Introduction
Osteoarthritis (OA), the most common multifactorial
degenerative joint disease in the elderly, is characterized by
progressive degeneration of the articular cartilage, changes in the
subchondral bone, osteophyte formation and synovial inflammation
(1). The etiology of OA involves
numerous mechanical and biochemical factors (2–5).
Healthy articular cartilage is essentially avascular
and is resistant to vascular invasion in vitro (6,7). In
OA, however, the invasion of blood vessels from the subchondral
bone is apparent even in the early stages of the disease and
subsequently leads to the loss of tidemark integrity (8–11).
Angiogenesis is dependent on a complex network, which is regulated
in a timely and sequential order to mediate blood vessel formation,
and vascular endothelial growth factor (VEGF) has been recognized
as a dominant mediator of this process (12). VEGF-dependent signaling in
embryonic development is important for the regulation of growth
plate morphogenesis and the coupling between cartilage and bone
formation (13). As healthy adult
cartilage is essentially avascular, VEGF is not expressed in normal
chondrocytes. However, several studies have revealed the expression
of VEGF and the corresponding receptors in OA (14–17).
Osteopontin (OPN) is a member of the small
integrin-binding ligand N-linked glycosylated protein family. It is
abundant in the extracellular matrix of mineralized tissues, such
as bone, where it mediates important cell-matrix and cell-cell
interactions (18,19). Upregulation of OPN has been
observed in human cartilage from patients with OA, and plasma and
synovial fluid OPN levels were increased in patients with primary
knee OA, which were shown to be correlated with more severe OA
(20,21). OPN may be thus involved in the
molecular pathogenesis of OA, contributing to the progressive
degeneration of articular cartilage. OPN is hypothesized to be
involved in the destruction of the cartilage matrix by inducing the
production of matrix metalloproteinases (MMPs) in articular
chondrocytes (22).
The aim of the present study was to investigate the
effect of OPN on VEGF levels in articular cartilage cells, and
evaluate the possible underlying mechanisms involved.
Materials and methods
Cell culture and treatment
A total of 12 Sprague-Dawley (SD) rats (age, 1 week)
were purchased from Wuhan University Center for Animal Experiment
(Wuhan, China). The study was approved by the institutional review
board of Wuhan Central Hospital, and all procedures complied with
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MD, USA). Briefly, articular
chondrocytes were isolated from the knee joint of SD rats,
cartilage tissues were obtained and cut into small sections as
previously described (23). The
cartilage slices were further dissociated enzymatically for 2 h
with 0.2% type II collagenase (Sigma-Aldrich, St. Louis, MO, USA)
at 37°C. The cells were collected and resuspended in culture medium
(Dulbecco's modified Eagle's medium/F12 supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin; Invitrogen Life Technologies, Carlsbad, CA, USA) and
cultured in a 37°C, humidified, 5% CO2 incubator. The
medium was replaced every other day until the cells reached 80%
confluence. The chondrocytes were confirmed by aggrecan and
collagen-II expression. The expression of aggrecan and collagen-II
were examined by immunohisto-chemistry. The chondrocytes were
confirmed by positive expression of aggrecan and collagen-II. The
cell viability was assessed using trypan blue staining
(Sigma-Aldrich). Cells were exposed to OPN for different durations
(0, 2, 8, 12 or 24 h) and dosages (0, 0.1, 0.25 or 0.5 µM).
