Introduction
Cancer is a prominent public health problem
worldwide, which has increasing incidence and mortality rates
(1). Progress has been made in
improving cancer therapy, with surgical resection, chemotherapy and
radiotherapy being the three major conventional modes of cancer
treatment (2). However, effective
treatment remains to be achieved for numerous types of tumors
(2). Biological treatment is a
novel model in comprehensive cancer treatment, which has received
extensive attention (3,4). Adoptive cellular immunotherapy (ACI)
is an important form of biological tumor therapy, which involves
the infusion of autologous or allogeneic immune cells in order to
enhance immune function in patients and in turn achieve antitumor
effects (5).
Cascade primed immune (CAPRI) cells and
cytokine-induced killer (CIK) cells have been used as novel
adoptive immunotherapy cells and are known to have different
strengths and biological characteristics (6). These cells have been widely used in
previous clinical studies; however, there have been no systematic
comparative evaluations of the two treatments (7,8).
Therefore, the present study aimed to compare the antitumor effects
of CAPRI and CIK cells in vitro, through investigating cell
morphology, proliferation, cytotoxic activity to tumor cells and
the ability of these cells to secrete cytokines. These methods of
comparison may be extended for the future detection of a variety of
cell lines and cytokines in order to better guide clinical
treatment.
Materials and methods
Materials and reagents
K562 human leukemia cells and MCF-7 human breast
cancer cells were purchased from the cell library of Cancer
Institute of Chinese Medical Sciences Academy (Beijing, China).
K562 and MCF-7 cells were cultured in RPMI 1640 medium (Beijing
Suolaibao Science and Technology Co., Ltd., Beijing, China) with
10% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT,
USA) at 37°C in 5% CO2. Low molecular weight heparin
injections were purchased from Qilu Pharmaceutical Co., Ltd (Jinan,
China). The substrate mix (BCIP/NBT Color Development Substrate)
was purchased from Promega Corp. (Madison, WI, USA). 96-well plates
and cell culture flasks were obtained from Beijing Aibo Biological
Engineering Co., Ltd. Recombinant human (rHu) interferon (INF)-γ
and rHu interleukin (IL)-2 were purchased from Peprotech Co. (Rocky
Hill, NJ, USA). Mouse monoclonal anti-human CD3 antibodies were
provided by Beijing Tonglihaiyuan Biotechnology Co., Ltd. (Beijing,
China). CAPRI cell culture patented reagents were obtained from the
Institute of Immunology, University of Munich (Munich, Germany).
Lymphocyte stratified fluid was purchased from Tianjin Haoyang
Biological Products Co., Ltd (Tianjin, China). IFN-γ and IL-2
ELISPOT kits were purchased from Shenzhen Dakewei Biotechnology
Co., Ltd. (Guangdong, China).
Instruments
Clean benches, portable autoclaves and the constant
temperature water bath were purchased from Shanghai Boxun Industry
and Commerce Co., Ltd. (Shanghai, China). The CO2
incubator was obtained from Sanyo Electric International Trading
Co., Ltd. (Shanghai, China) and the micropipettes were purchased
from Thermo Fisher Scientific Co., Ltd. (Shanghai, China). The
low-speed centrifuge (B40) was obtained from Hebei Anxin Baiyang
Centrifugal Machinery Factory (Hebei, China) and enzyme-linked
immunospot (ELISPOT; S5) analyzers were provided by Cellular
Technology, Ltd (Shaker Heights, OH, USA). The inverted microscope
(BDS200) was obtained from Chongqing Auto Optical Instruments Co.,
Ltd. (Chongqing, China) and the ELISA analyzer (RT2100c) was
obtained from Rayto Life and Analytical Science Co. (Shenzhen,
China).
Culture of CAPRI cells and CIK cells
The current study was approved by the Ethics
Committee of the Affiliated Hospital of Weifang Medical University
(Weifang, China) and written informed consent was obtained from the
patient/the patient’s families. A total of 50 ml blood was
extracted from three healthy volunteers and heparin (1 ml; 6250
U/ml) was added to prevent coagulation. The three specimens were
then stratified with lymphocyte fluid. Ficoll-Conray density
gradient centrifugation (50–60 ml peripheral blood, density
1.077±0.001 g/ml) was performed to obtain three separate
suspensions of peripheral blood mononuclear cells (PBMCs).
Following washing with 0.9% NaCl, RPMI 1640 medium was used to
adjust the PBMC suspension to 1×106cells/ml. Samples
were labeled (a, b and c) and the PBMC suspensions from each source
were divided into two parts as follows: a1, b1 and c1 for CAPRI
cell induction; and a2, b2 and c2 for CIK cell induction.
CAPRI cells induction
A total of 12 ml coating solution (Shanghai Weike
Co., Ltd., Shanghai, China) was added to three flasks, which were
then incubated at 4°C for 24 h. The coating solution was removed
and 20 ml saline (Shandong Qidu Pharmaceutical Co., Ltd., Shandong,
China) was added to each flask, which were then mixed uniformly and
left to stand for 2 min prior to saline removal. A total of 18 ml
complete medium (RPMI 1640 and 10% FBS) was added to each flask.
PBMC suspensions (12 ml; 1×106 cells/ml) of a1, b1 and
c1 were added to the flasks and incubated at 37°C in a 5%
CO2 incubator for 3 h. CAPRI patent reagent A (0.4 ml)
was added to each culture flask for 3 h at 25°C and the stimulated
culture substances were subsequently obtained.
