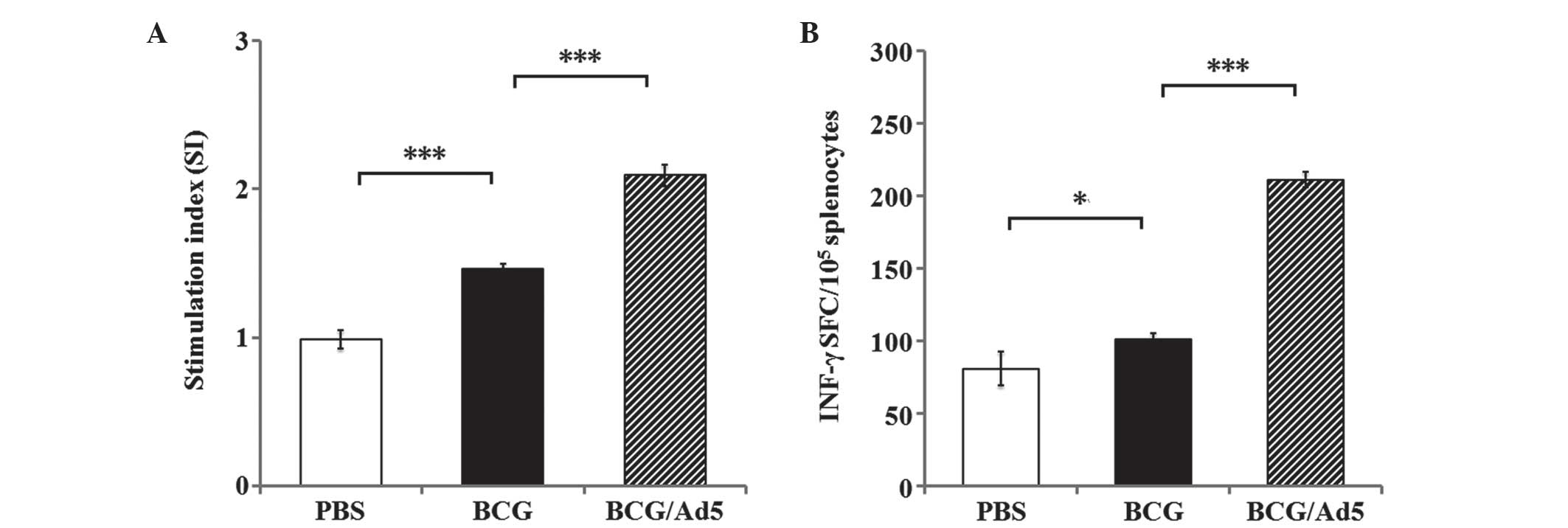
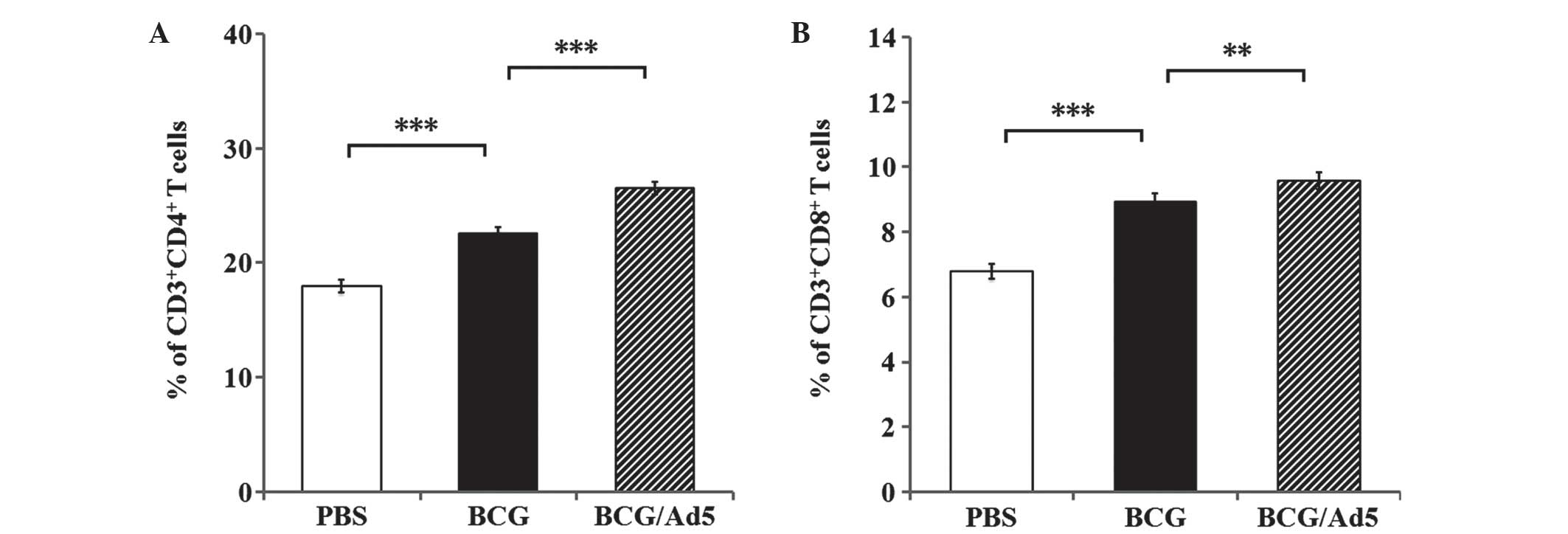
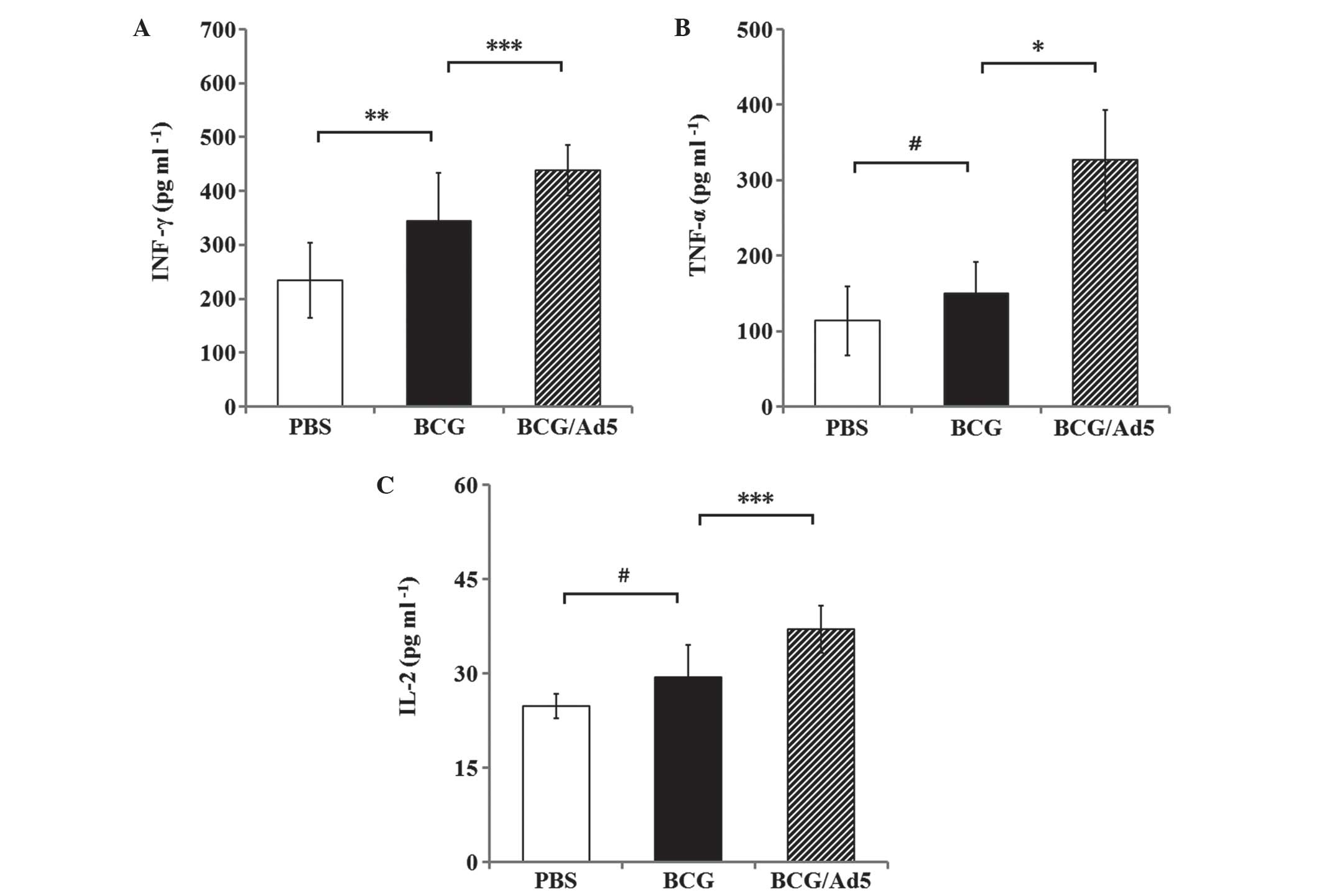
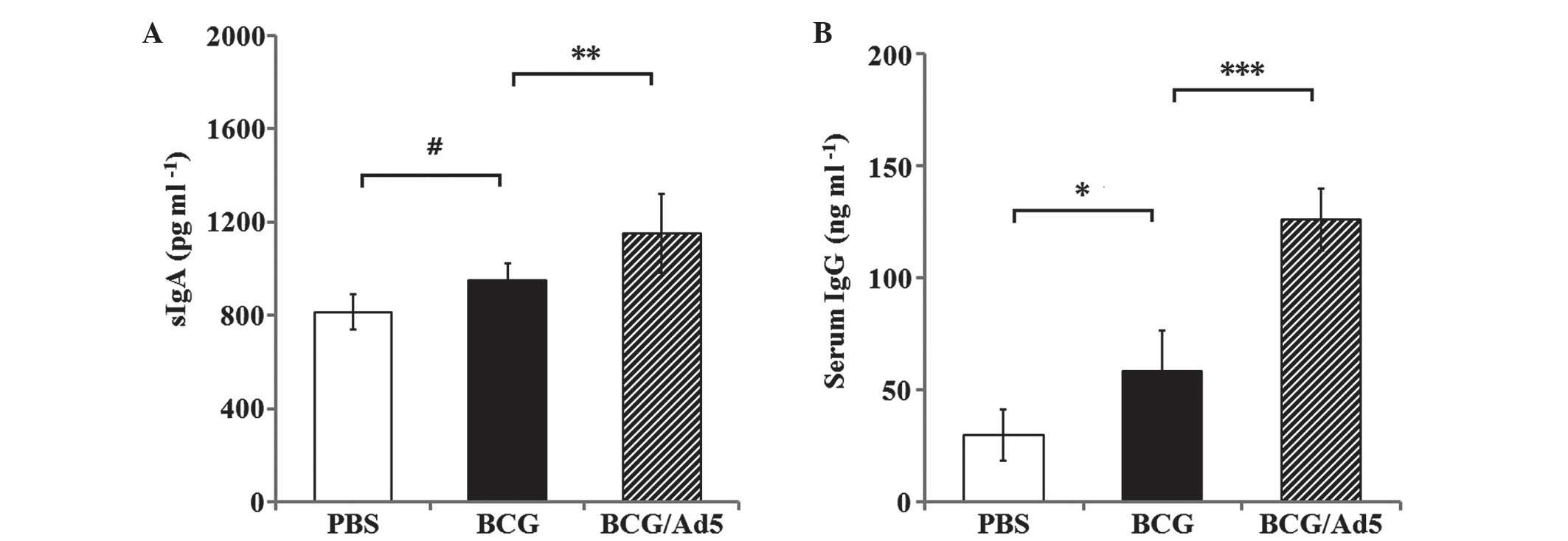
|
1
|
World Health Organization (WHO): Global
Tuberculosis Report 2012. WHO; Geneva, Switzerland: pp. 3062012
|
|
2
|
Churchyard GJ, Chaisson RE, Maartens G and
Getahun H: Tuberculosis preventive therapy: An underutilised
strategy to reduce individual risk of TB and contribute to TB
control. S Afr Med J. 104:339–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muwonge A, Malama S, Johansen TB, et al:
Molecular epidemiology, drug susceptibility and economic aspects of
tuberculosis in Mubende district, Uganda. PLoS One. 8:e647452013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awasthi S and Moin S: Effectiveness of BCG
vaccination against tuberculous meningitis. Indian Pediatr.
