Introduction
Cancer remains a significant cause of morbidity and
mortality (1), and there has been
extensive research into the development of antitumor agents. In
recent years, there has been an emergence of therapeutics directed
against specific signaling pathways that are critical for the onset
and progression of cancer.
Protein tyrosine kinases are involved in processes,
such as cell proliferation, survival, apoptosis, migration, and DNA
damage repair, and have therefore received attention as potential
novel anticancer agents. The epidermal growth factor receptor
(EGFR), which is a transmembrane receptor tyrosine kinase, is
involved in the proliferation of human tumors and is an effective
target for the treatment of cancer (2–5). The
present study aimed to identify novel potent indolin-2-one protein
tyrosine kinase inhibitors; indolin-2-one derivatives were
synthesized using sunitinib as a leading compound. A
quinolone-indolone conjugate QIC1
[9-Fluoro-3,7-dihydro-3-methyl-10-(4-methy
l-1-piperazinyl)-6-(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)
-7-oxo-2H-(1,4) oxazino(2,3,4-ij)quinoline], which
targeted EGFR, was synthesized.
The EGFR pathway comprises a number of downstream
signal transduction cascades, such as extracellular
signal-regulated kinase 1/2, protein kinase B (AKT) and signal
transducer and activator or transcription 3 (STAT3). Inhibition of
EGFR leads to downregulation of these signaling cascades, resulting
in the apoptosis of cancer cells (6). STAT3 is overexpressed and
constitutively activated in a number of types of cancer cells.
Blocking STAT3 signaling inhibits cell growth, induces apoptosis
and reduces tumor cell metastasis (7,8).
STAT3 regulates the expression of numerous genes, such as CyclinD1,
Bcl-2, Bax, Survivin, as well as other genes that regulate cell
cycle progression and cell proliferation (9,10).
STAT3 is proposed to be one of the most important oncoproteins and
a potential target for cancer therapy (11,12).
Within cancer cells, a particular metabolic pathway, which is
characterized by the anaerobic degradation of glucose even in the
presence of oxygen, leads to the production of large quantities of
lactate; a phenomenon known as the Warburg effect (13,14).
Clinical studies have confirmed that enhanced glucose degradation
occurs within tumors (15–17). Hexokinase II (HK2), which catalyzes
the first committed step of glycolysis, is overexpressed and
activated in a number of cancers. Therefore HK2 has also been
investigated as a target of cancer therapy (18). Furthermore, previous studies by
this group have demonstrated that STAT3 may regulate the expression
of HK2 (19–21).
Therefore, it was hypothesized that EGFR, STAT3 and
HK2 constitute a pathway that regulates cancer progression. The
present study investigated the activity of QIC1 against liver,
lung, cervix and breast cancer cells, in addition to the mechanisms
underlying the effect of QIC1 on the EGFR pathway, which result in
apoptosis in cancer cells.
Materials and methods
Cell lines, antibodies and reagents
MCF7 (human breast cancer), HepG2 (human
hepatocellular carcinoma), A549 (human lung cancer), HeLa (human
cervical cancer) cells and QSG7701 healthy human hepatocytes were
procured from the American Type Culture Collection (Manassas, VA,
USA). The following antibodies were obtained from Cell Signaling
Technology, Inc., (Danvers, MA, USA): Mouse monoclonal
anti-phosphorylated EGFR (pTyr1068-EGFR) (1:1,000; cat. no. 2236),
rabbit polyclonal anti-STAT3 (1:1,000; cat. no. 9132), rabbit
polyclonal anti-phosphorylated STAT3 (pTyr705-STAT3) (1:1,000; cat.
no. 9131), rabbit monoclonal anti-AKT (1:1,000; cat. no. 4685),
rabbit monoclonal anti-phosphorylated AKT (pSer473-AKT) (1:1,000;
cat. no. 4060), rabbit monoclonal anti-Bax (1:1,000; cat. no.
5023), rabbit monoclonal Bcl-2 (1:1,000; cat. no. 2870) and rabbit
monoclonal HK-2 (1:1,000; cat. no. 2867). Rabbit polyclonal
anti-EGFR (1:1,000; cat. no. 18986-1-AP), rabbit polyclonal
anti-β-actin (1:2,000; cat. no. 20536-1-AP) primary antibodies and
peroxidase-conjugated Affinipure goat anti-rabbit/mouse secondary
antibodies (1:10,000; cat. nos. SA00001-2 and SA00001-1) were
obtained from Proteintech Group, Inc. (Chicago, IL, USA).
EasyScript Two-Step RT-PCR SuperMix kit was obtained from TransGen
Biotech (Beijing, China). Propidium iodide (PI), RNase A and
Hoechst 33342 were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Triton X-100 was obtained from Amresco LLC (Solon, OH, USA).
TRIzol was obtained from Biyuntian biotech (Shanghai, China).
Agarose was purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA).
Effect of STAT3 small interfering (si)RNA
and HK-2 inhibitor on anticancer activity of QIC1
HepG2 cells were transfected with STAT3 siRNA or
treated with the HK-2 inhibitor, 3-BrPA for 6 h. Subsequently,
different concentrations of QIC1 (0, 1, 2 and 4 μM) was
added. After 48 h, an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay was performed to detect the cell viability to evaluate
whether QIC1 regulates cancer cell growth through the STAT3-HK2
pathway.
Cell culture and cell viability
assay
Cells were cultured in RPMI-1640 (Invitrogen Life
Technologies) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin in 5% CO2 at 37°C. The
quinolone-indolone conjugate, QIC1, was synthesized chemically by
aldol condensation of the quinolone C-3 position, in order to
introduce indolin-2-one. QIC1 was dissolved in DMSO
(Sigma-Aldrich). An MTT assay was used to detect cell viability and
cytotoxicity. MCF7, HepG2, HeLa, A549 and QSG7701 cells, cultured
in 96-well plates, were treated with final concentrations of 0.5,
1, 2 and 4 μM of QIC1 for 48 h. Treated cells were then
incubated in fresh medium containing MTT (0.5 mg/ml; Sigma-Aldrich)
at 37°C for 4 h followed by replacement with 150 μl DMSO.
