Introduction
Colorectal cancer is the third most common type of
cancer and is the third leading cause of cancer-associated
mortality in males and females in the United States (1). However, despite significant effort,
the molecular pathways involved, and the order of genetic events in
the genesis of colorectal cancer remain to be fully elucidated.
Caudal-related homeobox transcription factor 2 (CDX2) is a
transcription factor, which is specifically expressed in the adult
intestine. It is essential for the development and homeostasis of
the intestinal epithelium (2).
CDX2 is central in the regulation of the balance between
differentiation and proliferation of intestinal epithelial cells
(IECs) (3). Conditional
intestine-specific inactivation of the murine CDX2 gene has a
marked effect on the villus morphology and cytodifferentiation of
IECs (4). Previous chromatin
immunoprecipitation-sequencing data has revealed that CDX2 binds a
significantly higher number of target genes in differentiated IECs,
compared with proliferating cells (5,6). In
adult human tissue, a number of studies have identified the
involvement of CDX2 in regulating the expression of genes encoding
intestine-specific proteins, including sucrase-isomaltase (7), lactase (8,9),
calbindin-D9K (10,11), apolipoprotein B (12), claudin-2 (13) and mucin 2 (14,15).
Additionally, it has been demonstrated that CDX2 functions as a
tumor suppressor in the adult colon. Bonhomme et al revealed
that reduced expression of CDX2 accelerates tumor progression in a
mouse model of sporadic colorectal cancer (16). Furthermore, Aoki et al
confirmed these findings in a mouse model of familial adenomatous
polyposis (17). The role of CDX2
as a tumor suppressor is also supported by the observation that its
expression is decreased in human colorectal cancer, and reduced
expression of CDX2 is associated with poor overall survival rates
in patients with colorectal cancer (18–20).
Histopathological analyses have demonstrated that the expression of
CDX2 is low in invasive colorectal cancer cells, which localize at
the tumor/stroma interface, but is restored in metastases, at a
level corresponding to that of the primary tumor (21). These data suggest that decreased
expression of CDX2 is involved in tumor migration. In the present
study, the effects of the overexpression of CDX2 on the growth of
colon cancer was were investigated via subcutaneous implantation of
CDX2-overexpressing LoVo colon cancer cells, delivered using a
transfected eukaryotic expression vector, pEGFP-C1-CDX2, into a
nude mouse model.
Materials and methods
Cell line and culture
The LoVo human colon cancer cell line was purchased
from China Centre for Type Culture Collection (Shanghai, China).
The cells were cultured at 37°C in RPMI-1640 medium (Gibco Life
Technologies, Grand Island, NY, USA), supplemented with 10% fetal
bovine serum (Hyclone, Waltham, MA, USA) in a humidified atmosphere
of 5% CO2. The cells were detached using 0.25% trypsin
and 0.02% ethylenediaminetetraacetic acid (EDTA) (Boster Biological
Technology Co., Ltd., Wuhan, China).
Animals
Athymic nude male BALBC/c mice, weighing 15–18 g
(4–5 weeks old), were purchased from the Institute of Laboratory
Animal science, Chinese Academy of Medical Science (Beijing,
China). The mice were maintained in specific pathogen-free,
temperature-controlled (24°C) conditions, were housed separately
and were fed with sterilized food and autoclaved water, according
to the experimental animal guidelines (22). All animal procedures were approved
by the Committee on Animal Experimentation of Xi'an Jiaotong
University (Xi'an, China), and the procedures complied with the NIH
Guide for the Care and Use of Laboratory Animals (23).
Vector construction and transfection
CDX2 full-length cDNA was amplified using reverse
transcription-polymerase chain reaction (RT-PCR) using total RNA,
which was extracted according to the manufacturer's instructions
using TRIzol (Gibco Life Technologies) from human colorectal
carcinoma LoVo cells as a template. The resulting RNAs were treated
with RNase-free DNase (Promega Corporation, Madison, WI, USA) and
2.5 µg was reverse transcribed into cDNA using the RT-PCR
kit (Invitrogen Life Technologies, Carlsbad, CA, USA) according to
the manufacturer's instructions. The following primers were used:
Forward 5′-CCA ATA AGC TTA GGC AGC ATG GTG AGG TCT G-3′) and
reverse 5′-CCA ATG GAT CCC TGA GGA GTC TAG CAG AGT CCA C-3′. PCR
amplifications were performed in duplicate and the conditions were
as follows: Initial denaturation at 94°C for 2 min, then 35 cycles
of 94°C for 30 sec, 62°C for 30 sec and 72°C for 30 sec, followed
by an extension step at 72°C for 10 min. Full-length CDX2 cDNA
(1,055 bp) was then cloned into the HindII/BamHI
sites of the pEGFP-C1 eukaryotic expression vector, (Clontech
Laboratories, Inc., Mountain View, CA, USA). The LoVo cells were
transfected using Lipofectamine™ 2000 (Invitrogen Life
Technologies), according to the manufacturer's instructions. The
cells (1×105 cells/cm2 in 24-well plates)
were then grown in complete medium containing 250 mg/ml G418
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C. Subsequent to
transient transfection for 48 h, the cells were passaged at 1:10
(volume/volume) and cultured in medium supplemented with G418
(Sigma-Aldrich) at 600 µg/ml for 4 weeks. The survival
clones were selected and maintained in medium containing 300
µg/ml G418. The subclone cells expressing CDX2 were termed
the pEGFP-C1-CDX2 cells. CDX2 cloning was confirmed using western
blotting.
