Introduction
Hilar cholangiocarcinoma is a life threatening
malignancy, which is difficult to diagnose and is associated with
high mortality rates (1). Surgery,
either in the form of liver resection or liver transplantation, is
the only effective curative therapy for hilar cholangiocarcinoma
(2,3) and only 50–70% of the patients who
undergo surgery are candidates for curative resection (4), however, the recurrence rates remain
high even following curative resection (5). Adjuvant therapy, including
chemotherapy and radiation therapy, has not been confirmed to
reduce the risk of recurrence (6).
Therefore, identifying novel biomarkers, which are involved in
tumor development and progression may assist in improving
therapeutic strategies and patient outcomes.
Hepatoma-derived growth factor (HDGF), as a member
of the heparin-binding growth factors, was originally purified from
cultured media with the HuH-7 human hepatoma cell line (7,8).
Previous studies have demonstrated that HDGF is upregulated in
various types of malignancy and is predictive of a poor survival
outcome, and that HDGF possesses aggressive biological behaviors
in vitro, including proliferation, migration, invasiveness
and angiogenesis (9–12). However, the correlation between the
malignant behaviors and HDGF in the hilar cholangiocarcinoma cell
line remains to be fully elucidated.
The present study was designed to clarify the
malignant behaviors of the FRH0201 human hilar cholangiocarcinoma
cell line by using small interfering (si)RNA in vitro, and
aimed to provide novel experimental evidence for the targeted
therapy of human hilar cholangiocarcinoma.
Materials and methods
Cell lines and cell culture
The human FRH0201 hilar cholangiocarcinoma cell line
was provided by Professor Xiaopeng Wu (Qilu Hospital of Shandong
University, Jinan, China). The cells were incubated in Dulbecco's
modified Eagle' medium (DMEM), supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Invitrogen Life
Technologies, Carlsbad, CA, USA), at 37°C with 5%
CO2.
siRNA transfection
The sequence of the siRNA targeting HDGF
(5′-AACCGGCAGAAGGAGUACAAA-3′) used was adopted from a previous
study (10). The HDGF siRNA and
negative control siRNA were chemically synthesized by GenePharma
Co., Ltd. (Shanghai, China). The corresponding cells were divided
as follows: Control, negative-siRNA and HDGF-siRNA group. In
vitro transfections were performed using Lipofectamine RNAiMax
(Invitrogen Life Technologies), according to the manufacturer's
instructions. All siRNAs were dissolved in sterilized and
RNase-free water at a final concentration of 20 mM. Briefly, the
FRH0201 cells were seeded in a six-well plate at a density of
5×105 cells/well at 37°C with 5% CO2 and were
incubated until they reached 80% confluence. The cells were
incubated in the siRNA-Lipofectamine complex-containing medium for
6 h, following which the medium was replaced with DMEM containing
10% FBS at 37°C with 5% CO2. The cells were incubated
for 48 h and were then harvested for analysis of the mRNA and
protein expression levels of HDGF.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the FRH0201 cells
in 96-well plates (seeded at a density of 2×103/200
µl) using TRIzol reagent (Takara Bio, Inc., Tokyo, Japan).
The quantity of total RNA was 2 µg as per the instructions.
First strand cDNA was synthesized from the mRNA using a
Primescript™ RT reagent kit (Takara, Bio., Inc.), and the RT-qPCR
was performed using a SYBR Green PCR kit (Takara, Bio., Inc.) on a
Light Cycler system 2.0 (Roche Diagnostics, Mannheim, Germany). The
sequence of the primers (GenePharma Co., Ltd., Shanghai, China)
used were as follows: HDGF, sense 5′-CAGCCAACAAATACCAAGTCT-3′ and
antisense 5′-GTTCTCGATCTCCCACAGC-3′, and GAPDH, sense
5′-GGTGGTCTCCTCTGACTTCAACA-3′ and antisense
5′-GTTGCTGTAGCCAAATTCGTTGT-3′. The PCR conditions were as follows:
Initial denaturation at 95°C for 10 sec, followed by 45 cycles at
95°C for 5 sec, 60°C for 30 sec and 72°C for 10 sec. The
comparative threshold cycle (Ct) method (2−ΔΔCt) was
used to analyze the relative changes in gene expression and the
levels were normalized against GAPDH (13). The experiment was repeated twice
with triplicate measurements in each experiment.