Finally, the spectrophotometric absorbance of the samples in DMSO
was determined using a microplate reader (Sunrise™, Tecan Group
Ltd., Männedorf, Switzerland) at 570 nm.
Cell cycle analysis
Cell cycle analysis was performed using flow
cytometry. HepG2 and A549 cells treated with QIC1 (0, 1, 2 or 4
μM) for 48 h were collected and washed twice with ice-cold
PBS. Cells (1×106) were fixed in 75% ethanol at 4°C for
≥4 h. Cells were then washed twice with ice-cold PBS, followed by
incubation with DNA staining solution [(PI; 50 μg/ml), RNase
(50 μg/ml) and Triton-x-100 (0.5%)] for 20 min. Cell cycle
analysis was immediately performed using flow cytometry
(FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis analysis
Cell apoptosis was analyzed using an ArrayScan VTI
HCS 600-type high content live cell imaging system (Thermo Fisher
Scientific, Waltham, MA, USA). MCF7, HepG2 and A549 cells were
seeded in 96-well plates. After 12 h, the cells were treated with
QIC1 (0, 0.5, 1, 2 and 4 μM) for 48 h, and then washed with
ice-cold PBS. Cells were incubated with 5 mg/ml Hoechst 33342 for
30 min, and then incubated with 5 mg/ml PI for a further 1 h at
37°C. The cells were subsequently washed with ice-cold PBS. The
cell apoptosis analysis was immediately performed using the
ArrayScan VTI HCS 600-type high content live cell imaging system.
The second channel overall average fluorescence intensity (mean
fluorescence intensity; MFI) represents cells in late
apoptosis.
Western blotting
Cells treated with QIC1 were harvested in RIPA
buffer (50 mM Tris-HCl, pH 8.0; 150 mM sodium chloride; 1.0% NP-40;
0.5% sodium deoxycholate; and 0.1% SDS) with 10 μg/ml of the
protease inhibitor, PMSF (Sigma-Aldrich). Total cellular proteins
(40 μg) were separated using SDS-PAGE and subjected to
western blotting using primary antibodies against EGFR,
pTyr1068EGFR, STAT3, pTyr705-STAT3, AKt, pSer473-AKT, HK, Bcl-2,
Bax and β-actin (Cell Signaling Technology, Inc. and Proteintech
Group, Inc.). β-actin was used as loading control. Bands were
detected using an ECL detection system (AlphaImager; Proteinsimple,
San Jose, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total mRNA was extracted from cultured cells using
TRIzol. Reverse transcription and PCR reactions were performed
using an Easy Script Two-Step RT-PCR SuperMix kit (TransGen Biotech
company, Beijing, China). The primer pairs for RT-PCR were as
follows: Forward: 5′-ATGGTCAAGTGCTGGATG-3′ and reverse:
5′-GAGGAAGGTGTCGTCTATG-3′ for EGFR, forward:
5′-CCAAGGAGGAGGCATTCG-3′ and reverse: 5′-ACATCGGCAGGTCAATGG-3′ for
STAT3 and forward: 5′-GGTGCTGAGTATGTCATGGA-3′ and reverse:
5′-TTCAGCTCTGGGATGACCTT-3′ for GAPDH (both sets from Sangon Biotech
Co., Ltd., Shanghai, China). The PCR conditions were as follows:
