Introduction
Rheumatoid arthritis (RA) is a systemic, chronic
inflammatory disease characterized by synovial hyperplasia, joint
destruction and extraarticular manifestations, and it has a
significant impact on morbidity as well as mortality, leading to
hyperproliferation of synovial cells and tissue destruction
(1,2). Although it is thought that the
interaction of genetic, immunological and environmental factors
contributes to the disease (3),
the pathogenesis of RA, in particular the role of microRNAs
(miRNAs/miRs) in RA, has remained to be fully elucidated.
miRNAs are endogenous small (21–25 nucleotides),
single-stranded, non-coding RNAs that mediate mRNA cleavage,
translational repression and mRNA destabilization (4). To date, using various computational
and experimental approaches, >2,200 human miRNAs have been
registered in the miR Base database (release 19) (5). It has been reported that alterations
in miRNA expression levels impact expression of the target genes in
a 1.2-fold to 4-fold manner and thus represent a fine-tuning
mechanism regulating protein expression (6). Therefore, miRNAs are thought to have
a crucial role in regulating innate as well as adaptive immune
responses (7). Dysregulation of
miRNA in peripheral blood mononuclear cells (PBMCs), T lymphocytes,
synovial fibroblasts and osteoclasts, each of those considered a
key effector of joint destruction, has been shown to cause
inflammation, degradation of the extracellular matrix and invasive
behavior of resident cells (8–11).
Altered expression of circulating miRNAs has repeatedly been
reported to be associated with RA (12,13).
miR-221 is one of the miRNAs that is evolutionarily
conserved among vertebrates and abundantly expressed in plasma
(14,15). Recently, accumulating evidence has
demonstrated that miR-221 has a crucial role in cell growth,
oncogenesis, invasion, migration and drug resistance in tumor cells
(16–18). In addition, miR-221 also regulates
cytokine production (19). In
particular, a recent study showed that miR-221/222 was dysregulated
in synovial fibroblasts in RA (20). However, the role of miR-221 in RA
has remained elusive. Therefore, the present study analyzed the
association between miR-221 expression and the expression of
pro-inflammatory cytokines and a chemokine in fibroblast-like
synoviocytes (FLS). The effects of miR-221 on cell apoptosis,
migration as well as invasion in FLS were also examined.
Materials and methods
Patients and healthy controls
Fresh peripheral blood and synovial tissue specimens
were obtained from healthy donors (n=18) with no history of
autoimmune diseases or patients with rheumatoid arthritis (RA,
n=22) at the Department of Orthopedic Surgery, the Second Hospital
of Jilin University (Changchun, China). Ethical approval of the use
of human samples in this study was granted by the Ethics Committee
of Jilin University, and written informed consent was obtained from
all study participants. All RA patients fulfilled the American
College of Rheumatology criteria for classification of the disease
(21).
A 5-ml sample of peripheral venous blood from each
study participant was collected using EDTA-coated tubes (BD
Vacutainer 5 ml; BD Diagnostics, Franklin Lakes, NJ, USA) according
to standard procedures. The blood samples were then centrifuged at
1,000 xg for 10 min at 4°C, the serum was immediately separated
from the blood, and RNA was immediately extracted.
Cell culture
FLS were isolated from synovial tissues by enzymatic
digestion as previously described (22). FLS were grown in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) medium containing 10%
heat-inactivated fetal calf serum (FCS; Invitrogen Life
Technologies) supplemented with antibiotics (100 mg/ml streptomycin
and 100 U/ml penicillin; Sigma-Aldrich, St. Louis, MO, USA) in a
humidified incubator at 37°C in 5% CO2. Cultures of FLS
were subjected to experimental procedures at passages 4–6.
Cell transfection
miR-221 inhibitors and corresponding negative
controls (NC) were purchased from Ambion (Life Technologies, Thermo
Fisher Scientific, Waltham, MA, USA). The Oligofectamine™
Transfection Reagent from Invitrogen Life Technologies was used for
cell transfection according to the manufacturer’s instructions. The
final concentration of the miRNA inhibitor was 50 nM.
miRNA reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA of cells, serum or tissue, including
miRNAs, was extracted using the Qiagen miRNeasy mini kit (catalogue
no. 217004; Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. The purity and concentration of RNA
were determined by using a dual-beam ultraviolet spectrophotometer
(BioSpectrometer plus; Eppendorf, Hamburg, Germany). RNA was then
reversely transcribed into cDNA using an NCode VILO miRNA cDNA
Synthesis kit (Life Technologies). Real-time qPCR was performed
using Express SYBR Green ER qPCR Supermix (both from Life
Technologies) and an Applied Biosystems 7500 Thermocycler (Life
Technologies). For detection of miR-221 and U6 small nuclear RNA
expression, specific primers were obtained from Exiqon (Vedbaek,
Denmark), i.e. U6 snRNA PCR primer set (product no. 203907), and
LNA™ Homo sapiens-miR-221 PCR primer set (product no.
204532). The qPCR conditions were as follows: An initial 95°C for 4
min followed by 40 cycles of 95°C for 10 sec and 58°C for 30 sec. A
dissociation curve was established after each PCR in order to
verify amplification specificity. The integrity of the miRNA and
the efficiency of qPCR in each sample were confirmed by the
endogenous control U6 small nuclear RNA. Negative control
experiments were set without cDNA template. The data were analyzed
with SDS Relative Quantification Software version 2.06 (Life
Technologies).