Western blotting
For western blotting, ~1×107 untreated
cells, pEGFP-C1 cells and pEGFP-C1-CDX2 cells were harvested,
washed once with ice-cold phosphate-buffered saline (PBS; Boster
Biological Technology Co., Ltd.), resus-pended in 100–200 µl
lysis buffer (Sigma-Aldrich), containing 50 mM Tris, 150 mM NaCl, 5
mM EDTA, 5 mM ethylene glycol tetraacetic acid and 1% SDS (pH 7.5),
and then ultrasonicated (MS2 Minishaker; IKA-Works, Wilmington, NC,
USA) on ice until the solution became clear. The total protein
concentration was measured using the Bradford method (24), according to the manufacturer's
instructions (Sigma-Aldrich). The samples were heated at 100°C for
5 min with and equal volume of 2X SDS loading buffer (Shaanxi
Pioneer Biotech Co., Ltd., Xi'an, China), containing 125 mM
Tris-HCl (pH 6.8), 10% glycerol, 2% SDS, 5% 2-mercaptoethanol and
2.5 ml 0.0025% bromophenol blue, and were cooled on ice for 10~20
min. Total protein (80 µg) from each sample was resolved
using 8% or 10% SDS-PAGE to detect CDX2 (38 kDa) and β-actin (43
kDa), respectively. The protein was then transferred onto a
polyvinylidine difluoride membrane in transfer buffer, containing
25 mM Tris (pH 8.5), 200 mM glycerin, and 20% methanol) at 100 V
for 2 h. The proteins were detected using mouse monoclonal CDX2
antibody (cat. no. AM392-M; 1:500; BioGenex, San Ramon, CA, USA)
and mouse monoclonal β-actinantibody (cat. no. sc-47778; 1:1,000,
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as a loading
control. The membranes were incubated with primary antibodies
overnight at 4°C after blocking in 5% non-fat milk for 1 h at room
temperature, and were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit or goat anti-mouse
immunoglobulin G (Beijing ZhongShan Goldbridge Biotechnology Co,
Beijing, China). The proteins were visualized using
chemiluminescence luminol reagents (cat. no. sc-2048; Santa Cruz.
Biotechnology, Inc.).
Subcutaneous human colorectal cancer cell
xenograft growth and oncogenicity
A total of 18 nude mice were randomly divided into
three groups (n=6), comprising an untreated cell group, a group
inoculated with pEGFP-C1 cells and a group inoculated with
pEGFP-C1-CDX2 cells. The mice were subcutaneously inoculated in the
right hind lateral leg with cells (1×107/ml) in the
logarithmic growth period. At 10 days post-injection, tumor growth
was monitored every 2 days. The measurements of the tumor diameter
(a) and short diameter (b), measured using a vernier caliper
(Qingdao Tide Machine Tool Supply Co., Ltd., Qingdao, China), were
used to calculate the tumor volume, according to the formula a ×
b2 / 2, and tumor growth curves were drawn on the basis
of the average values of the tumor volume from each group. At the
end of the third week, the mice were sacrificed by cervical
dislocation, and the tumors were resected in order to measure the
final volumes and weights using a photoelectric balance (ACS-JL808
LED; Yongkang Jieli Weighing Apparatus Co., Ltd, Yongkang, China).
The tumor growth inhibition rate was determined using the volume
and weight measurements and the following formula: Inhibition rate
= (1 - tumor weight of transfectant / tumor weight of untreated
cells) × 100%. The tumor tissue of each group was stored in liquid
nitrogen. A total of 18 sections (0.25 cm3) of the
tissues were fixed with 10% formaldehyde (Boster Biological
Engineering Co., Ltd.) solution for subsequent immunohistochemical
analysis.
Immunohistochemical staining
The tumor tissues were fixed in 4% formaldehyde,
dehydrated using gradient ethanol, and embedded in paraffin (Xian
Chemical Reagents Instruments, Inc., Xian, P.R. China). Tissue
sections (4 µm) were deparaffinized in fresh xylene (Xian
Chemical Reagents Instruments, Inc.) and rehydrated through
sequential graded ethanols. Antigen retrieval was performed by
incubation with citrate buffer (10 mmol/l; pH 6.0) using a
microwave pressure cooker (Zhejiang Duobao Industrial & Trade
Co., Ltd., Ningbo, China) for 20 min. The slides were cooled for 20
min, incubated for 5 min with 3% hydrogen peroxide (Xian Chemical
Reagents Instruments, Inc.), washed in PBS-0.1% Triton X-100 (pH
7.6), blocked for 20 min in 20% normal goat serum (Boster
Biological Engineering Co., Ltd.), and incubated in an appropriate
antibody dilution for CDX2 (cat. no. MU392A-UC; 1:400; mouse
monoclonal; Biogenex, San Ramon, CA, USA) or MMP-2 (cat. no.
BA0569; 1:400; rabbit polyclonal; Boster Biological Technology Co.,
Ltd.) overnight at 4°C. The subsequent day, the slides were washed
in PBS-0.1% Triton X-100 and incubated for 30 min in a 1:200
dilution of biotinylated anti-mouse (cat. no. BA1001) or
anti-rabbit (cat. no. BA1003) secondary antibody. The ABC Elite kit
(Boster, Biological Technology Co., Ltd.), with
3,3′-diaminobenzidine development, was used to visualize antibody
binding, and the slides were subsequently counterstained with
hematoxylin (0.4%; Boster Biological Engineering Co., Ltd.).
