Introduction
Glioma is the most common and aggressive type of
brain tumor. Although patients undergo comprehensive treatment,
including maximal micro-neurosurgical resection, radiotherapy and
chemotherapy with temozolomide, tumor recurrence is almost
inevitable and the 5-year survival rate is <10% (1,2).
Several types of tumor, which have been considered by histology to
be equivalent have been identified through molecular and genetic
investigations to be teleologically and ontologically diverse,
suggesting that their treatment requires an increased understanding
of their biology and the use of a targeted therapeutic approach
(3). Poor prognosis is associated
with diffused infiltrative growth in the surrounding brain tissue.
SHP-2 is a ubiquitously expressed cytoplasmic protein tyrosine
phosphatase (PTP), encoded by the PTPN11 gene (4). Mutations in PTPN11 have been
identified in several human afflictions, including Noonan syndrome
(NS), Leopard syndrome and childhood hematologic malignancies
(5). Activating mutations in
PTPN11 have also been identified in solid tumors, including
lung cancer, liver carcinoma, colon cancer, neuroblastoma and
melanoma (6,7). The present study aimed to investigate
whether the inhibition of SHP-2 increases the radiosensitivity of
glioma cell lines. The results of the present study may improve the
understanding of the functions of SHP-2 in the pathogenesis of
glioma and assist in the development of future therapeutic
strategies for the treatment of glioma.
Materials and methods
Tissue collection
Glioma cases (n=21; 10 male; 11 female) were used in
the present study. Patient age ranged between 25 and 65 years, with
an average age of 42 years. Each patient underwent primary surgical
resection of glioma between 2008 and 2012. Normal brain tissue was
obtained from nine patients (five male and four female; age range,
18–55 years) who endured decompressive surgical procedures for
severe head injury within the same time period. This study was
approved by the Harbin Medical University (HMU) Ethics Committee
(Heilongjiang, China) and informed consent was obtained from the
patients. Pathological grading was performed, according to the 2007
WHO classification (8). The tumor
samples were consisted of three grade I, seven grade II, five grade
III and six grade IV. The pathological review was diagnosed at The
Fourth Affiliated Hospital of Harbin Medical University,
(Heilongjiang, China) by three pathologists and two neurosurgeons
as a routine study.
Immunoblot analysis
Glioma cells (5×106) were directly lysed
with radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was
performed by transfer of the proteins onto polyvinylidene fluoride
membranes (Schleicher & Schuell Microscience, Riviera Beach,
FL, USA) using a mini Trans-Blot apparatus (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Following blocking for 2 h, the membranes
were incubated overnight at 4°C with the following specific primary
human antibodies: Anti-SHP-2 (1:1,000; cat. no. 3752; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-Caspase 3
(1:1,000; cat. no. ab2302) Abcam, Cambridge, UK), anti-Bax
(1:1,000; cat. no. ab7977; Abcam), anti-Bcl2 (1:1,000; cat. no.
ab33862; Abcam) and anti-GAPDH (1:1,000; cat. no. ab9485; Abcam).
Following washing, the membrane was incubated with the appropriate
fluorescein-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (1:200; cat. no. ZF-0311; ZSGB-BIO, Beijing, China) for 1
h at room temperature. Following extensive washing, the signals
were visualized by enhanced chemilluminescence substrate (Pierce
Chemical, Rockford, IL, USA).
Cell culture, transient transfection and
radiation exposure
The U251, U87 and SHG44 human glioma cell lines were
obtained from the Shanghai Cell Collection (Shanghai, China). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all Gibco Life Technologies, Carlsbad, CA,
USA) at 37°C in a humidified incubator containing 5%
CO2. The specific SHP-2 small interfering (si)RNA
(SHP-2.si) was designed and synthesized by Invitrogen Life
Technologies (Shanghai, China). Once the cells reached 70–80%
confluence, the glioma cells were transfected with SHP-2.si using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer's instructions. Following 24 h
transfection, the glioma cells were exposed to different doses of
radiation at a dose rate of 200 Gy/min at room temperature. An X
linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA,
USA) was used for radiation treatment. Following irradiation (IR),
the glioma cells were returned immediately to a 37°C incubator.
Cell proliferation assay
The glioma cells were seeded into 96-well plates
(6,000 cells/well) and were transfected with SHP-2.si as described
above. Following incubation for 24 h, the 96-well plates were
irradiated with 0, 2, 4, 6 or 8 Gy and the glioma cells were
subsequently incubated for 24 or 48 h at 37°C, and cell
proliferation was analyzed using a cell counting kit (CCK)8 assay.
CCK8 solution (10 ml) was added to each well of the 96-well plates
and the plates were incubated at 37°C for 4 h prior to measuring
the absorbance at 450 nm using a plate reader (iMark microplate
absorbance reader; Bio-Rad Laboratories, Inc.).