ELISA. To quantify tumor necrosis factor
(TNF)-α, interleukin (IL)-6, IL-1β and chemokine (C-X-C motif)
ligand 16 (CXCL16) production in FLS, cells transfected with
miR-221 inhibitor and corresponding negative control, respectively,
were cultured for 8 h, followed by stimulation with 10 μg/ml
lipopolysaccharide (LPS; Sigma-Aldrich), and then incubated for 24
h. Twenty-four hours after LPS stimulation, culture supernatants
were harvested, and the concentrations of TNF-α, IL-6, IL-1β and
CXCL16 were measured using an ELISA kit for human TNF-α, IL-6,
IL-1β and CXCL16 (Genzyme, Cambridge, MA, USA) in a microplate
reader (Multiskan MK3; Thermo Fisher Scientific). The
concentrations of each were normalized relative to the total number
of cells.
Apoptosis analysis
The terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) assay was performed to determine the number of
apoptotic cells. In brief, cellular DNA fragmentation was measured
with the ApoTag Red in situ Apoptosis detection kit
(Chemicon International, EMD Millipore, Billerica, MA, USA)
according to the manufacturer’s instructions after FLS cells were
transfected with miR-221 inhibitor and corresponding negative
control for 24 h and exposed to LPS for 48 h. In order to quantify
the apoptotic cells, the TUNEL-positive cells were counted using a
confocal microscope (IX51; Olympus, Tokyo, Japan). The number of
apoptotic cells in five random high-power fields were counted and
expressed as a percentage of total cells (apoptotic fraction).
In addition, survivin and X-linked inhibitor of
apoptosis protein (XIAP) were also detected by western blot as an
additional indicator of apoptosis.
Cell migration and invasion assay
To assess the effect of miR-221 on FLS cell
migration, a wound-healing assay was performed. In brief, FLS cells
were seeded at a density of 5×103 cells/well in a
96-well plate after FLS were pre-treated with miR-221 inhibitor or
corresponding negative control (NC) for 24 h and exposed to LPS for
48 h. After the cells had formed a fluent monolayer, they were
observed under a fluorescent microscope (X41; Olympus). A linear
scratch was formed using a 10-μl pipette tip at 48 h after
transfection. Wounded monolayers were washed with
phosphate-buffered saline (PBS) to remove detached cells and
debris. Photomicrographs of ten random fields were obtained
(original magnification, ×100), and cells were counted to calculate
the average number of cells that had migrated.
For the in vitro invasion assay, Transwell
chamber experiments were performed using inserts coated using a
Matrigel basement membrane matrix (Matrigel; Becton Dickinson, La
Jolla, CA, USA). In brief, the Matrigel was diluted in serum-free
cold media, placed into upper chambers of a 24-well Transwell plate
and incubated at 37°C for 1 h. Cells were re-suspended with
serum-free DMEM media at a density of 5×104 cells/well
and incubated for 48 h to evaluate cell invasion. Cell invasion was
observed using an immunofluorescence microscope (X41; Olympus) by
counting the cells that had invaded into the bottom of the cell
culture insert. All experiments were performed in triplicate.
Measurement of VEGF production
VEGF production was determined by competitive ELISA.
In brief, FLS cells were treated with miR-221 inhibitor and
corresponding negative control for 24 h in 12-well plates and
subsequently exposed to LPS for 48 h. These culture media were
centrifuged to remove cell debris. Cell-free culture media were
collected at 8 h. Then PGE2 levels were measured using Human VEGF
ELISA kits (Yanyu, Shanghai, China) according to the manufacturer’s
instructions.
Western blot analysis
For western blot analyses, FLS cells were harvested
and rinsed once with ice-cold PBS and lysed in ice-cold cell lysis
buffer (Walterson, London, UK) containing complete protease
inhibitor cocktail (Sigma-Aldrich) following pre-treatment of FLS
with miR-221 inhibitor or corresponding negative control (NC) for
24 h and exposure to LPS for 48 h. The protein concentration was
determined using the bicinchoninic protein assay kit (KeyGen
Biotech, Nanjing, China) using a c-globulin standard curve. Equal
amounts of protein (20 μg/lane) from the cell lysates were
separated using 10–15% SDS-PAGE (Sigma-Aldrich) and transferred
onto nitrocellulose membranes (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The membrane was incubated for 2 h in PBS plus
0.1% Tween-20 (Sigma-Aldrich) and 5% non-fat skimmed milk to block
non-specific binding. The membranes were then incubated overnight
at 4°C with the following antibodies: Rabbit monoclonal
anti-survivin (1:1,000; cat. no. EP2880Y; Abcam, Cambridge, UK);
rabbit polyclonal anti-XIAP (1:1,000; cat. no. 2042s; Cell
Signaling Technology, Beverly, MA, USA); mouse monoclonal
anti-MMP-3 (1:1,000; cat. no. 69543; EMD Millipore) and mouse
monoclonal anti-MMP-9 (1:5,000; cat. no. sc-12759; Santa Cruz
Biotechnology, Inc.). Mouse monoclonal anti-GAPDH (1:10,000; cat.
no. sc-47724; Santa Cruz Biotechnology, Inc.) was used as a loading
control. The membranes were incubated with the appropriate
horseradish peroxidase-conjugated immunoglobulin G (anti-rabbit
1:10,000; cat. no. 7074S; Cell Signaling Technology; or anti-mouse
1:10,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 2 h
at room temperature, and protein bands were visualized using an
Enhanced Chemiluminescence reagent (Thermo Fisher Scientific).
