Introduction
Subacromial bursitis (SAB) is the main cause of pain
and dysfunction in shoulder tendinopathy, accounting for ~50% of
all cases of shoulder pain (1).
The majority of cases of SAB are associated with subacromial
impingement syndrome (SIS) and rotator cuff tears. The subacromial
bursa lies between the acromion and the rotator cuff, and the
friction between them is reduced predominantly by lubrication.
Therefore, high levels of exercise are likely to induce
inflammation in the shoulder area. Fibrocartilaginous metaplasia in
the rotator cuff has previously been reported (2,3).
Gotoh et al (4) observed
that, compared with unaffected individuals, patients with SIS often
presented with a chronic inflammatory response and increased
numbers of cluster of differentiation (CD)-2 and CD-11b mononuclear
cells in the subacromial bursa. Injection of corticosteroids into
the subacromial bursa provided a therapeutic effect, suggesting
that SAB occurs as a result of an important pathological change in
rotator cuff disease. However, an unavoidable problem in using
corticosteroids is their adverse effects. Crofford et al
(5) demonstrated that interleukin
(IL)-1 and tumor necrosis factor (TNF)-α are able to relieve pain
by mediating the expression of cyclooxygenase (COX)-2 and COX-1,
suggesting that subacromial bursa cells are important in SAB, by
secreting multiple cytokines and forming a network that regulates
the chronic inflammatory response, with TNF-α being key in this
inflammatory circuit (5).
Immunohistochemical studies have reported the presence of
anti-TNF-α antibody staining in >80% of SAB tissue, suggesting
that the expression of TNF-α in subacromial bursa cells may also
affect the metabolism of these cells, causing damage to the tendon
and cartilage (6).
There has been significant interest in using the
technique of small interfering RNA (siRNA) to potently suppress
genetic expression in a sequence-specific manner (7). In 2003, Song et al (8) demonstrated the therapeutic effect of
Fas-specific siRNA in mice with experimentally induced hepatitis.
Since then, numerous studies have been performed to investigate the
use of siRNA in various disease models (8–12).
In the field of locomotor diseases, Schiffelers et al
(13) reported that
luciferase-specific siRNA reduces luciferase activity in joints.
Our previous study also revealed that the fluorescence of the
synovium was decreased by green fluorescent protein (GFP)-specific
siRNA in the GFP rat (14). These
delivery methods for the expression of short hairpin (sh)RNA
include the direct application of naked siRNAs and lipid-based
delivery vehicles (15). However,
the application of these methods is limited by low transduction
efficiency, poor control of gene expression and short duration of
effect, particularly when it is necessary to generate long-term
gene silencing in vivo (16). However, these problems can be
addressed using the technique of lentivirus-mediated RNA
interference (RNAi) to achieve the desired therapeutic effect
(17). To the best of our
knowledge, no previous studies have used this technique in the
treatment of locomotor diseases, therefore, the present study aimed
to use the RNAi technique to silence and inhibit the expression of
TNF-α in subacromial bursa cells through local injection of the
lentivirus vector specifically targeting TNF-α into the rat
subacromial bursa. This may reveal a novel strategy for the gene
therapy of SAB.
Materials and methods
Ethical approval
The present study was approved by the Ethics
Committee of the Second Military Medical University (Shanghai,
China).
Animals
A total of 32 male Sprague-Dawley rats, aged 3
months and weighing 200–220 g (Shanghai Research Center for Model
Organisms, Shanghai, China) were housed under constant temperature
(21°C) and regular light (06:30–19:30 h)-dark (19:30–06:30 h)
cycles with ad libitum access to food and water at the
Animal Care and Veterinary Services Facility of Fudan University
(Shanghai, China), according to the guidelines of the International
Council for Laboratory Animal Science. The rats were allowed to
acclimate for 2 weeks prior to the initiation of the
experiment.