94°C for 5 min, followed by 38 cycles at 94°C for 30 sec, 56°C for
30 sec and 72°C for 1 min, an 72°C for 7 min using an Arktik PCR
system (Thermo Scientific, Waltham, MA, USA). mRNA expression of
GAPDH, EGFR and STAT3 was determined with density scanning
(AlphaImager; Proteinsimple, San Jose, CA, USA). Subsequently the
EGFR:GAPDH ratio and the STAT3:GAPDH (mRNAs were detected in the
same RT sample) in densitometric units was calculated and analyzed
using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Statistical analysis
Each analysis was repeated three times. Relative
cell viability is expressed as a percentage relative to the
untreated control cells. Error bars represent standard deviation.
Data were analyzed using analysis of variance for each two-group
comparison test. Statistical analyses were conducted using SPSS
17.0. P<0.05 was considered to indicate a statistically
significant difference.
Results
QIC1 inhibits the proliferation of cancer
cells and is a non-cytotoxic compound
The quinolone-indolone conjugate, QIC1, was
synthesized to target EGFR. It was hypothesized that QIC1 may
target human cancers via its effect on EGFR. The schematic
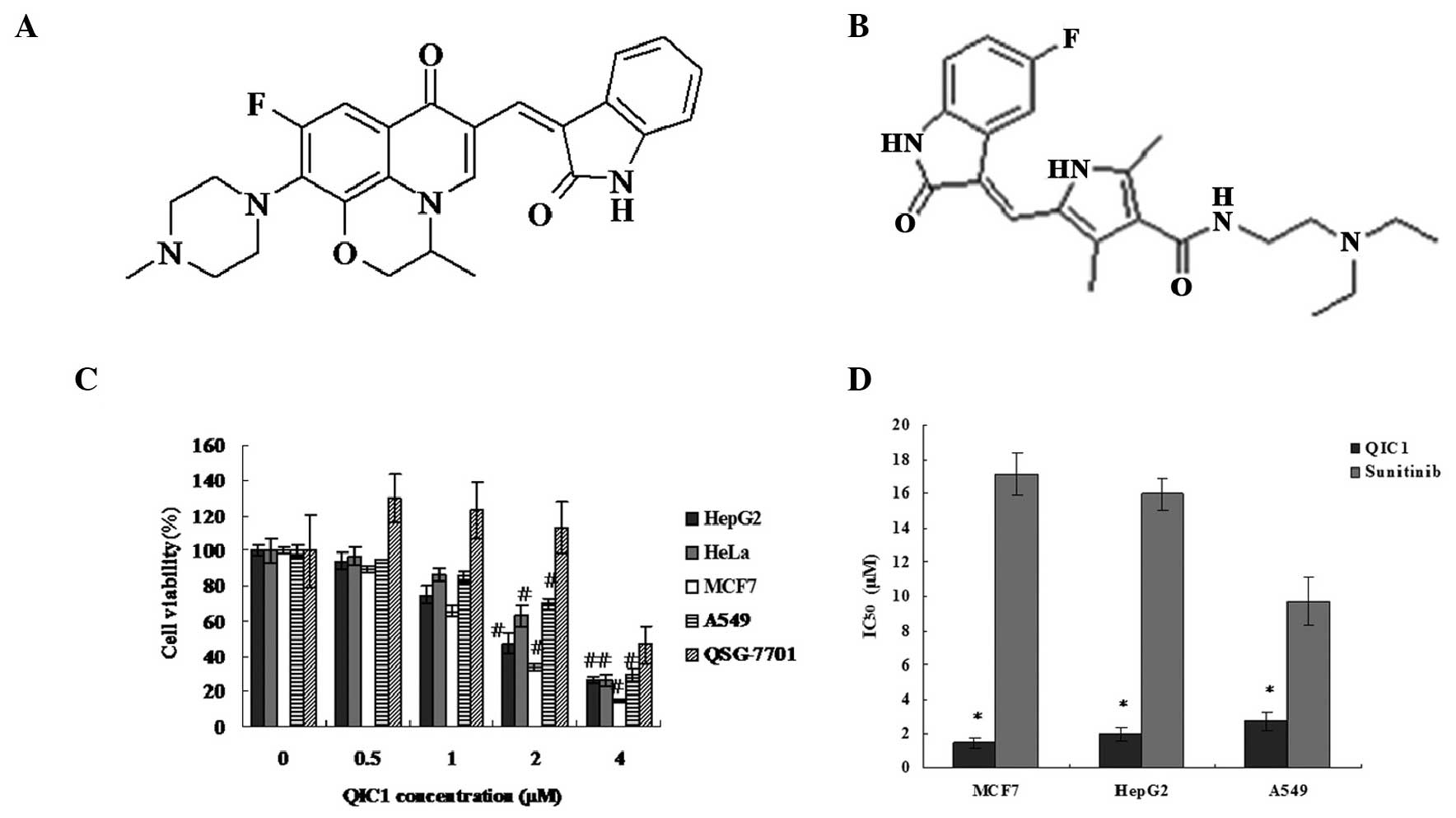
structural diagram of QIC1 is shown in Fig. 1A. The antiproliferative/survival
activity of QIC1 in liver, lung, cervix and breast cancer cells was
investigated and compared with that of the tyrosine kinase
inhibitor, sunitinib (schematic structural diagram shown in
Fig. 1B), which was used as a
positive control. The activity of QIC1 was examined in MCF7, HepG2,
HeLa and A549 cells, and QSG7701 healthy human hepatocytes, using
an MTT assay. Among these cell types, QIC1 induced a significant
dose-dependent decrease in cancer cell survival (Fig. 1C). In MCF7, HepG2, HeLa and A549
cells, the dose of QIC1 required to achieve 50% cell viability
(IC50) was 1.467, 1.994, 2.513 and 2.708 μM
respectively. Furthermore, QIC1 was shown to be a non-cytotoxic
compound as QIC1 (0.5, 1 and 2 μM) did not inhibit cell
growth in the healthy QSG7701 hepatocyte cell line (Fig. 1C). QIC1 exhibited a stronger
anticancer activity than that of sunitinib (Fig. 1D).
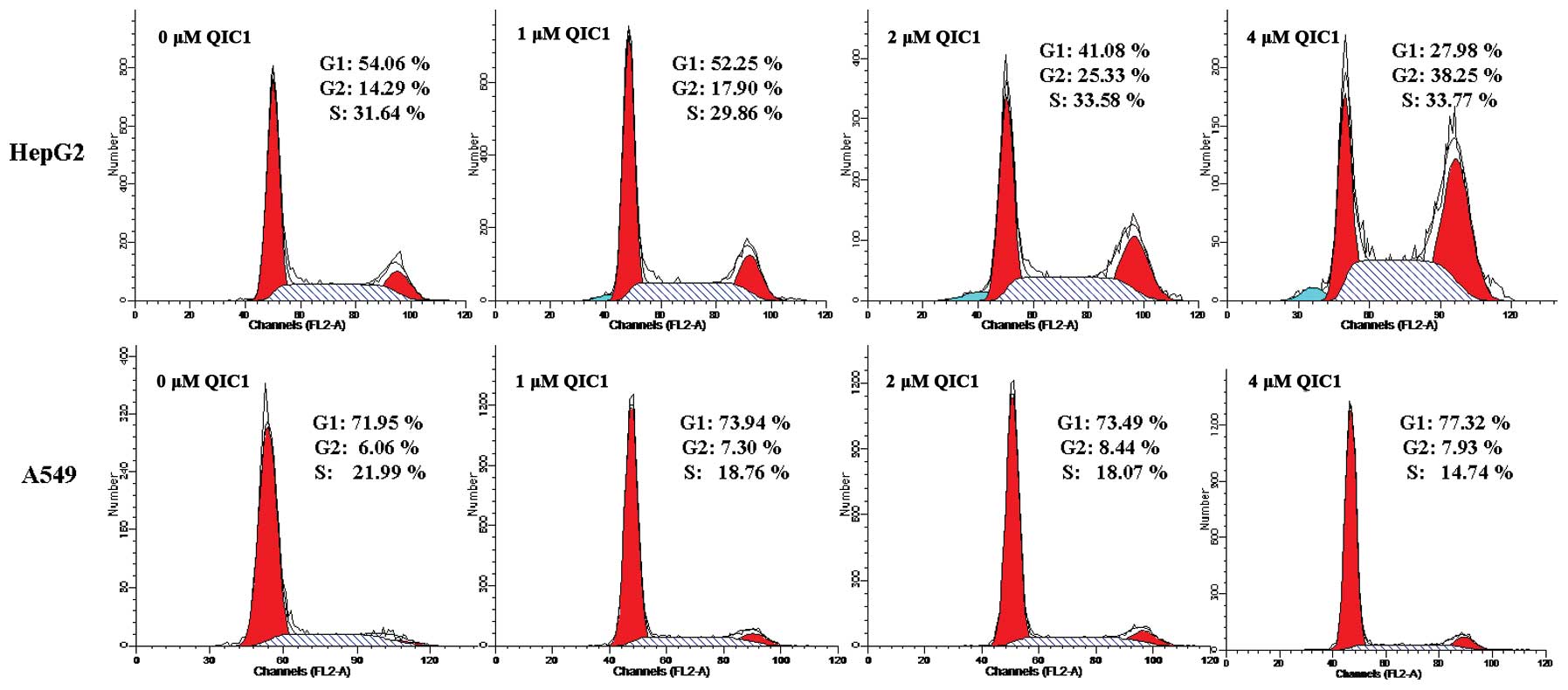
The effects of QIC1 on cell proliferation were also
examined using cell cycle analysis. There was a significant
accumulation of the G2/M (4N-DNA) cell population in HepG2 cells
treated with QIC1 (17.90, 25.33 and 38.25% following incubation
with 1, 2 and 4 μM, respectively) compared with untreated
cells (14.29%; Fig. 2). In A549
cells, the S phase cell population was decreased, which indicated
that QIC1 may inhibit DNA synthesis in A549 (Fig. 2). These observations suggest that
QIC1 may be a promising candidate with which to inhibit cancer cell
survival and proliferation.