Statistical analysis
Values are expressed as the mean ± standard
deviation of data from at least three independent experiments.
Student’s t-test or analysis of variance were used. All data
were analyzed using the SPSS® statistical package,
version 16.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism
version 5.01 (GraphPad Software, La Jolla, CA, USA) for
Windows®. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
miR-221 expression in serum and synovial
tissues is significantly elevated in RA patients
Using RT-qPCR analysis, miR-221 expression levels in
serum and synovial tissues of patients with RA and healthy controls
were determined. It was found that miR-221 expression in serum and
synovial tissues was significantly elevated in RA patients compared
with those in healthy controls (P<0.01; Fig. 1A and B). In addition, miR-221
expression in human FLS cells was determined. The results showed
that miR-221 in FLS cells was significantly upregulated by LPS
stimulation compared with that in normal FLS (without stimulation)
(P<0.01; Fig. 1C). To
investigate the role of miR-221 in RA, miR-221 inhibitor and
corresponding negative control were transfected into FLS cells
stimulated with LPS. RT-PCR analysis demonstrated that the
expression of miR-221 in cells transfected with miR-221 inhibitor
was decreased compared with that in cells transfected with negative
control (P<0.05; Fig. 1D).
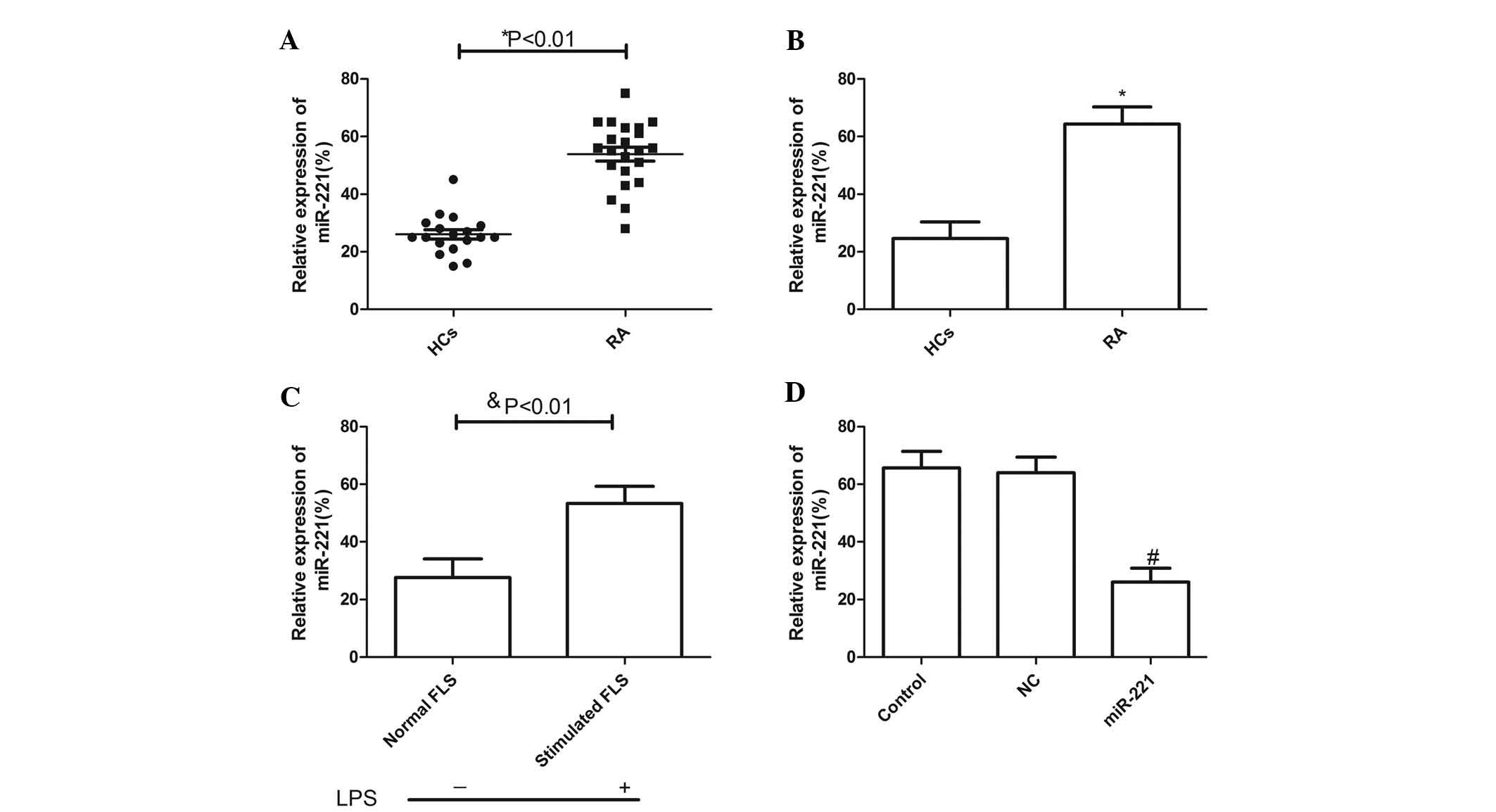 | Figure 1miR-221 expression is significantly
increased in serum and synovial tissues from patients with RA.