Lymphocyte stratified fluid (12 ml) was added to
each stimulated culture medium and then cultured at 37°C in a 5%
CO2 incubator for 16 h. The culture substances were
collected in 50 ml centrifuge tubes and centrifuged at 750 × g for
8 min. The supernatants were discarded and RPMI 1640 was used to
resuspend cells. Cells were then counted using a cell counting
plate under the inverted microscope and were subjected to further
centrifugation (800 × g for 12 min).
Complete medium was used to resuspend the cells and
CAPRI patent reagent B (0.4 ml) was added to each cell suspension,
which were then dispensed into flasks and cultured at 37°C in a 5%
CO2 incubator for 72 h. CAPRI cells were then collected
and used for subsequent experiments. One part were further cultured
(3×106 cells/ml; −80°C) to study the cells proliferative
capacity, the other groups (3×106 cells/ml) were frozen
(−80°C) till 14 days and the cytotoxic activity and cytokine
secretion levels of them were used to perform a comparative study
with CIK cells.
CIK cells induction
The three PBMC suspensions (a2, b2 and c2; 12 ml)
were transferred into sterile culture flasks and 18 ml complete
medium containing 10% FBS was then added. Samples were then
incubated at 37°C in a 5% CO2 incubator for 2 h of
static culture. IFN-γ (1,000 U/ml) was added and continuously
cultured for 24 h, 37°C, 5% CO2. IL-2 cytokines (300
U/ml) were then added with 50 ng/ml CD3 monoclonal antibody. Cells
passaged every 3 days and a further 300 U/ml IL-2 was added with
each passage. Samples were cultured at 37°C in a 5% CO2
incubator for 14 days to collect CIK cells. These cells were
subsequently used for determining proliferation, cytotoxic activity
and cytokine secretion.
Proliferation assay of CAPRI cells and
CIK cells
An inverted microscope was used to dynamically
observe the proliferation of three PBMC suspensions (a, b and c),
which were induced using the methods described above. On days 1, 3,
5 and 14 of culture, cells were stained using Trypan blue (Yocon
Bio-Technology Co., Ltd., Beijing, China) and counted in order to
determine proliferation and morphology. Experiments were performed
in triplicate.
Cytotoxic activity detection of effector
cells using a lactate dehydrogenase (LDH) release assay
Cell culture groups
CIK cells cultured for 14 days (a, b and c) and the
thawed CAPRI cells (a, b and c) were the effector cells. K562
leukemia cells and MCF-7 breast cancer cells were used as the
target cells. All experiments were performed in triplicate and
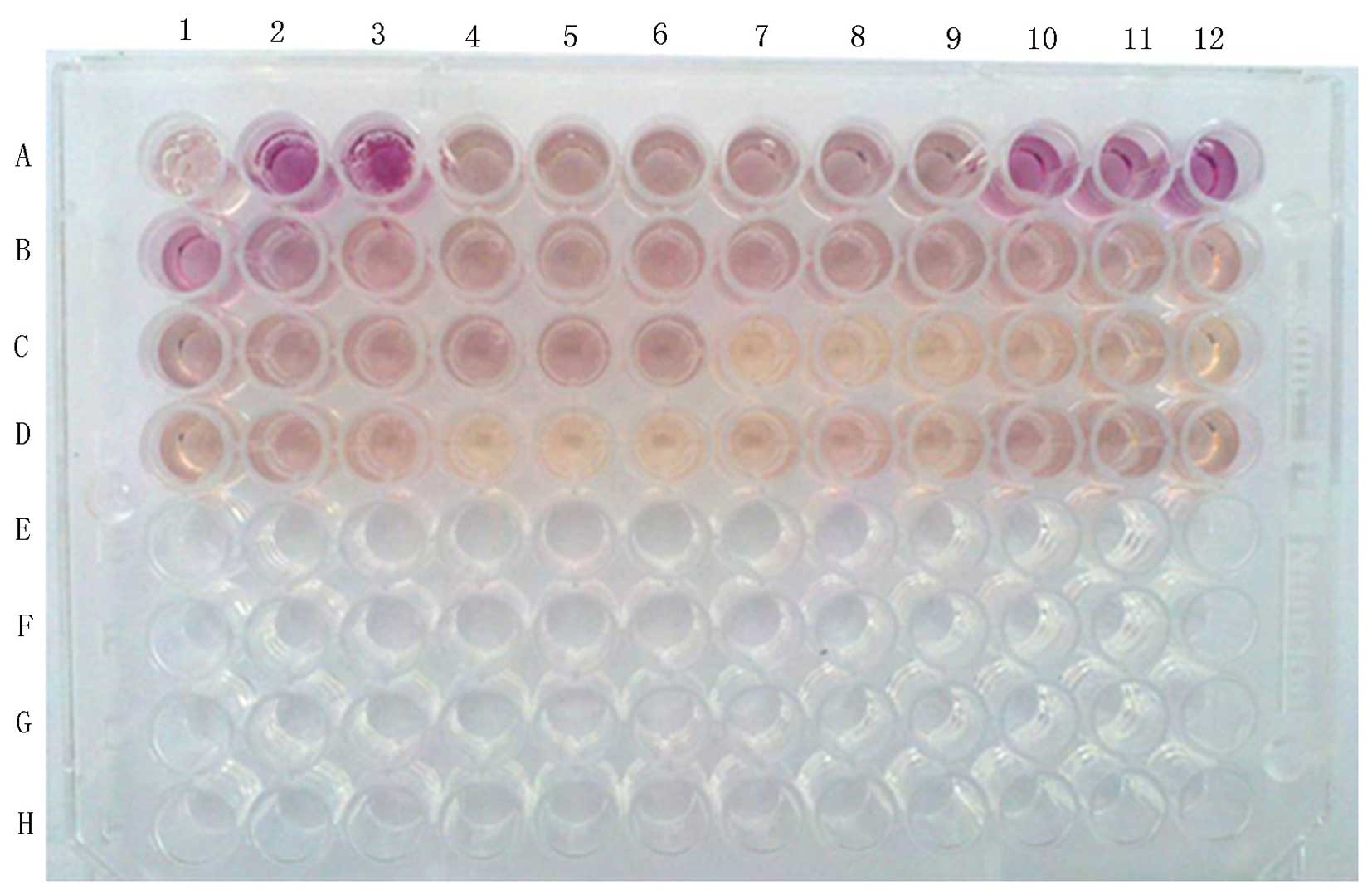
plating of the treatment groups is shown in Fig. 1. The following procedure was
performed separately for each of the two target cell lines.