Negative controls were included by replacement of the primary
antibody with PBS. Evaluation of the immunostaining of the CDX2 and
MMP-2 genes were performed simultaneously by two independent
observers in a blinded manner using an Olympus BX51 microscope
(Olympus, Center Valley, PA, USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Differences were assessed between the two groups using
a t-test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software, version 13.0 (SPSS Inc., Chicago, IL,
USA).
Results
Construction of the pEGFP-C1-CDX2
eukaryotic vector and overexpression of CDX2 in LoVo cells
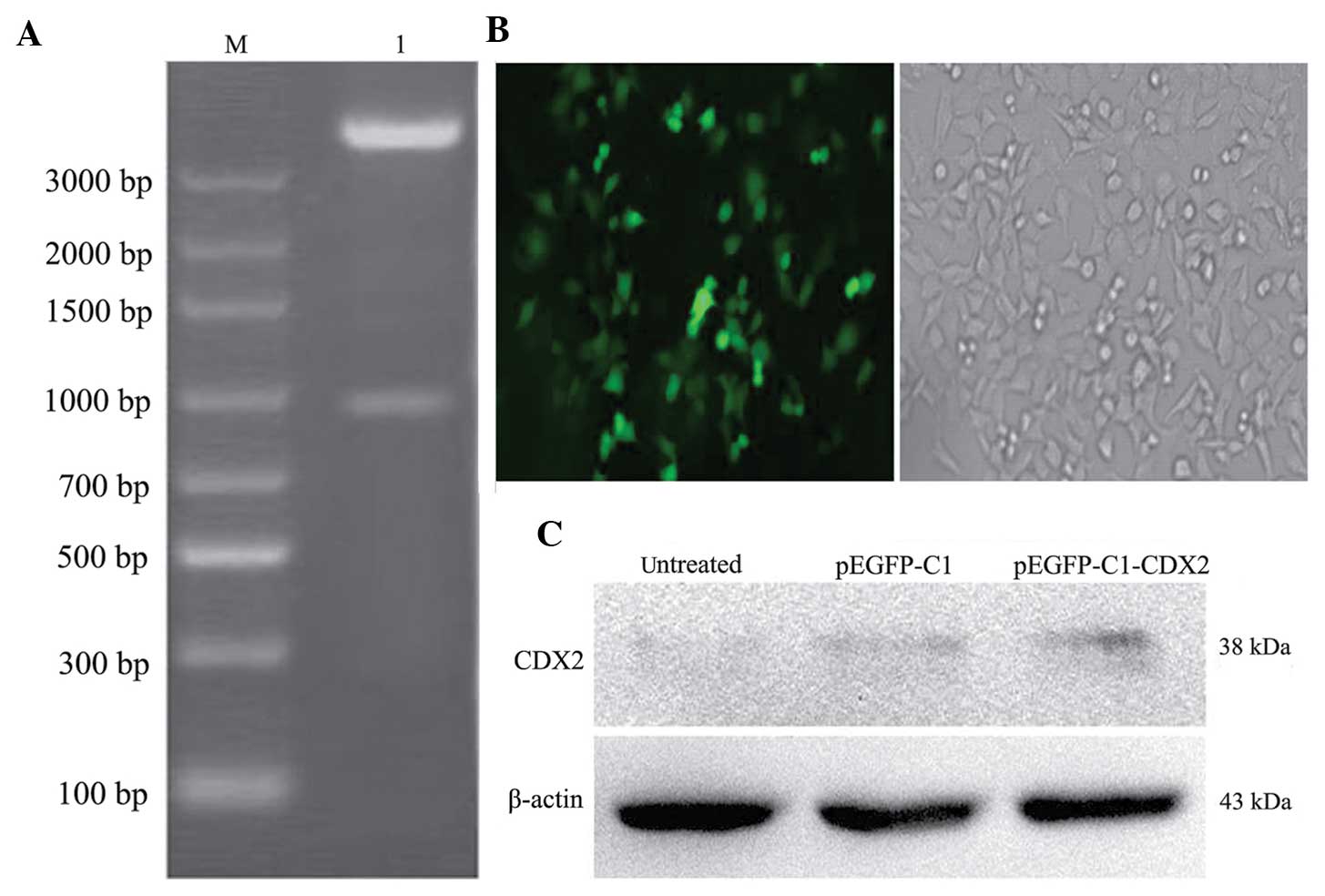
The pEGFP-C1-CDX2 recombinants were validated using
DNA sequencing analysis (data not shown) and restriction
endonuclease analysis (Fig. 1A).
None of the untransfected LoVo cells survived following G418 (250
µg/ml) selection for 2 weeks. The pEGFP-C1-CDX2- and
pEGFP-C1-transfected cells were continuously selected using G418
for 6 weeks, until a mono-clone was observed (Fig. 1B). The clones were then amplified,
to provide subclone pEGFP-C1 cells, pEGFP-C1-CDX2 cells and
untreated cells.
To investigate the protein expression levels of CDX2
in the untreated cells, pEGFP-C1 cells and pEGFP-C1-CDX2 cells, the
levels of CDX2 were measured using western blotting. The relative
expression levels of CDX2 to β-actin were determined. The protein
level of CDX2 in the pEGFP-C1-CDX2 cells was significantly higher
than those in the untreated cells and pEGFP-C1 cells (P<0.05).
The brightness of the CDX2 bands between the untreated and pEGFP-C1
cells exhibited no significant difference (P>0.05; Fig. 1C).