Western blot analysis
All the grouped cells were harvested and rinsed
twice with phosphate-buffered saline (PBS). The total cellular
protein was extracted using a Nuclear and Cytoplasmic Protein
Extraction kit (Beyotime Institute of Biotechnology, Shanghai,
China). Following extraction, the protein concentration was
measured using an Enhanced BCA Protein Assay kit (Beyotime
Institute of Biotechnology), and an equal quantity of protein (30
µg) from each group was subjected to 10% SDS-PAGE (Beyotime
Institute of Biotechnology). The proteins were subsequently
transferred onto polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with
Tris-buffered saline with Tween-20 (pH 7.6; Beyotime Institute of
Biotechnology), containing 5% non-fat milk for 2 h at room
temperature, the PVDF membranes were incubated with rabbit
anti-humanHDGF polyclonal antibody (1:100; Proteintech Group, Inc.,
Chicago, USA) and rabbit anti-human β-actin monoclonal antibody
(1:1,000; Santa Cruz Biochemistry, Inc., Santa Cruz, CA, USA)
overnight at 4°C. The membranes were subsequently incubated for 1 h
with horseradish peroxidase-conjugated secondary antibody (1:1,000;
Beyotime Institute of Biotechnology) at room temperature. The
immunoreactive bands were visualized using a Chemilluminescent ECL
Detection system (EMD Millipore), according to the manufacturer's
instructions. The intensity of each band was quantified using
ImageJ software version 1.43 (National Institutes of Health,
Bethesda, MD, USA). The experiment was repeated twice with
triplicate measurements in each experiment.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
Prior to siRNA transfection, 104 cells
were seeded into a 96-well plate (200 µl/well), with three
wells for each group. At 48 h post-transfection, MTT (5 mg/ml; 20
µl/well; Beyotime Institute of Biotechnology) was added and
the cells were cultured at 37°C for 4 h. Following discarding of
the supernatant, the cells were mixed with dimethyl sulfoxide (150
µl/well; Beyotime Institute of Biotechnology) for 10 min.
The absorbance of each well was measured at 570 nm (A570) using an
ultraviolet spectrophotometer, UV9100 (LabTech, Inc., Beijing,
China) and the cell proliferation rate was calculated.
Wound healing migration assay
Briefly, the cells in each group were seeded into a
12-well plate, at equal densities, in complete medium and were
incubated at 37°C with 5% CO2 until the cells had grown
to 80% confluence. Scratching wounds of an identical width were
created in the monolayer using a sterile pipette tip. The wells
were rinsed with PBS three times to remove floating cells and
debris, and the remaining cells were cultured in serum-free DMEM,
with the culture plates incubated at 37°C and in 5% CO2.
Following incubation, wound healing was measured and images were
captured with a light microscope Olympus IX81 (Olympus, Tokyo,
Japan) at 0, 8 and 16 h. The experiment was repeated twice with
triplicate measurements in each experiment.
Transwell invasion assay and migration
assay
The invasive capability of tumor cells was
determined using Matrigel-coated Transwell invasion chambers (8
µm pore size; BD Biosciences, Bedford, MA, USA). At 48 h
post-siRNA treatment, the cells in the groups were collected and
105 cells from each group were added to upper Transwell
chambers in 100 µl serum-free medium. The lower chamber was
filled with 500 µl DMEM, containing 10% FBS. Following
incubation at 37°C for 24 h, the cells that had invaded through the
membrane were fixed with methanol for 10 min, stained with Trypan
blue (Beyotime Institute of Biotechnology) for 10 min and counted
under a light microscope Olympus IX81 (Olympus). The migration
assay were performed in a similar manner, using a Transwell chamber
without Matrigel, and incubation conditions were 37°C for 16 h.
Triplicate measurements were performed in each experiment.