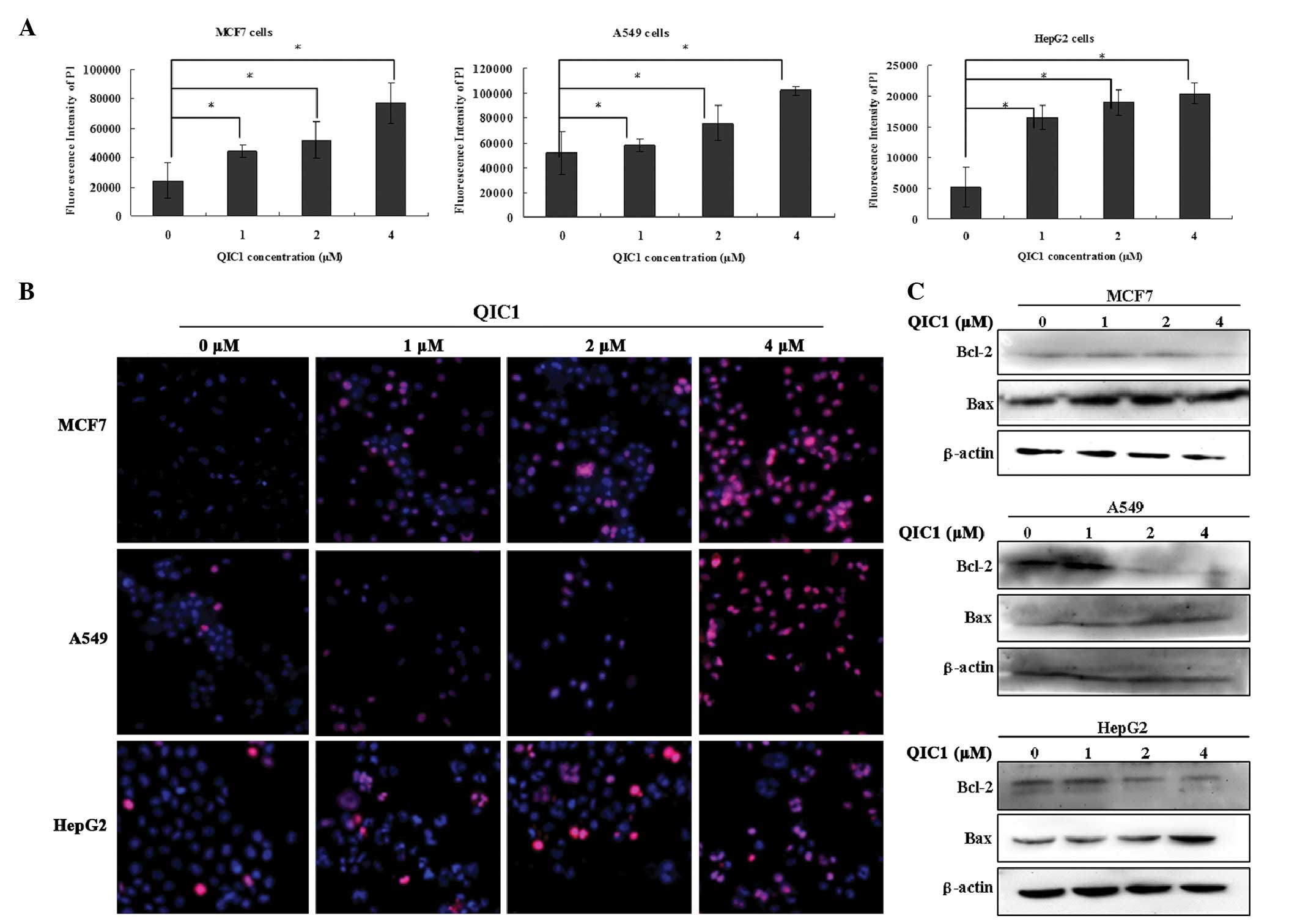
QIC1 induces cancer cell apoptosis
In subsequent experiments, the hypothesis that QIC1
results in cancer cell death by promoting apoptosis was
investigated. Cell apoptosis was detected using an ArrayScan VTI
HCS 600-type high content live cell imaging system. MCF7, HepG2 and
A549 cells underwent apoptotic death in a dose-dependent manner
when they were treated with QIC1 at doses of 1, 2 and 4 μM
for 48 h (Fig. 3A and B).
Furthermore, enhanced Bax expression and reduced Bcl-2 expression
was observed in HepG2, MCF7 and A549 cells that were treated with
QIC1 (1, 2 or 4 μM; Fig.
3C). These results supported the hypothesis of the induction of
an apoptotic response in MCF7, HepG2 and A549 cancer cells upon
treatment with QIC1.