Cell apoptosis analysis
To assess the induction of apoptosis, Annexin V and
propidium iodide (PI) double staining was performed using the
Annexin-V-FLUOS Staining kit (Roche Diagnostics, Shanghai, China).
Following transfection of SHP-2.si, the glioma cells were incubated
at 37°C for 24 h. The cells were subsequently irradiated at doses
of 0, 2 or 6 Gy. Following incubation for 24 h, the glioma cells
were stained with Annexin V and PI, and analyzed using a FACS
Calibur (BD Bioscience, Franklin Lakes, NJ, USA).
Cell invasion assay
Matrigel (BD Biosciences) was added to the upper
chamber of the Transwell apparatus with 8-µm pore size
membrane (Corning Costar, Corning, NY, USA). When the Matrigel had
solidified at 37°C, serum-free DMEM, containing 1×103
glioma cells in 100 µl was added into the upper chamber. The
lower chamber was loaded with 500 µl DMEM, containing 10%
FBS. Following incubation at 37°C for 24 h, the membranes coated
with Matrigel were wiped with a cotton swab and fixed using 100%
methanol for 10 min. The membranes with cells were soaked in 0.1%
crystal violet (Beyotime Institute of Biotechnology) for 10 min and
subsequently washed with distilled water. The number of cells
attached to the lower surface of the polycarbonate filter was
counted at a magnification of x400 under a light microscope
(Eclipse E200; Nikon Instruments, Inc., Melville, NY, USA). The
results are expressed as the mean of triplicate experiments.
