Introduction
Neonatal necrotizing enterocolitis (NEC) is a severe
intestinal disease that occurs predominantly in preterm infants and
is one of the leading causes of mortality in neonatal intensive
care units (NICUs). According to a previous study, the incidence of
NEC in live births was 1–3%, while the rate in preterm infants with
a birth weight of ≤1500 g was ~10% (1). Severe cases required surgical
treatment, and a mortality rate of ~25% was observed (1). However, preterm birth and low
birth-weight do not provide a full explanation on the incidence of
NEC, since even with exposure to similar environmental conditions
or interference, NEC occurred only in certain newborns
(particularly premature infants). In addition, each case exhibited
significantly different degrees of disease severity. In certain
cases, only minor bloating occurred, while in others, intestinal
perforation and necrosis developed rapidly. Therefore, certain
neonates may be more likely to develop NEC, possibly due to genetic
predisposition.
A previous study demonstrated that excessive
activation of the innate intestinal immune response and the
inflammatory cascade were involved in the pathogenesis of NEC
(2). Myeloid differentiation-2
(MD-2) and GM2 activator protein (GM2A) are members of the
MD-2-related lipid-recognition (ML) family. MD-2 is an important
component of the intestinal Toll-like receptor 4 (TLR4) innate
immune signaling pathway (3). MD-2
is an essential accessory molecule, required for the binding of
lipopolysaccharides (LPSs) by TLR4 (4). MD-2 has been demonstrated to bind
TLR4 and form a heterodimer, allowing the formation of a complete
binding site for LPS (5). Cells
expressing TLR4 alone, or TLR4 and mutational MD-2 exhibited a low
LPS reactivity (6). Thus, genetic
polymorphisms of the MD-2 gene promoter or exon site may
significantly affect the transcriptional activities of the MD-2
gene or affect the LPS-induced signal transduction (7), thereby producing an abnormal immune
response. GM2A is known to be an inflammatory regulator, with a
β-cup structure that is able to bind platelet activating factor
(PAF) and hydrolyze it into the inactive form, lyso-PAF (8). As an endogenous phospholipid that
regulates inflammation, PAF exhibits important biological
activities. PAF-mediated signaling cascades activate nuclear
factor-κB, signal transducers and activation of transcription 3
(STAT3), resulting in transcription and immune response, induced by
a series of inflammatory factors (9). Thus, PA F is hypothesized to be
closely associated with the development of NEC (10). MD-2 and GM2A have been proposed to
be associated with the occurrence of NEC (11,12);
however, no conclusive evidence exists demonstrating that the
expression or structural changes of these factors involved in the
pathogenesis of NEC. In the present study, a gene sequencing method
was used to resequence the MD-2 and GM2A exons, as well as the
rs11465996 locus region (located in the MD-2 gene promoter region)
of 42 neonates, which were diagnosed with NEC (NEC group). The aim
of the present study was to investigate the presence of genetic
polymorphisms in these gene fragments. Subsequently, a comparative
analysis was conducted between the functional polymorphic loci of
the NEC neonates and 83 neonates without NEC, who were born in the
same period (control group), in order to investigate the
association between genetic polymorphisms of the MD-2 and GM2A
genes and the incidence or severity of NEC.
Materials and methods
Clinical data
In total, 42 neonates diagnosed with NEC (Bell’s
stage≥II) at the NICU of Guangzhou Women and Children’s Medical
Center (Guangzhou, China) between June 2011 and May 2012 were
enrolled in the study. These infants were termed as the NEC group,
which included 25 full-term (59.5%) and 17 preterm infants (40.5%).
In total, 20 infants underwent surgery (47.6%; including 14
full-term and 6 preterm infants), while 22 did not (52.4%). The
gestational age range of the NEC group was 28–40 weeks, and the
body weight at birth ranged between 1,230 and 3,750 g. The
gestational age range of the control group was 31–40 weeks, and the
body weight at birth ranged between 1,400 and 3,750 g. Furthermore,
83 neonates without NEC, who had been hospitalized in the NICU
during the same period, were enrolled in this study. Follow-up
examination at 2 months after birth was used to exclude infants
with gastrointestinal inflammation or malformation. Samples from
these 83 neonates were used as the control group, which included 48
preterm and 35 full-term infants. Neonates with a history of
serious infections and anoxic asphyxia, as well as those with
congenital malformations in organs, including the brain, heart,
gastrointestinal tract, kidney and respiratory tract, were excluded
from this study.
