Introduction
Human ovarian cancer is the fourth most common type
of cancer among females worldwide, and ~90% of cases of ovarian
cancer are epithelial ovarian cancer (EOC) (1,2).
Despite the increases use of surgery and chemotherapy to treat EOC,
the outcome remains poor, with a 30–40% 5-year-survival rate in
patients with EOC, due to the lack of specific symptoms in the
early stages of the disease, and a lack of effective tumor
biomarkers and effective treatment strategies (3,4).
Therefore, identifying the molecular mechanisms of EOC
carcinogenesis may improve current understanding of the
pathogenesis of EOC and, ultimately, identify novel diagnostic
and/or prognostic markers.
MicroRNAs (miRs/miRNAs) are small (19–25 nt),
endogenous non-coding RNAs, and are novel post-transcriptional
regulators of gene expression through binding to the
3′-untranslated region of target genes via their own RNA-induced
silencing complexes (5–8). The actions of miRNAs are varied, as
they have been observed to be involved in a multitude of
physiological processes, including the cell cycle, metabolism,
angiogenesis, differentiation, apoptosis and proliferation
(9–14). Additionally, increasing reports
have indicated that miRNAs are involved in tumorigenesis and cancer
metastasis by regulating the expression of their target oncogenes
or tumor suppressor genes (15,16).
For example, miR-135a acts as a potential tumor suppressor
inhibiting EOC cell proliferation, migration and invasion by
decreasing the expression of the Homeobox A10 oncogene (17), and miR-124 markedly inhibits the
motility of ovarian cancer cells in vitro by downregulating
the protein expression of the sphingosine kinase 1 oncogene
(18).
An increasing number of reports have indicated that
a number miRNAs are involved in several aspects of the pathogenesis
of EOC, including cell cycle control, invasion, migration,
resistance to chemotherapy and radiotherapy, and cell apoptosis. It
has been reported that the miR-15a, miR-16 (19), miR-221/222 (20) and miR-200 families (7) are upregulated, while miRNA-34
(21), miR-124 (18), miR-145 (22) and miR-137 families (23) are downregulated in EOC. miR-137 is
located on chromosome 1p21.3 and lies across a large CpG island,
which is associated with certain diseases and has been found to be
abnormally expressed in lung cancer (24), oral squamous cell carcinoma
(25), gastric cancer cell lines
(26), colorectal cancer (27), neuroblastoma (28) and breast cancer (29). At present, only one study has
reported that miR-137 is differentially expressed between normal
ovarian and ovarian cancer tissues through quantitative polymerase
chain reaction (qPCR), which also found that miR-137 negatively
regulates the astrocyte elevated gene-1 (AEG-1) oncogene and
suppresses ovarian cancer cell proliferation and clonogenicity
(23). However, the function of
miR-137 in the apoptosis, migration and invasion of EOC cells
remains to be elucidated Validation of the function of miR-137 in
ovarian cancer, particularly in EOC is required, therefore, the aim
of the present study was to characterize the expression of miR-137,
identify its detailed function in EOC cells in vitro and
in vivo to determine its utility in EOC diagnosis and
therapy.
Patients and methods
Patients and tissue samples
The procedures used in the present study were
approved by the Ethical Committee of the Affiliated Hospital,
Changchun University of Chinese Medicine (Changchun, China). All
patients were informed and agreed to be involved in the present
study. All clinical investigations were performed according to the
principles detailed in the Declaration of Helsinki.
Malignant ovarian tumor tissues and their adjacent
non-tumorous samples were obtained from 30 consecutive patients
with EOC, who underwent curative ovarian resection between June
2012 and March 2014 at the Affiliated Hospital, Changchun
University of Chinese Medicine. Patients with a previous or
secondary malignancy, or who had previously undergone chemotherapy
or radiotherapy treatment were excluded from the investigation.
Clinicopathological information was collected from the clinical
data of patients diagnosed with OEC (as listed in Table I). The tumor tissues were staged
according to the tumor, node, metastasis staging system of the
Union for International Cancer Control (30).
 | Table ICorrelation between the relative
levels of miR-137 in ovarian epithelial tissues and
clinicopathological features of patients with ovarian epithelial
cancer. |
Table I
Correlation between the relative
levels of miR-137 in ovarian epithelial tissues and
clinicopathological features of patients with ovarian epithelial
cancer.
| Feature | Number of
patients | Relative level of
miR-137 (2−ΔΔCt) | P-value |
|---|
| Age (years) | | | |
| <55 | 18 | 4.586±0.435 | NS |
| ≥55 | 12 | 4.389±0.427 |
| Regional lymph
nodes | | | |
| Yes | 13 | 3.046±0.286 | P<0.01 |
| No | 17 | 4.912±0.792 |
| TNM clinical
stage | | | |
| I–II | 19 | 5.035±0.418 | P<0.01 |
| III–IV | 11 | 3.382±0.288 |
Cell lines and cell culture
The SKOV3 human ovarian cancer cell line, with a
high metastatic potential, (31)
was obtained from the Shanghai Institute of Cell Biology, China
Academy of Sciences (Shanghai, China). The HEY human ovarian cancer
cell line and normal human ovarian surface epithelial (OSE) cells
were obtained from American Type Culture Collection (Manassas, VA,
USA). The SKOV3 and HEY cells were cultured in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal
bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA, USA) in a
humidified atmosphere of 5% CO2 at 37°C. The OSE cells
were cultured in Dulbecco’s modified Eagle’s medium-F12 growth
medium (Sigma-Aldrich) with 15% FBS and 5% penicillin-streptomycin
(Sigma-Aldrich). The complete media was generally replaced every
2–3 days.
miRNA transfection
The miR-137 mimic and negative control miRNA
(mirVanaR miRNA mimic/inhibitor; Life Technologies, Grand
Island, NY, USA) were transiently transfected into the SKVO3
ovarian cell lines in 6-well plates using Oligofectamine™
transfection reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions, at a
concentration of 100 nM.