Three different effector-target ratios of 40:1,
20:1, 10:1 were used; effector cells (100 µl) concentrations
of 4×106, 2×106 and 1×106/ml were
added to each well with 100 µl target cells at a
concentration of 1×105/ml. CIK cells, B1-B9; and CAPRI
cells, C7-C12 and D1-D3.
Effector cells (100 µl/well) were plated at
concentrations of 4×106, 2×106 and
1×106/ml for each group (a, b and c) with 100 µl
culture medium in order to detect spontaneous cytotoxic activity.
CIK cells, B10–B12 and C1–C6; and CAPRI cells, D3–D12.
In order to determine maximum cytotoxic activity,
target cells (100 µl/well) were plated at a concentration of
1×105/ml into three wells (A1–A3) with 100 µl
culture medium. These cells were incubated at 37°C in a 5%
CO2 culture incubator, at 45 min prior to the end of
incubation, 20 µl/well cell lysate was added. In addition,
to determine the spontaneous cytotoxicity of target cells,
1×105/ml target cells (100 µl/well) were plated
into three wells (A4–A6) with 100 µl culture medium.
Blank control wells (A7–A9) consisted of 200
µl/well culture medium; in addition, the corrected volume
control wells consisted of 200 µl/well culture medium
(A10–A12), which were then incubated 20 µl/well cell lysate,
which was added at 45 min prior to the end of culture at 37°C in a
5% CO2 incubator.
Culture and supernatant
collection
Following centrifugation at 250 × g for 4 min, cells
were cultured at 37°C in a 5% CO2 incubator for 4–6 h.
At 45 min prior to the end of culture, the culture plates were
removed and 20 µl cell lysate was added to wells in the
target cells maximum cytotoxicity group and the corrected volume
control group. Cells in these two groups were then centrifuged at
250 × g for 4 min and further cultured for 45 min.
LDH measurement
Each group was centrifuged at 250 × g for 4 min and
50 µl supernatant per well was transferred into a separate
96-well plate with 50 µl/well substrate mix and incubated
for 30 min at room temperature in the dark. Stop solution (50
µl/well; Yanhui Bio-Technology Co., Ltd., Shanghai, China)
was then added and pigmented particles were broken up using an
oscillator (G560E; Scientific Industries, New York, NY, USA).
Absorbance was measured at 490 nm using an ELISA analyzer.
Results and calculation
Mean absorbance values were calculated for each
group. In order to obtain the corrected values for A (test), T
(target cells spontaneous release) and E (effector cells
spontaneous release), the mean absorbance value for the blank
control group was subtracted from the mean absorbance values for
the test, target cells spontaneous release and effector cells
spontaneous release groups. The mean absorbance values in target
cell maximum release group minus the mean absorbance values in
corrected volume group, and the corrected Tmax was obtained.
Cytotoxic activity (%)=(A−E−T)/(Tmax−T)×100%.
ELISPOT detection of IFN-γ and IL-2
secretion
CAPRI cells (a1, b1 and c1) and CIK cells (a2, b2
and c2) were transferred and the stimulating substance was added,
under aseptic conditions.
ELISPOT plates were precoated with 200
µl/well RPMI 1640 medium and incubated at room temperature
for 10 min. CAPRI or CIK cells at concentrations of
1×106/ml and 5×105/ml were then plated (100
µl/well). The positive and negative control groups for each
cell type were plated as follows: Negative control,
1×106 and 5×105 cells/ml with 10
µl/well RPMI 1640 medium with 10% FBS; positive control,
1×106 and 5×105 cells/ml with
phytohemagglutinin cell stimulator working fluid. The well plate
was then covered and incubated at 37°C in a 5% CO2
incubator for 20 h. Following incubation, the cells and medium were
removed and added to ice-cold deionized water (200 µl/well).
Hypotonic cell lysis was performed with Hypotonic Lysis Buffer
(Amresco LLC, Solon, OH, USA) at 4°C for 10 min. Cells were then
washed five times for 60 sec each with 1X washing buffer (200
µl/well; Shenzhen Dakewei Biotechnology Co., Ltd.) and then
dried on absorbent paper.