Establishment of the rotator cuff disease
model
The rotator cuff disease model was established by
subacrominal injection of 10 μl 3% carrageenan
(Sigma-Aldrich, St. Louis, MO, USA) eight times weekly for 2 weeks
as previously described (1). Based
on the physiological features of the animals, the injection site
was located in the subacromial space above the rotator cuff, to
ensure that repeated subacromial saline injections would not damage
the tendons (18). Using sterile
needles (Microlance 3, 27G3/4,0.4*19, No.20; Becton-Dickinson,
Drogheda, Ireland) and glass syringes (Wegene, Shanghai, China),
all injections were made aseptically in the left shoulder under
anesthesia with carbon dioxide, according to Soslowsky et al
(18). Of the 32 animals, 24 were
equally assigned to three groups: A lenti-virus TNF-α-RNAi group
(group A), in which the animals received a local injection of
lentivirus-RNAi l×107 tranducing units (TU) per rat in
the subacromial bursa; a lentivirus negative control (NC) group
(group B), in which the animals received a local injection of
lentivirus-NC 1×107 TU per rat in the subacromial bursa,
and a rotator cuff disease model group (group C), in which the
animals received a local injection of 100 ml phosphate-buffered
saline (PBS) per rat. All injections began on day 2 following
establishment of the disease model. The eight remaining animals
were injected with 10 μl PBS as a negative control of the
model animals (control).
RNAi design and small interference
(siRNA) plasmid construction
siRNAs targeting the TNF-α bases on the pLL3.7
vector (Wegene) were used. The sequence of the siRNAs targeting rat
TNF-α were as follows: Rattus norvegicus; GenBank: L00981.1;
TNF-α,
5′-TAATGGCATGGATCTCAAAGATTCAAGAGATCTTTGAGACCATGCCATTTTTTTTC-3′ and
5′-TCGAGAAAAAAAATGGCATGGATCTCAAAGATCTCTTGAATCTTTGAGATCCATGCCATTA-3′.
siRNA against rat TNF-α was synthesized by Invitrogen Life
Technologies (Carlsbad, CA, USA). The oligos were denatured for 10
min at 95°C, using the Applied Biosystems 7500 Real-Time Polymerase
Chain Reaction (PCR) system (Applied Biosystems, Foster City, CA,
USA) and annealed at room temperature for >30 min. The paired
oligos and pLL3.7 vectors, digested with XhoI and
HpaI, were ligated with T4 ligase at 16°C overnight.
Ligation was performed to transform the cells into stable competent
cells. The pLL3.7-TNF-α-siRNA plasmids were supplied by Shanghai
GenePharm Co., Ltd. (Shanghai, China). The plasmid containing TNF-α
siRNA was sequenced. A negative control (NC) siRNA containing a
scramble sequence was used with the following sequences:
5′-TGCCCTACCACCGAGGTCAATTCAAGAGATTGACCTCGGTGGTAGGGCTTTTTTC-3′ and
5-TCGAGAAAAAAGCCCTACCACCGAGGTCAATCTCTTGAATTGACCTCGGTGGTAGGGCA-3′.
The TNF-α siRNA-containing plasmid was sequenced and the
pLL3.7-TNF-α-siRNA was transfected into 293T cells (American Type
Culture Collection, Manassas, VA, USA) together with Δ8.9 and
vesicular stomatitis virus glyoprotein (VSVG) (Sigma-Aldrich) to
package virus particles with Ca2+ reagent. Briefly,
pLL3.7-TNF-α-siRNA (10 μg), Δ8.9 (8 μg) and VSVG (6
μg) were mixed with 94 μl CaCl2 (2 M;
Sigma-Aldrich), adjusted to 750 μl and were added dropwise
into 750 μl 2X Hepes-buffered saline. Following a 5 min
tranquilization step, the mixture was added to the 293T cells
pretreated with chloroquine (5 μm; Sigma-Aldrich). Following
an 8 h culture period at 37°C and a replacement of the medium, the
cells were cultured for an additional 48 h at 37°C, harvested and
centrifuged at 50,000 × g for 120 min at 10°C. The supernatant was
then discarded. The viral particles were pelleted, titrated by
series dilution and then used to infect the 293T cells in the
presence of polybrene 8 μg/ml (Sigma-Aldrich). The titer of
3–6×108 TU/ml was achieved routinely.
Administration of the lentivirus vector
in vivo
The animals in group A received a local injection of
lentivirus-RNAi l×107 TU/rat in the subacromial bursa
and animals in group B received a local injection of lentivirus-NC
1×107 TU/rat in the subacromial bursa. At 5 weeks
following injection, the animals were sacrificed by injection with
4% pentobarbital sodium (Wegene; 2 ml/kg) to obtain the sera and
the supra, infraspinatus and subacromial bursa specimens, which
were divided into two. The sera and half of the tissue specimens
were stored at −70°C and the remaining specimens were fixed in 4%
paraformaldehyde (Sigma-Aldrich) until use.