Reverse transcription (RT)-qPCR
The isolation of total RNA from the cells and
ovarian tissues was performed using QIAzol lysis reagent and an
miRNeasy mini kit (Qiagen, Valencia, CA, USA), according to the
manufacturer’s instructions. The RNA was reverse-transcribed using
a One Step Prime script miRNA cDNA synthesis kit (Qiagen) and then
quantified using RT-qPCR with SYBR Premix Ex Taq (Takara Bio Inc.,
Dalian, China). All the PCR reactions were detected using the ABI
7900 Fast system (Applied Biosystems, Foster City, CA, USA). The
primers used in the RT-qPCR reaction were as follows: miR-137,
sense 5′-GCGCTTATTGCTTAAGAATAC-3′ and antisense
5′-CAGTGCAGGGTCCGAGGT-3′; and U6, sense
5′-CTCGCTTCGGCAGCACATATACT-3′ and antisense
5′-ACGCTTCACGAATTTGCGTGTC-3′. Amplification of miR-137 and U6 was
performed with 1 cycle at 95°C for 5 min and 40 cycles of 95°C for
15 sec then 55°C for 60 sec. The expression levels of U6 were
determined as an internal control. The PCR efficiencies were
calculated using a relative standard curve, derived from a
complementary DNA mixture, and provided regression coefficients of
>0.95. The relative quantification of each miRNA was presented
as the fold change following normalization to the U6 RNA, with the
2−∆∆Ct equation using Rotor-Gene 6000 series software
1.7 (Qiagen). All experiments were performed in triplicate to
reduce curve-derived variance.
Cell proliferation and colony formation
assays
To measure the effect of miR-137 on cell
proliferation, a Cell Counting kit-8 (CCK-8) assay was performed
(Dojindo Laboratories, Kumamoto, Japan). Briefly, the SKOV3 cells
were seeded into 24-well plates at a density of 5×103
cells/well. The SKOV3 cells were them incubated in 10% CCK8 and
diluted in normal culture medium at 37°C until visual color
conversion occurred. The absorbance in each well was measured on a
Wellscan MK3 microplate reader set at 450 nm 24, 48, 72 and 96 h
after transfection using an enzyme-linked immunosorbent assay
(ELISA) reader (Thermo Labsystems, Helsinki, Finland). The
experiment was performed at least three times with similar
results.
For the colony formation assay, the cells were
seeded in 6-well plates at a low density (1×103
cells/well) and cultured for 7 days. Subsequently, the cells were
fixed with 4% para-formaldehyde for 20 min and were counted
following staining with 1% crystal violet (Sigma-Aldrich). The
experiments were performed in triplicate wells at least three
times.
Cell cycle and cell apoptosis assay
For analysis of the cell cycle, the SKOV3 cells were
transfected with either miR-137 or negative control miRNA. At 48 h
post-transfection, the cells were collected by trypsinisation,
washed with ice-cold phosphate-buffered saline (PBS; pH 7.2;
Sigma-Aldrich), and the fixed cells were then incubated with the
DNA binding dye propidium iodide (20 μg/ml; Sigma-Aldrich)
and RNase (1.0 mg/ml) for 30 min at 37°C in the dark, followed by
analysis using flow cytometry (FACSCalibur; BD Biosciences,
Mansfield, MA, USA). The experiments were performed in
triplicate.
For analysis of apoptosis, the SKOV3 cells were
collected and diluted to a concentration of 1×106
cells/ml, then washed three times with ice cold PBS 48 h
post-transfection. The cells were incubated with phycoerythrin
(PE)-Annexin-V and 7AAD using the PE Annexin-V apoptosis detection
kit I (BD Pharmingen, CA, USA), according to the manufacturer’s
instructions, and then analyzed using fluorescence-activated cell
sorting (FACS). The experiments were performed in triplicate. In
addition, caspase-3 activity was detected using an ELISA, as an
additional indicator of apoptosis.
Measurement of caspase-3 activity
The SKOV-3 cells were plated in 6-well plates and
transfected with the miR-137 mimics or corresponding negative
control. At 48 h post-incubation, the cells were harvested and
lysed using lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China) on ice for 20 min. The cell lysates were centrifuged
at 20,000 × g for 15 min at 4°C. The protein concentration was
measured using a Bradford protein assay kit (Beyotime Institute of
Biotechnology). The activation of caspase-3 activity in the SKOV-3
cells was determined using a caspase-3 activity assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer’s
instructions. The experiment was performed at least three times in
triplicate.
Wound-healing assays
The SKOV3 cells were grown to 80–90% confluence in
24-well plates and treated with miR-137 mimics or the corresponding
negative control. At 24 h post-transfection, linear scratch wounds
were created on the confluent cell monolayers using a 200 μl
pipette tip. To prevent the cells from entering the cell cycle
prior to wounding, the cells were maintained in serum-free
RPMI-1640 medium. To visualize the migrating cells and wound
healing, images were captured at 0 and 24 h. For visualization,
five field areas were randomly selected from each well, and three
wells of each cell group were analyzed.
Cell invasion assay
For the invasion assay, Transwell migration chambers
(8-μm pore filter; BD Biosciences) were coated with Matrigel
(BD Biosciences) and incubated at 37°C for 4 h, allowing it to
solidify. At 24 h post-transfection with the miR-137 mimics,
5×105 SKOV3 cells, suspended in serum-free RPMI-1640
medium, were added to the upper chamber and medium, containing 10%
FBS, was added to the lower chamber as a chemoattractant. After 48
h, the non-invading cells were gently removed using a cotton swab,
and the invasive cells located on the lower surface of the chamber
were stained with 0.1% crystal violet in 20% methanol
(Sigma-Aldrich). The level of invasiveness was determined by
counting the number of penetrating cells under a Nikon
phase-contrast microscope (Nikon TMS; Nikon, Tokyo, Japan) and
counted in >10 fields of view (magnification, ×200). The
invasion of the cells without any treatment was determined as
100%.