A biotin-labeled diluted antibody solution (100
µl; in ELISPOT kit) was added to each experimental well and
incubated at 37°C for 1 h. Cells were then washed, as above, and
dried on absorbent paper. Horseradish peroxidase-avidin diluted
antibody working solution (100 µl) was added to each
experimental well and incubated at 37°C for 1 h. Cells were then
washed, as above, and dried on absorbent paper.
Each experimental well was stained with
3-amino-9-ethylcarbazole working solution (100 µl/well;
Shenzhen Dakewei Biotechnology Co., Ltd.) and incubated at room
temperature in the dark for 25 min. In order to terminate staining,
liquid was poured out in each well, the base plate was opened and
deionized water (Xinyu, Shanghai, China) was used to wash the wells
five times. The plates were then placed at room temperature in the
dark, with the base closed, until they dried.
Spot count was then performed in the ELISPOT plates
and various parameters of spots were recorded in order to determine
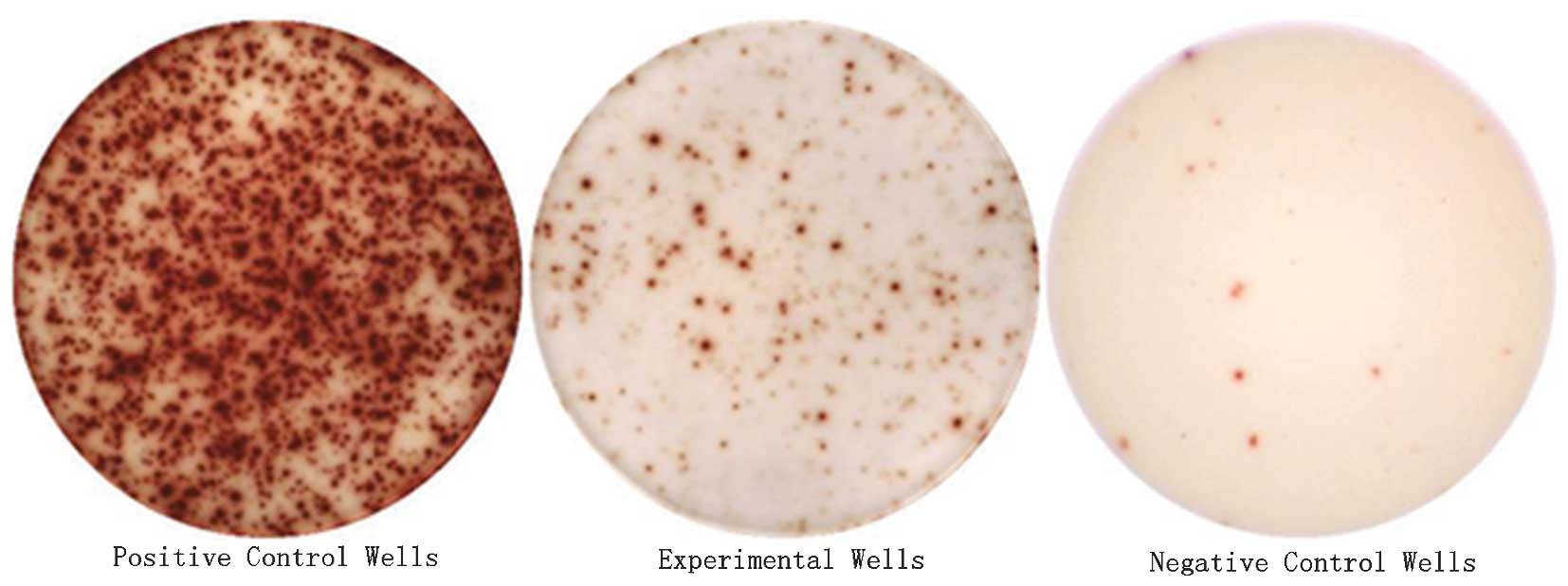
IFN-γ and IL-2 secretion levels. Brown spots indicated that the
cells had produced cytokines.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data analysis. Values are expressed as the mean ±
standard deviation. Independent samples t-tests were used for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Morphology and cell proliferation
activity of CAPRI cells and CIK cells
Cultured CAPRI cells began to proliferate within 2
days and entered the proliferation stage within 3 days. Following
14 days, cultured cells reached a density of
(6.32±1.23)×107. Cultured CIK cells began to proliferate
within 3 days and entered the proliferation stage within 5 days.
Following 14 days, cultured cells reached a density of
(60.21±6.08)×107. Following 3 days of culture, the
proliferation rate of CIK cells was significantly faster compared
with CAPRI cells; of note, at day 5, the proliferation rate of CIK
cells was ~3 times that of CAPRI cells (P<0.001); at day 14, CIK
cells had proliferated ~60 times compared with day 1, which was ~10
times that of CAPRI cells at day 14 (P<0.001) (Table I).
 | Table IProliferation comparison of CAPRI
cells and CIK cells. |
Table I
Proliferation comparison of CAPRI
cells and CIK cells.
