasing evidence suggests that changes in matrix
metalloproteinases are associated with apoptosis (12–14).
In a number of experimental systems, SDT has been shown to induce
apoptosis. Therefore, the present study aimed to detect several key
apoptosis related proteins, such as PARP, cleaved PARP,
procaspase-3, cleaved caspase-3 and cleaved caspase-9, to assess
whether MG-63 cells can be induced by SDT.
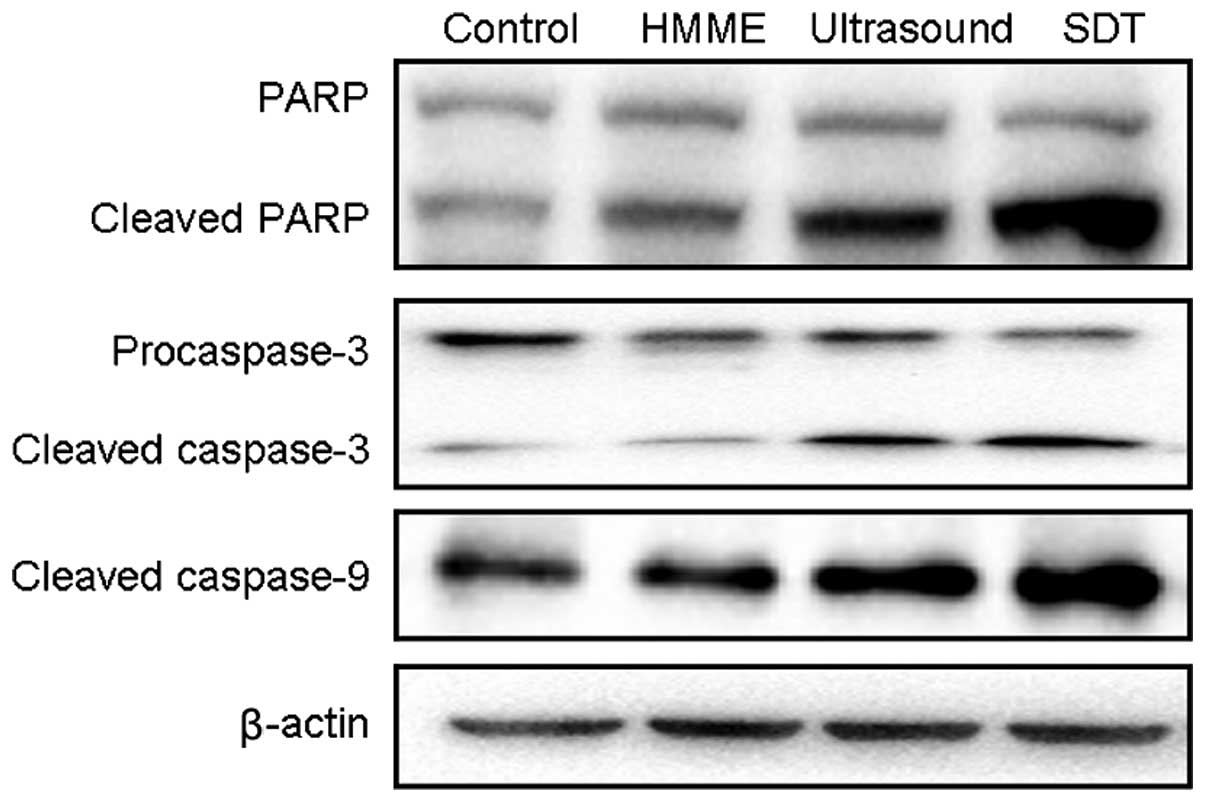
Cleaved PARP is a classical substrate of activated
caspase-3, and it can often be considered as a marker of apoptosis;
thus, it was used to indicate the occurrence of apoptosis. PARP
cleavage occurs at the early stages of apoptosis, as well as the
later stages. Fig. 5 shows that
cleaved PARP significantly increased following sonodynamic therapy
when compared with that in the ultrasound alone or HMME alone
group. Similarly, little cleaved caspase-9 (caspase-9 activation)
was measured in either the HMME alone or ultrasound irradiation
group, but typical 35 kDa caspase-9 cleavage was observed, and
accompanied by a decrease in the procaspase-9 level. In addition,
ultrasound irradiation in the presence of HMME resulted in more
obvious caspase-3 activation compared with the control, accompanied
by the decreased level of procaspase-3. These results showed that
sonodynamic therapy could induce apoptotic cell death of MG-63
cells, which is caspase dependent.