Western blot analysis
For the western blot analyses, 48 h
post-transfection, the cells were harvested and lysed via
incubation on ice for 30 min in lysis buffer, containing 25 mM
Tris-HCl (pH 8.0), 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1%
sodium dodecylsulfate (SDS) and 125 mM NaCl, containing complete
protease inhibitor cocktail (Roche, Mannheim, Germany). The protein
concentration was determined using a bicinchoninic acid protein
assay kit (KeyGEN Biotech, Nanjing, China) using a c-globulin
standard curve. Equal quantities of protein (20 μg/lane)
from the cell lysates were separated on 10% SDS-polyacrylamide gels
and transferred onto nitrocellulose membranes (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The membrane was
incubated for 2 h in PBS with 0.1% Tween-20 and 5% nonfat skim milk
to block nonspecific binding. Subsequently, the membranes were
incubated overnight at 4°C with the following antibodies: Mouse
polyclonal anti-human matrix metalloproteinase (MMP)-2 (1:1,000;
cat. no. 4022s; Cell Signaling Technology, Inc., Danvers, MA, USA)
and mouse monoclonal anti-human MMP-9 (1:2,000; cat. no. sc-12759;
Santa Cruz Biotechnology, Inc.). Mouse monoclonal anti-human GAPDH
(1:10,000; cat. no. sc-365062; Santa Cruz Biotechnology, Inc.) was
used as a loading control. The membranes were then incubated with
polyclonal goat anti-mouse horseradish peroxidase-conjugated
immunoglobulin G (1:10,000; cat. no. sc-8454; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature and the proteins
were detected using an enhanced chemiluminescence detection kit
(Thermo Fisher Scientific, Rockford, IL, USA) and then visualized
using a Molecular Imager® ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Tumor growth in vivo
A total of 30 male BALB/c mice (5–6-weeks old; 18–20
g), were purchased from HFK Bio-Technology, Co., Ltd. (Beijing,
China) and were maintained under specific pathogen-free conditions
with ad libitum access to food and water at room temperature
with 45–70% humidity. Subsequently, 2×106 of the SKOV3
cells stably overexpressing miR-137 or the corresponding negative
control cells were suspended in 50 μl PBS and injected into
the flanks of the mice (n=10). The mice were monitored weekly for
tumor growth. Tumor size was measured every week, and tumor volume
was calculated as 0.5236 × width2 × length. At 4 weeks
post-inoculation, the mice were sacrificed via cervical
dislocation. The tumors were resected and weighed, and a section of
each tumor was used to measure the levels of miR-137 using RT-qPCR.
All animal experiments were performed, according to the standards
of animal care as outlined in the Guide for the Care and Use of
Changchun University of Chinese Medicine, and were approved by the
Ethics Committees of the Affiliated Hospital, Changchun University
of Chinese Medicine.
Statistical analysis
The data are expressed as the mean ± standard
deviation of at least three independent experiments. Differences
between groups were compared using one-way analysis of variance or
a two-tailed Student’s t-test with SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA) and GraphPad Prism version 5.01 (GraphPad, San
Diego, CA, USA) software for Windows®. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-137 is reduced in
highly metastatic ovarian cancer cell lines and clinical tumor
tissues
To determine the expression levels of miR-137 in the
progression of ovarian cancer, the expression levels between
malignant ovarian tumor tissues and adjacent non-tumorous tissues
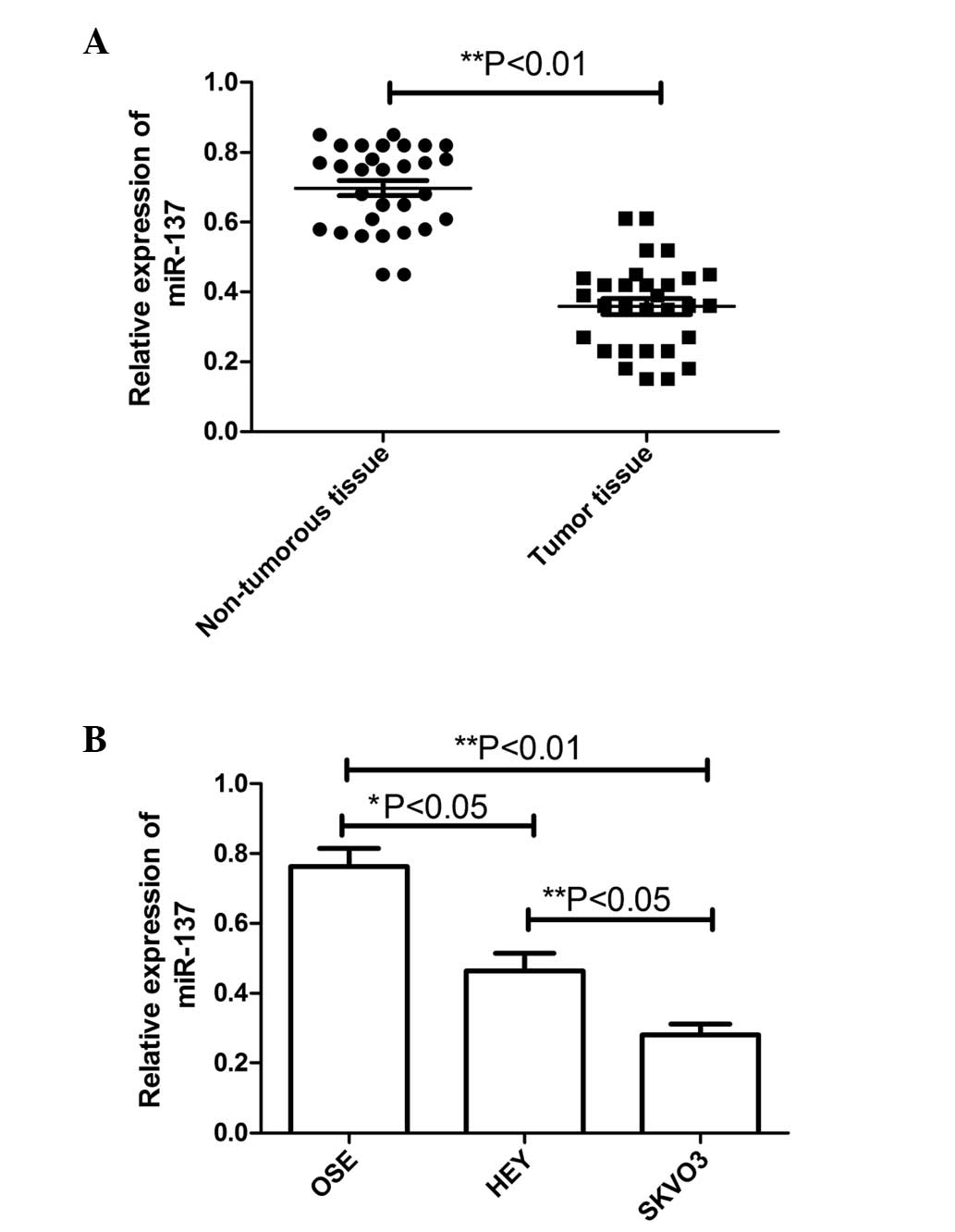
were compared using RT-qPCR. As shown in Fig. 1A, the results revealed that the
expression of miR-137 was decreased in the ovarian cancer samples
(P<0.01), compared with the non-tumorous tissue. Furthermore,
the correlation between the expression of miR-137 and various
clinicopathological factors, including patient age, presence of
lymph node metastasis and clinical stage, was examined (Table I). Patients with lymph node
metastases were found to exhibit significantly lower expression
levels of miR-137, compared with those without metastases
(P<0.01). The expression levels of miR-137 were also found to be
lower in patients with clinical stage III–IV carcinoma, compared
with stage I–II (P<0.01). No correlation was identified between
the expression of miR-137 and patient age.