To inhibit the phosphoinositide 3-kinase (PI3K)/AKT and
extracellular signal-regulated kinase (ERK)1/2 pathways, LY294002
(10 µM) and PD98059 (20 µM) were used respectively or
in combination. Each experiment was repeated at least 3 times.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from cells using the
RNeasy plus mini kit (Qiagen China Co., Ltd, Shanghai, China) and
reverse-transcribed into cDNA according to the manufacturer's
instructions. RT-qPCR was performed using the Applied Biosystems
7500 Real-time PCR system (Applied Biosystems, Foster City, CA,
USA), and the SYBR Green fluorescent dye (Invitrogen Life
Technologies) method was used to quantify the cDNA. β-actin was
used as the internal control. The relative contents of the copy
numbers of the target gene mRNA were then calculated using the
2−ΔΔCt method. All experiments were performed in
triplicate. The primers sequences were as follows: VEGF, forward
5′-TGTGAATGCAGACCAAAGAAAGA-3′ and reverse
5′-GCTTTCTCCGCTCTGAGCAA-3′; β-actin, forward
5′-GTCCACCGCAAATGCTTCTA-3′ and reverse 5′-TGCTGTCACCTTCACCGTTC-3′.
Target sequences were amplified at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min.
Western blotting
Cell lysates were prepared using cell lysis
radioimmunoprecipitation assay buffer (Sigma-Aldrich). The protein
concentrations were determined using the bicinchoninic acid protein
assay kit (Sigma-Aldrich). For each sample, a total of 40 µg
protein was separated using 10% SDS-PAGE. The separated proteins
were then transferred onto a polyvinylidene difluoride membrane
(Life Technologies, Carlsbad, CA, USA). Following blocking with 5%
non-fat milk in Tris-buffered saline containing 0.05% Tween-20
(TBST; Life Technologies), the membrane was incubated with the
following primary antibodies: Rabbit polyclonal anti-human VEGF
(1:1,000; cat. no. ab46154; Abcam, Cambridge, MA, USA), mouse