Vasculogenic mimicry (VM) assay
Immediately prior to use, 24-well plates were coated
with high-concentration Matrigel (BD Biosciences; 200
µl/well) and incubated at 37°C for 40 min until the Matrigel
was solid. The glioma cells were transfected and pre-incubated in
DMEM without serum overnight. The cells were lifted using 0.05%
trypsin (Beyotime Institute of Beyotechnology), which was
neutralized with DMEM, containing 10% FBS. The cells were
centrifuged at 800 × g for 5 min, resuspended and seeded onto the
Matrigel-coated wells at a density of 3×104 cells/well.
Photomicrographs were captured (OLS4100; Olympus Corporation,
Tokyo, Japan) following 16 h of incubation from each well and the
number of tubes (complete circular structures) was counted. The
mean of three readings of each well was used as the final reading
from that well.
Statistical analysis
All data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of SHP-2 in human glioma
tissues
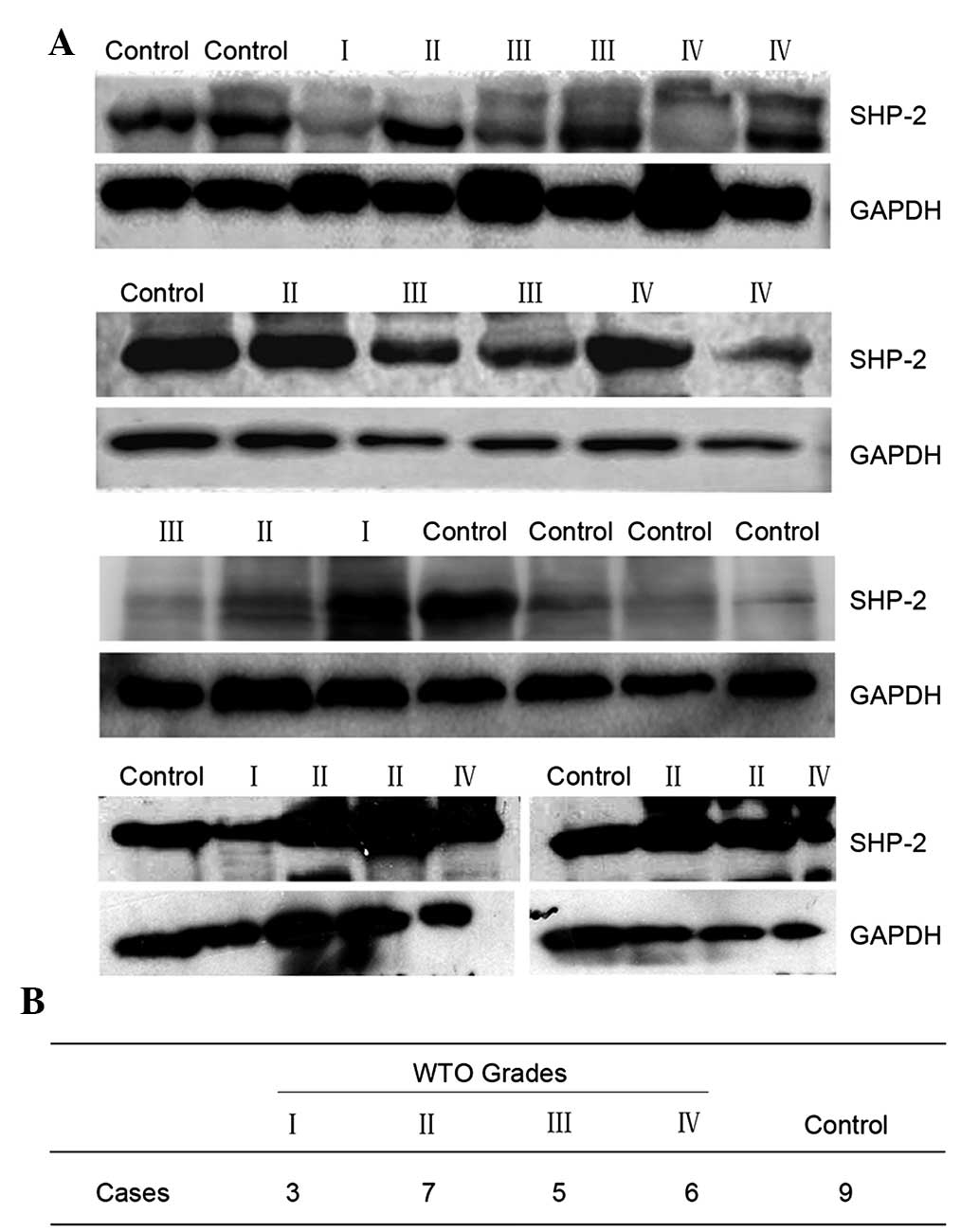
The expression of SHP-2 in the 21 cases of human
glioma and nine normal brain tissue samples was assessed by
immunoblotting. The results demonstrated that there was no
significant difference between the glioma tissue and the normal
brain tissue (Fig. 1).
Suppression of SHP-2 in combination with
IR improves the antiglioma effect in vitro
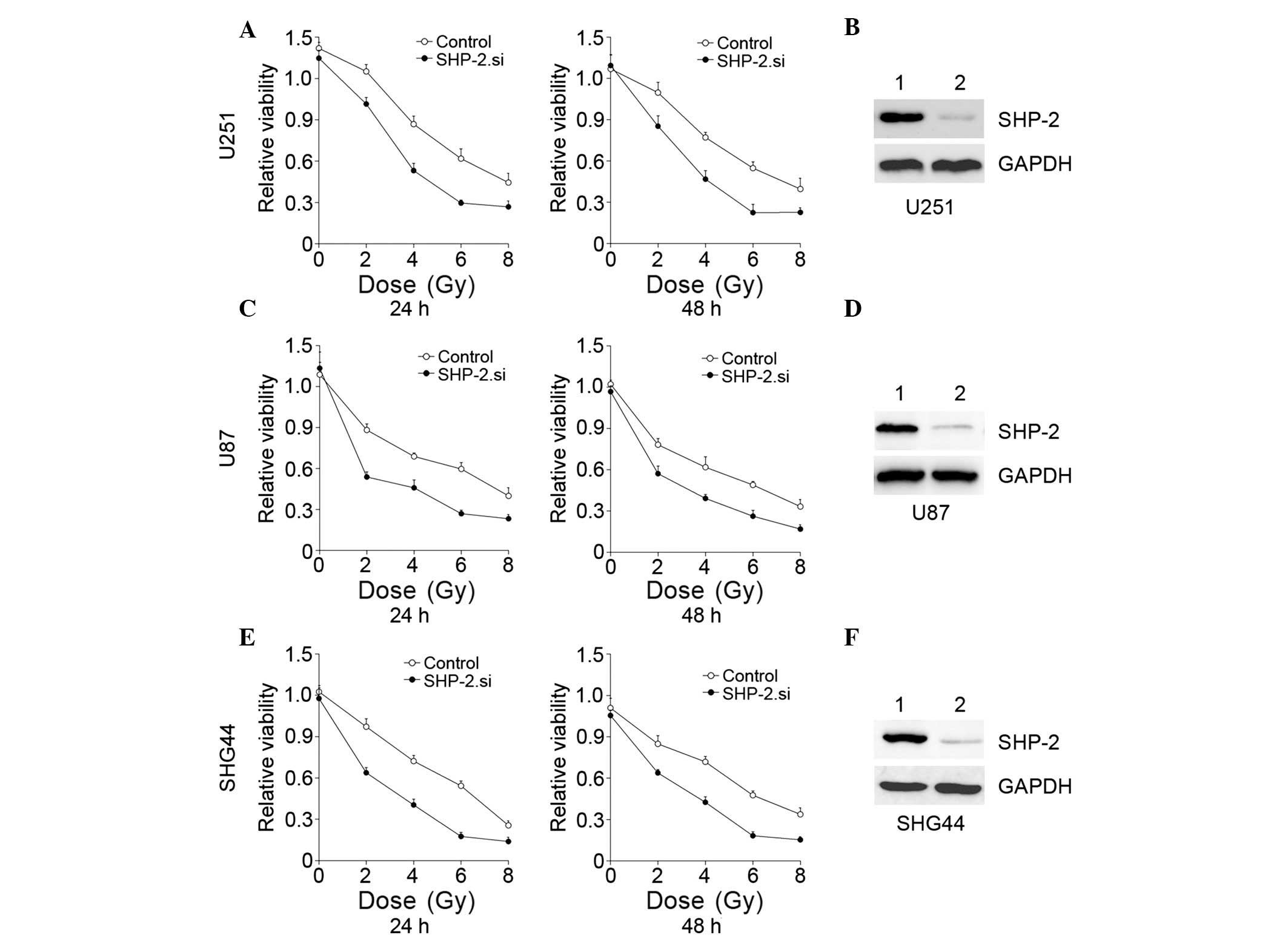
Glioma cell lines were transfected with SHP-2.si.