Specimen collection and processing
Peripheral venous and arterial blood (1 ml) was
collected from each infant in the NEC and control groups in an EDTA
anticoagulant tube (Yaohua Pharmaceutical Packing Co., Ltd., Laiwu,
China), following diagnosis with NEC or birth, respectively. The
blood samples were sub-packaged in 1.5 ml Eppendorf tubes
(Eppendorf AG, Hamburg, Germany) (250 μl in each tube) and
then stored at −80°C for subsequent experiments.
Extraction of genomic total DNA and
amplification of target fragments
A GD2311 Blood gDNA Miniprep kit (Biomiga, San
Diego, CA, USA) was used, and total DNA extraction of whole blood
samples was performed according to the manufacturer’s instructions.
Primer Premier 5.0 software (Premier Biosoft International, Palo
Alto, CA, USA) was then used to design the primers and amplify the
target fragments. The primers used in the PCR analysis are shown in
Table I and were synthesized by
Shenzhen BGI Tech Institute (Shenzhen, China).
 | Table IPrimer of each amplified fragment. |
Table I
Primer of each amplified fragment.
| Amplification
region | Primers (5′→3′) | Annealing temperature
(°C) |
|---|
| MD-2-1 | (F):
AAGAGGAAACAGTTGGATAGGA
(R): GAGAAAGATGACGCAGGGA | 55 |
| MD-2-2 | (F):
GCCACATTGCTGATGTCATT
(R): TGCTGTGTTAAGCCACAAAGA | 55 |
| MD-2-4 | (F):
AAAGCCTCTGAAATAGTAGCA
(R): ACAAACACTCTTGCCCAC | 55 |
| MD-2-pa | (F):
TATCTGGCCCTGTTCTGTCC
(R): ATGGTGGCACACACCTGTAA | 63.5 |
| GM2A-1 | (F):
CCGTTCCAGCCGCCTTCA
(R): TCTCAGCCAGACCCGCACA | 55 |
| GM2A-2 | (F):
CCAAAGGCCAATTAGGTCAG
(R): CTTCACAGTTCCCCAAGCAT | 55 |
| GM2A-3 | (F):
TGGTATGTTTGCCCTGGAAT (R): AGCCGCACAAGATGAGAGAC | 55 |
| GM2A-4 | (F):
ACAGTGCTATGGCCGTCTCT
(R): CCCTGGGCTATCAAGAACTG | 54 |
Gel electrophoresis and purification of
polymerase chain reaction (PCR) products
Using the PCR products, 2% agarose gel
electrophoresis (horizontal electrophoresis EPS 300, horizontal
electrophoresis tank and Gel Doc XR gel imaging system; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was performed and the
results were compared with the marker (DL700 DNA Marker; Takara Bio
Inc., Otsu, Japan) in order to verify whether the amplified
products had the same sizes as the target fragments. A 96-well
purification plate (EMD Millipore, Billerica, MA, USA) was
subsequently used to purify the PCR products, according to the
manufacturer’s instructions. Briefly, the PCR products were added
to the 96-well purification plate alongside 100 μl
ddH2O, and incubated for 10 min at room temperature. The
plate was then dried using a vacuum pump for 10 min. A further 40
μl ddH2O was added to the plate, and the plate
was incubated at room temperature for 10 min. The purified products
were then transferred to another 96-well plate.
Gene sequencing
The PCR amplification products were sequenced by the
Shenzhen BGI Tech Institute using the Sanger method with the ABI
3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Quantitative data are expressed
as the mean ± standard deviation and enumerated data are expressed
as ratios. Comparisons between pairs of binary data were performed
using the χ2 test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Detection of polymorphic loci in the MD-2
exon region
Among the 42 infants with NEC, detection of the
functional polymorphic loci was not possible in the MD-2 gene exon
region, as exon 3 was smaller, with only 53 bp. However, no studies
in the Single Nucleotide Polymorphism database (dbSNP; http://www.ncbi.nlm.nih.gov/SNP/; accessed May,
2012) or the associated literature have reported polymorphisms in
this region. As the fragment is small, the SNP database was unable
to identify associated functional SNPs.