Histopathological analysis
The supra, infraspinatus and subacromial bursae were
fixed in 4% paraformaldehyde for 2 days, dehydrated, paraffin
embedded, sliced parallel to the collagen bundles into 3 μm
sections and stained with hematoxylin and eosin (HE; Sigma-Aldrich)
and Van Gieson's stain (Sigma-Aldrich) for routine histological
evaluation. A total of eight 100×100 μm visual fields were
selected randomly in each section, and the total numbers of
inflammatory cells were recorded under a microscope (Leica DMI3000
B, Wetzlar, Germany).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from 1.0 g tissue and the
neuronal cells using TRIzol® reagent from Invitrogen
Life Technologies, according to the manufacturer's instructions.
Total RNA was quantified by measuring absorbance at 260 nm using a
Beckman DU640 spectrophotometer (Beckmann Coulter, Fullerton, CA,
USA). Reverse transcriptase was used to generate cDNA using 5
μg RNA and oligo(dT) primers, according to manufacturer's
instructions (Invitrogen Life Technologies). The reactions were run
on a Real-Time PCR system (Applied Biosystems 7500 PCR) with the
following cycle conditions: 95°C for 15 sec, 45 cycles at 95°C for
5 sec and at 60°C for 30 sec. A standard curve for TNF-α was
generated by using serially diluted total RNA from neuronal cells
and the spinal cord to quantitate relative mRNA levels of TNF-α.
The sequences of the TNF-α gene primers (Sangon Biotechnology Co.,
Ltd., Shanghai, China) were as follows: Forward
5′-TGTCTGTGCCTCAGCCTCTTC-3′ and reverse
5′-TTTGGGGAACTTCTCCTCCTTG-3′, with a product of 110 bp. GAPDH
served as a normalization control. The primers sequences for GAPDH
were as follows: Forward 5′-TGGAGAAACCTGCCAAGTATGA′-3 and reverse
5′-TGGAAGAATGGGAGTTGCTGT-3′, with a product of 135 bp. The relative
expression of mRNA was calculated using the 2−ΔΔCt
method (19).
Western blotting
The tissues were washed with cold PBS containing 2
mM EDTA (Sigma-Aldrich) and lysed with denaturing SDS-PAGE sample
buffer (United States Biological, Salem, MA, USA) using standard
methods. Briefly, the cell pellets were resuspended in 100
μl lysis buffer (20 mM Tris. Cl pH 7.9, 1 mM EDTA, 5%
Glycerol) with an equal volume of 0.5 mm glass beads (Biospec
products, Inc., Bartlesville, OK, USA) and vortexed for 10 min at
4°C. The total protein concentration was determined using a protein
assay kit (Thermo Fisher Scientific) and analyzed with AlphaView SA
software (Cell Biosciences, Inc., San Jose, CA, USA). The final
concentration of protein in each sample was adjusted to 2 mg/ml.
The protein lysates were separated by 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes (Millipore, Bedford, MA,
USA), incubated with primary antibodies and blocked with 5% bovine
serum albumin (BSA; Sigma-Aldrich). The primary antibodies
(1:1,000) used were TNF-α (cat. no. bs-2081R; Bioss, Woburn, MA,
USA), NF-κB (cat. no. 4790; Cell Signaling Technology, Danvers, MA,
USA), matrix metalloproteinase (MMP)-1 (cat. no. MAB13439;
Millipore), MMP-9 (cat. no. BS-1241; Bioworld, St Louis Park, MN,
USA), COX-1 (cat. no. BS1075; Bioworld), COX-2 (cat. no. MAB3462;
Millipore), and stromal cell-derived growth factor-1 (SDF-1; cat.
no. 3740S; Cell Signaling Technology). The membranes were washed
with Tris-buffered saline (Sigma-Aldrich) three times and incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin G antibody (1:5,000; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at room temperature for 2 h. Signal detection
was performed using an enhanced chemiluminescence system (GE
Healthcare, Waukesha, WI, USA). The protein levels in the tissues
were quantified by densitometry and normalized to GAPDH, with the
levels of the phospho-Ras homolog gene family, member A (RhoA)
normalized to the total levels of RhoA.