Expression of miR-221 in (A) serum and (B) synovial tissues of 18
HCs and in the 22 patients with RA was measured by qPCR. (C) After
stimulation with LPS, miR-221 mRNA levels of FLS cells were
evaluated by qPCR. (D) After transfection with miR-221 inhibitor
and corresponding NC, miR-221 mRNA levels in FLS cells stimulated
with LPS were determined by qPCR. Values are expressed as the mean
± standard deviation. *P<0.01 vs. HCs,
$P<0.01 vs. Normal, #P<0.01 vs. NC. NC,
negative control; RA, rheumatoid arthritis; miR, microRNA; FLS,
fibroblast-like synoviocytes; qPCR, quantitative polymerase chain
reaction; HC, healthy control; LPS, lipopolysaccharide. |
miR-221 inhibitor decreases LPS-induced
pro-inflammatory cytokines and CXCL16 expression in FLS
To examine whether downregulation of miR-221 had any
effect on pro-inflammatory cytokines and a chemokine in FLS cells
stimulated with LPS, ELISAs were performed to quantify TNF-α, IL-6,
IL-1β and CXCL16 levels. It was found that transfection of miR-221
inhibitor significantly inhibited the expression of TNF-α, IL-6 and
IL-1β and CXCL16 upregulated by LPS (P<0.01; Fig. 2A–D). These results suggested that
downregulation of miR-221 decreased TNF-α, IL-6 and IL-1β and
CXCL16 production in FLS cells stimulated with LPS.
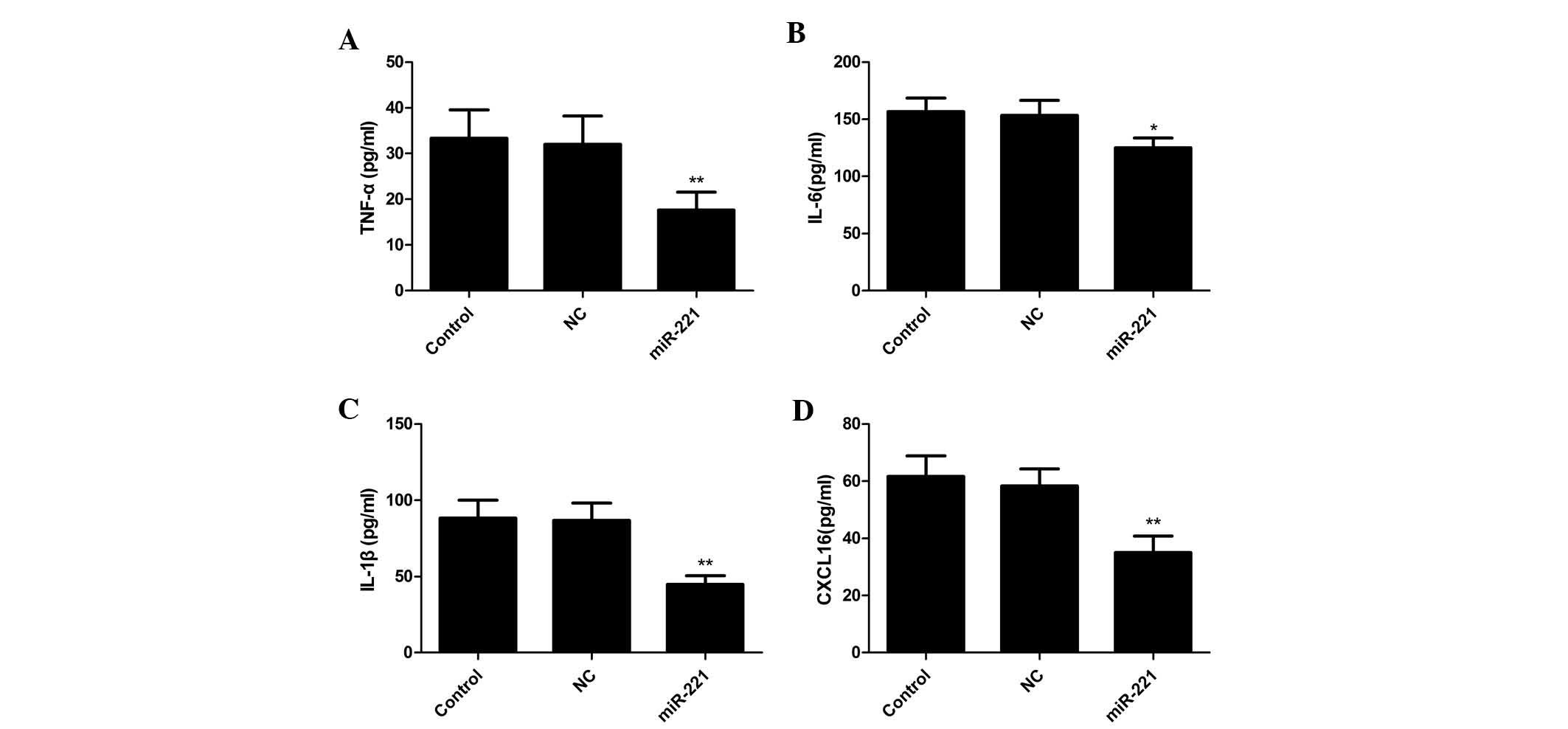 | Figure 2Suppression of LPS-induced expression
of pro-inflammatory cytokines in FLS by miR-221 inhibitor. Cells
were transfected with miR-221 or corresponding NC and then
stimulated with 10 mg/ml LPS. 24 h later, the culture supernatants
were collected and concentrations of (A) TNF-α, (B) IL-6, (C) IL-1β
and (D) CXCL16 were determined by ELISA. Values are expressed as