Polymorphic loci of the MD-2 promoter
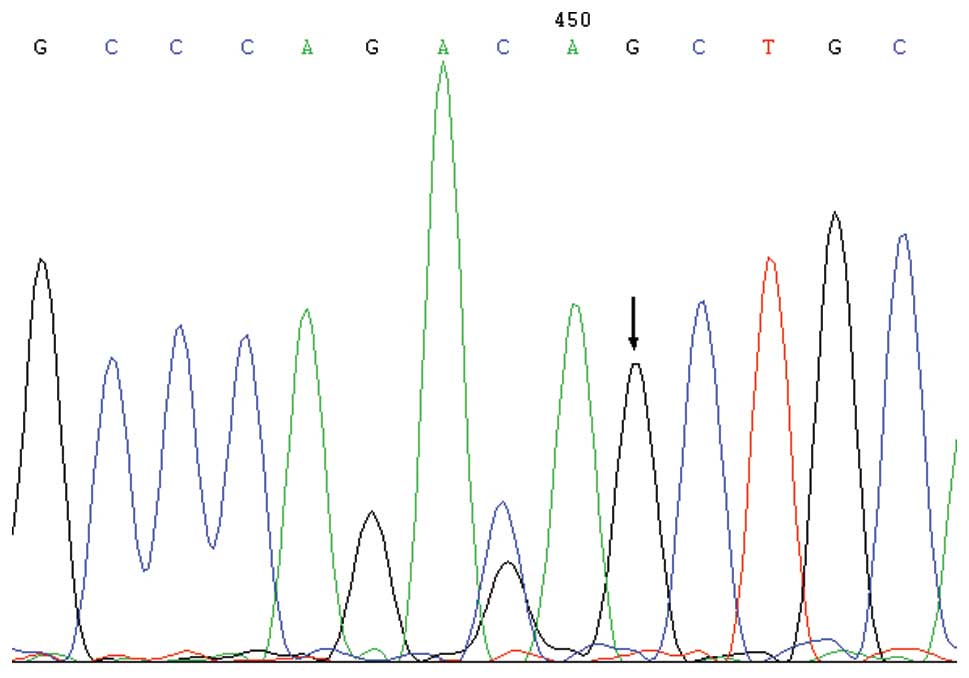
region C-1625 G (rs11465996)
Two genotypes, namely C/C and C/G, were detected in
the rs 11465996 locus of the NEC and control groups, where G was
the low frequency allele (Fig. 1).
The C/G genotype frequencies in the NEC and control groups were
found to be 38.1 and 30.1%, respectively, with no statistically
significant difference between these groups. In addition, the
frequencies of the G allele in the two groups were found to be 19.0
and 15.1%, respectively. The frequencies of the C/G genotype and G
allele detected in the present study were lower compared with those
previously reported for the Chinese population in the dbSNP (40.0
and 22.2%, respectively) (http://www.ncbi.nlm.nih.gov/snp/). Amongst the
neonates with NEC who underwent surgery, the C/G genotype frequency
was 55%, which was significantly different compared with the
control group (30.1%; P=0.036). Thus, the G allele may be a risk
factor for the development of NEC [odds ratio (OR), 1.66; 95%
confidence interval (CI), 1.092–3.054]. In the NEC group, the
frequency of the C/G genotype was found to be higher in the
neonates who had undergone surgery compared with the non-operated
neonates, as well as in the full-term infants compared with the
preterm infants; however, these differences were not statistically
significant (P>0.05; Table
II).
 | Table IIDistributions of the C/G genotype and
G allele in each group. |
Table II
Distributions of the C/G genotype and
G allele in each group.
| Group | Cases (n) | Genotype (cases, n)
| C/G genotype
frequency (%) | G allele frequency
(%) |
|---|
| C/C | C/G |
|---|
| Control | 83 | 58 | 25 | 30.1 | 15.1 |
| NEC | 42 | 26 | 16 | 38.1a | 19.0 |
| NEC surgery | 20 | 9 | 11 | 55.0b | 27.5 |
| NEC non-surgery | 22 | 16 | 6 | 27.3c | 13.6 |
| NEC full-term
infants | 25 | 14 | 11 | 44.0d | 22.0 |
| NEC preterm
infants | 17 | 12 | 5 | 29.4e | 14.7 |
Polymorphisms in the GM2A gene
A total of three missense mutations with high
frequencies were detected in the GM2A exon region. Furthermore, a
polymorphic locus with abnormal frequency was detected in the
intron region. The positions of these loci, the frequency of
low-frequency alleles and the frequency of genotypes carrying the
low frequency allele in the various groups are shown in Table III.
 | Table IIILow frequency allele frequencies and
genotype frequencies that carried the low frequency allele among
the groups. |
Table III
Low frequency allele frequencies and
genotype frequencies that carried the low frequency allele among
the groups.