Following incubation for 24 h, the cells were exposed to IR at
different doses (0, 2, 4, 6 or 8 Gy), and the cell proliferation
was analyzed by a CCK8 assay at different time points (24 and 48
h). The results demonstrated that cell proliferation was
significantly suppressed by the downregulation of SHP-2 in
combination with IR (Fig. 2). IR
inhibited glioma cell proliferation and in the SHP-2. si groups,
the inhibitory effects were markedly increased compared with
treatment with IR alone. A significant difference was observed
between SHP-2.si and the control groups of each glioma cell line,
particularly 24 h following IR. The results indicated that the
suppression of SHP-2 improved the antiglioma effect of IR.
Inhibition of SHP-2 in glioma cell lines
upregulates IR-induced cell apoptosis
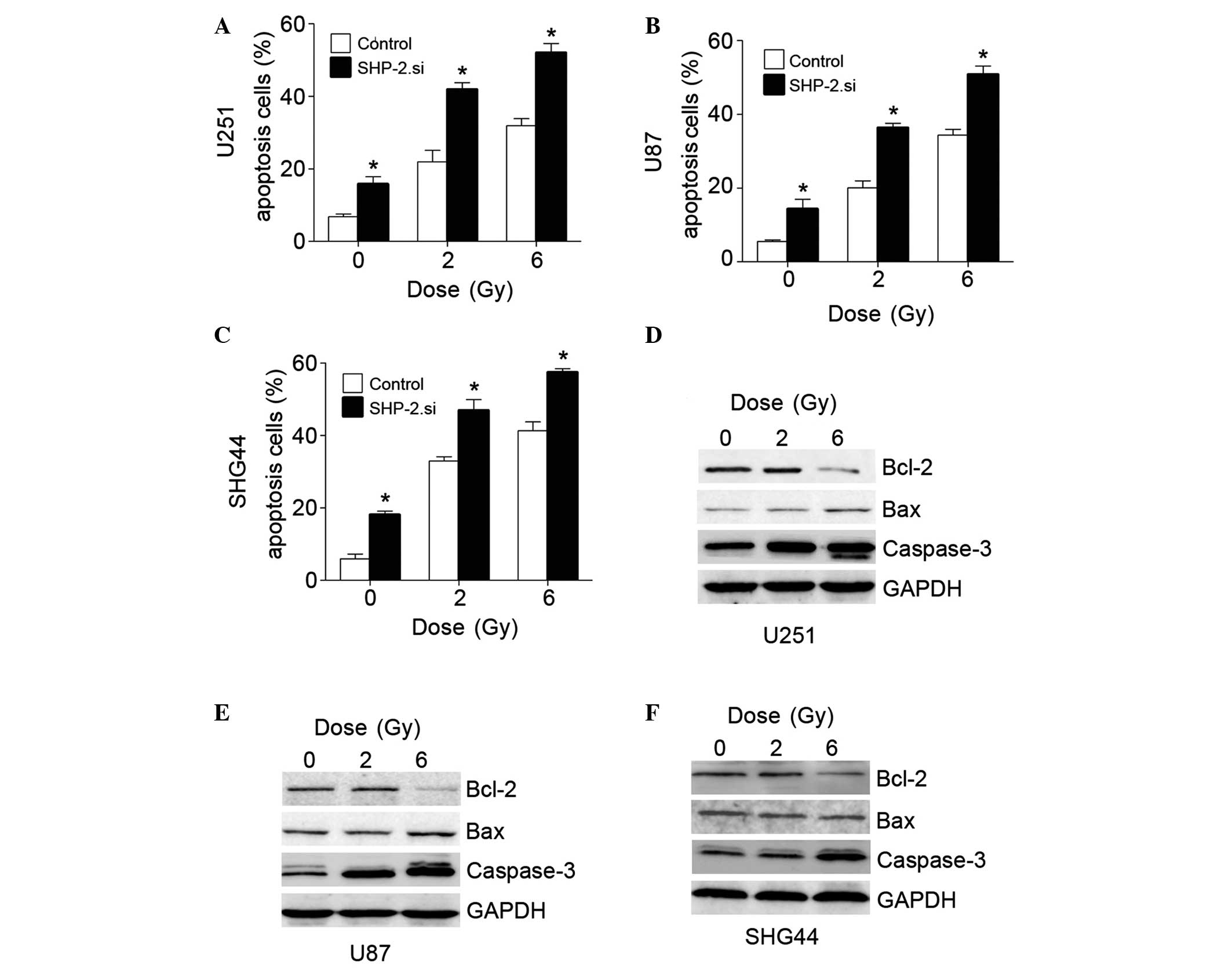
Glioma cell lines were transfected with SHP-2.si and
exposed to IR (0, 2 or 6 Gy). Following incubation for 24 h, cell
apoptosis was assessed by FACS. The apoptotic rate increased in the
SHP-2.si groups, which was significantly difference compared with
the control group (P<0.05). Additional proteins associated with
cell apoptosis, including Bcl-2, Bax and Caspase-3, were assessed.