monoclonal anti-human PI3K and rabbit polyclonal anti-human
phospho-PI3K (1:500; cat. nos. ab182651 and ab189403; Abcam),
Rabbit polyclonal anti-human pan-AKT and phosphor-AKT1 (1:1,000;
cat. no. ab8805 and ab66138; Abcam) and mouse monoclonal anti-human
β-actin (1:2,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) in TBST at 4°C overnight. The membrane
was then washed three times with TBST and incubated with the
horseradish peroxidase-conjugated secondary antibody (1:3,000;
Santa Cruz Biotechnology, Inc.) in TBST for 2 h at room
temperature. The membrane was washed again, followed by
visualization using an enhanced chemiluminescence substrate
(Sigma-Aldrich). The densitometric data were obtained using Image J
software (National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS version
13.0 software (SPSS, Inc., Chicago, IL, USA). A one-way analysis of
variance was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
OPN increases the expression of VEGF in a
dose- and time-dependent manner
To investigate whether OPN regulates the expression
of VEGF in chondrocytes, the expression of VEGF was analyzed
initially in chondrocytes treated with OPN at different
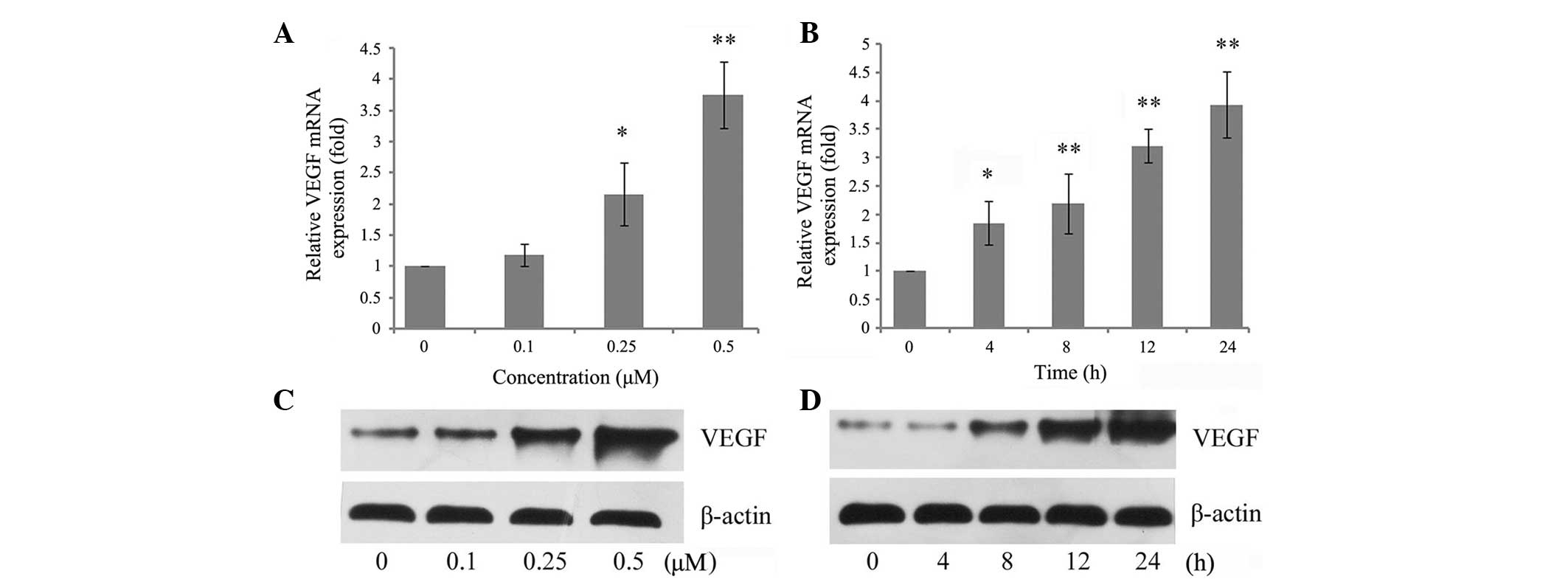
concentrations and time-durations. As shown in Fig. 1A, OPN increased VEGF mRNA
expression in a dose-dependent manner. Incubation of chondrocytes
with 0.25 µM OPN resulted in a statistically significant
increase in the relative level of VEGF, while incubation with 0.5
µM OPN resulted in an almost 4-fold increase. It was then
established whether there was a time-dependent regulation and
chondrocytes were incubated with 0.5 µM OPN. It was found
that VEGF mRNA levels were increased substantially by 4 h and
relatively high levels persisted for 24 h after stimulation with
OPN (Fig. 1B). In addition, VEGF
expression was assessed using western blot analysis. The protein