Growth of xenograft tumors in nude
mice
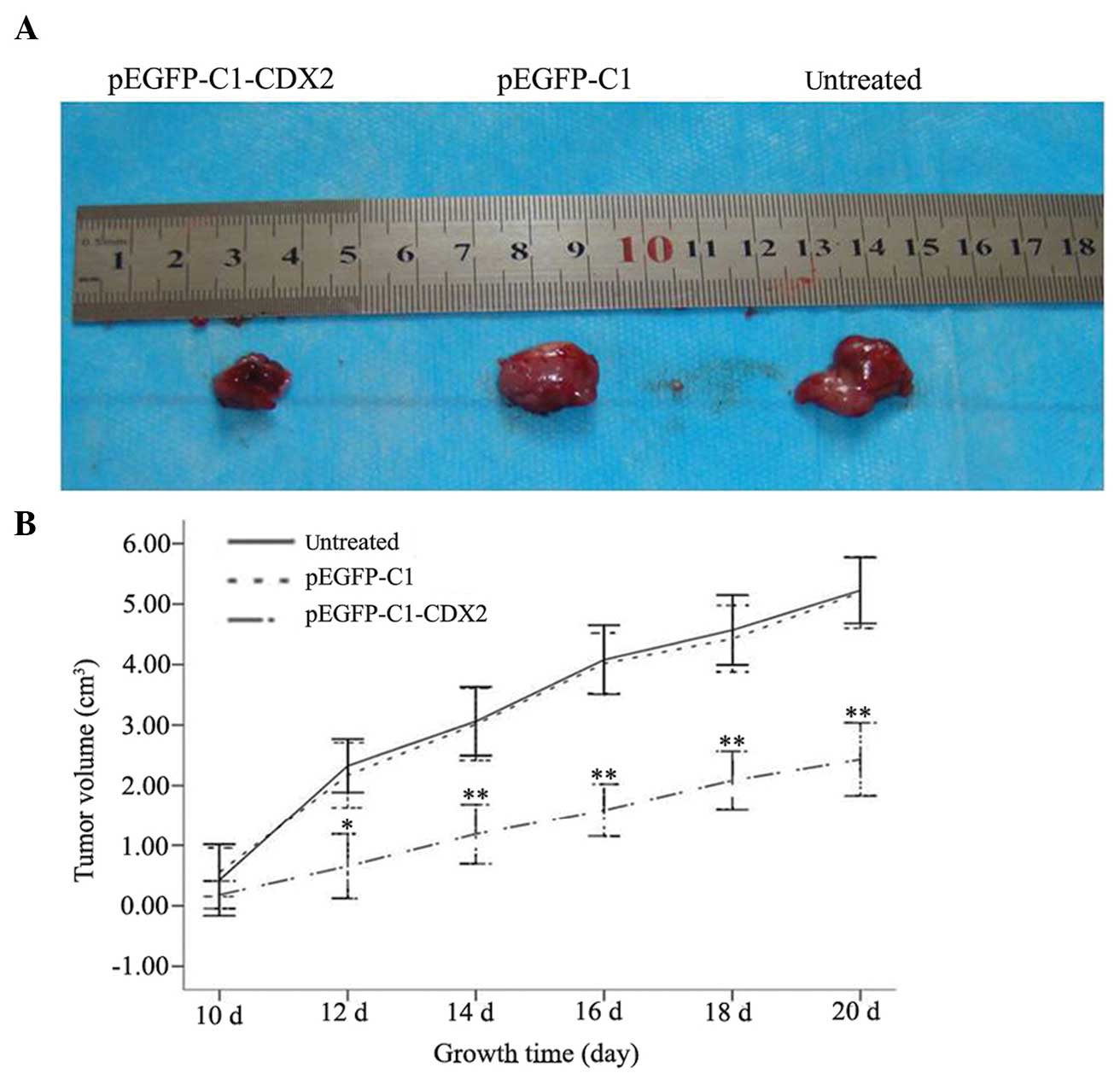
Within an average of 10 days, a 3- to 4-mm diameter
tumor developed at the subcu taneous injection sites of the right
hind lateral leg of the nude mice, with a 100% tumor formation
rate. From day 12, the tumor volumes between the pEGFP-C1-CDX2 cell
group and the untreated cell and pEGFP-C1 cell groups were
significantly different (P<0.05) and by day 20, tumor volumes of
5.22±0.27, 5.19±0.30 and 2.43±0.30 cm3 were recorded in
the untreated, pEGFP-C1 and pEGFP-C1-CDX2 cell groups, respectively
(Fig. 2A). Observations during the
10 day period were performed and a tumor growth curve was plotted
(Fig. 2B). The nude mice in each
group were healthy, fed a normal diet and exhibited no toxicity
throughout the feeding process during the inhibition of LoVo
proliferation by overexpressing CDX2. Until sacrifice of the nude
mice on day 21, the average weights of the transplanted tumors in
the untreated, pEGFP-C1 and pEGFP-C1-CDX2 groups were 0.62±0.22,
2.10±0.78 and 2.56±0.76 g, respectively. The tumor weight in the
pEGFP C1 CDX2 group was significnatly lighter than the other two
groups (*P<0.05). The rate of inhibition of tumor
weight in the pEGFP-C1-CDX2 cells group was 75.79%, which was
increased compared with the pEGFP-C1 group (17.79%) (Table I).
 | Table ITumor parameters following the 10-day
period prior to sacrifice of the nude mice. |
Table I
Tumor parameters following the 10-day
period prior to sacrifice of the nude mice.