The pro-apoptotic proteins, Bax and Caspase-3, were upregulated and
the anti-apoptotic protein, Bcl-2, was downregulated in the
SHP-2.si groups (Fig. 3). These
results suggested that SHP-2.si transfection induced cell
apoptosis.
siRNA-mediated silencing of SHP-2
suppresses the invasive ability of glioma cells in vitro
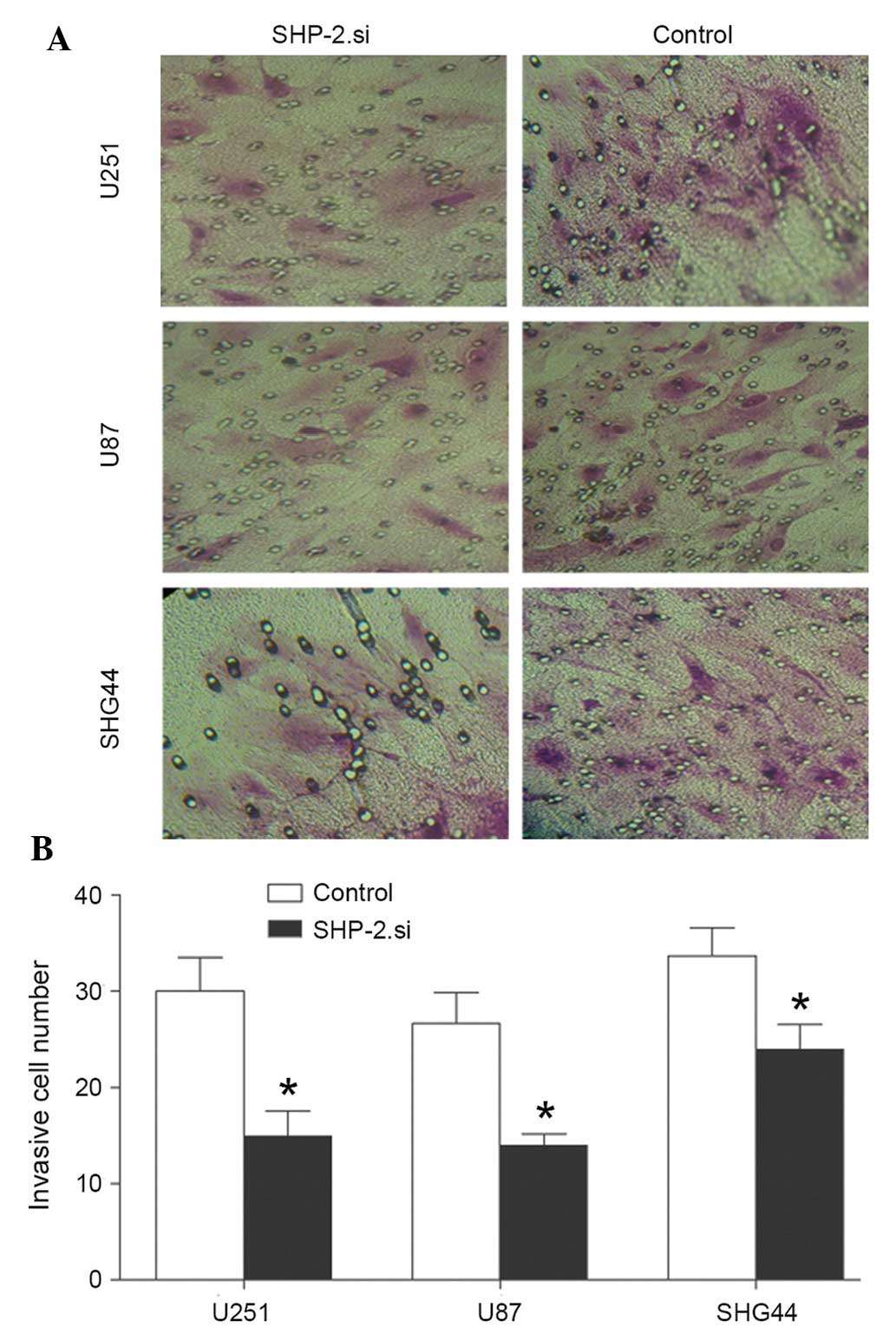
Following transfection with SHP-2.si, an invasion
assay was performed in the glioma cell lines. The results of
Matrigel Transwell analysis demonstrated that the invasive capacity
of glioma cells was significantly reduced by transfection with
SHP-2.si (Fig. 4). The number of
cells in the SHP-2.si group passing through the Matrigel was
significantly lower compared with the control group. The results
demonstrated that siRNA-mediated SHP-2 silencing suppressed the
metastasis and inhibited the invasion of glioma cells.