Immunohistochemistry
The rat tissue sections (5 μm) were treated
with 0.1 M PB:methanol (1:1) (Sigma-Aldrich) and 1% hydrogen
peroxide (Sigma-Aldrich) for 20 min, followed by incubation with
0.1 M phosphate buffer (PB) with 1% BSA and 0.3% Triton X-100
(Wegene) for 1 h. Subsequently, the sections were incubated
overnight at 4°C with mouse anti-rat-fibro-nectin primary antibody
(Santa Cruz Biotechnology, Inc.) in 0.1 M PB with 0.3% BSA and 0.3%
Triton X-100, followed by a biotinylated mouse secondary antibody
and the Avidin-biotin Complex-HRP kit (Thermo Fisher Scientific,
Inc., Wilmington, MA, USA) for 2 h. Specific binding was visualized
with diaminobenzidine (Santa Cruz Biotechnology, Inc.). The tissue
was then dehydrated, covered with mounting solution and observed
under an Eclipse E200 optical microscope (Nikon, Tokyo, Japan).
ELISA
The serum protein level of TNF-α was determined
using an ELISA kit (R&D Systems, Minneapolis, MN, USA),
according to the manufacturer's instructions. The results were
normalized to the number of rats per group. The data are presented
as mean ± standard error of the mean from four independent
experiments, which were performed in triplicate.
Statistical analysis
The parametric data were compared using
multi-variable two-way analysis of variance followed by Tukey's
post hoc test for comparison between several independent groups.
P<0.05 was considered to indicate a statistically significant