Discussion
Apoptosis is a complex mechanism that regulates cell
numbers and is essential throughout the life of all metazoan
animals. Caspase activation is the most fundamental pathway among
different types of biochemical events in cells undergoing apoptosis
(15–20). The induction of apoptosis may be
the most effective defense against cancer (20). Recent studies have revealed that
low intensity ultrasound combined with sonosensitizers can induce
apoptosis in a number of cancer cell types (21–26).
The majority of those studies are irradiated by selecting cell
types, sonosensitizers and the ultrasound exposure parameters
(21–26). This study demonstrated that SDT
therapy mediated by HMME has significant antitumor effects on MG-63
human osteosarcoma cells.
The localization of sonosensitizer is important in
the treatment of tumors, due to the short half life and short
diffusion distance of the majority of radicals derived from the
sonosensitizer produced during SDT (27). In order to detect the intracellular
localization of HMME in MG-63 cells, cells were co-loaded with
HMME, MT-G mitochondria probe and Ho nucleus dye, and their
corresponding fluorescence was imaged by inverted confocal laser
scanning microscopy. Fig. 3 shows
that the fluorescence of HMME corresponded well with that of MT-G
but not with Ho, which suggests that HMME is predominantly
localized in the mitochondria of MG-63 cells. The cell survival
rate was then detected by MTT 6 h following SDT treatment. Data as
shown in Fig. 2B shows that the
proposed ultrasound-induced cell damage was markedly enhanced by 20
μg/ml HMME (P<0.01), whereas HMME alone did not exhibit
significant cytotoxic effects on MG-63 cells. Fig. 5 shows that cleaved PARP
significantly increased after sonodynamic therapy when compared
with treatment with ultrasound alone or HMME alone. Similarly,
little cleaved caspase-9 (caspase-9 activation) was measured in
either the HMME alone or ultrasound irradiation group, but typical
35 kDa caspase-9 cleavage was measured, and was accompanied by a
decrease in the procaspase-9 level. In addition, ultrasound
irradiation with the presence of HMME resulted in more notable
caspase-3 activation compared with the control, accompanied by a
decrease in the level of procas-pase-3.
Mitochondrial damage may be the predominant reason
for HMME-SDT-induced cytotoxicity as HMME primarily accumulated in
the mitochondria of MG-63 cells, suggesting that the mitochondria
are the target of SDT. As the major energy generators,
mitochondrial-mediated apoptosis occurs in response to a wide range
of stimuli.
There are two major apoptotic pathways: The external
(receptor-mediated) and the intrinsic (mitochondria-mediated). The
intrinsic pathway of apoptosis occurs through internal and external
stimulation, including a number of mediators, which can promote or
inhibit the process (28). The
most representative regulators of the mitochondria-mediated pathway
are p53, an inducer of apoptosis, and Bcl-2, a molecule with the
opposite effect (29–31). The mitochondria-caspase signaling
pathway was activated following SDT and ultrasound to promote the
expression of pro-apoptotic proteins, such as caspase-3 and Bax in
cancer cells (12).
In another study, histidine significantly reduced
the generation of nitrogen oxides and activation of caspase-3
during sonodynamically-induced apoptosis, suggesting that active
species, such as singlet oxygen are important in the sonodynamic
induction of apoptosis (32). It
has been reported that SDT induced apoptosis in cancer cells
partially through a Ca2+ dependent pathway. SDT can
induce cell apoptosis through ion channels. The intensity of bubble
activity determines intracellular Ca2+ level (33) and is closely associated with cell
membrane damage and repair. In addition, certain cells maintain
high levels of Ca2+ long after ultrasound exposure,
which indicates the complete loss of cell membrane integrity
(34). An increase in calcium
level is the key process underlying cell apoptosis (35).