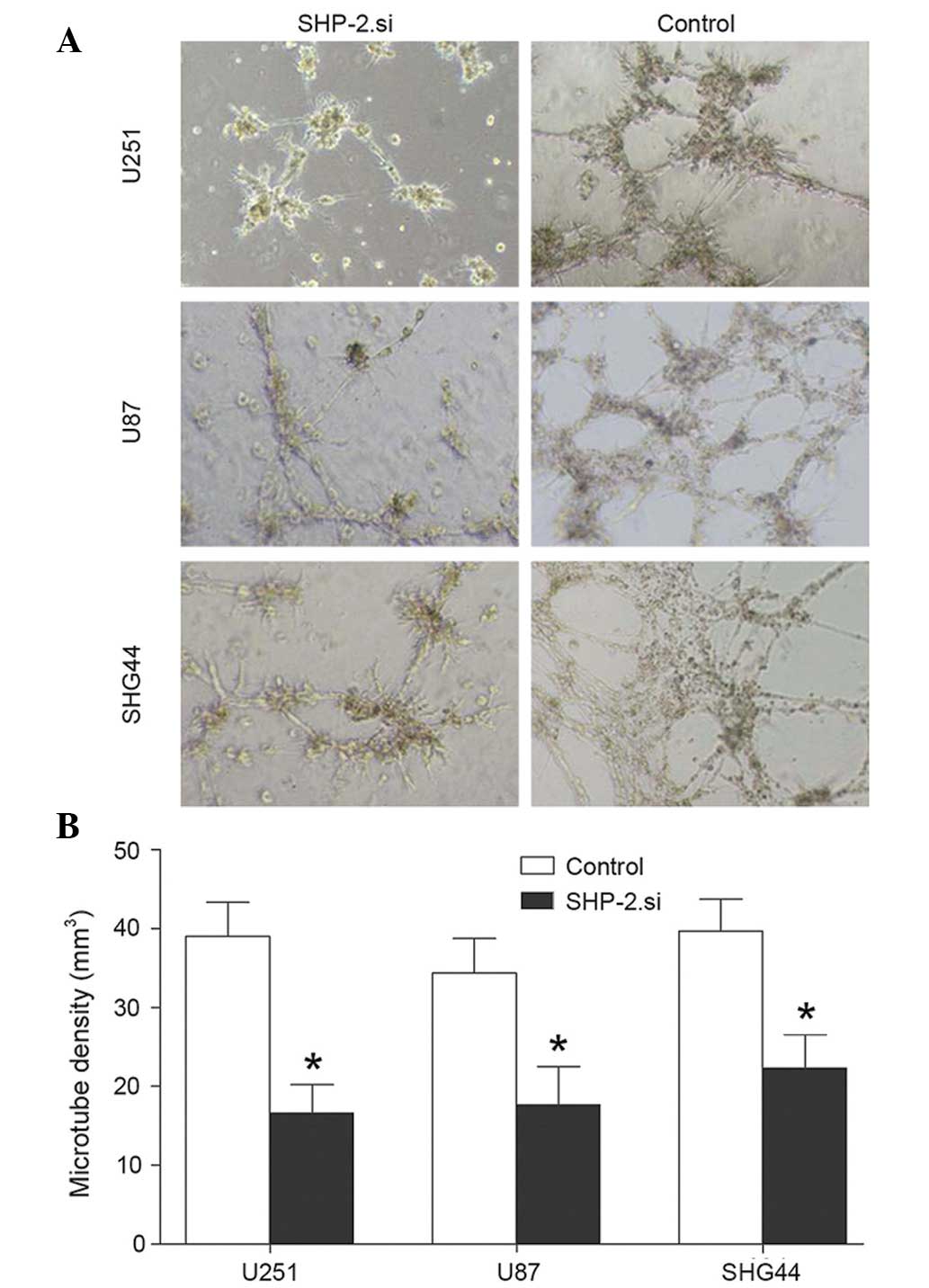
Downregulation of SHP-2 inhibits VM in
glioma cell lines
A VM assay was conducted in the SHP-2.si and the
control groups of each glioma cell line. The VM assay revealed that
the tube formation capacity of glioma cells was inhibited following
transfection with SHP-2.si and the microtube density was
significantly lower compared with the control group (Fig. 5).