level was consistent with the mRNA level of VEGF, which also
exhibited a dose- and time-dependent regulation by OPN (Fig. 1C and D).
PI3K/AKT and ERK1/2 pathways are
activated by OPN
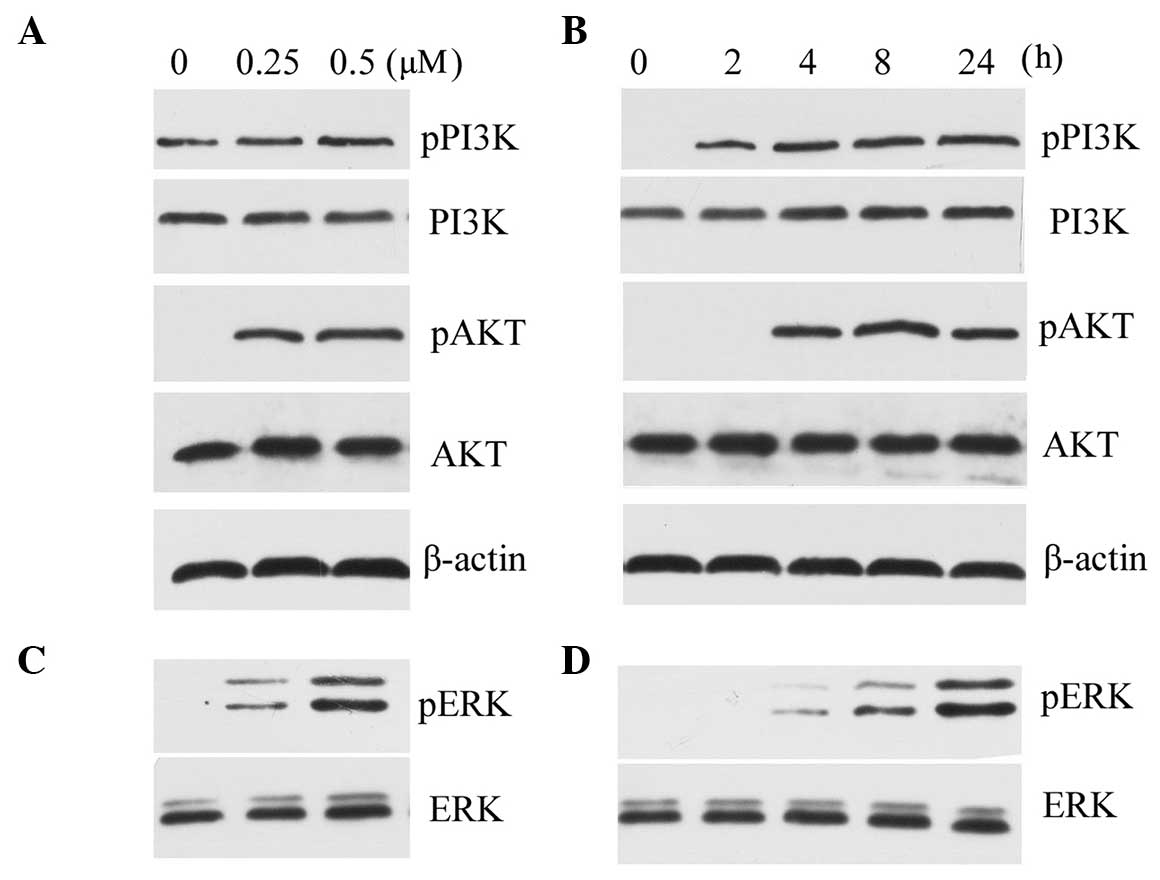
To investigate the signaling pathway involved, the
phosphorylation of PI3K/AKT and ERK1/2 was assessed. PI3K/AKT
activation was observed following exposure to 0.25 µM and
0.5 µM OPN, in a dose-dependent manner (Fig. 2A). The OPN-induced phosphorylation
of PI3K/AKT was also found following exposure to OPN for 4 h, and
lasted for 24 h (Fig. 2B). A
concentration-dependent effect of OPN on chondrocytes causing
ERK1/2 phosphorylation was also observed (Fig. 2C). The time course for the
phosphorylation of ERK1/2 was almost the same as for PI3K (Fig. 2D).
Blocking PI3K/AKT and ERK1/2 pathways
inhibits VEGF production
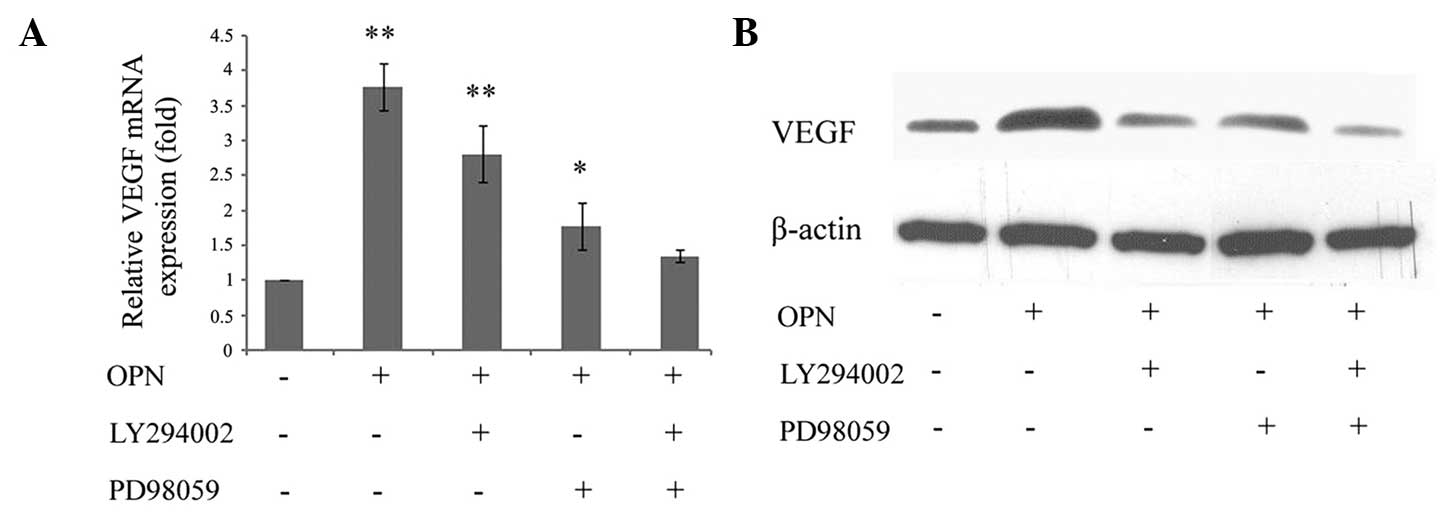
To determine whether OPN induced VEGF expression
through the PI3K/AKT and ERK1/2 signaling pathways, the cells were
treated with LY294002 and PD98059. It was identified that
OPN-induced VEGF mRNA was significantly inhibited by either
LY294002 or PD98059. While a single pathway inhibitor may partially
reduce VEGF mRNA expression, the combination of the two inhibitors
exhibited a synergistic effect and was able to completely reverse
the increase (Fig. 3A). These data
indicated that the PI3K/AKT and ERK1/2 signaling pathways may have
an important function in OPN-induced VEGF expression in
chondrocytes. In addition, VEGF expression was assessed using
western blotting. The protein level was consistent with the mRNA
level of VEGF, which also exhibited PI3K/AKT- and ERK1/2-dependent
regulation by OPN (Fig. 3B).
Discussion
In pathological conditions, such as OA, damaged
articular cartilage is frequently covered with and invaded by