| Day of culture | CAPRI cells
(×107) | CIK cells
(×107) | t-value | P-value |
|---|
| 1 | 1.21±0.21 | 1.16±0.20 | 0.447 | 0.661 |
| 3 | 2.33±0.56 | 3.19±1.14 | 2.033 | 0.059 |
| 5 | 3.61±1.16a | 10.39±4.24 | 4.629 | <0.001 |
| 14 | 6.32±1.23a | 60.21±6.08 | 26.057 | <0.001 |
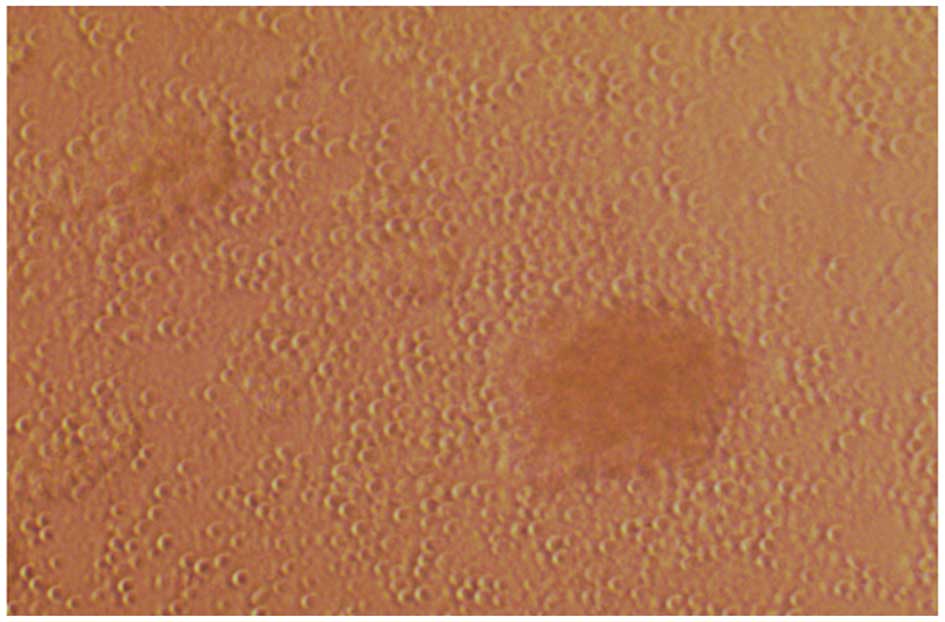
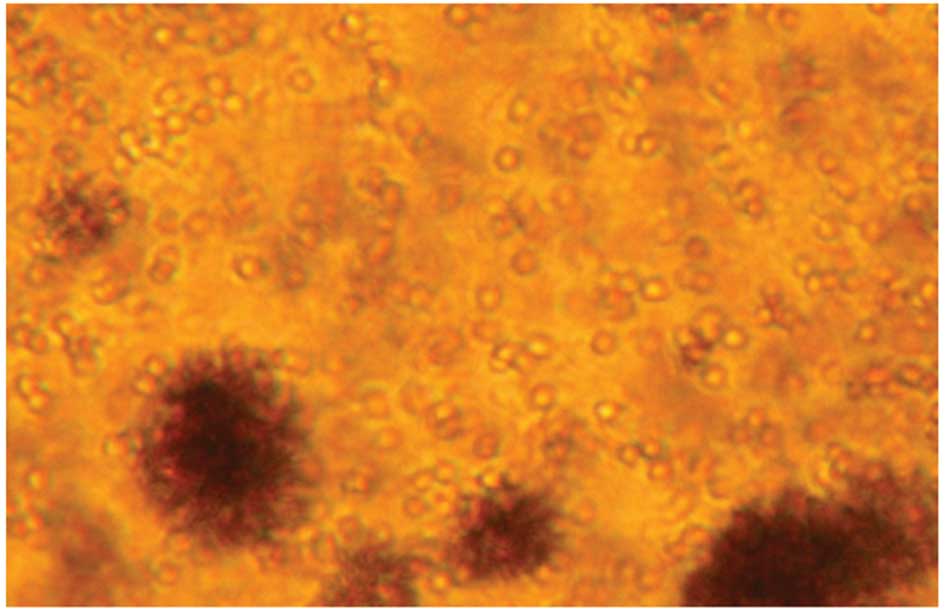
Cells were harvested and examined under an inverted
microscope, which revealed that cells grew as colonies in
suspension (Figs. 2 and 3). Cell volume was markedly increased
over time and the survival rate of cultured cells was >95%. Of
note, CAPRI cell patent reagents instructions described that the
cultured cells should be harvested for clinical treatment on day 5
(6); however, since the present
study was a comparative to CIK cells, the experimental culture was
performed for 14 days.
Cytotoxic activity of CAPRI and CIK cells
against K562 leukemia and MCF-7 breast cancer cells
The results of the cytotoxic activity of CAPRI and
CIK cells against K562 cells are shown in Table II. These results demonstrated that
the two cell types exhibited cytotoxicity on K562 cells. The
cytotoxic activity of CAPRI cells were 55.1±3.25 and 35.0±2.65% at
effector-target ratios of 40:1 and 20:1, respectively, which were
significantly reduced compared with the corresponding values of the
CIK cells, 60.0±3.03 and 39.7±3.42% (P=0.004 and 0.005,
respectively). No significant difference was observed in cytotoxic
activity at an effector-target ratio of 10:1 between the two groups
(P=0.056).
 | Table IICytotoxic activity of CAPRI cells and
CIK cells against K562 leukemia cells. |
Table II
Cytotoxic activity of CAPRI cells and
CIK cells against K562 leukemia cells.
| Effector-target
ratio | CAPRI cells
(%) | CIK cells (%) | t-value | P-value |
|---|
| 40:1 | 55.1±3.25a | 60.0±3.03 | 3.310 | 0.004 |
| 20:1 | 35.0±2.65a | 39.7±3.42 | 3.265 | 0.005 |
| 10:1 | 28.5±2.36 | 30.6±1.72 | 2.062 | 0.056 |
The results of the cytotoxic activity of CAPRI and
CIK cells against MCF-7 cells are shown in Table III. These results revealed that
the two cell types exerted cytotoxic effects on MCF-7 cells.