Discussion
In glioma, radiotherapy is the most common treatment
following microsurgery. For several solid tumors, IR is a treatment
tool, which offers a clear survival benefit (9). However, glioblastoma multiforme and
pancreatic carcinoma represent types of tumor, which are resistant
to conventional radiochemotherapeutical regimes (10). A previous study indicated that
glioma demonstrates low sensitivity to radiation therapy and that
glioma cells have the capacity of repairing IR-induced apoptosis
(11). Glioma progression and
resistance to radiotherapy has been associated with angiogenesis
(12–14). Neovascularization has long been
implicated as a salient feature of glioma progression. Therefore
anti-angiogenic therapy may be useful to increase the
radiosensitivity of glioma (15).
Despite intensive research and development of novel targeted
therapies, the prognosis for patients with these types of tumor
remains poor (16,17). Novel therapeutic approaches are
therefore required.
Neovascularization is the most important feature of
glioma. High-grade gliomas are among the most vascular of all solid
types of tumor, and vascular proliferation is a pathological
hallmark of glioma (18). Glioma
resistance to radiotherapy and chemotherapy leads to poor clinical
outcomes and this resistance was demonstrated to be associated with
the tumor microenvironment (19,20).
Decades of research improved the understanding of the tumorigenesis
of gliomas at the molecular level, through the identification of
VEGF and its associated pathways, novel therapeutic targets may be
developed (21). Therefore,
anti-angio-genic therapies are used in patients with glioma in
combination with chemotherapy and radiotherapy (22,23).
The most well established anti-angiogenic therapy is bevacizumab
(24,25). Anti-angiogenic agents are
hypothesized to be significant in the treatment of glioma in the
future.
SHP-2 is a protein phosphatase identified in the
early 1990s, which is expressed in all tissue types (26). It contains two tandem SH2 domains,
a PTP domain and a COOH-terminal hydrophobic tail with two tyrosine
phosphorylation sites. SHP-2 is a positive signaling component
downstream of growth factor, cytokine and extracellular matrix
receptors, and is important in regulating cell growth,
transformation, differentiation and migration. Protein
phosphorylation and dephosphorylation are fundamental cellular
events mediated by kinases and phosphatases, respectively, which
govern a host of cell functions, including growth, division,
adhesion and motility (27).
Mutations in PTPs and/or altered expression of PTPs may contribute
to cancer, autoimmune disorders and inflammation. Previous studies
demonstrated that SHP-2 is involved in the RAS-MAPK, PI3K-AKT, JNK,
JAK-STAT, NF-κB, RHO and NFAT signaling pathways (27,28).
It has been revealed that SHP-2 is important in tumor proliferation
and invasion in several types of cancer, including glioma. Zhan
et al (7) revealed that
SHP-2 is required for EGFRvIII oncogenic transformation in human
glioblastoma cells. SHP-2/PTPN11 mediates glioma genesis driven by
PDGFRA and INK4A/ARF aberrations in mice and humans (29). The present study demonstrated that
inhibition of SHP-2 increases the antiglioma effect of IR by
suppressing the proliferation of, and upregulating the apoptosis of
glioma cells. Previous studies have demonstrated that SHP-2
increases cell migration and angiogenesis in NS and leukemia. Since
the invasion and abundant vasculature are the biological features
of glioma, the present study investigated the invasion and
angiogenesis ability of glioma cells when SHP-2 is downregulated.
The results demonstrated that silencing of SHP-2 inhibited the
migratory ability and VM of glioma cells. However, the expression
of SHP-2 was not upregulated in human glioma tissues, which
indicated that SHP-2 may control the biological functions of glioma
by other means, for instance, the downstream cell signaling
pathways of SHP-2, including the Ras-MAPK, PI3K-AKT, JAK-STAT,
NF-κB and NFAT signaling pathways.
These findings suggested that SHP-2 is important in
glioma radiosensitivity by regulating cell proliferation,
apoptosis, migration and neoangiogenesis. Thus, SHP-2 may be a
potential therapeutic target for the treatment of human glioma in
the future.
Acknowledgments
This study was partially supported by the WU JIEPING
Clinical Research Fund (no. 320.6750.1330). The authors would like
to thank all patients involved in the present study.
References
|
1
|
Stupp R, Hegi ME, Mason WP, et al: Effects
of radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JH, Bae Kim Y, Han JH, et al:
Pathologic diagnosis of recurrent glioblastoma: morphologic,
immunohistochemical and molecular analysis of 20 paired cases. Am J
Surg Pathol. 36:620–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janbazian L, Karamchandani J and Das S:
Mouse models of glioblastoma: lessons learned and questions to be
answered. J Neurooncol. 118:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matozaki T, Murata Y, Saito Y, et al:
Protein tyrosinephosphatase SHP-2: a proto-oncogene product that
promotes Ras activation. Cancer Sci. 100:1786–1793. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng H, Alter S and Qu CK: SHP-2 tyrosine
phosphatase in human diseases. Int J Clin Exp Med. 2:17–25.