The levels of miR-137 were further detected in two
ovarian cancer cell lines (SKOV3 and HEY) and normal human OSE
cells using RT-qPCR. Compared with the OSE cells, the levels of
miR-137 were significantly reduced, to different extents, in the
SKOV3 cells and HEY cells (Fig.
1B), with markedly lower levels in the SKOV3 cells with a high
metastatic potential, in particular. These results suggested that
the expression of miR-137 was significantly decreased in the human
ovarian cancer specimens and cell lines, and may be involved in EOC
metastasis.
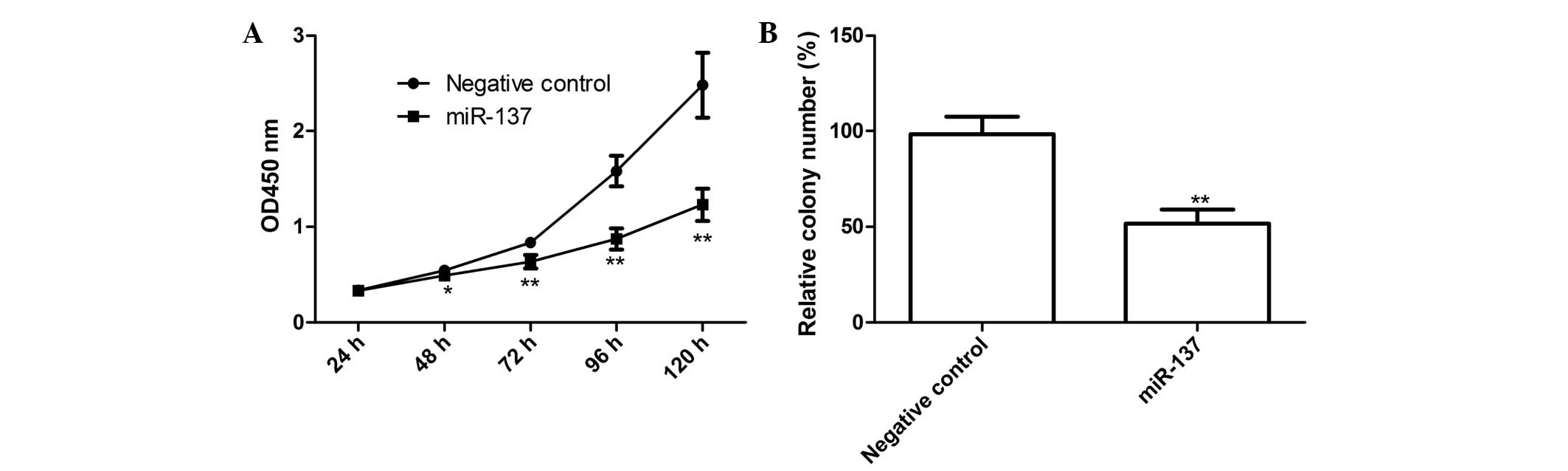
miR-137 inhibits EOC cell proliferation
and colony formation
In view of the reduced expression of miR-137 in
SKOV3 cells, the effect of miR-137 on the proliferation of SKOV3
cells was examined. The miR-137 mimic and corresponding negative
control were transfected into the SKOV3 cells, following which
CCK-8 assays were performed. As shown in Fig. 2A, the viability of the SKOV3 cells
was markedly decreased following transfection with the miR-137
mimic (P<0.05), compared with the negative control. The effect
of the miR-137 mimic on cell proliferation was observed from day 2,
becoming more marked on days 3 and 4 (P<0.01; Fig. 2A).
In addition, the present study determined the effect
of miR-137 on cell colony formation, which demonstrated that the
cells transfected with the miR-137 mimics exhibited significantly
inhibited cell colony formation, compared with cells transfected
with the negative control (P<0.01; Fig. 2B). These findings suggested that
overexpression of miR-137 markedly inhibited cell proliferation and
colony formation in the SKOV3 cells.