| Polymorphic
locus | Position | Mutation type | Low frequency
allele frequency (%)
| Genotype
frequencies that carried the low frequency allele (%)
|
|---|
| NEC group | Control group | Surgery group | Full-term infants
group | NEC group | Control group | Surgery group | Full-term infants
group |
|---|
| rsl048719 | Exon1 | m |
23.8a |
10.9b | 17.5 | 24.0 |
38.1e |
20.5d | 30.0 | 40.0 |
| rs153477 | Exon2 | m | 58.8 | 66.2 | 63.2 | 66.7 | 80.0 | 88.2 | 84.2 | 91.7 |
| rs1048723 | Ter | – | 23.8 | 34.3 | 30.0 | 32.0 | 42.9 | 49.4 | 50.0 | 56.0 |
| rs2075783 | Intron | – |
23.8e |
10.8f | 27.5 | 32.0 |
42.9? |
13.3h | 45.0 | 56.0 |
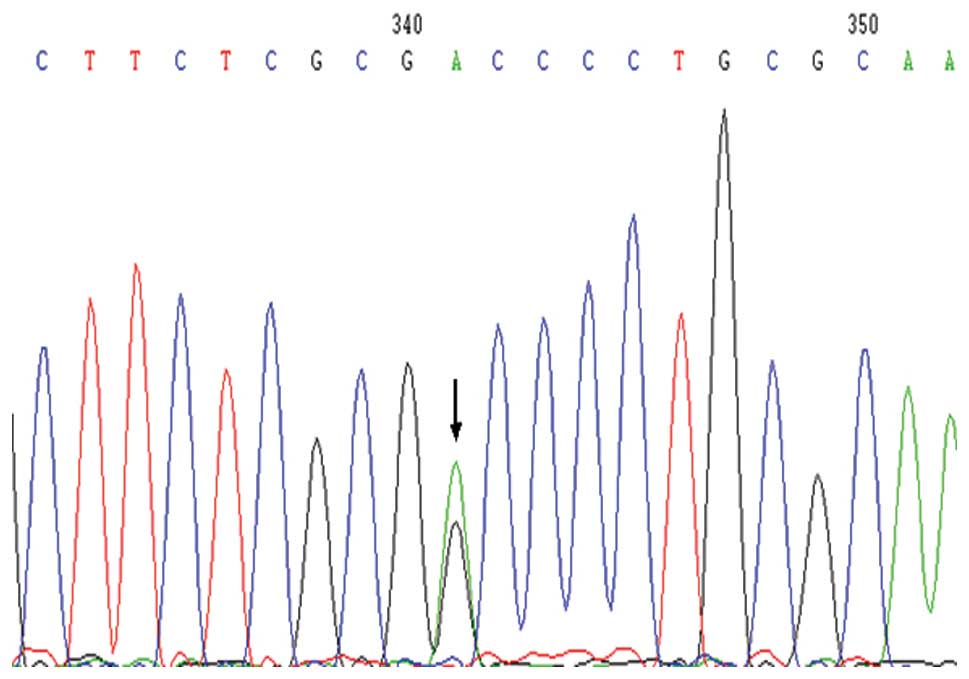
rs1048719 (G>A) was the single nucleotide
polymorphism (SNP) locus of the exon 1 region, where A was the low
frequency allele (Fig. 2). In the
NEC Group, the frequencies of the A allele and the genotypes
carrying the A allele were significantly increased, when compared
with the control group (P=0.008 and 0.038, respectively). The A/A
and A/G genotypes, which carry the A allele, may be associated with
the pathogenesis of NEC (OR, 1.857; 95% CI, 1.037–3.326). In the
NEC surgery group and the control group, the frequency of genotypes
carrying the A allele (six cases) was higher compared with the
control group, although the difference was not statistically
significant (P=0.364). However, the A allele frequency of the
full-term infants with NEC was significantly higher compared with
the control group (χ2=5.374, P= 0.020).
rs153477 (A>G) was the SNP locus of exon 2 and
possessed a missense mutation. The frequencies of the G allele and
genotypes carrying the G allele in this locus exhibited no
statistically significant differences among the various groups (in
the Chinese population, A was the low frequency allele; (http://www.ncbi.nlm.nih.gov/snp/)). The A allele
of the rs1048723 (A>G) locus was a stop codon nucleotide in one
of the variants of GM2A. In another variant of GM2A, this locus was
located in the coding region. In the present study, the
polymorphism of this locus did not exhibit a statistically
significant difference among the groups (P>0.05).