Enzyme-linked immunosorbent assay
(ELISA)
The cells from all the groups were seeded into
six-well plates (5×105 cells/well) and cultured in
serum-free DMEM for 24 h at 37°C with 5% CO2. The
supernatant was collected, centrifuged at 1,000 × g for 15 min at
4°C, filtered through a 0.22 mm filter (EMD Millipore) and stored
at −80°C until use. Additionally, the supernatant levels of
vascular endothelial growth factor (VEGF) were detected using a
VEGF ELISA kit (Pierce Biotechnology, Rockford, IL, USA), to
recognize VEGF165 and VEGF121. The ELISA was performed, according
to the manufacturer's instructions. The concentrations of VEGF in
the supernatant was measured in duplicate.
Statistical analysis
All results are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The differences between
groups were assessed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
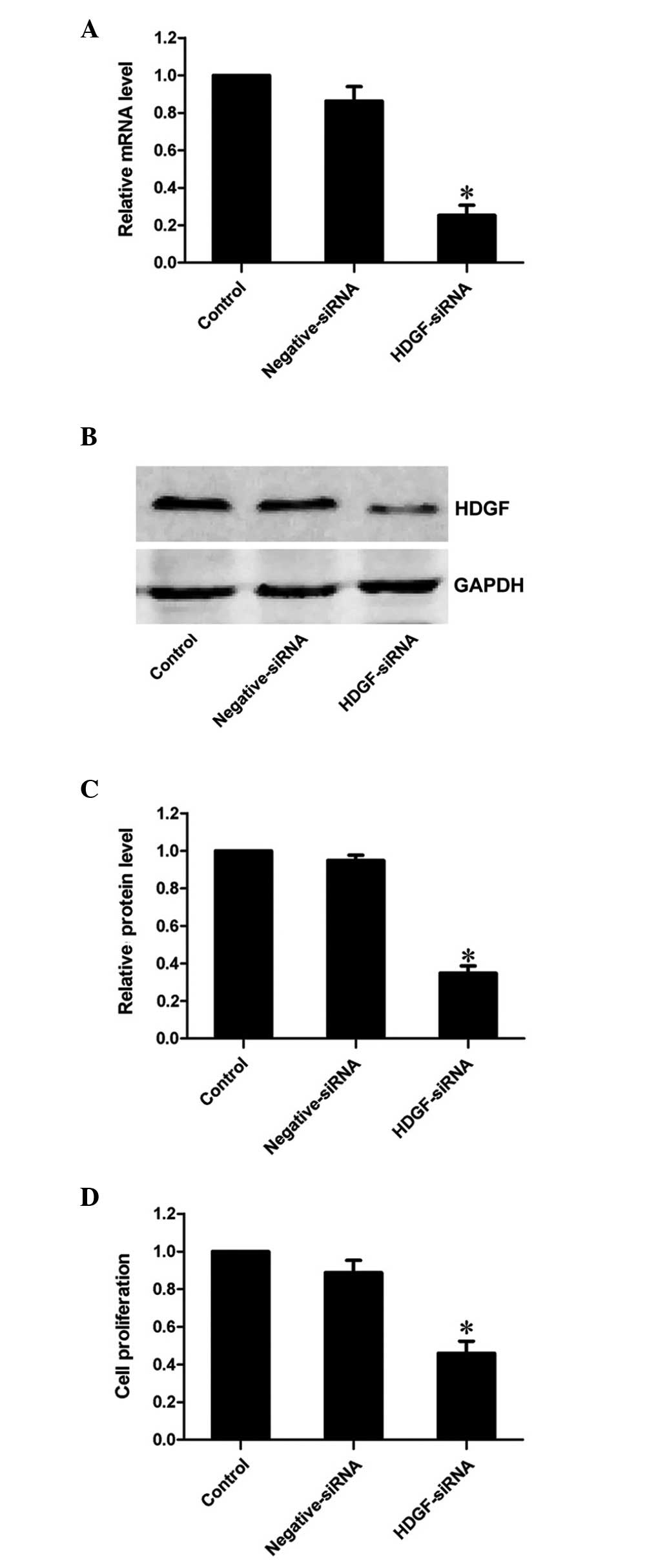
HDGF siRNA effectively suppresses the
mRNA and protein expression levels of HDGF
siRNA targeting HDGF was used to decrease the mRNA
expression levels of HDGF in the FRH0201 cells. RT-qPCR revealed
that the mRNA expression of HDGF was markedly decreased to 25.3% by
the HDGF siRNA at 48 h post-transfection, compared with the control
group (P<0.01; Fig. 1A).