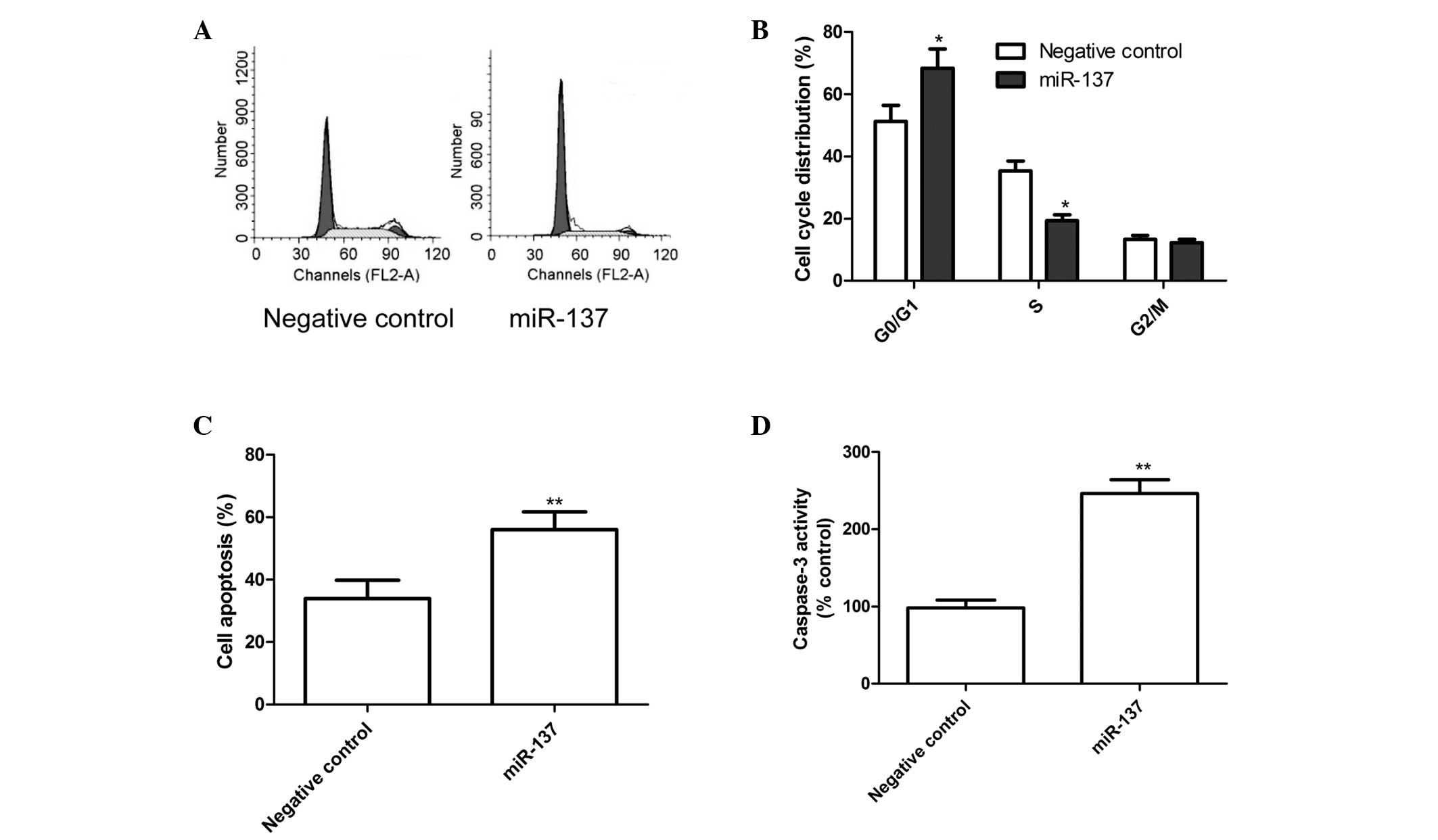
miR-137 induces G1 arrest and cell
apoptosis
In order to determine the effects of miR-137 on the
cell cycle, FACScan flow cytometry assays were performed. The
results revealed that the G1-phase cell population was increased in
the cells transfected with the miR-137 mimics, compared with the
cells transfected with the negative control (P<0.05; Fig. 3A and B).
The role of miR-137 in SKOV3 cell apoptosis was also
assessed, which revealed that apoptosis was significantly induced
in the SKOV3 cells transfected with the miR-137 mimics, compared
with cells transfected with the negative control (P<0.01;
Fig. 3C). Finally, the effects of
miR-137 on caspase-3 activity were analyzed using an ELISA. As
shown in Fig. 3D, caspase-3
activity in the cells transfected with the miR-137 mimics was
significantly increased compared with those transfected with the
negative control (P<0.01).