Cytotoxic activity of CAPRI cells were 71.5±3.06, 56.0±3.76 and
40.2±2.90% at effector-target ratios of 40:1, 20:1 and 10:1,
respectively, which were significantly increased compared with the
corresponding values of CIK cells, 65.4±3.86, 49.5±3.91 and
36.1±3.73% (P=0.002, 0.003 and 0.02, respectively).
 | Table IIICytotoxic activity of CAPRI cells and
CIK cells against MCF-7 breast cancer cells. |
Table III
Cytotoxic activity of CAPRI cells and
CIK cells against MCF-7 breast cancer cells.
| Effector-target
ratio | CAPRI cells
(%) | CIK cells (%) | t-value | P-value |
|---|
| 40:1 | 71.5±3.06a | 65.4±3.86 | 3.729 | 0.002 |
| 20:1 | 56.0±3.76a | 49.5±3.91 | 3.563 | 0.003 |
| 10:1 | 40.2±2.90a | 36.1±3.73 | 2.582 | 0.020 |
IFN-γ and IL-2 secretion levels of CAPRI
and CIK cells
As shown in Fig. 4,
each spot-forming cell (SFC) represented a measured cytokine
secretion of CAPRI or CIK cells. ELISPOT analysis results are
quantified in Tables IV and
V. CAPRI and CIK cells each
produced high levels of IFN-γ and IL-2 secretion. ELISPOT detection
was performed at two different cell concentrations
(1×106 and 5×105 cells/ml). In CAPRI cells,
the number of IFN-γ SFCs detected were 126.2±10.31 and 48.8±10.99,
respectively, which were significantly lower compared with the
corresponding values in CIK cells, 409.3±7.76 and 159.3±15.45
(P<0.001) (Table IV). The
number of IL-2 SFCs in CAPRI cells were 325.1±16.24 and 113.8±11.29
at concentrations of 1×106 and 5×105
cells/ml, respectively, which were increase compared with the
corresponding values in CIK cells, 212.0±16.58 and 70.7±10.57
(P<0.001) (Table V).
 | Table IVNumber of IFN-γ spot-forming cells at
different cell densities of CAPRI cells and CIK cells. |
Table IV
Number of IFN-γ spot-forming cells at
different cell densities of CAPRI cells and CIK cells.
| Cell density | CAPRI cells | CIK cells | t-value | P-value |
|---|
|
1×106/ml | 126.2±10.31a | 409.3±7.76 | 65.833 | <0.001 |
|
5×105/ml | 48.8±10.99a | 159.3±15.45 | 17.494 | <0.001 |
 | Table VNumber of IL-2 spot-forming cells at
different cell densities of CAPRI cells and CIK cells. |
Table V
Number of IL-2 spot-forming cells at
different cell densities of CAPRI cells and CIK cells.
| Cell density | CAPRI cells | CIK cells | t-value | P-value |
|---|
|
1×106/ml | 325.1±16.24a | 212.0±16.58 | 14.621 | <0.001 |
|
5×105/ml | 113.8±11.29a | 70.7±10.57 | 8.362 | <0.001 |
Discussion
Developments in the fields of molecular tumor
biology, cell biology and immunology of cancer have resulted in a
more comprehensive and in-depth understanding of tumors (9). Adoptive immunotherapy of cancer cells
is a novel model of comprehensive treatment, which has an
increasingly important role in cancer therapy (10). However, issues for the clinical use
of this model require addressing, including differing effects of
CAPRI and CIK cell therapies in different cell types, the balance
between the use of adoptive immunotherapy and conventional
treatments of surgery, chemotherapy and radiotherapy to achieve the
highest combination efficacy as well as the method of activating
the suppression status of the body’s immune system (11). CAPRI and CIK cell therapies are
prominent adoptive cell immunotherapies, which are currently widely
used in clinical practice (12).
CAPRI cells are chain autologous activated immune
cells, the effector cells of which include natural killer (NK)
cells, NK-like T cell lymphocytes (NKT cells), dendritic cells,
CD4+ T helper (Th) cells and CD8+ cytotoxic T
lymphocytes (CTLs) (6). Th cells
and CTLs accounted for 80% of the major cytotoxic activity of CAPRI
cells and the remaining effector cells accounted for 20% (6). CIK cells simultaneously express two
types of membrane protein molecules, CD3+ and
CD56+, and are also known as NKT cells; these cells were
reported to have the oncolytic advantage of T lymphocytes as well
as the non-major histocompatibility complex (MHC)-restricted
antitumor activity of NK cells (13). The potent antitumor activities of
CIK cells primarily proceed via the following four mechanisms:
Direct cytotoxic effects on tumor cells (14); induced proliferation of T cells to
CTL (15); induced apoptosis of
tumor cells (16); and production
of cytokines with oncolytic effects (17).