| Cell group | Tumor weight
(g) | Inhibition rate
(%) |
|---|
| pEGFP-C1-CDX2 | 0.62±0.22 | 75.79 |
| pEGFP-C1 | 2.10±0.78* | 17.79 |
| Untreated | 2.56±0.76* | – |
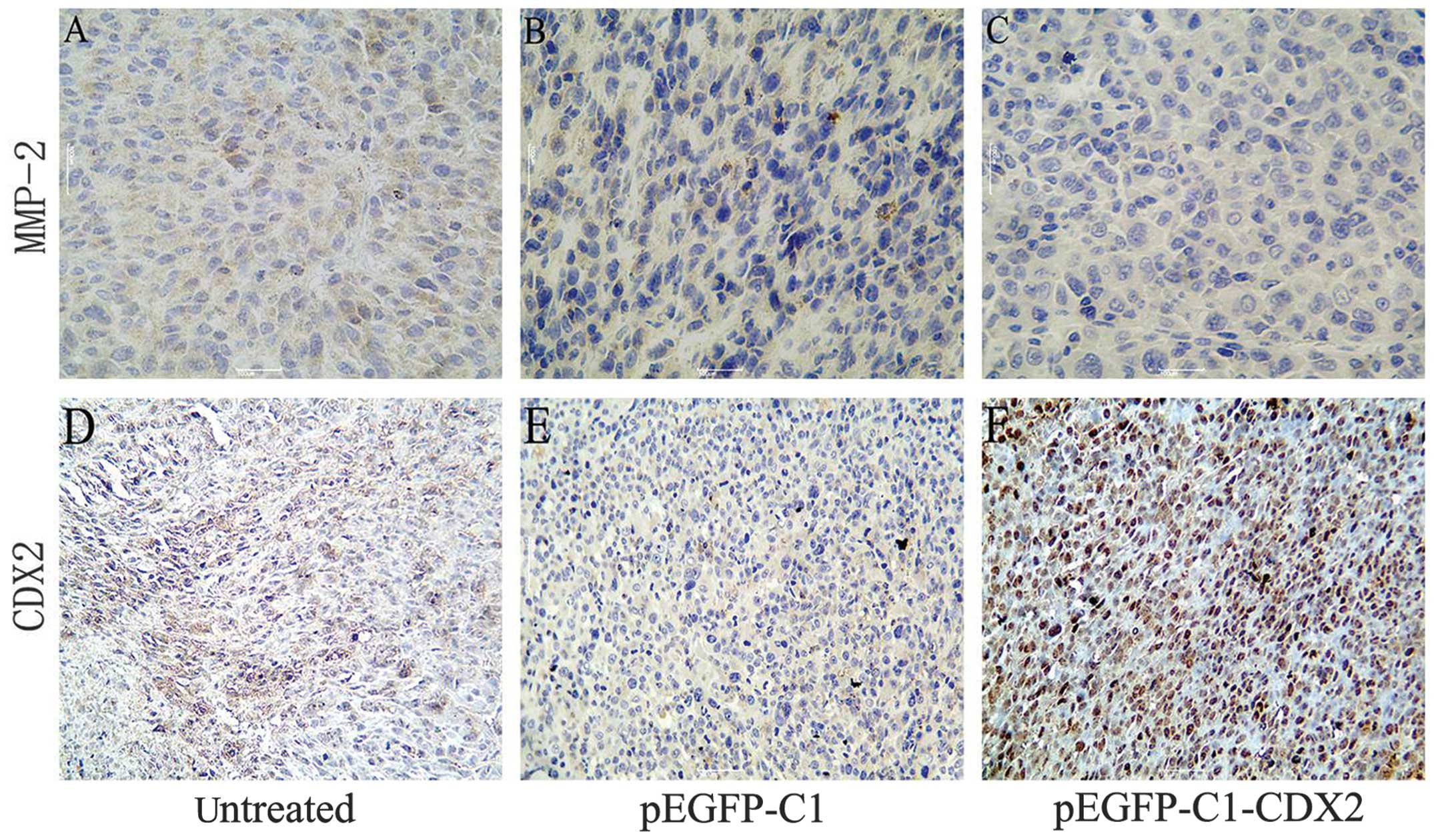
Immunohistochemical analysis of the
protein expression levels of CDX2 and MMP-2 in the xenograft
tumors
The protein expression levels were also investigated
using immunohistochemical staining of the xenograft tumor tissues.
An increase in the expression of CDX2, which was distributed in the
nucleus, was observed in the stably pEGFP-C1-CDX2-transfected cells
(brown staining in Fig. 3). In
addition, the immunohistochemical staining revealed a decrease in
the protein expression of MMP-2 in the pEGFP-C1-CDX2 cell group,
compared with the untreated and pEGFP-C1 cell groups.
Discussion
CDX2 is a member of the caudal homeobox gene family.
It was identified over 10 years ago in Drosophila
melanogaster. CDX2 is essential in intestinal epithelial cell
proliferation, differentiation, formation and maintenance of
columnar morphology and polarization (25,26),
and is an intestinal epithelial-specific marker with high
sensitivity in the human body. Excluding the intestinal epithelium,
the expression of CDX2 is seldom detected in the normal epithelium
of other systems (27). CDX2 is a
tumor suppressor gene in the formation and development of tumors,
however, its antitumor mechanism remains to be fully elucidated.