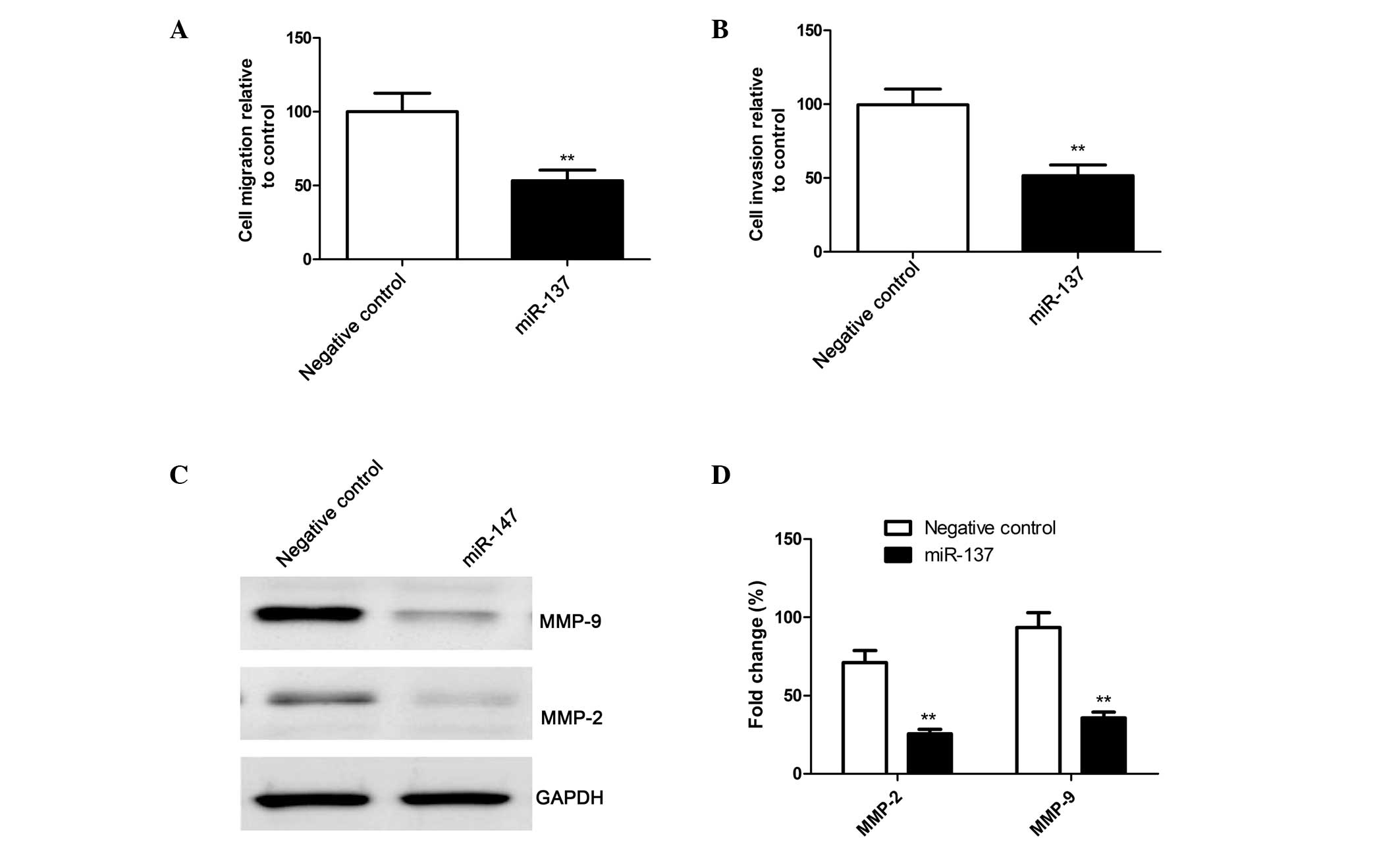
miR-137 inhibits cell migration and
invasion in SKOV3 cells
To ascertain the inhibitory effect of miR-137 on
cell motility in vitro, a wound-healing assay was performed.
A scratch was introduced into confluent monolayers of SKOV3 cells
transfected with the miR-137 mimic or the corresponding negative
control, and the time-dependent movement of the cells into the
injured area was monitored microscopically. After 24 h, the SKOV3
cells transfected with the miR-137 mimics exhibited significantly
decreased migration, compared with the cells transfected with the
negative control (P<0.01; Fig.
4A).
The ability of miR-137 to reduce the invasiveness of
SKOV3 cells was further investigated using a Transwell system
assay. The results demonstrated that overexpression of miR-137
significantly inhibited cell invasion (P<0.01; Fig. 4B).
Furthermore, the effects of miR-137 on the
expression levels of the cell invasion relevant proteins, MMP-2 and
MMP-9, were analyzed using western blot analysis As shown in
Fig. 4C and 4D, compared with the cells transfected
with the negative control, the protein expression levels of MMP-2
and MMP-9 were significantly decreased in the cells transfected
with the miR-137 mimics (P<0.05).
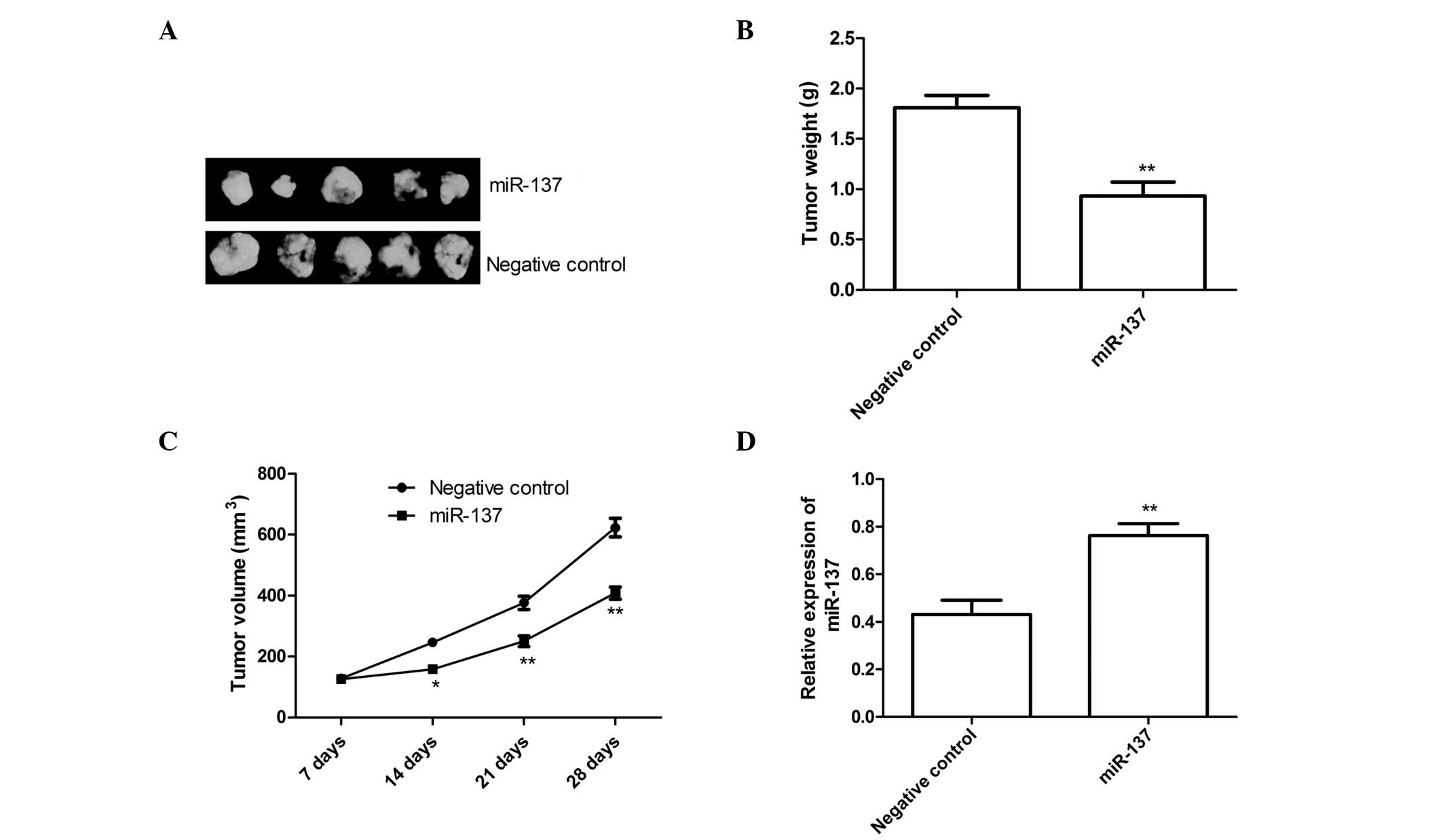
miR-137 suppresses tumor growth in a nude
mouse model
To further examine the association between miR-137
and tumorigenesis in vivo, SKOV3 cells stably overexpressing
miR-137 or negative control were injected subcutaneously into nude
mice (n=10). The tumor volume was measured once a week, and mice
were sacrificed 28 days after tumor cell implantation. As shown in
Fig. 5A, the
miR-137-overexpressing tumors were significantly smaller than
tumors in the negative control group. The average volume and weight
of the miR-137-overexpressing tumors were significantly reduced,
compared with the negative control group (P<0.05; Fig. 5B and 5C). The level of miR-137 in the grafted
tumor tissues was also examined using RT-qPCR. The RT-qPCR analysis
revealed that the expression levels of miR-137 were significantly
increased in the groups treated with the miR-137 mimics compared
with the negative control treatment group (P<0.01; Fig. 5D). These results suggested that the
overexpression of miR-137 may inhibit tumor growth of EOC cells
in vivo.
Discussion
In the present study, the results revealed that
miR-137 was downregulated in ovarian cancer cell lines and tumor
tissues, compared with normal OSE cells and non-tumorous ovarian
tissues. Furthermore, the results also demonstrated that miR-137
inhibited EOC cell migration and invasion, which may be involved in
the development of ovarian cancer metastasis. It was also
demonstrated that miR-137 suppressed EOC tumor growth in nude mice.
Therefore, it was hypothesized that low expression levels of
miR-137 may be important in the progression of EOC.
It has been well-established that miR-137 is
downregulated in various types of cancer (23–29).
Several studies have reported that miR-137 may act as a tumor
suppressor and inhibit cell proliferation, migration and invasion,
and induce cell cycle arrest and apoptosis. Chen et al