The antitumor mechanisms of CIK cells have been
established; however, CAPRI cells are a novel method of treatment,
for which the antitumor mechanisms are relatively undetermined
(18). It was speculated that
CAPRI cells were T lymphocytes, with comparable tumor cell-killing
mechanisms to CIK cells. In the present study, a classic experiment
was performed in order to compare the cytotoxic activity of CAPRI
and CIK cells against K562 leukemia cells and MCF-7 breast cancer
solid tumor cells. Previous studies have demonstrated that IFN-γ
and IL-2 may be used alone in clinical treatment and as an inducer
of cell therapy (19,20). Therefore, in the present study, the
applications of these two cytokines were selected for ELISPOT
technique detection in order to determine the indirect antitumor
effect of CIK and CAPRI cells.
CAPRI cells were reported to require synergy of
human leukocyte antigen (HLA)-I and HLA-II expression for
successful tumor cell lyses (21).
This demonstrated the complete interdependence of Th cells and
CTLs. Generation of CTLs was demonstrated to be dependent on the
interactions between α-β T cell receptors, peptide-MHCs and
antigen-presenting cell surface molecules of HLA-II tumor
immunogenic peptides (22).
However, the complete dissolve of tumor cells may be dependent on
the interaction of HLA-I- and HLA-II-type antigens (22). CAPRI cells were reported to enhance
HLA-I and HLA-II expression on the surface of solid tumors;
however, K562 cells were not induced to express leukocyte antigen
and K562 cell lysis was primarily mediated by activated NKT cells
in PBMC culture (23,24). The results of the present study
demonstrated that CAPRI cells exerted potent and specific cytotoxic
effects on breast cancer cells of solid tumors, whereas CIK cells
had strong non-specific cytotoxic activity. These results may have
practical significance for the clinical use of these two cell types
for adoptive immunotherapy.
In the present study, ELISPOT analysis revealed that
IFN-γ secretion levels of CIK cells were higher than those of CAPRI
cells, whereas IL-2 secretion levels of CAPRI cells were higher
than those of CIK cells. However, the secretion levels for IFN-γ
and IL-2 were relatively high in the two cell types. CAPRI and CIK
cells have different types of effector cells; therefore, their
antitumor mechanisms may also differ. IL-2 is primarily produced by
CD4+ and CD8+ T cells, which make up 80% of
the CAPRI effector cells, while IFN-γ is predominantly produced by
CD8+ T cells and NK cells, of which NK cells are the
major effector cells of CIK cells (25). The cytotoxic mechanisms of the two
cell types involve the tumor suppressing and cytotoxic effects of
inhibitory cytokines, which are secreted by effector cells of CAPRI
and CIK cells (26). The present
study demonstrated that CAPRI and CIK exerted immunomodulatory
effects via the secretion of IFN-γ and IL-2, which was an important
aspect of the cytotoxic activity evaluation. The detection
techniques used in the present study may be applied to clinical
work, as patients may receive cytokines detection tests prior to
and following adoptive cell immunotherapy in order to evaluate
therapeutic effectiveness.
The key principle of tumor cell adoptive
immunotherapy is to have sufficient quantities of immune cells with
strong cytotoxic activity (27).
These technical cycles are short (5 days) and have a high
specificity; i addition, cells can be cryopreserved following
harvesting, which provides convenience for clinical use.
The results of the current study demonstrated that
the cell density of CAPRI and CIK cells was
(6.32±1.23)×107 and (60.21±6.08)×107,
respectively, following treatment for 14 days. The cell
proliferative activity of CAPRI cells was significantly lower than
that of CIK cells (P<0.001). Compared with the 1st day, the
proliferative rate of CAPRI was 3 times higher at the 5th and 6
times higher at the 14th days, while CIK was 10 times higher at the
5th and 60 times higher at the 14th days.
In conclusion, the experimental results of the
present study demonstrated that CAPRI cells have a weaker cytotoxic
effect on NK target cells of K562 leukemia cells compared with
their potent cytotoxic effect on the NK-insensitive solid tumors
MCF-7 cells. These experimental methods may be used as a model to
further explore the cytotoxic activity of tumor cells in other
entities and to provide guidance for future clinical studies and
treatment options. The cytotoxic activity of CIK cells is not MHC
restricted, however CAPRI cells filter MHC of cells, which remedies
the deficiency of CIK cells treatment. The ELISPOT technique may be
used to detect the secretion of cytokines for these two cell types
prior to and following treatment for clinical efficacy assessment.
Since the culture time and antitumor mechanisms between CAPRI and
CIK cells differed, this may offer the possibility for combination
of the two cell types for antitumor therapy; however, further
studies and clinical trials are required in order to confirm the
effectiveness of this combined therapy.
Acknowledgments
The present study was supported by the construction
funds from Oncology Medical Key Laboratory of Shandong
Province.
References
|
1
|
Dong Z, Qiao Y, Li L, et al: Report of
Chinese cancer control strategy. Bull Chin Cancer. 11:250–260.
2002.
|
|
2
|
Tang ZY: Modern Oncology. 2nd. Shanghai
Medical University Press; Shanghai: pp. 513–516. 2000
|
|
3
|
Han BH: Tumor Biological Immune Targeted
Therapy. 1st. Shanghai Science and Technology Publishing House;
Shanghai: pp. 47–52. 2006
|
|
4
|
Hao: Solid Tumor Cellular Immunotherapy.