Similarly, western blot analysis demonstrated that the protein
expression of HDGF was inhibited to 34.9% of that observed in the
control group (P<0.01; Fig. 1B and
C). This confirmed that HDGF siRNA effectively inhibited the
expression of its target gene, HDGF, at the transcriptional and
translational levels.
Downregulation of the expression of HDGF
decreases the proliferative ability of FRH0201 cells
The results of the MTT assay revealed that the
proliferative ability of the FRH0201 cells treated with HDGF siRNA
was 46.0% of that observed in the control cells 48 h
post-transfection (P<0.01; Fig.
1D).
Downregulation of the expression of HDGF
inhibits the migratory ability of FRH0201 cells
In the present study, a cell wound healing migration
assay and a Transwell migration assay were used to detect the
migratory ability of tumor cells in vitro. In the cell wound
healing migration assay, no statistical difference was observed
between the groups, although the migratory ability was decreased 8
h post-transfection with HDGF siRNA. By contrast, the migratory
ability of the HDGF siRNA group was significantly decreased at 16 h
post-transfection, and was 41.7% of that observed in the control
cells (P<0.05: Fig. 2). In
addition, the Transwell migration assay confirmed in,
three-dimensional culture medium, that the migration of the cells
transfected with HDGF siRNA was inhibited and the number of
migrating cells was 26.5% of that observed in the control cells
(P<0.01; Fig. 3).
Downregulation of the expression levels
of HDGF inhibits the invasive ability of FRH0201 cells
The Transwell invasion assay revealed that the
invasive ability of the cells transfected with HDGF siRNA was
inhibited. The number of invading cells was 22.7% of that observed
in the control cells (P<0.01; Fig.
3).
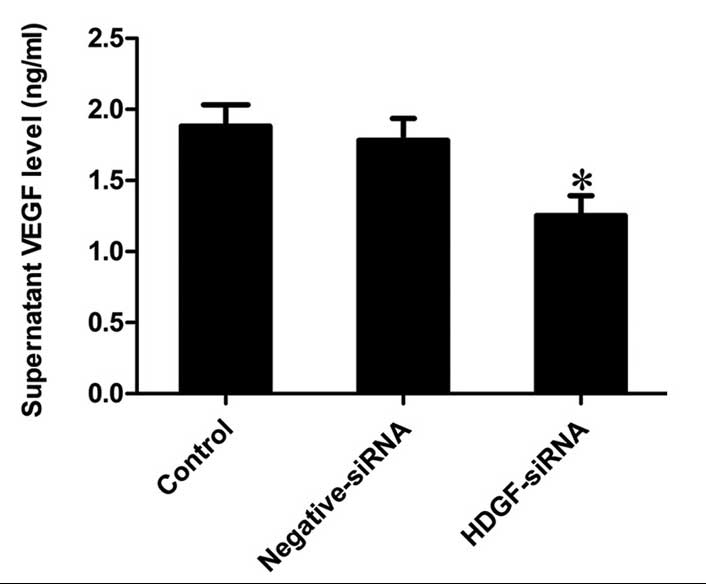
Downregulation of the protein expression
of HDGF inhibits the secretion of VEGF in FRH0201 cells
The levels of VEGF, which were detected in the
supernatants of the different groups revealed that the secretion of
VEGF was significantly inhibited by HDGF siRNA, the level of which
was 66.51% of that observed in the control supernatant (P<0.001;
Fig. 4).
Discussion
The potential capacities of malignant tumors,
including sustaining proliferative signaling, enabling replicative
immortality, inducing angiogenesis, and activating invasion and
metastasis, make its therapeutic strategies more complicated and
difficult (14). Therefore,
identifying and screening potential molecular targets or tumor
biomarkers involved in one or more malignant processes is
beneficial for tumor diagnosis and therapy, and for improving
patient survival rates and prognosis.
As a nuclear-targeted mitogen, HDGF has mitogenic
activity for various cells following translocation into the
nucleus, including hepatic, gastric, lung cancer cells,
fibroblasts, endothelial cells, smooth muscle cells and neuronal
cells (7,15–20).