(32) demonstrated that miR-137
deregulation is common in glioma, and that restoration of its
function inhibits cell proliferation and invasion by targeting
cyclooxygenase (COX)-2. Liu et al (33) found that miR-137 suppresses cell
growth and metastasis in hepato-cellular carcinoma by directly
targeting AKT2. Bi et al (34) found that the ectopic expression of
miR-137 inhibits cell proliferation, induces cell apoptosis and
suppresses cell migration and invasion in the A549 non-small cell
lung cancer cell line by targeting paxillin. In the present study,
it was observed that the expression level of miR-137 was low in
ovarian cancer tissues and even lower in the metastatic ovarian
tissues. In agreement with these results, Guo et al
(23) demonstrated that miR-137 is
downregulated in ovarian cancer tissues compared with normal
ovarian samples, and that miR-137 negatively regulates the AEG-1
oncogene and suppresses ovarian cancer cell growth in vitro
and in vivo. However, the role of miR-137 in ovarian cancer
cell migration and invasion has not been investigated, to the best
of our knowledge. Invasion and metastasis are two important
attributes of malignant types of cancer, which are the cause of the
high mortality rate in EOC (35).
To the best of our knowledge, there are no previous reports
regarding the role of miR-137 in migration and invasion in EOC. In
this context, the present study revealed that the enforced
expression of miR-137 inhibited the migration and invasion of
ovarian cancer cells by inhibiting the expression of MMP-2 and
MMP-9 expression, suggesting that miR-137 is important as a tumor
suppressor in the motility of ovarian cancer cells.
It is important to note that one miRNA can exert
different functions by targeting multiple mRNAs (36). To date, specific genes have been
identified as gene targets of miR-137 in different tumors,
including COX-2 in glioblastoma multiforme (28), cyclin-dependent kinase-6 in glioma
cell lines (37) and lung cancer
(38), Related to testes-specific,
vespid and pathogenesis protein-1 in glioblastoma (39), C-terminal binding protein 1 in
melanoma cells (40),
estrogen-related receptor α in breast cancer (29,41),
cell division control protein 42 homolog in gastric cancer cells
(32), colorectal cancer cells
(42) and lung cancer (38) and c-Met, Y box binding protein 1,
enhancer of zeste homolog 2 and microphthalmia-associated
transcription factor in melanoma cells (43). Therefore, the present study
hypothesized that miR-137 decreased cell proliferation,
clonogenicity, migration and invasion, and induced G1 arrest and
cell apoptosis via targeting multiple genes.
In conclusion, the findings of the present study
demonstrated that miR-137 was downregulated in ovarian cancer cell
lines and tumor tissues, compared with normal OSE cells and
non-tumorous ovarian tissues, and that the overexpression of
miR-137 inhibited cell proliferation, clonogenicity, migration, and
invasion; induced G1 arrest and cell apoptosis in vitro and
suppressed tumor growth in vivo. These findings suggested
that miR-137 may be a potential therapeutic target for the
treatment of EOC.