QIC1 inhibits the EGFR-STAT3-HK2
pathway
Previous studies have indicated that downregulation
of either the expression or the activity of EGFR contributes to the
inhibition of cell proliferation and the induction of apoptosis in
cancer cells (22,23). In the present study, the effect of
QIC1 on the expression and activity of EGFR was measured in MCF7,
HepG2 and A549 cell lines. Notably, decreased EGFR activity and
expression was observed in all three cell lines exposed to QIC1, in
a dose-dependent manner (Fig. 4A).
It has also been reported that certain downstream components of the
EGFR signaling pathway, such as activated AKT and STAT3, are
involved in cell survival and apoptosis (24–26).
Therefore, the expression and activation status of these downstream
signaling molecules was also investigated. Reduced expression and
activity of AKt and STAT3 was observed in the three cancer cells
treated with QIC1 (Fig. 4B).
Previous studies by this group, have indicated that STAT3 may
regulate the expression of HK2 in HepG2 cells. HK2 catalyzes the
first committed step of glycolysis, an important metabolic pathway
in cancer cells, which provides essential energy for the growth and
proliferation of cancer cells. It has been shown to be
overexpressed and activated in a number of cancers. The
downregulation of HK2 inhibits the growth and proliferation of
cancer cells. Therefore, the present study examined the effect of
QIC1 on the expression status of HK2 in MCF7, HepG2 and A549 cell
lines. Notably, decreased HK2 expression was observed in the cell
lines exposed to QIC1, and this decrease occurred in a
dose-dependent manner (Fig. 4C).
In order to further confirm whether QIC1 regulates cancer cell
growth through the EGFR-STAT3-HK2 pathway, cell growth status was
measured after cancer cells were pretreated with STAT3 siRNA:
Sense: 5′-GCAACAGAUUGCCUGCAUUdTdT-3′ and antisense:
5′-AAUGCAGGCAAUCUGCAUU-3′), and the HK2 inhibitor, 3-bromine
pyruvate (3-BrPA; Adamas-Beta, Shanghai, China). IT was observed
that the anticancer activity of QIC1 was counteracted by the
administration of STAT3 siRNA and 3-BrPA (Fig. 4D). Furthermore, STAT3 siRNA
decreased the expression of HK2 in these cells (Fig. 4E). These results indicated that the
downregulation of the EGFR-STAT3-HK2 pathway may be one if the