36:455–460. 1999.
|
|
5
|
Nuttall JJ, Davies MA, Hussey GD and Eley
BS: Bacillus Calmette-Guérin (BCG) vaccine-induced complications in
children treated with highly active antiretroviral therapy. Int J
Infect Dis. 12:e99–e105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bolger T, O’Connell M, Menon A and Butler
K: Complications associated with the bacille Calmette-Guérin
vaccination in Ireland. Arch Dis Child. 91:594–597. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McShane H and Hill A: Prime-boost
immunisation strategies for tuberculosis. Microbes Infect.
7:962–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean G, Whelan A, Clifford D, et al:
Comparison of the immunogenicity and protection against bovine
tuberculosis following immunization by BCG-priming and boosting
with adenovirus or protein based vaccines. Vaccine. 32:1304–1310.
2014. View Article : Google Scholar
|
|
9
|
Hoft DF, Blazevic A, Stanley J, et al: A
recombinant adenovirus expressing immunodominant TB antigens can
significantly enhance BCG-induced human immunity. Vaccine.
30:2098–2108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perez de Val B, Villarreal-Ramos B,
Nofrarías M, et al: Goats primed with Mycobacterium bovis BCG and
boosted with a recombinant adenovirus expressing Ag85A show
enhanced protection against tuberculosis. Clin Vaccine Immunol.
19:1339–1347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dou J, Wang Y, Yu F, et al: Protection
against Mycobacterium tuberculosis challenge in mice by DNA vaccine
Ag85A-ESAT-6-IL-21 priming and BCG boosting. Int J Immunogenet.
39:183–190. 2012. View Article : Google Scholar
|
|
12
|
Cervantes-Villagrana AR, Hernández-Pando
R, Biragyn A, et al: Prime-boost BCG vaccination with DNA vaccines
based in β-defensin-2 and mycobacterial antigens ESAT6 or Ag85B
improve protection in a tuberculosis experimental model. Vaccine.
31:676–684. 2013. View Article : Google Scholar
|
|
13
|
Xing Z and Lichty BD: Use of recombinant
virus-vectored tuberculosis vaccines for respiratory mucosal
immunization. Tuberculosis. 86:211–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lasaro MO and Ertl HC: New insights on
adenovirus as vaccine vectors. Mol Ther. 17:1333–1339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Thorson L, Stokes RW, et al:
Single mucosal, but not parenteral, immunization with recombinant
adenoviral-based vaccine provides potent protection from pulmonary
tuberculosis. J Immunol. 173:6357–6365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Deng G, Li M, Liu X and Wang Y:
Roles of mucosal immunity against Mycobacterium tuberculosis
infection. Tuberc Res Treat. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cripps AW, Kyd JM and Foxwell AR: Vaccines
and mucosal immunisation. Vaccine. 19:2513–2515. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Deng G, Li M, et al: A recombinant
adenovirus expressing CFP10, ESAT6, Ag85A and Ag85B of
Mycobacterium tuberculosis elicits strong antigen-specific immune
responses in mice. Mol Immunol. 62:86–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Peng P, Miao S, et al:
Recombinant Mycobacterium smegmatis expressing an ESAT6-CFP10
fusion protein induces anti-mycobacterial immune responses and
protects against Mycobacterium tuberculosis challenge in mice.