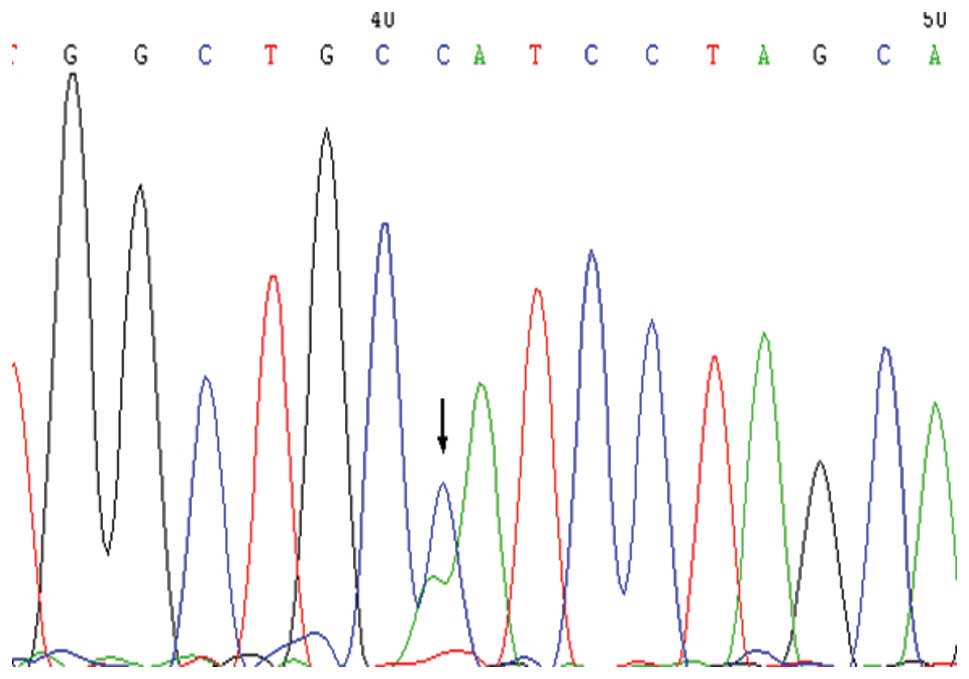
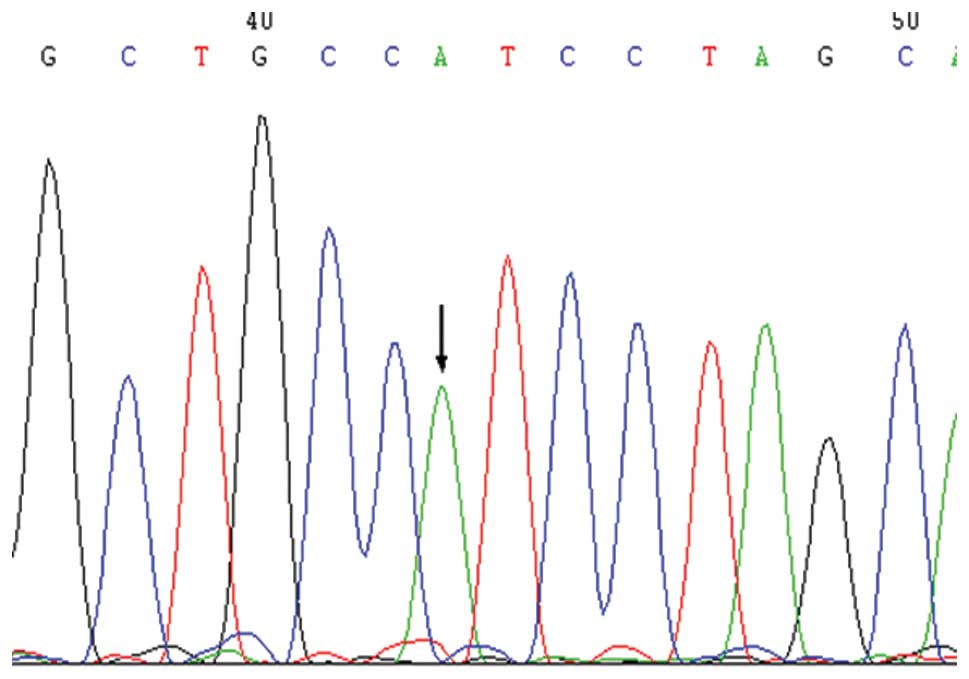
Within the intron region that was upstream of exon
4, the rs2075783 (A>C) polymorphic locus was detected, which
exhibited statistically significant differences as compared with
the NEC group (Figs. 3 and
4). In this locus, C was the low
frequency allele. The frequency of genotypes carrying the C allele
in the NEC group exhibited a statistically significant difference
compared with the control group (χ2=13.717, P<0.001).
Thus, genotypes carrying the C allele may be a risk factor for the
development of NEC (OR, 3.234; 95% CI, 1.685–6.206). Furthermore,
the frequency of genotypes carrying the C allele was significantly
higher in the NEC surgery and the full-term infants groups compared
with the control group (P<0.05).