CDX2 may be involved in the arrest of cell differentiation,
abnormal decreased proliferation and altered cell adhesion. Our
previous study demonstrated that the positive rate of CDX2 was
69.4% in colorectal cancer, and 95.0% in normal colorectal tissues,
suggesting that the expression of CDX2 was high in the colorectal
tissues. In addition, the expression of CDX2 was significantly
higher in the normal colorectal tissues, compared with the
colorectal cancer tissues. With the decreased degree of colorectal
cancer differentiation, the protein expression of CDX2 appeared
reduced. The protein expression of CDX2 was also markedly lower in
the lymph node metastasis group than in the non-lymph node
metastasis group. In addition, CDX2 expression is reduced in Dukes
A-D colorectal cancer (28). These
results indicated that CDX2 possibly acts in the development of
colorectal cancer. The expression of CDX2 is correlated with the
degree of colorectal cancer malignancy, and decreased expression of
CDX2 indicates an increased degree of tumor malignancy and
increased invasion and metastasis, which has been observed in
previous studies (19,29). The above-described results suggest
that decreased expression of CDX2 is the predominant reason for
differentiation arrest or loss of tumor cells, and increased
expression levels of CDX2 possibly inhibit the growth and
metastasis of tumor cells. However, the above-mentioned studies
were focused predominantly on immunohistochemical expression and
in vitro molecular biological effects, while few studies
have investigated the in vivo effects.
The present study focused on in vivo
experiments in nude mice. Stably-transfected pEGFP-C1-CDX2 cells,
negative control pEGFP-C1 cells and untreated LoVo cells were
injected into BALB/c nude mice. Subcutaneous tumor formation was
detected within 10 days. With prolonged duration, tumor growth was
slower in the pEGFP-C1-CDX2 group compared with the pEGFP-C1 and
untreated control groups, with statistical significance. However,
no significant difference in tumor growth was observed between the
pEGFP-C1 and untreated cells groups. These results confirmed that
the overexpression of CDX2 in LoVo cells repressed the growth of
transplanted tumors.
The present study revealed that the overexpression
of CDX2 inhibited the proliferation of LoVo tumor cells in
vivo. However, the results of our previous in vitro
investigation demonstrated that the inhibitory effect on the LoVo
cells was not significantly altered following overexpression of
CDX2, with no suppression of cell division, indicating that CDX2
may not be involved in the proliferation of colon cancer cells
(30). The conflicting results
between the present in vivo experiments and previous in
vitro experiments may be due to a number of reasons During the
in vitro experiments, CDX2 was transiently expressed in the
LoVo cells. MTT assays were used to measure the effects of CDX2 on
tumor cell proliferation within 72 h, as well as on the cell cycle
and apoptosis. The in vivo experiments were performed over a
loner time-period, indicating that the inhibitory effects of CDX2
on colorectal cancer were time-dependent and the short-term effects
were not significant, but CDX2 may inhibit tumor growth over a
longer duration. In addition, in vivo experiments are more
representative of the microenvironment of human body, which is
composed of tumor cells, various host cells, extracellular matrix
and abundant secreted factors. The extracellular matrix affects
tumor cell proliferation, invasion and metastasis by affecting the
gene expression of CDX2 (31–33).
Additionally, hypoxic microenvironments commonly occur in solid
tumors, and hypoxia impacts the growth of these tumors by affecting
the gene expression of CDX2 (28,34).
However, the conclusion of the present study was consistent with
our previous conclusion that low gene expression levels of CDX2 in
colorectal cancer accelerates the malignant growth of colorectal
tumors (28).
Invasion and metastasis are basic biological
characteristics of malignant tumor cells, and >80% patients with
cancer succumb to mortality from these. Tumor cells have to degrade
the extracellular matrix and basement membrane during local
invasion and distant metastasis. Degradation of the extracellular
matrix depends on predominantly on various proteolytic enzymes
(35). Of the MMPs, MMP-2 is
important in tumor invasion and metastasis due to the ability of
MMP-2 to specifically degrade collagen IV (36). A previous study verified that MMP-2
affects the immune response and extracellular matrix remodeling,
promotes formation of tumor angiogenesis and contributes to tumor
metastasis (37). Numerous studies
have confirmed that increased expression of MMP-2 is positively
correlated with the invasion and metastasis of human gastric
cancer, colon cancer and breast cancer (38–43).
In the present study, protein expression of CDX2 was observed in
the tumor tissues following inoculation with the pEGFP-C1-CDX2
cells. In addition, the protein expression of MMP-2 was
significantly reduced, suggesting that CDX2 suppressed the invasion
and metastasis of tumor cells, possibly by downregulating the
expression of MMP-2, which was also demonstrated in the results of
our previous in vitro investigation (31).
In conclusion, CDX2 exhibited notable inhibitory
effects on the progression of colorectal cancer. CDX2 may be an
important tumor suppressor, which can be used as a novel index to
screen and monitor colorectal cancer, and may provide a novel
strategy for targeted cancer therapy.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (grant. nos. 81101874,
81172362 and 81172359), the Science and Technology Project of
Shaanxi Province (grant. no. 2011-K12-19), the Co-ordinative and
Innovative Plan Projects of the Science and Technology in Shaanxi
Province (grant. no. 2013KTCQ03-08) and the Clinical Innovation
Fund of the First Affiliated Hospital of Xi'an Jiaotong University
(grant. nos. 2ZD12 and 12ZD21).
Abbreviations:
|
CDX2
|
caudal-related homeobox protein 2
|
|
IECs
|
intestinal epithelial cells
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
CRC
|
colorectal cancer
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beck F, Chawengsaksophak K, Waring P,
Playford RJ and Furness JB: Reprogramming of intestinal
differentiation and intercalary regeneration in Cdx2 mutant mice.