The downregulation of HDGF suppresses cancer cell growth and
invasion, induces apoptosis, and may function as a tumor survival
factor (21,22). Previously, a systematic proteomic
investigation of human metastatic hepatocellular carcinoma (HCC)
cell lines demonstrated that the HDGF protein was one of the
metastasis-associated proteins, and that knockdown of HDGF induced
cell apoptosis in metastatic HCC cells (23). Therefore, HDGF may act as an
oncogene, being important in tumor pathogenesis and progression,
and may be a key target for future therapy. However, the roles of
HDGF protein in the malignant biological behaviors of the human
hilar cholangiocarcinoma cell line, including proliferation,
migration, invasion and angiogenesis remain to be elucidated.
Tumorigenesis and progression is the result of cell
proliferation and apoptosis in a condition of disequilibrium
(24). Several studies have
indicated that, following nuclear translocation, HDGF promotes the
proliferation of numerous tumor cells, whereas HDGF interference,
knockdown or neutralizing antibodies significantly increase the
rate of apoptosis and inhibit proliferation (25–27).
The results of the present study demonstrated that, following
downregulation of the HDGF protein by HDGF siRNA, the proliferative
capacity of the FRH0201 cells was significantly decreased.
Tumor cell migration, invasion and metastasis is a
complicated process involving multiple steps and factors (28). Although primary tumors can be
effectively controlled or cured with surgery, radiotherapy,
chemotherapy and other local therapies, these methods fail to
manipulate disease progression for disseminated tumors, which is an
important cause of malignancy-associated mortality (29). Therefore, inhibiting the process of
tumor cell migration and invasion is an important therapeutic
strategy. Previous studies have demonstrated that knockdown of the
expression of HDGF inhibits tumor cell migration and invasion in
hepatic, lung and prostate carcinoma (12,21,30).
Our previous study demonstrated that tumor tissues with high
expression levels of HDGF exhibit increased invasive abilities,
compared with tissues expressing a low level HDGF. Similarly,
patients with increased expression of HDGF have been observed to
have a significantly poorer outcome, compared with those exhibiting
low expression levels of HDGF (31). In the present study it was
confirmed that inhibition of the protein expression of HDGF
significantly decreased the migration and invasion of FRH0201
cells.
It is generally accepted that tumor growth, invasion
and metastasis require angiogenesis, and that vascularization is
closely associated with tumor invasion and patient prognosis
(32). HDGF, a member of the
heparin-binding growth factor family, exhibits a wide range of
biological functions and can promote tumor neovascular formation
and development as a potential endothelial mitogen (19,33).
Previous studies have demonstrated that high expression levels of
HDGF activate the extracellular signal-regulated kinase pathway and
upregulate the secretion of VEGF (34–36).
In addition, another study revealed that HDGF is a potent
endothelial mitogen in vivo and regulates endothelial cell
migration by mechanisms distinct from VEGF (11). Okuda et al (37) reported that HDGF-induced tumor
formation and angiogenesis in vivo involves the direct
angiogenic activity and induction of VEGF secretion. In the present
study downregulation of the expression of HDGF decreased the
secretion of VEGF, which demonstrated that HDGF may induce
angiogenesis partially by the action of VEGF.
In conclusion, the present study demonstrated that
HDGF was important in promoting the malignant biological behaviors
(proliferation, migration and invasion) of the FRH0201 hilar
cholangiocarcinoma cell line, and inhibition of the expression of
HDGF downregulated the malignant biological behaviors. These
results provide novel insights and indicate the potential clinical
use of HDGF as an effective therapeutic target for hilar
cholangiocarcinoma by inhibiting proliferation, migration and
invasion of cancer cells. Further investigation of the mechanism
underlying the action of HDGF in hilar cholangiocarcinoma is
required.
Acknowledgments
This study was supported by the Promotive Research
fund for Excellent Young and Middle-aged Scientisits of Shandong
Province (grant. no. BS2013YY029), the Natural Science Foundation
of China (grant. no. 81302123) and the China Postdoctoral Science
Foundation funded project (grant. nos. 201003781 and
20080441310).