the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. control. NC, negative control; miR,
microRNA; FLS, fibroblast-like synoviocytes; TNF, tumor necrosis
factor; IL, interleukin.; LPS, lipopolysaccharide; CXCL16,
chemokine (C-X-C motif) ligand 16. |
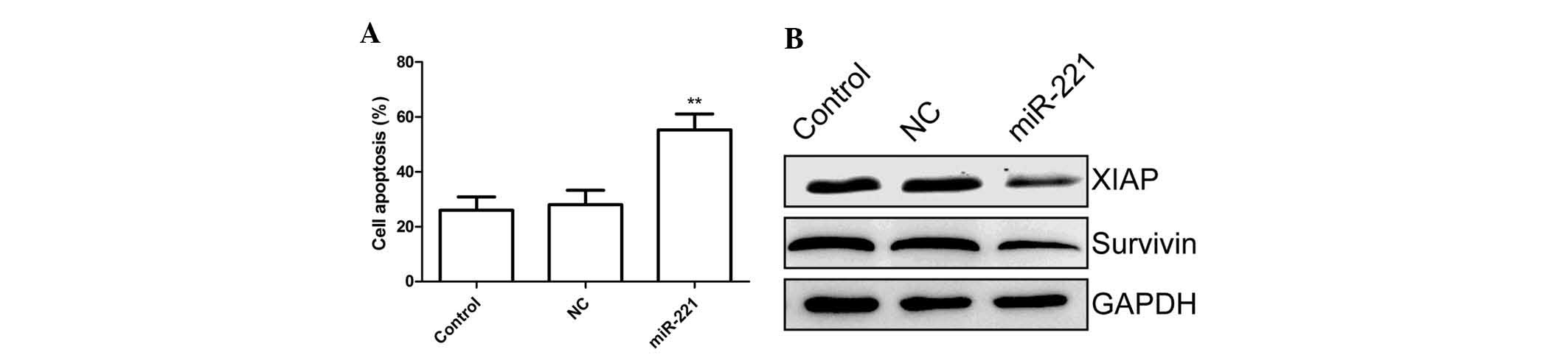
miR-221 induces cell apoptosis of FLS
stimulated with LPS
In order to examine whether the miR-221 has any
effect on cell apoptosis, a TUNEL assay was performed. FLS cells
were treated for 24 h with the miR-221 inhibitor and corresponding
negative control and then subsequently exposed to LPS for 48 h,
followed by TUNEL assay. The results showed that miR-221 inhibitor
markedly increased the percentage of apoptotic cells compared to
that in the untreated control group and negative control treatment
group (P<0.01; Fig. 3A).
To explore the possible mechanism of the
pro-apoptotic effect of miR-221, survivin and XIAP expression were
determined by western blot. The results are shown in Fig. 3B, demonstrating that the miR-221
inhibitor significantly decreased survivin and XIAP expression
compared with that in the control and negative control treatment
groups.
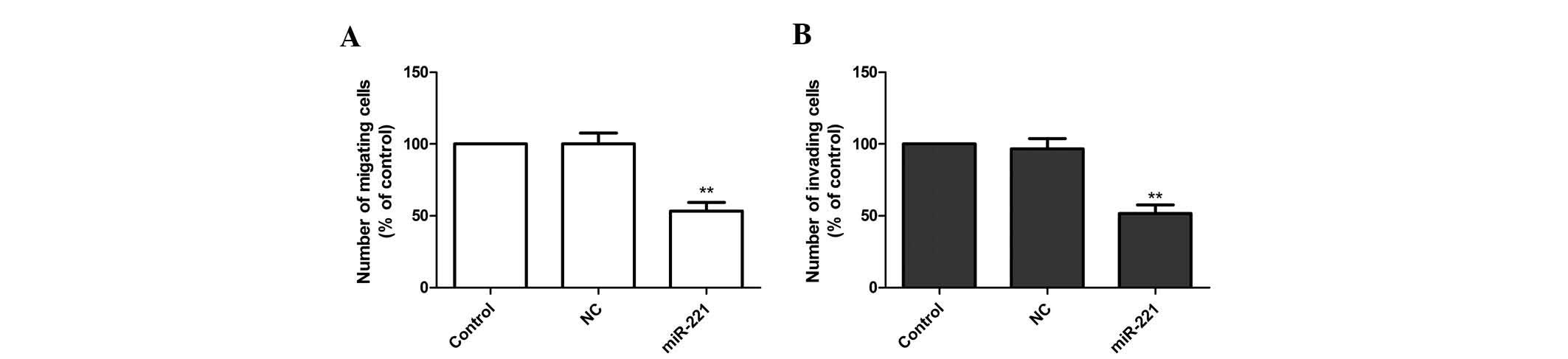
Inhibition of miR-221 reduces FLS
migration and invasion of FLS stimulated with LPS
In order to further illustrate the effects of
miR-221 on FLS, cell migration and invasion were evaluated. FLS
cells stimulated with LPS were transfected with miR-221 inhibitor
and corresponding negative control, followed by wound healing and
Transwell assays. The results demonstrated that the miR-221
inhibitor significantly attenuated the migration and invasion of
FLS (P<0.01; Fig. 4A and
B).