Discussion
NEC is a severe gastrointestinal disease that occurs
in the neonatal period and an important cause of neonatal morbidity
and mortality resulting from disorders of the gastrointestinal
tract (13). Although
epidemiological studies and other fundamental research have greatly
improved the understanding of the disease, the exact cause of NEC
remains unclear, while no fully effective methods of prevention or
treatment exist currently (14). A
further issue, leading to uncertainty in clinical practice, is that
NEC also occurs in full-term infants. Although there have been
advances in the management of preterm infants, no significant
reduction was observed in the incidence of NEC, indicating that
neonates developing NEC may possess a genetic predisposition. A
number of studies have been conducted, demonstrating the roles of
genetic factors in the pathogenesis of NEC. For instance, Bhandari
et al (15) performed a
multicenter retrospective study in twins. The results demonstrated
that the genetic background may affect the morbidity associated
with NEC. Furthermore, numerous studies have investigated the
association between polymorphisms of inflammatory cytokines and NEC
(16). The present study
investigated the association among polymorphisms in the MD-2 gene
and the incidence and severity of NEC.
MD-2 is an important cofactor in the TLR signaling
pathway and an important component of the CD14-TLR4/M D - 2
receptor complex, which is able to identify components of the
bacterial cell wall (17).
Previous studies have demonstrated that the polymorphic locus,
rs11465996 (C>G), in the MD-2 promoter region-1625 position
significantly affects the efficiency of MD-2 transcription
(6,7,18). A
mutation in the exon 1 region (amino acid no. 35; Thr35Ala) may
weaken the signal that is activated by LPS (19), and the G56R polymorphism may
decrease the binding ability of endotoxin (20).
In the present study, no polymorphic loci with
abnormal frequency were detected in the MD-2 gene exon region in
neonates with NED, indicating that the incidence of NEC may not be
associated with structural changes in the MD-2 protein. Hamann
et al (19) and Vasl et
al (20) revealed that
missense mutations in the MD-2 gene exon region lead to variations
in the MD-2 structure, which resulted in a decrease in the binding
capacity of the CD14-TLR4/MD-2 receptor complex towards LPS,
thereby weakening the LPS-induced signal. However, the mutations
reported in the aforementioned studies were very rare and may not
be associated with the development of NEC.
The polymorphic locus of rs11465996 (C>G) in the
MD-2 gene promoter region-1625 position has also been investigated
in previous studies (6,7,18).
Changes in allele frequency of this locus may significantly affect
the transcription efficiency of the MD-2 gene. Zeng et al
(6) identified that, in trauma
patients, the rs11465996 polymorphic locus was significantly
correlated with a higher incidence of sepsis and multiple organ
dysfunction (MOD) scores. In addition, Gu et al (7) demonstrated that polymorphism of this
locus may affect the activity of the MD-2 promoter. In individuals
carrying the G allele, LPS-stimulation resulted in a significant
increase in the expression levels of whole blood leukocyte MD-2
mRNA and tumor necrosis factor-α. In the Chinese Han population,
this polymorphic locus was shown to be associated with MOD and
sepsis following severe trauma (18). In the present study, the frequency
of the C/G genotype in the NEC and control groups was lower
compared with the frequency reported in the dbSNP; however, the
difference between these two groups was not statistically
significant. However, when compared with the control group, the
frequency of this genotype was significantly higher in the severe
cases of NEC (neonates who had undergone surgery; P=0.036). This
indicated that the G allele of this locus (C/G genotype) is
associated with the severity of NEC. In the 42 neonates with NEC,
the frequency of the C/G genotype was higher in the surgery group
compared with the non-surgery group, and in the full-term infants
group compared with the preterm infants group; however, these
differences were not statistically significant. These results
indicated that the polymorphism of C-1625 G in the MD-2 gene
promoter region may be associated with the severity of NEC. Based
on the results of the present and previous studies, this may be
associated with the polymorphism effect on the transcriptional
activities of the MD-2 gene. The abnormal transcription of MD-2
gene may influence the innate immune function of enteric ducts,
reducing the effectiveness of the immune regulation of external
pathogen stimuli during the process of intestinal flora
colonization process and leading to an abnormally amplified
inflammatory response. However, the exact underlying mechanism
remains unclear and further investigations of the genetics and
mechanism are required.