2009.PubMed/NCBI
|
|
6
|
Bentire-Alj M, Paez JG, David FS, et al:
Activating mutations of the noonan syndrome-associated SHP2/PTPN11
gene in human solid tumors and adult acute myelogenous leukemia.
Cancer Res. 64:8816–8820. 2004. View Article : Google Scholar
|
|
7
|
Zhan Y, Counelis GJ and O'Rourke DM: The
protein tyrosine phosphatase SHP-2 is required for EGFRvIII
oncogenic transformation in human glioblastoma cells. Exp Cell Res.
315:2343–2357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumors of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maddirela DR, Kesanakurti D, Gujrati M, et
al: MMP-2 suppression abrogates irradiation-induced microtubule
formation in endothelial cells by inhibiting αvβ3-mediated
SDF-1/CXCR4 signaling. Int J Oncol. 42:1279–1288. 2013.PubMed/NCBI
|
|
10
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plusconcomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kargiotis O, Geka A, Rao JS, et al: Review
effects of irradiation on tumor cell survival, invasion and
angiogenesis. J Neurooncol. 100:323–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prise KM, Schettino G, Folkard M, et al:
Review new insights on cell death from radiation exposure. Lancet
Oncol. 6:520–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beal K, Abrey LE and Gutin PH:
Antiangiogenic agents in the treatment of recurrent or newly
diagnosed glioblastoma: analysis of single-agent and combined
modality approaches. Radiat Oncol. 6:22011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hands JR, Abel P, Ashton K, et al:
Investigating the rapid diagnosis of gliomas from serum samples
using infrared spectroscopy and cytokine and angiogenesis factors.
Anal Bioanal Chem. 405:7347–7355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brem S, Cotran R and Folkman J: Tumor
angiogenesis: a quantitative method for histologic grading. J Natl
Cancer Inst. 48:347–356. 1972.PubMed/NCBI
|
|
16
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine formetastatic pancreatic cancer. N
Engl J Med. 364:1817–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milanović D, Firat E, Grosu AL, et al:
Increased radiosensitivity and radiothermosensitivity of human
pancreatic MIA PaCa-2 and U251 glioblastoma cell lines treated with
the novel Hsp90 inhibitor NVP-HSP990. Radiat Oncol. 28:422013.
View Article : Google Scholar
|
|
18
|
Hardee ME and Zagzag D: Mechanisms of
glioma-associated neovascularization. Am J Pathol. 181:1126–1141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zagzag D, Amirnovin R, Greco MA, et al:
Vascular apoptosis andinvolution in gliomas precede
neovascularization: a novel concept for glioma growth and
angiogenesis. Lab Invest. 80:837–849. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamszus K, Ulbricht U, Matschke J, et al:
Levels of soluble vascular endothelial growth factor (VEGF)
receptor 1 in astrocytic tumors and its relation to malignancy,
vascularity and VEGF-A. Clin Cancer Res. 9:1399–1405.
2003.PubMed/NCBI
|
|
22
|
Reiss Y, Machein MR and Plate KH: The role
of angiopoietins during angiogenesis in gliomas. Brain Pathol.
15:311–17. 2005. View Article : Google Scholar
|
|
23
|
Rong Y, Durden DL, Van Meir EG, et al:
'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic
feature that links vascular pathology, hypoxia and angiogenesis. J
Neuropathol Exp Neurol. 65:529–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norden AD, Young GS, Setayesh K, et al:
Bevacizumab for recurrent malignant gliomas: efficacy, toxicity and
patterns of recurrence. Neurology. 70:779–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narayana A, Kelly P, Golfinos J, et al:
Antiangiogenic therapy using bevacizumab in recurrent high-grade
glioma: impact on local control and patient survival. J Neurosurg.
110:173–80. 2009. View Article : Google Scholar
|
|
26
|
Freeman RM Jr, Plutzky J and Neel BG:
Identification of a human srchomology 2-containing
protein-tyrosine-phosphatase: a putative homolog of Drosophila
corkscrew. Proc Natl Acad Sci USA. 89:11239–11243. 1992. View Article : Google Scholar
|
|
27
|
Barr AJ: Protein tyrosine phosphatases as
drug targets: strategies and challenges of inhibitor development.
Future Med Chem. 2:1563–1576. 2010. View Article : Google Scholar
|
|
28
|
Díaz ME, González L, Miquet JG, et al:
Growth hormone modulation of EGF-induced PI3K-Akt pathway in mice
liver. Cell Signal. 24:514–523. 2012. View Article : Google Scholar
|
|
29
|
Liu KW, Feng H, Bachoo R, et al:
SHP-2/PTPN11 mediates glioma genesis driven by PDGFRA and INK4A/ARF
aberrations in mice and humans. J Clin Invest. 121:905–917. 2011.
View Article : Google Scholar : PubMed/NCBI
|