Inhibition of miR-221 decreases the
expression of VEGF-A, MMP-1 and MMP-3 in human RA-FLS
To illustrate the possible underlying mechanism of
the effect of miR-221 on the migration and invasion, the effect of
miR-221 inhibition on levels of VEGF, MMP-3 and MMP-9 was assessed
in FLS stimulated with LPS. The cells were pre-treated with miR-221
inhibitor and corresponding negative control for 24 h followed by
stimulation with LPS for 48 h. VEGF production was measured by
ELISA, revealing that VEGF excretion in the supernatant of the
miR-221 group was significantly decreased compared with that in the
control and negative control groups (P<0.05; Fig. 5A). Finally, the supernatants were
assayed for MMP-3 and MMP-9 by western blot analysis. It was found
that the miR-221 inhibitor substantially decreased the levels of
MMP-3 and MMP-9 in the supernatants compared with those in the
controls and negative control group (Fig. 5B). These results may imply that the
miR-221 inhibitor attenuated migration and invasion in FLS cells
stimulated by LPS via inhibiting VEGF, MMP-3 and MMP-9
expression.
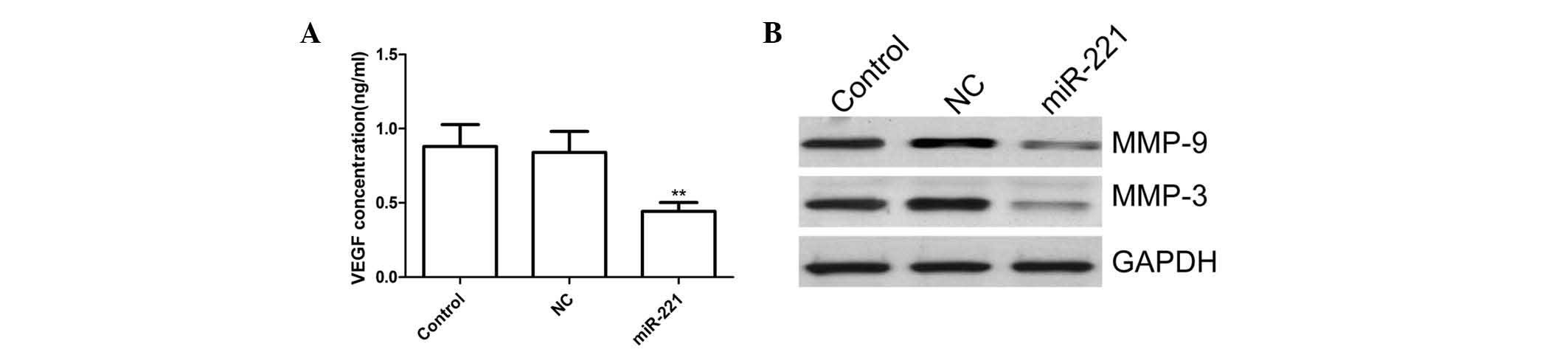 | Figure 5miR-221 inhibitor decreases secretion
of VEGF, MMP-3 and MMP-9 by FLS stimulated by LPS. (A) FLS were
pre-treated with miR-221 inhibitor or corresponding NC for 24 h and
exposed to human LPS for 48 h, then VEGF production was measured
using ELISA. (B) FLS were pre-treated with miR-221 inhibitor or
corresponding NC for 24 h and exposed to human LPS for 48 h, then
MMP-3 and MMP-9 protein expression were measured by western blot.
Values are expressed as the mean ± standard deviation.
*P<0.05, and **P<0.01 vs. control. NC,
negative control; miR, microRNA; FLS, fibroblast-like synoviocytes;
LPS, lipopolysaccharide; VEGF, vascular endothelial growth factor;
MMP, matrix metalloproteinase. |
Discussion
miRNAs are of interest due to their critical role as
fine regulators of gene expression at the post-transcriptional
level within cells in numerous diseases, which makes them potential
targets in the treatment of numerous diseases. For RA accumulating
evidence has demonstrated that the expression of miRNAs, including
miR-146a, miR-155, miR-16, miR-23b, miR-203, miR-124a, miR-346,
miR-223 as well as miR-34a was altered in synovial fibroblasts,
peripheral blood mononuclear cells, and T cells from RA patients
(8–12,23–29).