Scand J Immunol. 72:349–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Y, Wu X, Zhang J, et al:
Immunogenicity and therapeutic effects of Ag85A/B chimeric DNA
vaccine in mice infected with Mycobacterium tuberculosis. FEMS
Immunol Med Microbiol. 66:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pydi SS, Bandaru AR, Venkatasubramanian S,
et al: Vaccine for tuberculosis: up-regulation of IL-15 by Ag85A
and not by ESAT-6. Tuberculosis (Edinb). 91:136–139. 2011.
View Article : Google Scholar
|
|
22
|
Sibley L, Reljic R, Radford DS, et al:
Recombinant Bacillus subtilis spores expressing MPT64 evaluated as
a vaccine against tuberculosis in the murine model. FEMS Microbiol
Lett. 358:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi S, Yu L, Sun D, Liu J and Hickey AJ:
Rational design of multiple TB antigens TB10.4 and TB10.4-Ag85B as
subunit vaccine candidates against Mycobacterium tuberculosis.
Pharm Res. 27:224–234. 2010. View Article : Google Scholar
|
|
24
|
Berthet FX, Rasmussen PB, Rosenkrands I,
Andersen P and Gicquel B: A Mycobacterium tuberculosis operon
encoding ESAT-6 and a novel low-molecular-mass culture filtrate
protein (CFP-10). Microbiology. 144(Pt 11): 3195–3203. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon SV, Brosch R, Billault A, Garnier
T, Eiglmeier K and Cole ST: Identification of variable regions in
the genomes of tubercle bacilli using bacterial artificial
chromosome arrays. Mol Microbiol. 32:643–655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Behr MA, Wilson MA, Gill WP, et al:
Comparative genomics of BCG vaccines by whole-genome DNA
microarray. Science. 284:1520–1523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis KN, Liao R, Guinn KM, et al:
Deletion of RD1 from Mycobacterium tuberculosis mimics Bacille
Calmette-Guerin attenuation. J Infect Dis. 187:117–123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pym AS, Brodin P, Brosch R, Huerre M and
Cole ST: Loss of RD1 contributed to the attenuation of the live
tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium
microti. Mol Microbiol. 46:709–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You Q, Wu Y, Jiang D, et al: Immune
responses induced by heterologous boosting of recombinant bacillus
Calmette-Guerin with Ag85B-ESAT6 fusion protein in levamisole-based
adjuvant. Immunol Invest. 41:412–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan W, Dong N, Zhang L, et al:
Immunogenicity and protective efficacy of a tuberculosis DNA
vaccine expressing a fusion protein of Ag85B-Esat6-HspX in mice.
Vaccine. 30:2490–2497. 2012. View Article : Google Scholar
|
|
31
|
Esparza-González SC, Troy A, Troudt J, et
al: Recombinant adenovirus delivery of calreticulin-ESAT-6 produces
an antigen-specific immune response but no protection against a
Mycobacterium tuberculosis challenge. Scand J Immunol. 75:259–265.