GM2A is another member of the ML family, which binds
and inhibits PAF, thereby inhibiting PAF stimulation-induced
intracellular calcium release. As an endogenous
inflammation-regulatory phospholipid, PAF is known to be important
in a variety of pathophysiological processes, including
inflammation, allergic reactions, asthma, tumor formation and
apoptosis (21). Caplan et
al (22) demonstrated that the
plasma PAF levels of infants with NEC increased, while the level of
PAF acetylhydrolase (PAF-AH) was significantly reduced. PAF-AH
deficiency has also been observed in preterm infants (23). Soliman et al (24) demonstrated that PAF induced the
expression of TLR4 in rat intestinal mucosa, as well as the mRNA
and protein expression levels of TLR4 in intestinal epithelial
cells. In addition, PAF was found to promote the secretion of IL-8
and induce the phosphorylation and nuclear translocation of the
epithelial cell STAT3, thus initiating the transcription of
pro-inflammatory cytokines.
Previous studies have hypothesized that GM2A may
inhibit PAF (25,26). In addition, Rigat et al
(12) investigated the effects of
GM2A in animal models of NEC and demonstrated that the
administration of recombinant GM2A inhibited lethal rat intestinal
necrotic injury induced by LPS and PAF. The administration of
recombinant GM2A was found to significantly reduce the intestinal
tissue damage and was also involved in the maintenance of the
integrity of the intestinal tight junctions. These functions also
implied that GM2A was involved in the prevention of NEC. Whether
the interaction of GM2A with PAF is affected as a result of changes
to its structure or expression, thereby leading to the development
of diseases, including NEC, has not yet been reported.
In the present study, an SNP locus with abnormally
increased frequency was identified in the GM2A coding region,
namely rs1048719 (G>A), which was located in exon 1. The
mutation detected at this locus was a missense mutation, which led
to variations in amino acids. Statistical analysis demonstrated
that the frequency of the genotypes carrying the low frequency A
allele, in the NEC group was significantly higher compared with the
control group (P<0.05). Thus, the polymorphism at this locus may
be associated with the development of NEC (OR, 1.857; 95% CI,
1.037–3.326), and the A allele may increase susceptibility to this
disease. Since the G>A mutation of this locus resulted in
variations to the codon (missense mutation), the increase in
susceptibility to NEC, induced by the polymorphism at this locus,
may be due to changes in the structure of GM2A that decrease the
binding and hydrolytic abilities of GM2A to PAF. In addition, an
abnormally amplified inflammatory response may be mediated,
resulting in the development of NEC. However, this mechanism
remains hypothetical, and further investigation is required to
establish whether and how a variation to a single base may result
in significant changes in the spatial structure of GM2A.
Furthermore, due to the sequencing technology used,
100–200 bp fragments in the upstream and downstream regions of the
target fragment were also included when amplifying the fragments of
interest. The results revealed that the rs2075783 polymorphism in
the intron region exhibited statistically significant differences
between the NEC and control groups (P<0.001), and that the
frequency of the genotypes carrying the C allele was significantly
higher in the full-term NEC group compared with the preterm NEC
group (P=0.014). This was a notable result, as this locus is not
directly involved in the coding of the GM2A protein. However,
whether this polymorphism directly affects the transcription of the
GM2A gene or impacts upon the regulation of other genes, thus
increasing the NEC susceptibility, is unclear. To the best of our
knowledge, no studies have been conducted on the function of this
SNP locus. In fact, >95% genes of human genome were found to be
functional; however, our understanding of genes continues to
exhibit limitations, demonstrating that further research is
required.
In conclusion, the present study indicated that the
polymorphism, rs11465996, in the MD-2 gene promoter region is
associated with the severity of NEC and that the rs1048719
polymorphism in the GM2A gene exon 1 and the rs2075783 polymorphism
in the intron region are associated with the occurrence of NEC.
However, studies using larger samples are required in order to
verify these associations. Furthermore, the development of NEC
cannot be explained by a single gene. Further investigation is
required into the interactions between various polymorphisms, as
well as innate immune and inflammatory factors, and how these are
able to singly and synergistically affect the development of
NEC.
References
|
1
|
Garland SM, Tobin JM, Pirotta M, et al:
The ProPrems trial: investigating the effects of probiotics on late
onset sepsis in very preterm infants. BMC Infect Dis. 11:210–215.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu P, Sodhi CP and Hackam DJ: Toll-like
receptor regulation of intestinal development and inflammation in
the pathogenesis of necrotizing enterocolitis. Pathophysiology.
21:81–93. 2014. View Article : Google Scholar :
|
|
3
|
Kim SY, Koo JE, Seo YJ, et al: Suppression
of Toll-like receptor 4 activation by caffeic acid phenethyl ester
is mediated by interference of LPS binding to MD2. Br J Pharmacol.