It has been reported that this abnormal miRNA expression is
associated with abnormal innate immunity (23,24),
inflammation (23), cell
proliferation (24) and cell
apoptosis (29). Thus, the
suppression of overexpressed miRNAs or reconstitution of the
expression by restoration of silenced miRNAs is a therapeutic
strategy in the treatment of RA (30). Similar to these studies, the
present study showed that miR-221 expression in serum and synovial
tissues of patients with RA was higher than that in healthy
controls, and that downregulation of miR-221 significantly
suppressed the expression of pro-inflammatory cytokines and a
chemokine, as well as induced cell apoptosis in FLS cells
stimulated with LPS.
It has been shown that miR-221 has critical roles in
the regulation of cell proliferation, cell cycle, migration and
invasion by regulating target genes, and that miR-221 dysregulation
is causally involved in the initiation and progression of cancer
(17,18). It has also been suggested that
miR-221 contributes to CD4+ T-cell function (31). This indicated that miR-221 is
relevant to RA pathogenesis and that it possibly has a crucial role
in RA development and progression. To test this hypothesis, the
present study analyzed the association between miR-221 expression
and pro-inflammatory cytokines and chemokine production in
fibroblast-like synoviocytes (FLS). The effects of miR-221 on cell
apoptosis, migration as well as invasion in FLS were also
determined. The results showed that the miR-221 inhibitor
significantly suppressed the expression of pro-inflammatory
cytokines and a chemokine, induced cell apoptosis, as well as
inhibited migration and invasion in FLS cells stimulated with LPS.
These data suggested that miR-221 may be involved in the initiation
and progression of RA.
IL-17, TNF-α and IL-1β are important cytokines in
the pathogenesis of RA and regulate the cellular immune responses
in numerous dimensions, including the generation of T-cell memory,
controlling the development and function of regulatory T cells as
well as modulating the secretion of cytokines (32–35).
Thus, in the present study, it was also determined whether the
expression of miR-221 affected inflammatory conditions in human
FLS. The results showed that transfection with miR-221 inhibitor to
FLS cells stimulated by LPS inhibited the expression of TNF-α, IL-6
and IL-1β, suggesting that the miR-221 inhibitor may have great
potential for use as a therapeutic tool in the treatment of RA
patients resistant to anti-cytokine therapies.
It is known that synovial cells produce MMP-3 in RA,
which has a major role in the cartilage destruction in joints
(36). In addition, endogenous
MMP-2 or MMP-9 contribute to synovial fibroblast survival,
proliferation, migration and invasion in RA (37). The present study showed that an
miR-221 inhibitor suppressed VEGF, MMP3 and MMP-9 protein
expression, and inhibited migration and invasion in FLS stimulated
by LPS. These findings indicated that the observed attenuated FLS
migration and invasion by the miR-221 inhibitor may be caused via
inhibiting VEGF-A, MMP3 and MMP-9 protein expression.
In conclusion, the findings of the present study
provided evidence that miR-221 expression was increased in patients
with RA, and that inhibition of miR-221 suppressed pro-inflammatory
cytokines, induced cell apoptosis, as well as attenuated migration
and invasion via inhibiting VEGF, MMP3 and MMP-9 expression in FLS
cells stimulated by LPS. These findings suggested that miR-221 is a
potential target in the treatment of RA.
Acknowledgments
This work was supported by the Science and
Technology Research and Innovation Team Fund of Jilin province (no.
JL2014054).
References
|
1
|
Feldmann M, Brennan FM and Maini RN:
Rheumatoid arthritis. Cell. 85:307–310. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo X, Tsai LM, Shen N and Yu D: Evidence
for microRNA-mediated regulation in rheumatic diseases. Ann Rheum
Dis. 69(Suppl 1): i30–i36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozomara A and Griffiths-Jones S: miR
Base: integrating microRNA annotation and deep-sequencing data.
Nucleic Acids Res. 39:D152–D157. 2011. View Article : Google Scholar
|
|
6
|
O’Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar
|
|
7
|
Pauley KM, Cha S and Chan EK: MicroRNA in
autoimmunity and autoimmune diseases. J Autoimmun. 32:189–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stanczyk J, Pedrioli DM, Brentano F, et
al: Altered expression of MicroRNA in synovial fibroblasts and
synovial tissue in rheumatoid arthritis. Arthritis Rheum.
58:1001–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niimoto T, Nakasa T, Ishikawa M, et al:
MicroRNA-146a expresses in interleukin-17 producing T cells in
rheumatoid arthritis patients. BMC Musculoskelet Disord.
11:2092010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stanczyk J, Ospelt C, Karouzakis E, et al:
Altered expression of microRNA-203 in rheumatoid arthritis synovial
fibroblasts and its role in fibroblast activation. Arthritis Rheum.
63:373–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu MC, Yu CL, Chen HC, Yu HC, Huang HB and
Lai NS: Increased miR-223 expression in T cells from patients with
rheumatoid arthritis leads to decreased insulin-like growth
factor-1 mediated interleukin-10 production. Clin Exp Immunol.
177:641–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurowska-Stolarska M, Alivernini S,
Ballantine LE, et al: MicroRNA-155 as a proinflammatory regulator
in clinical and experimental arthritis. Proc Natl Acad Sci USA.
108:11193–11198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakasa T, Shibuya H, Nagata Y, Niimoto T
and Ochi M: The inhibitory effect of microRNA-146a expression on
bone destruction in collagen-induced arthritis. Arthritis Rheum.