SDT affects the expression of numerous genes which
provides novel insights into the molecular mechanisms of SDT for
the treatment of tumors (36).
In conclusion, this study shows that apoptosis
participates in SDT-induced cell death in MG-63 cells. The relative
percentages of cells undergoing apoptosis following SDT could be
experimentally manipulated. The existing research results can
provide important insight into SDT-induced cell apoptosis and also
indicated that HMME-SDT may be a potential therapeutic approach in
the management of OS.
Acknowledgments
This study was supported in part by funding from
Heilongjiang Provincial Health Bureau (grant no. 2012–690),
Heilongjiang province science and technology plan and technological
project (grant no. GC10C303-4), Harbin technological innovative
personnel and special fund project (grant no. 2012RFXXS066).
References
|
1
|
Klein MJ and Siegal GP: Osteosarcoma:
anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma: conventional treatment vs. gene therapy. Cancer Biol
Ther. 8:106–117. 2009. View Article : Google Scholar
|
|
3
|
Won KY, Lee CH, Kim YW and Park YK:
Primary giant-cell-rich osteosarcoma of the urinary bladder:
usefulness of osteocalcin and osteonectin immunohistochemical
staining and literature review. Pathology. 43:161–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibaguchi H, Tsuru H and Kuroki M:
Sonodynamic cancer therapy: a non-invasive and repeatable approach
using low-intensity ultrasound with a sonosensitizer. Anticancer
Res. 31:2425–2429. 2011.PubMed/NCBI
|
|
5
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy-a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.PubMed/NCBI
|
|
6
|
Lv Y, Fang M, Zheng J, et al:
Low-intensity ultrasound combined with 5-aminolevulinic acid
administration in the treatment of human tongue squamous carcinoma.
Cell Physiol Biochem. 30:321–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barati AH, Mokhtari-Dizaji M, Mozdarani H,
Bathaie SZ and Hassan ZM: Treatment of murine tumors using
dual-frequency ultrasound in an experimental in vivo model.
Ultrasound Med Biol. 35:756–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Fan H, Wang Z, Zheng J and Cao W:
Potentiation of scutellarin on human tongue carcinoma xenograft by
low-intensity ultrasound. PLoS One. 8:e594732013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai WK, Shen E and Hu B: The induction of
the apoptosis of cancer cell by sonodynamic therapy: a review. Chin
J Cancer Res. 24:368–373. 2012. View Article : Google Scholar
|
|
10
|
Wang X, Liu Q, Wang Z, et al: Role of
autophagy in sonodynamic therapy-induced cytotoxicity in S180
cells. Ultrasound Med Biol. 36:1933–1946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaruga E, Salvioli S, Dobrucki J, et al:
Apoptosis-like, reversible changes in plasma membrane asymmetry and
permeability and transient modifications in mitochondrial membrane
potential induced by curcumin in rat thymocytes. FEBS Lett.
433:287–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang W, Liu Q, Zhang J, Cao B, Zhao P and
Qin X: In vitro activation of mitochondria-caspase signaling
pathway in sonodynamic therapy-induced apoptosis in sarcoma 180
cells. Ultrasonics. 50:567–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duvshani-Eshet M, Benny O, Morgenstern A
and Machluf M: Therapeutic ultrasound facilitates antiangiogenic
gene delivery and inhibits prostate tumor growth. Mol Cancer Ther.
6:2371–2382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie F, Xu HX, Lu MD, Wang Y and Tang Q:
Anti-angiogenic gene therapy for hepatocellular carcinoma mediated
by micro-bubble-enhanced ultrasound exposure: an in vivo
experimental study. J Drug Target. 16:389–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuentes-Prior P and Salvesen GS: The
protein structures that shape caspase activity, specificity,
activation and inhibition. Biochem J. 384:201–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribe EM, Serrano-Saiz E, Akpan N and Troy
CM: Mechanisms of neuronal death in disease: defining the models
and the players. Biochem J. 415:165–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denecker G, Ovaere P, Vandenabeele P and
Declercq W: Caspase-14 reveals its secrets. J Cell Biol.