168:1933–1945. 2013. View Article : Google Scholar :
|
|
4
|
Shibata T, Motoi Y, Tanimura N, et al:
Intracellular TLR4/MD-2 in macrophages senses Gram-negative
bacteria and induces a unique set of LPS-dependent genes. Int
Immunol. 23:503–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akashi S, Saitoh S, Wakabayashi Y, et al:
Lipopolysaccharide interaction with cell surface Toll-like receptor
4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med.
198:1035–1042. 2013. View Article : Google Scholar
|
|
6
|
Zeng L, Zhang AQ, Gu W, et al:
Identification of haplotype tag SNPs within the whole myeloid
differentiation 2 gene and their clinical relevance in patients
with major trauma. Shock. 37:366–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu W, Shan YA, Zhou J, et al: Functional
significance of gene polymorphisms in the promoter of myeloid
differentiation-2. Ann Surg. 246:151–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wright CS, Mi LZ, Lee S and Rastinejad F:
Crystal structure analysis of phosphatidylcholine-GM2-activator
product complexes: evidence for hydrolase activity. Biochemistry.
44:13510–13521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caplan MS, Simon D and Jilling T: The role
of PAF, TLR and the inflammatory response in neonatal necrotizing
enterocolitis. Semin Pediatr Surg. 14:145–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frost BL and Caplan MS: Necrotizing
enterocolitis: pathophysiology, platelet-activating factor and
probiotics. Semin Pediatr Surg. 22:88–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan KL, Wong KF and Luk JM: Role of
LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis:
pathogenesis and therapeutic implications. World J Gastroenterol.
15:4745–4752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rigat B, Yeger H, Shehnaz D and Mahuran D:
GM2 activator protein inhibits platelet activating factor signaling
in rats. Biochem Biophys Res Commun. 385:576–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackam DJ, Good M and Sodhi CP: Mechanisms
of gut barrier failure in the pathogenesis of necrotizing
enterocolitis: Toll-like receptors throw the switch. Semin Pediatr
Surg. 22:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jain L: Necrotizing enterocolitis
prevention: art or science? Clin Perinatol. 40:xiii–xv. 2013.
|
|
15
|
Bhandari V, Bizzarro MJ, Shetty A, et al:
Familial and genetic susceptibility to major neonatal morbidities
in preterm twins. Pediatrics. 117:1901–1906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prencipe G, Azzari C, Moriondo M, et al:
Association between mannose-binding lectin gene polymorphisms and
necrotizing enterocolitis in preterm infants. J Pediatr
Gastroenterol Nutr. 55:160–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visintin A, Iliev DB, Monks BG, et al:
Md-2. Immunobiology. 211:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu W, Shan YA, Liu Q, et al: Relationship
of myeloid differentiation-2 gene promoter polymorphisms with
susceptivity of complications after severe trauma in Chinese Han
population. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 29:484–487.
2007.In Chinese. PubMed/NCBI
|
|
19
|
Hamann L, Kumpf O, Muller M, et al: A
coding mutation within the first exon of the human MD-2 gene
results in decreased lipopolysac-charide-induced signaling. Genes
Immun. 5:283–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vasl J, Prohinar P, Gioannini TL, et al:
Functional activity of MD-2 polymorphic variant is significantly
different in soluble and TLR4-bound forms: decreased endotoxin
binding by G56R MD-2 and its rescue by TLR4 ectodomain. J Immunol.
180:6107–6115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izumi T and Shimizu T: Platelet-activating
factor receptor: gene expression and signal transduction. Biochim
Biophys Acta. 1259:317–333. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caplan MS, Sun XM, Hseuh W and Hageman JR:
Role of platelet activating factor and tumor necrosis factor-alpha
in neonatal necrotizing enterocolitis. J Pediatr. 116:960–964.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caplan M, Hsueh W, Kelly A and Donovan M:
Serum PAF acetylhydrolase increases during neonatal maturation.
Prostaglandins. 39:705–714. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soliman A, Michelsen KS, Karahashi H, et
al: Platelet-activating factor induces TLR4 expression in
intestinal epithelial cells: implication for the pathogenesis of
necrotizing enterocolitis. PLoS One. 5:e150442010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rigat B, Reynaud D, Smiljanic-Georgijev N
and Mahuran D: The GM2 activator protein, a novel inhibitor of
platelet-activating factor. Biochem Biophys Res Commun.
258:256–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimada Y, Li YT and Li SC: Effect of GM2
activator protein on the enzymatic hydrolysis of phospholipids and
sphingomyelin. J Lipid Res. 44:342–348. 2003. View Article : Google Scholar : PubMed/NCBI
|