Beijing: People’s Medical Publishing House; Beijing: pp. 3–4.
2010
|
|
5
|
Sha W: Clinical Tumor Biological
Immunotherapy. 1st. Tianjin Science and Technology Press; Tianjin:
pp. 142–144. 2006
|
|
6
|
Laumbacher B, Gu S and Wank R: Activated
monocytes prime naïve T cells against autologous cancer: vigorous
cancer destruction in vitro and in vivo. Scand J Immunol.
75:314–328. 2012. View Article : Google Scholar
|
|
7
|
Ren H, Xing S, Li D, et al: The
proliferation profile in vitro and anti-tumor effects of CIK cells
in vivo and in vitro. Chin J Cancer Biother. 6:17–21. 1999.
|
|
8
|
Morse MA, Glay TM and Lyerly HK: Current
status of adoptive immunotherapy of malignancies. Expert Opin Biol
Ther. 2:237–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tai G: Tumor immunotherapy: Progresses and
trends. Chin J Cancer Biother. 16:427–430. 2009.
|
|
10
|
Anne C, Eaton Armstrong David, Ewing
Joanne C, et al: Cellular immunotherapy for cancer. BMJ Chinese
Edition. 3:331–335. 2003.
|
|
11
|
Yang JJ, Xu XX and Qian G: Research
progress on tumor biochemotherapy. Int J Respir. 30:1137–1141.
2010.
|
|
12
|
Qian QJ and Wu MC: Adoptive cell therapy
of cancer-An old story with a new twist. Chin J Cancer Biother.
18:1–6. 2011.
|
|
13
|
Pan CC, Huang ZL, Li W, Zhao M, Zhou QM,
Xia JC and Wu PH: Serum alpha-fetoprotein measurement in predicting
clinical outcome related to autologous cytokine-induced killer
cells in patients with hepatocellular carcinoma undergone minimally
invasive therapy. Chin J Cancer. 29:596–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Yu JP, Cao S, Wei F, Zhang P, An XM,
Huang ZT and Ren XB: CD4+ CD25+ regulatory T cells decreased the
antitumor activity of cytokine-induced killer (CIK) cells of lung
cancer patients. J Clin Immunol. 27:317–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mehta BA, Schmidt-Wolf IG, Weissman IL and
Negrin RS: Two pathways of exocytosis of cytoplasmic granule
contents and target cell killing by cytokine-induced CD3+ CD56+
killer cells. Blood. 86:3493–3499. 1995.PubMed/NCBI
|
|
16
|
Ren H, Xing SX, Xu GW, et al: In vitro
proliferation of CIK and kill tumor in vivo and in vitro activity
of experimental study. Chinese journal of tumor biological
treatment. 6:17–21. 1999.
|
|
17
|
Linn YC, Wang SM and Hui KM: Comparative
gene expression profiling of cytokine-induced killer cells in
response to acute myloid leukemic and acute lymphoblastic leukemic
stimulators using oligonucleotide arrays. Exp Hematol. 33:671–681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui CG, Meng F, Wang XH, et al:
Preparation and cytotokic effects of cytokine-induced killer cells
on different tumor cell lines in vitro. J Chin Med Univ.
34:210–211. 2005.
|
|
19
|
Shirai A, Homes K and Klinman D: Detection
and quantitation of cells secreting IL-6 under physiologic
conditions in BALB/c mice. J Immunol. 150:793–799. 1993.PubMed/NCBI
|
|
20
|
Yang J, Lemas VM, Flinn IW, Krone C and
Ambinder RF: Application of the ELISPOT assay to the
characterization of CD8(+) responses to Epstein-Barr virus
antigens. Blood. 95:241–248. 2000.
|
|
21
|
Garrido F and Ruiz-Cabello F: MHC
expression on human tumors - its relevance for local tumor growth
and metastasis. Semin Cancer Biol. 2:3–10. 1991.PubMed/NCBI
|
|
22
|
van der Merwe PA and Davis SJ: Molecular
interactions mediating T cell antigen recognition. Annu Rev
Immunol. 21:659–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verneris MR, Kornacker M, Mailänder V and
Negrin RS: Resistance of ex vivo expanded CD3+CD56+ T cells to
Fas-mediated apoptosis. Cancer Immunol Immunothr. 49:335–345. 2000.
View Article : Google Scholar
|
|
24
|
Schmidt-Wolf IG, Finke S, Trojaneck B, et
al: Phase I clinical study applying autologous immunological
effector cells transfected with the interleukin-2 gene in patients
with metastatic renal cancer, colorectal cancer and lymphoma. Br J
Cancer. 81:1009–1016. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang HN, Qi YC and Wang YD: Character and
advancement of cytokine-induced kill cells. Tum J World. 4:240–242.
2005.
|
|
26
|
Lei J, Qian M, Wu ZL, Zhang SQ and Yan YC:
Study on cytokine expression profiles of CIK cells in vitro. Chin J
Immunol. 21:589–593. 2005.
|
|
27
|
Laport GG, Sheehan K, Baker J, et al:
Adoptive immunotherapy with cytokine-induced killer cells for
patients with relapsed hematologic malignancies after allogeneic
hematopoietic cell transplantation. Biol Blood Marrow Transplant.
17:1679–1687. 2011. View Article : Google Scholar : PubMed/NCBI
|