Acknowledgments
The present study was supported provided by The
Science and Technology Research and Innovation team funded by Jilin
province (grant no. JL2012048).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heintz AP, Odicino F, Maisonneuve P, et
al: Carcinoma of the ovary. FIGO 26th annual report on the results
of treatment in gynecological cancer. Int J Gynaecol Obstet.
95(Suppl 1): 161–192. 2006. View Article : Google Scholar
|
|
4
|
Wang M, He Y, Shi L and Shi C:
Multivariate analysis by Cox proportional hazard model on prognosis
of patient with epithelial ovarian cancer. Eur J Gynaecol Oncol.
32:171–177. 2011.PubMed/NCBI
|
|
5
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV, Visone R, Di Leva G, et al:
MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iorio MV and Croce CM: MicroRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin J, Huang S, Wu S, et al: MicroRNA-423
promotes cell growth and regulates G (1)/S transition by targeting
p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis.
32:1641–1647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stevanato L and Sinden JD: The effects of
microRNAs on human neural stem cell differentiation in two- and
three-dimensional cultures. Stem Cell Res Ther. 5:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghosh G, Subramanian IV, Adhikari N, et
al: Hypoxia-induced microRNA-424 expression in human endothelial
cells regulates HIF-alpha isoforms and promotes angiogenesis. J
Clin Invest. 120:4141–4154. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schommer C, Palatnik JF, Aggarwal P, et
al: Control of jasmonate biosynthesis and senescence by miR319
targets. PLoS Biol. 6:e2302008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang W, Jiang Y, Mu X, Xu L, Cheng W and
Wang X: MiR-135a functions as a tumor suppressor in epithelial
ovarian cancer and regulates HOXA10 expression. Cell Signal.
26:1420–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharya R, Nicoloso M, Arvizo R, et
al: MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer.
Cancer Res. 69:9090–9095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong F, Li Y, Xu Y and Zhu L: Prognostic
significance of serum microRNA-221 expression in human epithelial
ovarian cancer. J Int Med Res. 41:64–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corney DC, Hwang CI, Matoso A, et al:
Frequent downregulation of miR-34 family in human ovarian cancers.
Clin Cancer Res. 16:1119–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Wang Q, Yu M, Wu N and Wang H:
MicroRNA-145 function as a cell growth repressor by directly
targeting c-Myc in human ovarian cancer. Technol Cancer Res Treat.
13:161–168. 2014.
|
|
23
|
Guo J, Xia B, Meng F and Lou G: miR-137
suppresses cell growth in ovarian cancer by targeting AEG-1.
Biochem Biophys Res Commun. 441:357–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, Ma L, Zhang Y, Ji F and Jin F:
MicroRNA-137 down-regulates KIT and inhibits small cell lung cancer
cell proliferation. Biomed Pharmacother. 68:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ando T, Yoshida T, Enomoto S, et al: DNA
methylation of microRNA genes in gastric mucosae of gastric cancer
patients: its possible involvement in the formation of epigenetic
field defect. Int J Cancer. 124:2367–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Zhao X, Zhou Y and Hu Y: miR-124,
miR-137 and miR-340 regulate colorectal cancer growth via
inhibition of the Warburg effect. Oncol Rep. 28:1346–1352.
2012.PubMed/NCBI
|
|
28
|
Chen L, Wang X, Wang H, et al: miR-137 is
frequently down-regulated in glioblastoma and is a negative
regulator of Cox-2. Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Li Y, Lou G, et al: MiR-137
targets estrogen-related receptor alpha and impairs the
proliferative and migratory capacity of breast cancer cells. PLoS
One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mehta SP, Jose P, Mirza A, Pritchard SA,
Hayden JD and Grabsch HI: Comparison of the prognostic value of the
6th and 7th editions of the Union for International Cancer Control
TNM staging system in patients with lower esophageal cancer
undergoing neoadjuvant chemotherapy followed by surgery. Dis
Esophagus. 26:182–188. 2013. View Article : Google Scholar
|
|
31
|
Wu B, Li S, Sheng L, et al: Metformin
inhibits the development and metastasis of ovarian cancer. Oncol
Rep. 28:903–908. 2012.PubMed/NCBI
|
|
32
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu LL, Lu SX, Li M, et al:
FoxD3-regulated microRNA-137 suppresses tumour growth and
metastasis in human hepatocellular carcinoma by targeting AKT2.
Oncotarget. 5:5113–5124. 2014.PubMed/NCBI
|
|
34
|
Bi Y, Han Y, Bi H, Gao F and Wang X:
miR-137 impairs the proliferative and migratory capacity of human
non-small cell lung cancer cells by targeting paxillin. Hum Cell.
27:95–102. 2014. View Article : Google Scholar
|
|
35
|
Hainaut P and Plymoth A: Targeting the
hallmarks of cancer: towards a rational approach to next-generation
cancer therapy. Curr Opin Oncol. 25:50–51. 2013. View Article : Google Scholar
|
|
36
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu X, Li Y, Shen H, et al: miR-137
inhibits the proliferation of lung cancer cells by targeting Cdc42
and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar
|
|
39
|
Bier A, Giladi N, Kronfeld N, et al:
MicroRNA-137 is down-regulated in glioblastoma and inhibits the
stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013.PubMed/NCBI
|
|
40
|
Deng Y, Deng H, Bi F, et al: MicroRNA-137
targets carboxyl-terminal binding protein 1 in melanoma cell lines.
Int J Biol Sci. 7:133–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Y, Li Y, Lou G, et al: MiR-137
targets estrogen-related receptor alpha and impairs the
proliferative and migratory capacity of breast cancer cells. PLoS
One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu M, Lang N, Qiu M, et al: miR-137
targets Cdc42 expression, induces cell cycle G1 arrest and inhibits
invasion in colorectal cancer cells. Int J Cancer. 128:1269–1279.
2011. View Article : Google Scholar
|
|
43
|
Luo C, Tetteh PW, Merz PR, et al: miR-137
inhibits the invasion of melanoma cells through downregulation of
multiple oncogenic target genes. J Invest Dermatol. 133:768–775.
2013. View Article : Google Scholar
|