References
|
1
|
Khans A, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar
|
|
2
|
Ortner MA and Dorta G: Technology insight:
Photodynamic therapy for cholangiocarcinoma. Nat Clin Pract
Gastroenterol Hepatol. 3:459–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang BY, Lu Y, Dong Q, Sun CD and Mu P:
Surgical treatment and prognostic analysis of 93 cases of hilar
cholangiocarcinoma. Am J Med Sci. 339:221–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M,
Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D and Blumgart
LH: Staging, resectability and outcome in 225 patients with hilar
cholangiocarcinoma. Ann Surg. 234:507–517; discussion 517–519.
2001. View Article : Google Scholar
|
|
5
|
Patel T: Cholangiocarcinoma. Nat Clin
Pract Gastroenterol Hepatol. 3:33–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jarnagin WR and Shoup M: Surgical
management of cholangiocarcinoma. Semin Liver Dis. 24:189–199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura H, Izumoto Y, Kambe H, Kuroda T,
Mori T, Kawamura K, Yamamoto H and Kishimoto T: Molecular cloning
of complementary DNA for a novel human hepatoma-derived growth
factor. Its homology with high mobility group-1 protein. J Biol
Chem. 269:25143–25149. 1994.PubMed/NCBI
|
|
8
|
Nakamura H, Kambe H, Egawa T, Kimura Y,
Ito H, Hayashi E, Yamamoto H, Sato J and Kishimotos: Partial
purification and characterization of human hepatoma-derived growth
factor. Clin Chim Acta. 183:273–284. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okuda Y, Nakamura H, Yoshida K, Enomoto H,
Uyama H, Hirotani T, Funamoto M, Ito H, Everett AD, Hada T, et al:
Hepatoma-derived growth factor induces tumorigenesis in vivo
through both direct angiogenic activity and induction of vascular
endothelial growth factor. Cancer Sci. 94:1034–1041. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Ren H, Yuan P, Lang W, Zhang L
and Mao L: Down-regulation of hepatoma-derived growth factor
inhibits anchorage-independent growth and invasion of non-small
cell lung cancer cells. Cancer Res. 66:18–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Everett AD, Narron JV, Stoops T, Nakamura
H and Tucker A: Hepatoma-derived growth factor is a pulmonary
endothelial cell-expressed angiogenic factor. Am J Physiol Lung
Cell Mol Physiol. 286:L1194–L1201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Z, He Y, Wang S, Zhang A, Zhao P, Gao
C and Cao B: Various effects of hepatoma-derived growth factor on
cell growth, migration and invasion of breast cancer and prostate
cancer cells. Oncol Rep. 26:511–517. 2011.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Everett AD and Bushweller J: Hepatoma
derived growth factor is a nuclear targeted mitogen. Curr Drug
Targets. 4:367–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Everett AD, Stoops T and McNamara CA:
Nuclear targeting is required for hepatoma-derived growth
factor-stimulated mitogenesis in vascular smooth muscle cells. J
Biol Chem. 276:37564–37568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kishima Y, Yamamoto H, Izumoto Y, Yoshida
K, Enomoto H, Yamamoto M, Kuroda T, Ito H, Yoshizaki K and Nakamura
H: Hepatoma-derived growth factor stimulates cell growth after
translocation to the nucleus by nuclear localization signals. J
Biol Chem. 277:10315–10322. 2002. View Article : Google Scholar
|
|
18
|
Zhou Z, Yamamoto Y, Sugai F, Yoshida K,
Kishima Y, Sumi H, Nakamura H and Sakodas: Hepatoma-derived growth
factor is a neurotrophic factor harbored in the nucleus. J Biol
Chem. 279:27320–27326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Everett AD, Lobe DR, Matsumura ME,
Nakamura H and McNamara CA: Hepatoma-derived growth factor
stimulates smooth muscle cell growth and is expressed in vascular
development. J Clin Invest. 105:567–575. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ooi BN, Mukhopadhyay A, Masilamani J, Do
DV, Lim CP, Cao XM, Lim IJ, Mao L, Ren HN, Nakamura H, et al:
Hepatoma-derived growth factor and its role in keloid pathogenesis.