difference in a two-tailed test. Comparisons between two groups
were performed using Student's t-test. Data are expressed as the
mean ± standard error of the mean. Data were analyzed using SPSS
version 16.0 statistical software (SPSS, Inc., Chicago, IL,
USA).
Results
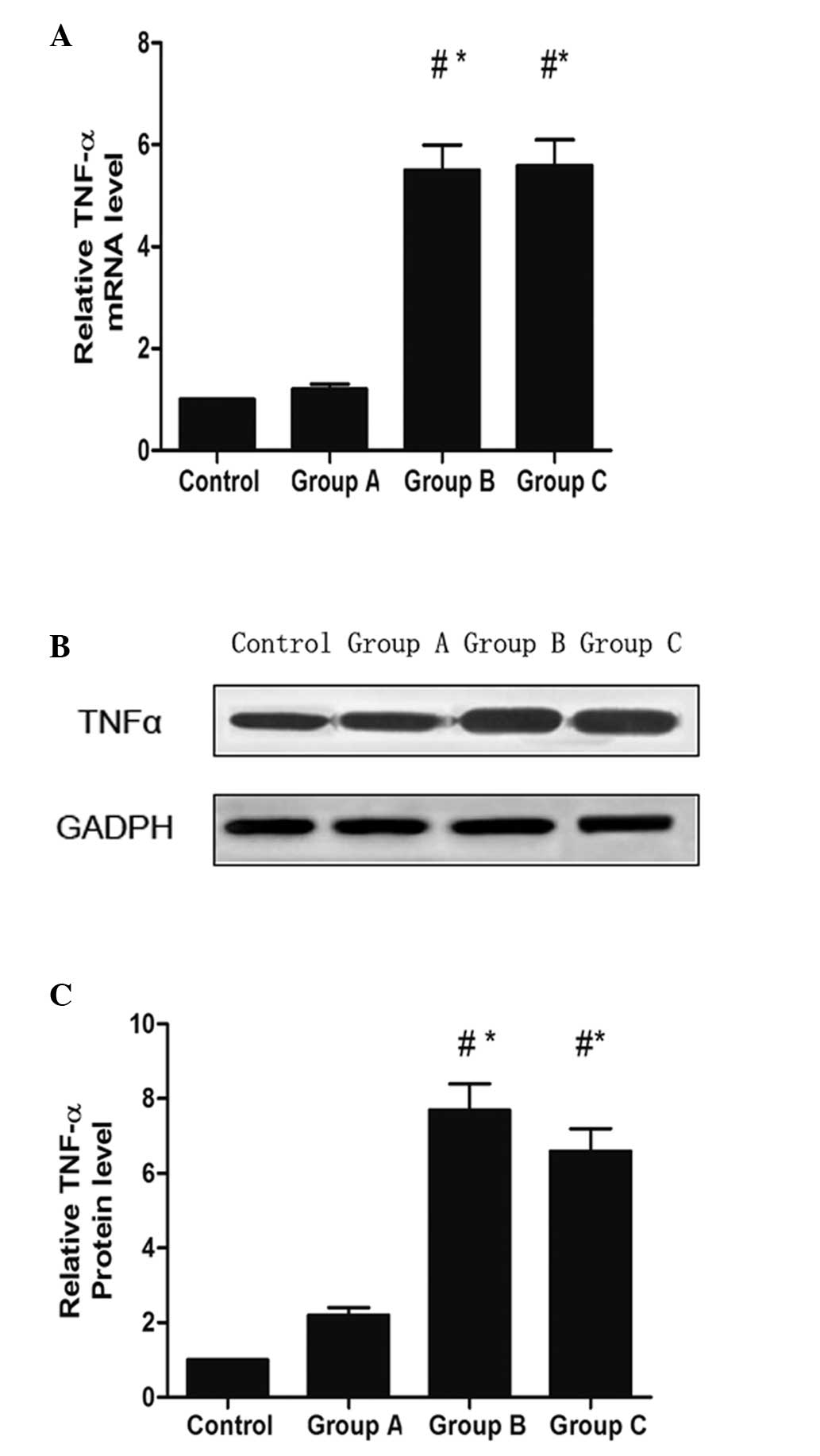
Gene-silencing effects of siRNA in
vivo
At 5 weeks following the injection of siTNF-α, the
levels of mRNA from the tendon of animals in the the rotator cuff
disease model were determined by RT-qPCR to compare the in
vivo gene-silencing effect of TNF-α among groups A, B and C.
The expression of TNF-α in the subacromial bursa of group A was
reduced compared with the expression levels in groups B and C
(Fig. 1A). Western blot analysis
revealed that the protein expression of TNF-α in group A was
significantly decreased compared with the levels observed in groups
B and C (Fig. 1B and C).
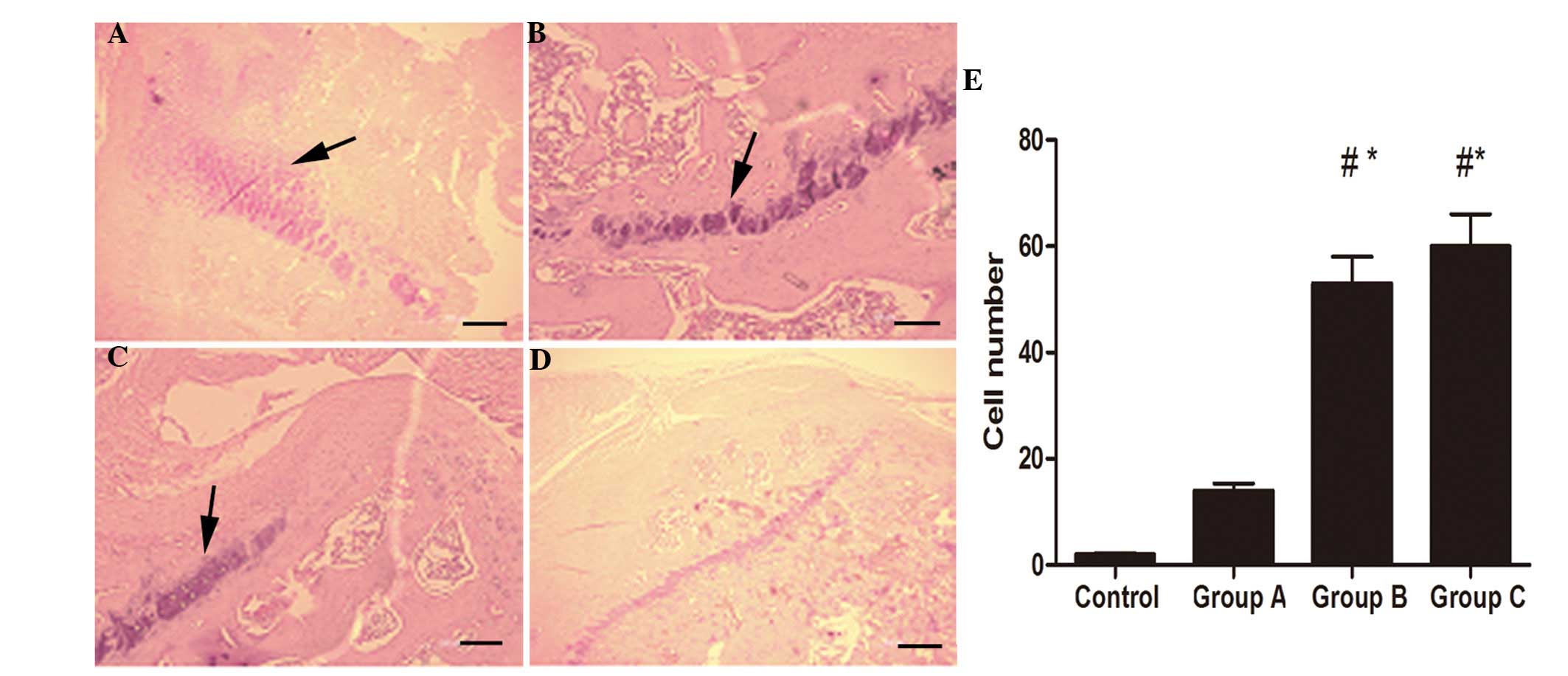
Histopathological evaluation
In the animals in the control group, which received
normal saline, no inflammatory cells were observed in the
subacromial space. Inflammatory cell infiltration was observed in
12.5±9.8 cells/visual field in group A vs. 53.2±8.7 and 57.8±8.9
cells/visual field in groups B and C. The inflammatory response was
predominantly characterized by infiltration of relatively large
quantities of lymphocytes and several neutrophils on the tendon
surface (Fig. 2).