mechanisms underlying the effect of QIC1.
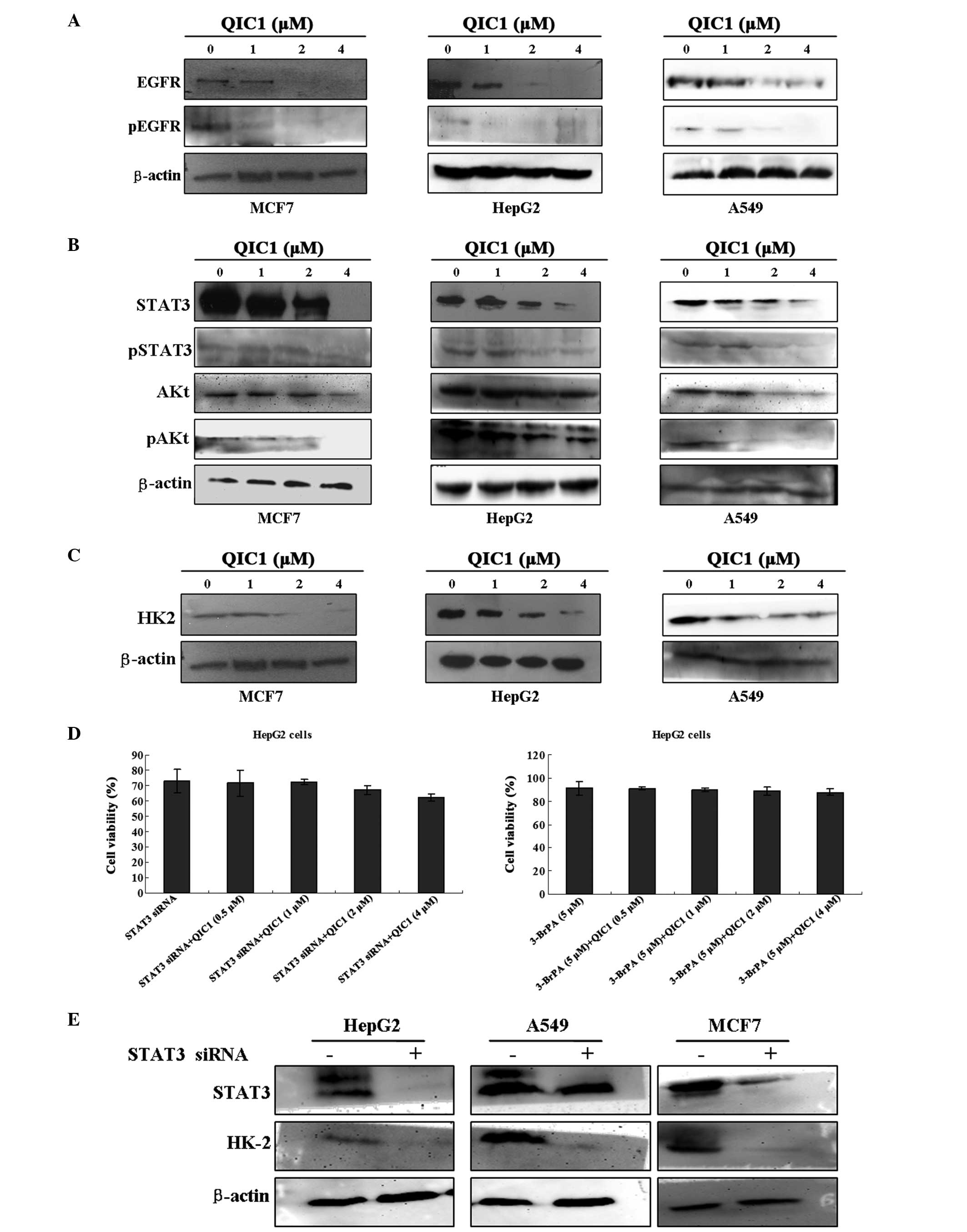 | Figure 4Evaluation of the inhibitory effect of
QIC1 on the EGFR-STAT3-HK2 pathway. (A) Changes in EGFR and
Phospho-EGFR levels were examined in MCF7, HepG2 and A549 cells
following QIC1 treatment for 48 h by western blotting. (B) Whole
cell lysates were prepared from cells treated with QIC1 for 48 h
and immunoblotted to assess normal and activated forms of STAT3 and
AKT. (C) Changes in the level of HK2 in QIC1-treated MCF7, HepG2
and A549 cells for 48 h were examined by western blotting. (D) The
anticancer activity of QIC1 was counteracted by STAT3 siRNA and the
HK2 inhibitor, 3-BrPA, using an MTT assay. (E) STAT3 siRNA. Changes
in the expression of HK2 in MCF7, HepG2 and A549 cells following
treatment with STAT3 siRNA for 48 h were detected by western
blotting. QIC1, quinolone-indolone conjugate; EGFR, epidermal
growth factor receptor; STAT3, signal transducer and activator of
transcription 3; HK2, hexokinase II; AKT, protein kinase B; 3-BrPA,
3-bromine pyruvate; siRNA, small interfering RNA. |
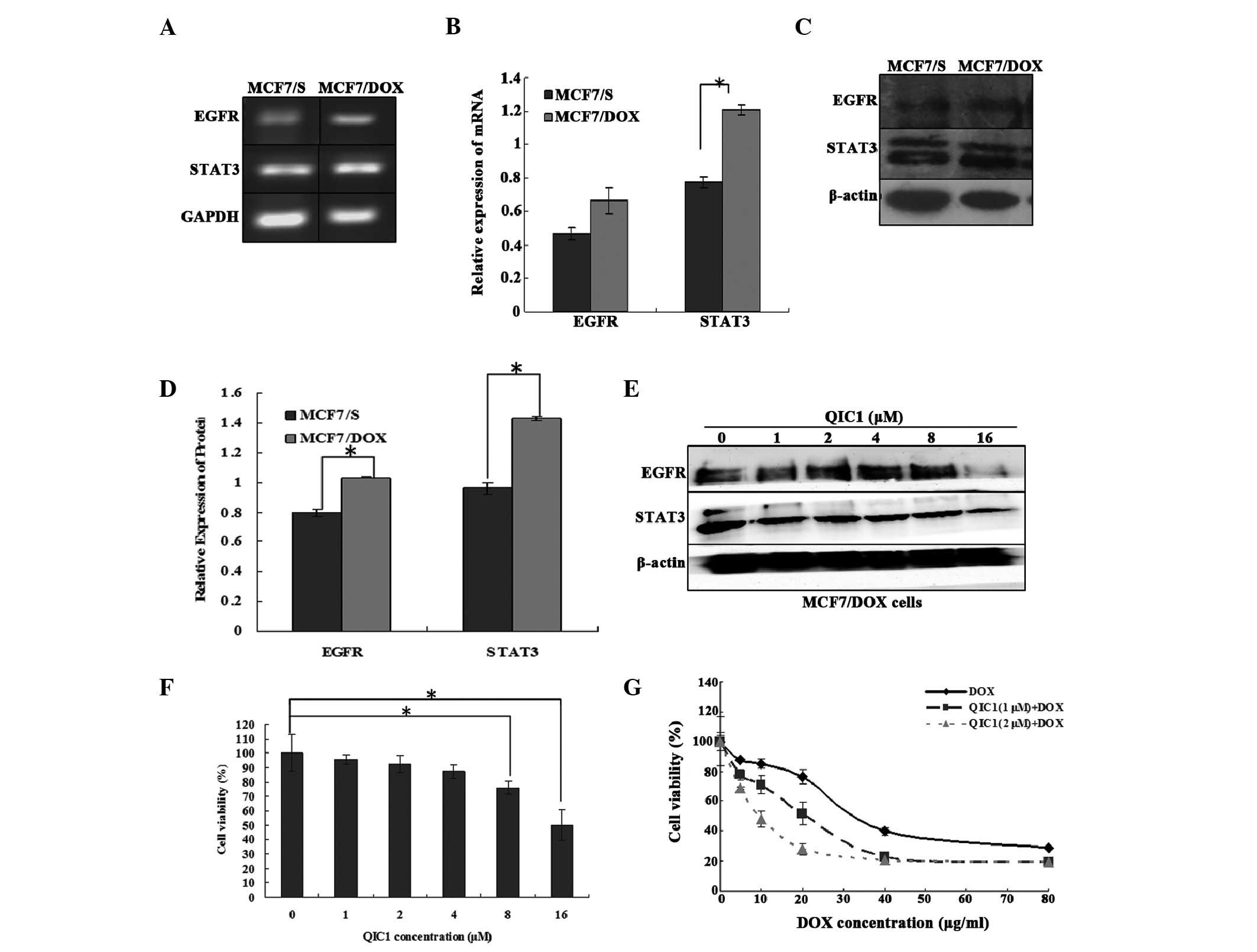
QIC1 reverses drug resistance in human
breast cancer MCF7/DOX cells
The results suggested that QIC1 may downregulate the
expression and activity of EGFR and STAT3. Furthermore, a previous
study demonstrated that the overexpression of STAT3 induces
multidrug resistant in cancer cells and consequently, that STAT3
may be an effective target with which to counter
multidrug-resistant cancer (27).
Therefore, the expression of STAT3 and its upstream gene, EGFR, was
measured in doxorubicin-resistant human breast cancer MCF7/DOX
cells, and the involvement of QIC1 in the MCF7/DOX cells was also
investigated. It was found that STAT3 as well as its upstream gene,
EGFR, were overexpressed in the MCF7/DOX cells (Fig. 5A–D). QIC1 may downregulate the
level of the STAT3 and EGFR proteins (Fig. 5E) in MCF7/DOX cells. An MTT assay
demonstrated that QIC1 inhibited MCF7/DOX cell proliferation in a
dose-dependent manner, and that non-toxic (cell survival rate
>90%) doses of QIC1 (1 μM, 2 μM) enhanced the
anticancer activity of doxorubicin in the MCF7/DOX cells,
consequently reversing the drug resistance of MCF7/DOX cells
(Fig. 5F and G). Therefore, QIC1
may also reverse multi-drug resistance in tumor cells.