granulation tissue with a high level of vascularization. These
observations in the pathophysiological conditions suggest the
involvement of angiogenic factors in the process of OA (24,25).
OPN has become a focus in OA pathogenesis research
in previous decades. Previous studies have revealed a close
association between OPN and OA (20,21).
Recent studies have also revealed that VEGF is important in the
degenerative process of OA (13,14,17).
VEGF has marked angiogenic activity with specific mitogenic and
chemotactic actions on endothelial cells. In the present study, the
data indicated that OPN enhanced the expression of VEGF in
chondrocytes. A previous study revealed that OPN promotes the
expression of MMP13 (22), while
VEGF inhibited the expression of aggrecan and type II collagen
(26). OPN and VEGF may destroy
the framework of articular cartilage. This process of extracellular
matrix degradation is required for angiogenesis and therefore
accelerates the angiogenic function of VEGF in cartilage.
To the best of our knowledge, this is the first
study in which OPN has been associated with VEGF in OA. The
signaling pathway that may connect OPN and VEGF was further
investigated. The signaling pathways involved in the function of
OPN appear to vary among different cell types. For instance, the
PI3K pathway has been observed to be induced by OPN in breast
cancer cells (27), while the
nuclear factor-κB signaling cascade was involved in OPN-induced
tumor cell migration and epithelial cell motility in prostate
cancer (28). In lymphocytes, the
mitogen-activated protein kinase/ERK-mediated signaling pathway was
associated with OPN-induced migration and motility (29). In the present study, the PI3K/AKT
and ERK1/2 signaling pathways were activated following
administration of OPN and persisted for at least 24 h, and
OPN-induced phosphorylation occured in a dose-dependent manner.