180:451–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinon F and Tschopp J: Inflammatory
caspases and inflammasomes: master switches of inflammation. Cell
Death Differ. 14:10–22. 2007. View Article : Google Scholar
|
|
19
|
Lamkanfi M, Festjens N, Declercq W, Vanden
Berghe T and Vandenabeele P: Caspases in cell survival,
proliferation and differentiation. Cell Death Differ. 14:44–55.
2007. View Article : Google Scholar
|
|
20
|
Lipponen P: Apoptosis in breast cancer:
relationship with other pathological parameters. Endocr Relat
Cancer. 6:13–16. 1999. View Article : Google Scholar
|
|
21
|
Wang P, Xu CS, Xu J, Wang X and Leung AW:
Hypocrellin B enhances ultrasound-induced cell death of
nasopharyngeal carcinoma cells. Ultrasound Med Biol. 36:336–342.
2010. View Article : Google Scholar
|
|
22
|
Wang P, Xu C, Xia X, et al: Mitochondrial
damage in nasopharyngeal carcinoma cells induced by ultrasound
radiation in the presence of hypocrellin B. J Ultrasound Med.
29:43–50. 2010.
|
|
23
|
Tang W, Liu Q, Wang X, Wang P, Zhang J and
Cao B: Potential mechanism in sonodynamic therapy and focused
ultrasound induced apoptosis in sarcoma 180 cells in vitro.
Ultrasonics. 49:786–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Y, Xia X, Xu C, et al:
5-Aminolaevulinic acid enhances ultrasound-induced mitochondrial
damage in K562 cells. Ultrasonics. 50:777–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XB, Liu QH, Mi N, et al:
Sonodynamically induced apoptosis by protoporphyrin IX on
hepatoma-22 cells in vitro. Ultrasound Med Biol. 36:667–676. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai S, Hu S and Wu C: Apoptotic effect of
sonodynamic therapy mediated by hematoporphyrin monomethyl ether on
C6 glioma cells in vitro. Acta Neurochir (Wien). 151:1655–1661.
2009. View Article : Google Scholar
|
|
27
|
Yumita N, Umemura S, Magario N, Umemura K
and Nishigaki R: Membrane lipid peroxidation as a mechanism of
sonodynamically induced erythrocyte lysis. Int J Radiat Biol.
69:397–404. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JW, Ryter SW and Choi AM: Functional
significance of apoptosis in chronic obstructive pulmonary disease.
COPD. 4:347–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schuler M and Green DR: Mechanisms of
p53-dependent apoptosis. Biochem Soc Trans. 29:684–688. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis-the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin DA and Elkon KB: Mechanisms of
apoptosis. Rheum Dis Clin North Am. 30:441–454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yumita N, Okudaira K, Momose Y and Umemura
S: Sonodynamically induced apoptosis and active oxygen generation
by gallium-porphyrin complex, ATX-70. Cancer Chemother Pharmacol.
66:1071–1078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Juffermans LJ, Dijkmans PA, Musters RJ,
Visser CA and Kamp O: Transient permeabilization of cell membranes
by ultrasound-exposed microbubbles is related to formation of
hydrogen peroxide. Am J Physiol Heart Circ Physiol.
291:H1595–H1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumon RE, Aehle M, Sabens D, et al:
Spatiotemporal effects of sonoporation measured by real-time
calcium imaging. Ultrasound Med Biol. 35:49–506. 2009. View Article : Google Scholar
|
|
35
|
Hutcheson JD, Schlicher RK, Hicks HK and
Prausnitz MR: Saving cells from ultrasound-induced apoptosis:
quantification of cell death and uptake following sonication and
effects of targeted calcium chelation. Ultrasound Med Biol.
36:1008–1021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tabuchi Y, Takasaki I, Zhao QL, et al:
Genetic networks responsive to low-intensity pulsed ultrasound in
human lymphoma U937 cells. Cancer Lett. 270:2862008. View Article : Google Scholar : PubMed/NCBI
|