Discussion
Currently, chemotherapy remains an important
therapeutic approach for the majority of types of cancer. However,
this approach is not always fully effective, either due to an
initial lack of response by the tumor, or as a result of the
development of drug resistance over time. Thus, there is a
continued need to develop alternative effective treatment agents.
Previous studies have shown that quinolone derivatives and indolone
derivatives exhibit anticancer activity in human cancer cell lines
(28,29). Therefore, the quinolone-indolone
conjugate, QIC1, was synthesized by introducing indolin-2-one to
the quinolone C-3 position. The present study focused on the effect
of QIC1 on the EGFR pathway in cancer cells.
It was observed that QIC1 inhibited proliferation
and induced apoptosis in HepG2, MCF7 and A549 cancer cell lines.
The present results also demonstrated that QIC1 is a non-cytotoxic
compound. The primary apoptotic signal transduction cascades
generally converge onto a common pathway that regulates proteins
involved in cell survival, such as Bcl-2, and in cell death, such
as Bax. Reduced expression of Bax has been reported in breast
cancer and is hypothesized to be responsible for the development of
relative drug resistance (30,31).
In the present study, a dose-dependent increase in Bax level and a
decrease in Bcl-2 expression was observed following treatment with
QIC1, which suggested that QIC1 is likely to promote apoptosis.
The EGFR signaling pathway is required for the
enhanced proliferation and survival of cancer cells. In the current
study, the expression of EGFR in HepG2, MCF7 and A549 cancer cell
lines was measured. The results demonstrated a prominent decrease
in EGFR and phospho-EGFR level in all cell lines that were treated
with QIC1. AKT and STAT3, which are downstream molecules of the
EGFR pathway, are generally found to be constitutively active in
the majority of cancers (32–35).
The present study demonstrated reduced activity of these two
molecules following treatment with QIC1. These results indicated
that the inhibition of EGFR and its downstream molecules, may
affect the viability of HepG2, MCF7 and A549 cells. HK2 is
overexpressed and activated in a number of types of cancer, which
facilitates the growth and proliferation of cancer cells. Decreased
HK2 expression was observed in all cell lines exposed to QIC1. A
previous study also found that HK2 may be regulated by STAT3.
Therefore, the present study sought to further confirm whether QIC1
regulates cancer cell proliferation and apoptosis through the
EGFR-STAT3-HK2 pathway. The current results showed that the
anticancer activity of QIC1 is counteracted by the administration
of STAT3 siRNA and 3-BrPA.
Additional experiments were designed to investigate
the effects of QIC1 in doxorubicin-resistant MCF7/DOX human breast
cancer cells. It was found that STAT3 as well as its upstream gene,
EGFR, were overexpressed in MCF7/DOX cells, and that QIC1
downregulated the level of STAT3 and EGFR proteins. It was also
demonstrated that QIC1 inhibited MCF7/DOX cell proliferation in a
dose-dependent manner, and that non-toxic (cell survival rate
>90%) doses of QIC1 enhanced the anticancer activity of
doxorubicin in MCF/DOX cells. The present study provides evidence
that QIC1 may reverse multidrug resistance in cancer cells.
In conclusion, QIC1 appears to be a promising novel
anticancer agent, which exhibits high efficacy in cancer cells. It
inhibits cancer cell growth by targeting the EGFR-STAT3-HK pathway,
thus reversing multidrug resistance in cancer cells.
Acknowledgments
This study was supported by a grant from the Key
Science and Technology Fund of Henan Province in China (grant no.
122102310558).
References
|
1
|
Shehata MA and Karim NA: Influenza
vaccination in cancer patients undergoing systemic therapy. Clin
Med Insights Oncol. 8:57–64. 2014.PubMed/NCBI
|
|
2
|
Lo HW, Hsu SC and Hung MC: EGFR signaling
pathway in breast cancers: From traditional signal transduction to
direct nuclear translocalization. Breast Cancer Res Treat.
95:211–218. 2006. View Article : Google Scholar
|
|
3
|
Ribeiro FA, Noguti J, Oshima CT and
Ribeiro DA: Effective targeting of the epidermal growth factor
receptor (EGFR) for treating oral cancer: A promising approach.
Anticancer Res. 34:1547–1552. 2014.PubMed/NCBI
|
|
4
|
Zahonero C and Sánchez-Gómez P:
EGFR-dependent mechanisms in glioblastoma: Towards a better
therapeutic strategy. Cell Mol Life Sci. 71:3465–3488. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arfaoui A, Kriaa L, Znaidi N, Gritli S,
Bouacha H, Zermani R and Rammeh S: Over-expression of EGFR is
closely correlated to poor prognosis in Tunisian patients with
non-small cell lung adenocarcinoma. J Immunoassay Immunochem.
35:256–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su JC, Lin KL, Chien CM, Chuang PW, Chang
LS and Lin SR: Concomitant inactivation of the epidermal growth
factor receptor, phosphatidylinositol 3-kinase/Akt and Janus
tyrosine kinase 2/signal transducer and activator of transcription
3 signalling pathways in cardiotoxin III-treated A549 cells. Clin
Exp Pharmacol Physiol. 37:833–840. 2010.PubMed/NCBI
|
|
7
|
Subramaniam A, Shanmugam MK, Ong TH, Li F,
Perumal E, et al: Emodin inhibits growth and induces apoptosis in
an orthotopic hepatocellular carcinoma model by blocking activation
of STAT3. Br J Pharmacol. 170:807–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Z, Tang Y, Fang J, Zhou Z, Xing Z, et
al: Simvastatin inhibits renal cancer cell growth and metastasis
via AKT/mTOR, ERK and JAK2/STAT3 pathway. PLoS One. 8:e628232013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weerasinghe P, Garcia GE, Zhu Q, Yuan P,
Feng L, et al: Inhibition of Stat3 activation and tumor growth
suppression of non-small cell lung cancer by G-quartet
oligonucleotides. Int J Oncol. 31:129–136. 2007.PubMed/NCBI
|
|
10
|
Zhang X, Yue P, Page BD, Li T, Zhao W, et
al: Orally bioavailable small-molecule inhibitor of transcription
factor Stat3 regresses human breast and lung cancer xenografts.