These findings suggested that the signaling cascades may be
associated with OPN-induced VEGF expression. In addition, when
blocking the activation of these two pathways using a PI3K
inhibitor and ERK inhibitor, OPN-induced VEGF expression was
significantly decreased. While a single pathway inhibitor partially
reduced VEGF mRNA expression, the combination of the two inhibitors
completely reversed the effect. These data indicated that OPN
enhanced the expression of VEGF through phosphorylation of PI3K/AKT
and ERK1/2.
In conclusion, the present results revealed an
association between OPN and VEGF in articular cartilage. It was
observed that OPN treatment resulted in an elevated expression of
VEGF at the gene level and protein level, and the effect was
induced through activation of the PI3K/AKT and ERK1/2 signaling
pathways. Additional studies are required to reveal the mechanism
of action of OPN in cartilage angiogenesis and cartilage
destruction.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171706) and the
Shanghai Municipal Natural Science Foundation (grant no.
11ZR1427400).
References
|
1
|
Gonzalez A: Osteoarthritis year 2013 in
review: Genetics and genomics. Osteoarthritis Cartilage.
21:1443–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barg A, Pagenstert GI, Hugle T, Gloyer M,
Wiewiorski M, Henninger HB and Valderrabano V: Ankle
osteoarthritis: Etiology, diagnostics and classification. Foot
Ankle Clin. 18:411–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandell LJ: Etiology of osteoarthritis:
Genetics and synovial joint development. Nat Rev Rheumatol.
8:77–89. 2012.PubMed/NCBI
|
|
4
|
Soder S and Aigner T: Osteoarthritis.
Etiology, typing, staging and histological grading. Pathologe.
32:183–192. 2011.In German.
|
|
5
|
Michael JW, Schluter-Brust KU and Eysel P:
The epidemiology, etiology, diagnosis and treatment of
osteoarthritis of the knee. Dtsch Arztebl Int. 107:152–162.
2010.PubMed/NCBI
|
|
6
|
Bara JJ, Johnson WE, Caterson B and
Roberts S: Articular cartilage glycosaminoglycans inhibit the
adhesion of endothelial cells. Connect Tissue Res. 53:220–228.
2012. View Article : Google Scholar
|
|
7
|
Hyc A, Osiecka-Iwan A, Jóźwiak J and
Moskalewski S: The morphology and selected biological properties of
articular cartilage. Ortop Traumatol Rehabil. 3:151–162. 2001.
|
|
8
|
Ashraf S, Mapp PI and Walsh DA:
Contributions of angiogenesis to inflammation, joint damage and
pain in a rat model of osteoarthritis. Arthritis Rheum.
63:2700–2710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pesesse L, Sanchez C and Henrotin Y:
Osteochondral plate angiogenesis: A new treatment target in
osteoarthritis. Joint Bone Spine. 78:144–149. 2011. View Article : Google Scholar
|
|
10
|
Mapp PI and Walsh DA: Mechanisms and
targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev
Rheumatol. 8:390–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang QY, Dai J, Kuang B, Zhang J, Yu SB,
Duan YZ and Wang MQ: Osteochondral angiogenesis in rat mandibular
condyles with osteoarthritis-like changes. Arch Oral Biol.
57:620–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nowak DG, Amin EM, Rennel ES,
Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ,
Woolard J, Ladomery MR, et al: Regulation of vascular endothelial
growth factor (VEGF) splicing from pro-angiogenic to
anti-angiogenic isoforms: A novel therapeutic strategy for
angiogenesis. J Biol Chem. 285:5532–5540. 2010. View Article : Google Scholar :
|
|
13
|
Lingaraj K, Poh CK and Wang W: Vascular
endothelial growth factor (VEGF) is expressed during articular
cartilage growth and re-expressed in osteoarthritis. Ann Acad Med
Singapore. 39:399–403. 2010.PubMed/NCBI
|
|
14
|
Jansen H, Meffert RH, Birkenfeld F,
Petersen W and Pufe T: Detection of vascular endothelial growth
factor (VEGF) in moderate osteoarthritis in a rabbit model. Ann
Anat. 194:452–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamairi F, Utsumi H, Ono Y, Komorita N,
Tanaka M and Fukunari A: Expression of vascular endothelial growth
factor (VEGF) associated with histopathological changes in rodent
models of osteoarthritis. J Toxicol Pathol. 24:137–142. 2011.