Proc Natl Acad Sci USA. 96:7318–7323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olsen AK, Boyd M, Danielsen ET and
Troelsen JT: Current and emerging approaches to define intestinal
epithelium-specific transcriptional networks. Am J Physiol
Gastrointest Liver Physiol. 302:G277–G286. 2012. View Article : Google Scholar
|
|
4
|
Gao N, White P and Kaestner KH:
Establishment of intestinal identity and epithelial-mesenchymal
signaling by Cdx2. Dev Cell. 16:588–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verzi MP, Shin H, He HH, Sulahian R, Meyer
CA, Montgomery RK, Fleet JC, Brown M, Liu XS and Shivdasani RA:
Differentiation-specific histone modifications reveal dynamic
chromatin interactions and partners for the intestinal
transcription factor CDX2. Dev Cell. 19:713–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boyd M, Hansen M, Jensen TG, Perearnau A,
Olsen AK, Bram LL, Bak M, Tommerup N, Olsen J and Troelsen JT:
Genome-wide analysis of CDX2 binding in intestinal epithelial cells
(Caco-2). J Biol Chem. 285:25115–25125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suh E, Chen L, Taylor J and Traber PG: A
homeodomain protein related to caudal regulates intestine-specific
gene transcription. Mol Cell Biol. 14:7340–7351. 1994.PubMed/NCBI
|
|
8
|
Troelsen JT, Mitchelmore C, Spodsberg N,
Jensen AM, Norén O and Sjöström H: Regulation of lactase-phlorizin
hydrolase gene expression by the caudal-related homoeodomain
protein Cdx-2. Biochem J. 322:833–838. 1997.PubMed/NCBI
|
|
9
|
Fang R, Santiago NA, Olds LC and Sibley E:
The homeodomain protein Cdx2 regulates lactase gene promoter
activity during enterocyte differentiation. Gastroenterology.
118:115–127. 2000. View Article : Google Scholar
|
|
10
|
Lambert M, Colnot S, Suh E, L'Horset F,
Blin C, Calliot ME, Raymondjean M, Thomasset M, Traber PG and
Perret C: cis-Acting elements and transcription factors involved in
the intestinal specific expression of the rat calbindin-D9K gene:
binding of the intestine-specific transcription factor Cdx-2 to the
TATA box. Eur J Biochem. 236:778–788. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colnot S, Romagnolo B, Lambert M, Cluzeaud
F, Porteu A, Vandewalle A, Thomasset M, Kahn A and Perret C:
Intestinal expression of the calbindin-D9K gene in transgenic mice.
Requirement for a Cdx2-binding site in a distal activator region. J
Biol Chem. 273:31939–31946. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SY, Nagy BP, Brooks AR, Wang DM,
Paulweber B and Levy-Wilson B: Members of the caudal family of
home-odomain proteins repress transcription from the human
apolipoprotein B promoter in intestinal cells. J Biol Chem.
271:707–718. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakaguchi T, Gu X, Golden HM, Suh E,
Rhoads DB and Reinecker HC: Cloning of the human claudin-2
5′-flanking region revealed a TATA-less promoter with conserved
binding sites in mouse and human for caudal-related homeodomain
proteins and hepatocyte nuclear factor-1alpha. J Biol Chem.
277:21361–21370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto H, Bai YQ and Yuasa Y:
Homeodomain protein CDX2 regulates goblet-specific MUC2 gene
expression. Biochem Biophys Res Commun. 300:813–818. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mesquita P, Jonckheere N, Almeida R,
Ducourouble MP, Serpa J, Silva E, Pigny P, Silva FS, Reis C,
Silberg D, Van Seuningen I and David L: Human MUC2 mucin gene is
transcriptionally regulated by Cdx homeodomain proteins in
gastrointestinal carcinoma cell lines. J Biol Chem.
278:51549–51556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonhomme C, Duluc I, Martin E,
Chawengsaksophak K, Chenard MP, Kedinger M, Beck F and Domon-Dell
C: The Cdx2 homeobox gene has a tumour suppressor function in the
distal colon in addition to a homeotic role during gut development.
Gut. 52:1465–1471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoki K, Tamai Y, Horiike S, Oshima M and
Taketo MM: Colonic polyposis caused by mTOR-mediated chromosomal
instability in Apc+/Delta716 Cdx2+/-compound mutant mice. Nat
Genet. 35:323–330. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bakaris S, Cetinkaya A, Ezberci F and
Ekerbicer H: Expression of homeodomain protein CDX2 in colorectal
adenoma and adenocarcinoma. Histol Histopathol. 23:1043–1047.
2008.PubMed/NCBI
|
|
19
|
Choi BJ, Kim CJ, Cho YG, Song JH, Kim SY,
Nam SW, Lee SH, Yoo NJ, Lee JY and Park WS: Altered expression of
CDX2 in colorectal cancers. APMIS. 114:50–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong KD, Lee D, Lee Y, Lee SI and Moon HY:
Reduced CDX2 expression predicts poor overall survival in patients
with colorectal cancer. Am Surg. 79:353–360. 2013.PubMed/NCBI
|
|
21
|
Brabletz T, Spaderna S, Kolb J, Hlubek F,
Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C,
Kirchner T and Freund JN: Down-regulation of the homeodomain factor
cdx2 in colorectal cancer by collagen type I: an active role for
the tumor environment in malignant tumor progression. Cancer Res.