J Cell Mol Med. 14:1328–1337. 2010. View Article : Google Scholar
|
|
21
|
Meng J, Xie W, Cao L, Hu C and Zhe Z:
shRNA targeting HDGF suppressed cell growth and invasion of
squamous cell lung cancer. Acta Biochim Biophys Sin (Shanghai).
42:52–57. 2010. View Article : Google Scholar
|
|
22
|
Tsang TY, Tang WY, Tsang WP, Co NN, Kongs
K and Kwok TT: Mechanistic study on growth suppression and
apoptosis induction by targeting hepatoma-derived growth factor in
human hepatocellular carcinoma HepG2 cells. Cell Physiol Biochem.
24:253–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu Y, Shen H, Yu H, Zhong F, Zhang Y,
Zhang C, Zhao J, Li H, Chen J, Liu Y, et al: Systematic proteomic
analysis of human hepotacellular carcinoma cells reveals molecular
pathways and networks involved in metastasis. Mol Biosyst.
7:1908–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carson DA and Ribeiro JM: Apoptosis and
disease. Lancet. 341:1251–1254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsang TY, Tang WY, Tsang WP, Co NN, Kongs
K and Kwok TT: Downregulation of hepatoma-derived growth factor
activates the Bad-mediated apoptotic pathway in human cancer cells.
Apoptosis. 13:1135–1147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren H, Chu Z and Mao L: Antibodies
targeting hepatoma-derived growth factor as a novel strategy in
treating lung cancer. Mol Cancer Ther. 8:1106–1112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao F, Dong W and Fan L: Apoptosis of
human colorectal carcinoma cells is induced by blocking
hepatoma-derived growth factor. Med Oncol. 27:1219–1226. 2010.
View Article : Google Scholar
|
|
28
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valastyans and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar
|
|
30
|
Zhou Y, Zhou N, Fang W and Huo J:
Overexpressed HDGF as an independent prognostic factor is involved
in poor prognosis in Chinese patients with liver cancer. Diagn
Pathol. 5:582010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YF, Zhao R, Guo S, Wang XQ, Lian PL,
Chen YG and Xu KS: Expression and clinical significance of
hepatoma-derived growth factor as a prognostic factor in human
hilar cholangio-carcinoma. Ann Surg Oncol. 18:872–879. 2011.
View Article : Google Scholar
|
|
32
|
Maeda K, Chung YS, Takatsukas, Ogawa Y,
Sawada T, Yamashita Y, Onoda N, Kato Y, Nitta A, Arimoto Y, et al:
Tumor angiogenesis as a predictor of recurrence in gastric
carcinoma. J Clin Oncol. 13:477–481. 1995.PubMed/NCBI
|
|
33
|
Oliver JA and Al-Awqati Q: An endothelial
growth factor involved in rat renal development. J Clin Invest.
102:1208–1219. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao J, Xu Z, Fang Y, Wang H, Xu J, Ye J,
Zhengs and Zhu Y: Hepatoma-derived growth factor involved in the
carcinogenesis of gastric epithelial cells through promotion of
cell proliferation by Erk1/2 activation. Cancer Sci. 99:2120–2127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee KH, Choi EY, Kim MK, Lee SH, Jang BI,
Kim TN, Kims W, Kims W, Songs K, Kim JR, et al: Hepatoma-derived
growth factor regulates the bad-mediated apoptotic pathway and
induction of vascular endothelial growth factor in stomach cancer
cells. Oncol Res. 19:67–76. 2010. View Article : Google Scholar
|
|
36
|
Ooi BN, Mukhopadhyay A, Masilamani J, Do
DV, Lim CP, Cao XM, Lim IJ, Mao L, Ren HN, Nakamura H, et al:
Hepatoma-derived growth factor and its role in keloid pathogenesis.
J Cell Mol Med. 14:1328–1337. 2010. View Article : Google Scholar
|
|
37
|
Okuda Y, Nakamura H, Yoshida K, Enomoto H,
Uyama H, Hirotani T, Funamoto M, Ito H, Everett AD, Hada T, et al:
Hepatoma-derived growth factor induces tumorigenesis in vivo
through both direct angiogenic activity and induction of vascular
endothelial growth factor. Cancer Sci. 94:1034–1041. 2003.
View Article : Google Scholar : PubMed/NCBI
|