Proc Natl Acad Sci USA. 109:9623–9628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kortylewski M and Yu H: Stat3 as a
potential target for cancer immunotherapy. J Immunother.
30:131–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai WT, Cheng AL, Shiau CW, Huang HP,
Huang JW, et al: Signal transducer and activator of transcription 3
is a major kinase-independent target of sorafenib in hepatocellular
carcinoma. J Hepatol. 55:1041–1048. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg’s contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hernández JF, Urueña CP, Cifuentes MC,
Sandoval TA, Pombo LM, Castañeda D, Asea A and Fiorentino S: A
Petiveria alliacea standardized fraction induces breast
adenocarcinoma cell death by modulating glycolytic metabolism. J
Ethnopharmacol. 153:641–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen WL, Wang JH, Zhao AH, Xu X, Wang YH,
et al: A distinct glucose metabolism signature of acute myeloid
leukemia with prognostic value. Blood. 124:1645–1654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venmar KT, Kimmel DW, Cliffel DE and
Fingleton B: IL4 receptor α mediates enhanced glucose and glutamine
metabolism to support breast cancer growth. Biochim Biophys Acta.
1853:1219–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Zhang S, Li Y, Tang Z and Kong W:
Hexokinase 2 overexpression promotes the proliferation and survival
of laryngeal squamous cell carcinoma. Tumour Biol. 35:3743–3753.
2014. View Article : Google Scholar
|
|
19
|
Wang TX, Liu YH and Shi XY: Oridonin
induces apoptosis of HepG2 cells via downregulating STAT3-HK II
pathway. Chinese Pharmacological Bulletin. 30:397–402. 2014.In
Chinese.
|
|
20
|
Li J, Liu T, Zhao L, Chen W, Hou H, et al:
Ginsenoside 20(S)-Rg3 inhibits the Warburg effect through STAT3
pathways in ovarian cancer cells. Int J Oncol. 2:775–781. 2015.
|
|
21
|
Li Z, Li X, Wu S, Xue M and Chen W: Long
non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase
2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci.
105:951–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moiseeva EP, Heukers R and Manson MM: EGFR
and Src are involved in indole-3-carbinol-induced death and cell
cycle arrest of human breast cancer cells. Carcinogenesis.
28:435–445. 2007. View Article : Google Scholar
|
|
23
|
Chen J, Wang W, Wang H, Liu X and Guo X:
Combination treatment of ligustrazine piperazine derivate DLJ14 and
adriamycin inhibits progression of resistant breast cancer through
inhibition of the EGFR/PI3K/Akt survival pathway and induction of
apoptosis. Drug Discov Ther. 8:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi H and Wang HG: The protein
kinase PKB/Akt regulates cell survival and apoptosis by inhibiting
Bax conformational change. Oncogene. 20:7779–7786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le Gall M, Chambard JC, Breittmayer JP,
Grall D, Pouysségur J and Van Obberghan Schilling E: The p42/p44
MAP kinase pathway prevents apoptosis induced by anchorage and
serum removal. Mol Biol Cell. 11:1103–1112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grandis JR, Drenning SD, Chakraborty A,
Zhou MY, Zeng Q, et al: Requirement of Stat3 but not Stat1
activation for epidermal growth factor receptor-mediated cell
growth in vitro. J Clin Invest. 102:1385–1392. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tai WT, Cheng AL, Shiau CW, Liu CY, Ko CH,
et al: Dovitinib induces apoptosis and overcomes sorafenib
resistance in hepatocellular carcinoma through SHP-1-mediated
inhibition of STAT3. Mol Cancer Ther. 11:452–463. 2012. View Article : Google Scholar
|
|
28
|
Wicke L, Engels JW, Gambari R and Saab AM:
Synthesis and antiproliferative activity of quinolone nucleosides
against the human myelogenous leukemia k-562 cell line. Arch Pharm
(Weinheim). 346:757–765. 2013. View Article : Google Scholar
|
|
29
|
Shou KJ, Li J, Jin Y and Lv YW: Design,
synthesis, biological evaluation, and molecular docking studies of
quinolone derivatives as potential antitumor topoisomerase I
inhibitors. Chem Pharm Bull (Tokyo). 61:631–636. 2013. View Article : Google Scholar
|
|
30
|
Bargou RC, Wagener C, Bommert K, Mapara
MY, Daniel PT, et al: Overexpression of the death-promoting gene
bax-alpha which is downregulated in breast cancer restores
sensitivity to different apoptotic stimuli and reduces tumor growth
in SCID mice. J Clin Invest. 97:2651–2659. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bargou RC, Daniel PT, Mapara MY, Bommert
K, Wagener C, et al: Expression of the bcl-2 gene family in normal
and malignant breast tissue: Low bax-alpha expression in tumor
cells correlates with resistance towards apoptosis. Int J Cancer.
60:854–859. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blando JM, Carbajal S, Abel E, Beltran L,
Conti C, et al: Cooperation between Stat3 and Akt signaling leads
to prostate tumor development in transgenic mice. Neoplasia.
13:254–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin J, Tang H, Jin X, Jia G and Hsieh JT:
p53 regulates Stat3 phosphorylation and DNA binding activity in
human prostate cancer cells expressing constitutively active Stat3.
Oncogene. 21:3082–3088. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vangala Jr, Dudem S, Jain N and Kalivendi
SV: Regulation of PSMB5 protein and β subunits of mammalian
proteasome by constitutively activated signal transducer and
activator of transcription 3 (STAT3): Potential role in
bortezomib-mediated anticancer therapy. J Biol Chem.
289:12612–12622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu K, Chang Q, Lu Y, Qiu P, Chen B, et al:
Gefitinib resistance resulted from STAT3-mediated Akt activation in
lung cancer cells. Oncotarget. 4:2430–2438. 2013.PubMed/NCBI
|