64:6973–6977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
American Psychological Association
Committee on Animal Research Ethics: Guidelines for ethical conduct
in the care and use of nonhuman animals in research. American
Psychological Association; Washington, DC, USA: 2012
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Collection: Reports funded by
National Institutes of Health; Washington, DC, USA: 2011
|
|
24
|
Hammond JB and Kruger NJ: The bradford
method for protein quantitation. Methods Mol Biol. 3:25–32.
1988.PubMed/NCBI
|
|
25
|
Kawai H, Tomii K, Toyooka S, Yano M,
Murakami M, Tsukuda K and Shimizu N: Promoter methylamine down
regulates CDX2 expression in colorectal carcinomas. Oncol Rep.
13:547–551. 2005.PubMed/NCBI
|
|
26
|
Keller MS, Ezaki T, Guo RJ and Lynch JP:
Cdxl or Cdx2 expression activates E-cadherin-ediated cell-cell
adhesion and compaction in human colo 205 cells. Am J Physiol
Castrointest Liver Physiol. 287:G104–G114. 2004. View Article : Google Scholar
|
|
27
|
Werling RW, Yaziji H, Bacchi CE and Gown
AM: CDX2, a highly sensitive and specific marker of adenocarcinomas
of intestinal origin: an immunohistochemical survey of 476 primary
and metastatic carcinomas. Am J Surg Pathol. 27:303–310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng J, Sun X, Wang W and Lu S:
Hypoxia-inducible factor-1α modulates the down-regulation of the
homeodomain protein CDX2 in colorectal cancer. Oncol Rep.
24:97–104. 2010.PubMed/NCBI
|
|
29
|
Dang LH, Chen F, Ying C, Chun SY, Knock
SA, Appelman HD and Dang DT: CDX2 has tumorigenic potential in the
human colon cancer cell lines Lovo and SW48. Oncogene.
25:2264–2272. 2006. View Article : Google Scholar
|
|
30
|
Zheng JB, Sun XJ, Li SS, Wang W, Ren HL,
Tian Y, Lu SY and Du JK: Effects of homeodomain protein CDX2
expression on the proliferation and migration of lovo colon cancer
cells. Pathol Oncol Res. 17:743–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gross I, Duluc I, Benameur T, Calon A,
Martin E, Brabletz T, Kedinger M, Domon-Dell C and Freund JN: The
intestine-specific homeobox gene Cdx2 decreases mobility and
antagonizes dissemination of colon cancer cells. Oncogene.
27:107–115. 2008. View Article : Google Scholar
|
|
32
|
Benahmed F, Gross I, Guenot D, Jehan F,
Martin E, Domon-Dell C, Brabletz T, Kedinger M, Freund JN and Duluc
I: The microenvi-ronment controls CDX2 homeobox gene expression in
colorectal cancer cells. Am J Pathol. 170:733–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Derbal-Wolfrom L, Pencreach E, Saandi T,
Aprahamian M, Martin E, Greferath R, Tufa E, Choquet P, Lehn JM,
Nicolau C, Duluc I and Freund JN: Increasing the oxygen load by
treatment with myo-inositol trispyrophosphate reduces growth of
colon cancer and modulates the intestine homeobox gene Cdx2.
Oncogene. 32:4313–4318. 2013. View Article : Google Scholar
|
|
35
|
Jiang WG, Sanders AJ, Katoh M, et al:
Tissue invasion and metastasis: Molecular, biological and clinical
perspectives. Semin Cancer Biol. View Article : Google Scholar
|
|
36
|
Murray GI: Matrix metalloproteinasers: a
multifunctional group of molecules. J Pathol. 195:135–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal progression and metastasis.
Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
38
|
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS,
Di GH, Liu G, Li FM, Ou ZL, et al: Prognostic value of matrix
metalloproteinases (MMP-2 and MMP-9) in patients with lymph
nodenegative breast carcinoma. Breast Cancer Res Treat. 88:75–85.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barbarosos A, Biacchi D, Bolognese A,
Galati G, Izzo L, Risuleo G and Tartaglia E: Molecular
epidemiologic analysis of the levels of metalloproteinases and
cyclooxygenase-2 in colorectal cancer. Supp Tumor. 4:S2062005.
|
|
40
|
Baker EA and Leaper DJ: The plasminogen
activator and matrix metalloproteinase systems in colorectal
cancer: relationship to tumor pathology. Eur J Cancer. 39:981–988.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bodey B, Bodey B Jr, Siegel SE and Kaiser
HE: Prognostic significance of matrix metalloproteinase expression
in colorectal carcinoma. In vivo (Athens, Greece). 14:659–666.
2000.
|
|
42
|
Curran S, Dundas SR, Buxton J, Leeman MF,
Ramsay R and Murray GI: Murray matrix metalloproteinase/tissue
inhibitors of matrix metalloproteinase phenotype identifies poor
prognosis colorectal cancers. Clinic Can Res. 10:8229–8234. 2004.
View Article : Google Scholar
|
|
43
|
Pesta M, Holubec L Jr, Topolcan O, Cerna
M, Rupert K, Holubec LS, Treska V, Kormunda S, Elgrova L, Finek J
and Cerny R: Quantitative estimation of matrix metalloproteinases 2
and 7 (MMP-2, MMP-7) and tissue inhibitors of matrix
metal-loproteinases 1 and 2 (TIMP-1, TIMP-2) in colorectal
carcinoma tissue samples. Anticancer Res. 25:3387–3391.
2005.PubMed/NCBI
|