2012. View Article : Google Scholar
|
|
32
|
Lin CW, Su IJ, Chang JR, Chen YY, Lu JJ
and Dou HY: Recombinant BCG coexpressing Ag85B, CFP10 and
interleukin-12 induces multifunctional Th1 and memory T cells in
mice. APMIS. 120:72–82. 2012. View Article : Google Scholar
|
|
33
|
Betts G, Poyntz H, Stylianou E, et al:
Optimising immunoge-nicity with viral vectors: mixing MVA and
HAdV-5 expressing the mycobacterial antigen Ag85A in a single
injection. PLoS One. 7:e504472012. View Article : Google Scholar
|
|
34
|
Dietrich J, Andersen C, Rappuoli R,
Doherty TM, Jensen CG and Andersen P: Mucosal administration of
Ag85B-ESAT-6 protects against infection with Mycobacterium
tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J
Immunol. 177:6353–6360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dou J, Tang Q, Yu F, et al: Investigation
of immunogenic effect of the BCG priming and Ag85A-GM-CSF boosting
in Balb/c mice model. Immunobiology. 215:133–142. 2010. View Article : Google Scholar
|
|
36
|
Lu D, Garcia-Contreras L, Muttil P, et al:
Pulmonary immunization using antigen 85-B polymeric microparticles
to boost tuberculosis immunity. AAPS J. 12:338–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wakeham J, Wang J, Magram J, et al: Lack
of both types 1 and 2 cytokines, tissue inflammatory responses and
immune protection during pulmonary infection by Mycobacterium bovis
bacille Calmette-Guerin in IL-12-deficient mice. J Immunol.
160:6101–6111. 1998.PubMed/NCBI
|
|
38
|
Williams A, Reljic R, Naylor I, et al:
Passive protection with immunoglobulin A antibodies against
tuberculous early infection of the lungs. Immunology. 111:328–333.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borrero R, García Mde L, Canet L, et al:
Evaluation of the humoral immune response and cross reactivity
against Mycobacterium tuberculosis of mice immunized with liposomes
containing glycolipids of Mycobacterium smegmatis. BMC Immunol.
14(Suppl 1): 132013. View Article : Google Scholar
|
|
40
|
Rodrigues LC, Pereira SM, Cunha SS, et al:
Effect of BCG revaccination on incidence of tuberculosis in
school-aged children in Brazil: the BCG-REVAC cluster-randomised
trial. Lancet. 366:1290–1295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dantas OM, Ximenes RA, de Albuquerque Mde
F, et al: A case-control study of protection against tuberculosis
by BCG revaccination in Recife, Brazil. Int J Tuberc Lung Dis.
10:536–541. 2006.PubMed/NCBI
|
|
42
|
Basaraba RJ, Izzo AA, Brandt L and Orme
IM: Decreased survival of guinea pigs infected with Mycobacterium
tuberculosis after multiple BCG vaccinations. Vaccine. 24:280–286.
2006. View Article : Google Scholar
|
|
43
|
Buddle B, Wedlock D, Parlane N, et al:
Revaccination of neonatal calves with Mycobacterium bovis BCG
reduces the level of protection against bovine tuberculosis induced
by a single vaccination. Infect Immun. 71:6411–6419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seder RA and Hill AV: Vaccines against
intracellular infections requiring cellular immunity. Nature.
406:793–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woodworth JS, Wu Y and Behar SM:
Mycobacterium tuberculosis-specific CD8+T cells require perforin to
kill target cells and provide protection in vivo. J Immunol.
181:8595–8603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma M, Sharma S, Roy S, Varma S and
Bose M: Pulmonary epithelial cells are a source of interferon-γ in
response to Mycobacterium tuberculosis infection. Immunol Cell
Biol. 85:229–237. 2007.PubMed/NCBI
|
|
49
|
Bean AG, Roach DR, Briscoe H, et al:
Structural deficiencies in granuloma formation in TNF gene-targeted
mice underlie the heightened susceptibility to aerosol
Mycobacterium tuber- culosis infection, which is not compensated
for by lymphotoxin. J Immunol. 162:3504–3511. 1999.PubMed/NCBI
|
|
50
|
Mazanec MB, Nedrud JG, Kaetzel CS and Lamm
ME: A three-tiered view of the role of IgA in mucosal defense.