View Article : Google Scholar
|
|
16
|
Murata M, Yudoh K and Masuko K: The
potential role of vascular endothelial growth factor (VEGF) in
cartilage: How the angiogenic factor could be involved in the
pathogenesis of osteoarthritis? Osteoarthritis Cartilage.
16:279–286. 2008. View Article : Google Scholar
|
|
17
|
Matsumoto T, Cooper GM, Gharaibeh B,
Meszaros LB, Li G, Usas A, Fu FH and Huard J: Blocking VEGF as a
potential approach to improve cartilage healing after
osteoarthritis. J Musculoskelet Neuronal Interact. 8:316–317.
2008.
|
|
18
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: Role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakraborty G, Jain S, Behera R, Ahmed M,
Sharma P, Kumar V and Kundu GC: The multifaceted roles of
osteopontin in cell signaling, tumor progression and angiogenesis.
Curr Mol Med. 6:819–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao SG, Li KH, Zeng KB, Tu M, Xu M and Lei
GH: Elevated osteopontin level of synovial fluid and articular
cartilage is associated with disease severity in knee
osteoarthritis patients. Osteoarthritis Cartilage. 18:82–87. 2010.
View Article : Google Scholar
|
|
21
|
Honsawek S, Tanavalee A, Sakdinakiattikoon
M, Chayanupatkul M and Yuktanandana P: Correlation of plasma and
synovial fluid osteopontin with disease severity in knee
osteoarthritis. Clin Biochem. 42:808–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu M, Zhang L, Zhao L, Gao S, Han R, Su D
and Lei G: Phosphorylation of osteopontin in osteoarthritis
degenerative cartilage and its effect on matrix metalloprotease 13.
Rheumatol Int. 33:1313–1319. 2013. View Article : Google Scholar
|
|
23
|
Chen Q, Liu SQ, Du YM, Peng H and Sun LP:
Carboxymethyl-chitosan protects rabbit chondrocytes from
interleukin-1beta-induced apoptosis. Eur J Pharmacol. 541:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashraf S and Walsh DA: Angiogenesis in
osteoarthritis. Curr Opin Rheumatol. 20:573–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mapp PI, Avery PS, McWilliams DF, Bowyer
J, Day C, Moores S, Webster R and Walsh DA: Angiogenesis in two
animal models of osteoarthritis. Osteoarthritis Cartilage.
16:61–69. 2008. View Article : Google Scholar
|
|
26
|
Chen XY, Hao YR, Wang Z, Zhou JL, Jia QX
and Qiu B: The effect of vascular endothelial growth factor on
aggrecan and type II collagen expression in rat articular
chondrocytes. Rheumatol Int. 32:3359–3364. 2012. View Article : Google Scholar
|
|
27
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin stimulates cell motility and nuclear factor
kappaB-mediated secretion of urokinase type plasminogen activator
through phosphatidylinositol 3-kinase/Akt signaling pathways in
breast cancer cells. J Biol Chem. 278:28593–28606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jain S, Chakraborty G and Kundu GC: The
crucial role of cyclooxygenase-2 in osteopontin-induced protein
kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate
tumor progression and angiogenesis. Cancer Res. 66:6638–6648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Z, Dai J, Fan K, Wang H, Ji G, Li B,
Zhang D, Hou S, Qian W, Zhao J, et al: A novel functional motif of
osteopontin for human lymphocyte migration and survival. Mol
Immunol. 45:3683–3692. 2008. View Article : Google Scholar : PubMed/NCBI
|