63:1582–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawaguchi T, Komatsu S, Ichikawa D, et al:
Clinical impact of circulating miR-221 in plasma of patients with
pancreatic cancer. Br J Cancer. 108:361–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao R, Wu J, Jia W, et al: Plasma miR-221
as a predictive biomarker for chemoresistance in breast cancer
patients who previously received neoadjuvant chemotherapy.
Onkologie. 34:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ergun S, Arman K, Temiz E, et al:
Expression patterns of miR-221 and its target caspase-3 in
different cancer cell lines. Mol Biol Rep. 41:5877–5881. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Yang Y, Gan R, et al:
Downregulation of mir-221 and mir-222 restrain prostate cancer cell
proliferation and migration that is partly mediated by activation
of SIRT1. PLoS One. 9:e988332014. View Article : Google Scholar
|
|
18
|
Ye X, Bai W, Zhu H, et al: MiR-221
promotes trastuzumab-resistance and metastasis in HER2-positive
breast cancers by targeting PTEN. BMB Rep. 47:268–273. 2014.
View Article : Google Scholar :
|
|
19
|
Brown PN and Yin H: PNA-based microRNA
inhibitors elicit anti-inflammatory effects in microglia cells.
Chem Commun (Camb). 49:4415–4417. 2013. View Article : Google Scholar
|
|
20
|
Pandis I, Ospelt C, Karagianni N, et al:
Identification of microRNA-221/222 and microRNA-323-3p association
with rheumatoid arthritis via predictions using the human tumour
necrosis factor transgenic mouse model. Ann Rheum Dis.
71:1716–1723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aletaha D, Neogi T, Silman AJ, et al: 2010
Rheumatoid arthritis classification criteria: an american college
of rheumatology/european league against rheumatism collaborative
initiative. Arthritis Rheum. 62:2569–2581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshioka Y, Kozawa E, Urakawa H, et al:
Suppression of hyaluronan synthesis alleviates inflammatory
responses in murine arthritis and in human rheumatoid synovial
fibroblasts. Arthritis Rheum. 65:1160–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semaan N, Frenzel L, Alsaleh G, et al:
miR-346 controls release of TNF-α protein and stability of its mRNA
in rheumatoid arthritis via tristetraprolin stabilization. PLoS
One. 6:e198272011. View Article : Google Scholar
|
|
24
|
Nakamachi Y, Kawano S, Takenokuchi M, et
al: MicroRNA-124a is a key regulator of proliferation and monocyte
chemoattractant protein 1 secretion in fibroblast-like synoviocytes
from patients with rheumatoid arthritis. Arthritis Rheum.
60:1294–1304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pauley KM, Satoh M, Chan AL, et al:
Upregulated miR-146a expression in peripheral blood mononuclear
cells from rheumatoid arthritis patients. Arthritis Res Ther.
10:R1012008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu S, Pan W, Song X, et al: The microRNA
miR-23b suppresses IL-17-associated autoimmune inflammation by
targeting TAB2, TAB3 and IKK-α. Nat Med. 18:1077–1086. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakasa T, Miyaki S, Okubo A, et al:
Expression of microRNA-146 in rheumatoid arthritis synovial tissue.
Arthritis Rheum. 58:1284–1292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fulci V, Scappucci G, Sebastiani GD, et
al: miR-223 is over-expressed in T-lymphocytes of patients affected
by rheumatoid arthritis. Hum Immunol. 71:206–211. 2011. View Article : Google Scholar
|
|
29
|
Niederer F, Trenkmann M, Ospelt C, et al:
Down-regulation of microRNA-34a* in rheumatoid arthritis
synovial fibroblasts promotes apoptosis resistance. Arthritis
Rheum. 64:1771–1779. 2012. View Article : Google Scholar
|
|
30
|
Filková M, Jüngel A, Gay RE and Gay S:
MicroRNAs in rheumatoid arthritis: potential role in diagnosis and
therapy. Bio Drugs. 26:131–141. 2012.
|
|
31
|
Kuipers H, Schnorfeil FM and Brocker T:
Differentially expressed microRNAs regulate plasmacytoid vs.
conventional dendritic cell development. Mol Immunol. 48:333–340.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 Cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Croft M: The TNF family in T cell
differentiation and function - unanswered questions and future
directions. Semin Immunol. 26:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schenten D, Nish SA, Yu S, et al:
Signaling through the adaptor molecule MyD88 in CD4+ T
cells is required to overcome suppression by regulatory T cells.
Immunity. 40:78–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nish SA, Schenten D, Wunderlich FT, et al:
T cell-intrinsic role of IL-6 signaling in primary and memory
responses. Elife. 3:e019492014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding L, Guo D, Homandberg GA, Buckwalter
JA and Martin JA: A single blunt impact on cartilage promotes
fibronectin fragmentation and upregulates cartilage degrading
stromelysin-1/matrix metalloproteinase-3 in a bovine ex vivo model.
J Orthop Res. 32:811–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue M, McKelvey K, Shen K, et al:
Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial
fibroblast survival, inflammation and cartilage degradation.
Rheumatology (Oxford). 53:2270–2279. 2014. View Article : Google Scholar
|