Immunol Today. 14:430–435. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Williams R and Gibbons R: Inhibition of
bacterial adherence by secretory immunoglobulin A: a mechanism of
antigen disposal. Science. 177:697–699. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tjärnlund A, Rodríguez A, Cardona PJ, et
al: Polymeric IgR knockout mice are more susceptible to
mycobacterial infections in the respiratory tract than wild-type
mice. Int Immunol. 18:807–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Olivares N, Marquina B, Mata-Espinoza D,
et al: The protective effect of immunoglobulin in murine
tuberculosis is dependent on IgG glycosylation. Pathog Dis.
69:176–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mosmann T and Coffman R: TH1 and TH2
cells: Different patterns of lymphokine secretion lead to different
functional properties. Annu Rev Immunol. 7:145–173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lamichhane A, Azegamia T and Kiyonoa H:
The mucosal immune system for vaccine development. Vaccine.
32:6711–6723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goonetilleke NP, McShane H, Hannan CM,
Anderson RJ, Brookes RH and Hill AV: Enhanced immunogenicity and
protective efficacy against Mycobacterium tuberculosis of bacille
Calmette-Guérin vaccine using mucosal administration and boosting
with a recombinant modified vaccinia virus Ankara. J Immunol.
171:1602–1609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen L, Wang J, Zganiacz A and Xing Z:
Single intranasal mucosal Mycobacterium bovis BCG vaccination
confers improved protection compared to subcutaneous vaccination
against pulmonary tuberculosis. Infect Immun. 72:238–246. 2004.
View Article : Google Scholar :
|
|
58
|
Santosuosso M, McCormick S, Zhang X,
Zganiacz A and Xing Z: Intranasal boosting with an
adenovirus-vectored vaccine markedly enhances protection by
parenteral Mycobacterium bovis BCG immunization against pulmonary
tuberculosis. Infect Immun. 74:4634–4643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu F, Wang J, Dou J, et al:
Nanoparticle-based adjuvant for enhanced protective efficacy of DNA
vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis
infection. Nanomedicine. 8:1337–1344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ndiaye BP, Thienemann F, Ota M, et al:
Safety, immunogenicity, and efficacy of the candidate tuberculosis
vaccine MVA85A in healthy adults infected with HIV-1: A randomised,
placebo-controlled, phase 2 trial. Lancet Respir Med. 3:190–200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Croyle MA, Patel A, Tran KN, et al: Nasal
delivery of an adenovirus-based vaccine bypasses pre-existing
immunity to the vaccine carrier and improves the immune response in
mice. PLoS One. 3:e35482008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lemiale F, Kong WP, Akyurek LM, et al:
Enhanced mucosal immunoglobulin A response of intranasal adenoviral
vector human immunodeficiency virus vaccine and localization in the
central nervous system. J Virol. 77:10078–10087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Richardson JS, Abou MC, Tran KN, Kumar A,
Sahai BM and Kobinger GP: Impact of systemic or mucosal immunity to
adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs.
J Infect Dis. 204(Suppl 3): 1032–1042. 2011. View Article : Google Scholar
|
|
64
|
Santosuosso M, Zhang X, McCormick S, Wang
J, Hitt M and Xing Z: Mechanisms of mucosal and parenteral
tuberculosis vaccinations: adenoviral-based mucosal immunization
preferentially elicits sustained accumulation of immune protective
CD4 and CD8 T cells within the airway lumen. J Immunol.
174:7986–7994. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shim BS, Stadler K, Nguyen HH, et al:
Sublingual immunization with recombinant adenovirus encoding
SARS-CoV spike protein induces systemic and mucosal immunity
without redirection of the virus to the brain. Virol J. 9:2152012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kaufman DR, Bivas-Benita M, Simmons NL,
Miller D and Barouch DH: Route of adenovirus-based HIV-1 vaccine
delivery impacts the phenotype and trafficking of vaccine-elicited
CD8+ T lymphocytes. J Virol. 84:5986–5996. 2010. View Article : Google Scholar : PubMed/NCBI
|