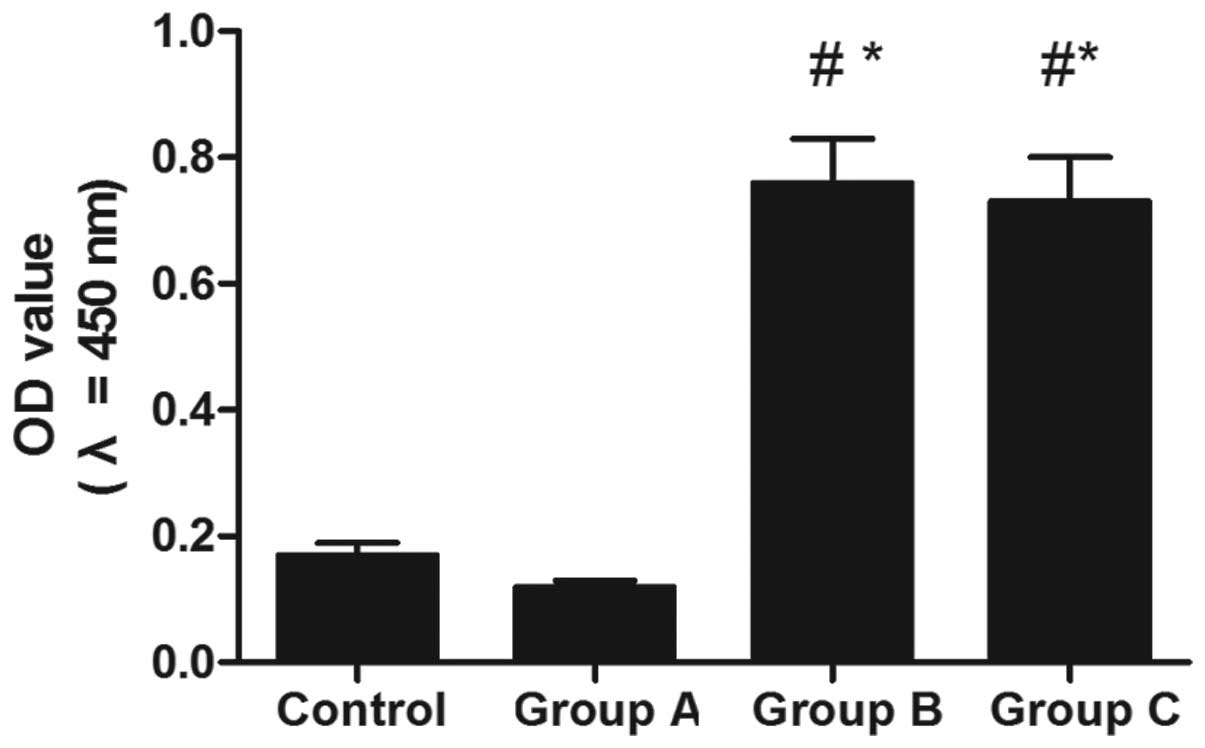
Microscopic evaluation of the tendon
Fibronectin was analyzed in order to detect signs of
an inflammatory response (Fig. 3).
A significant difference was identified in the optical densities of
the expression of fibronectin among groups A, B and C
(P<0.001).
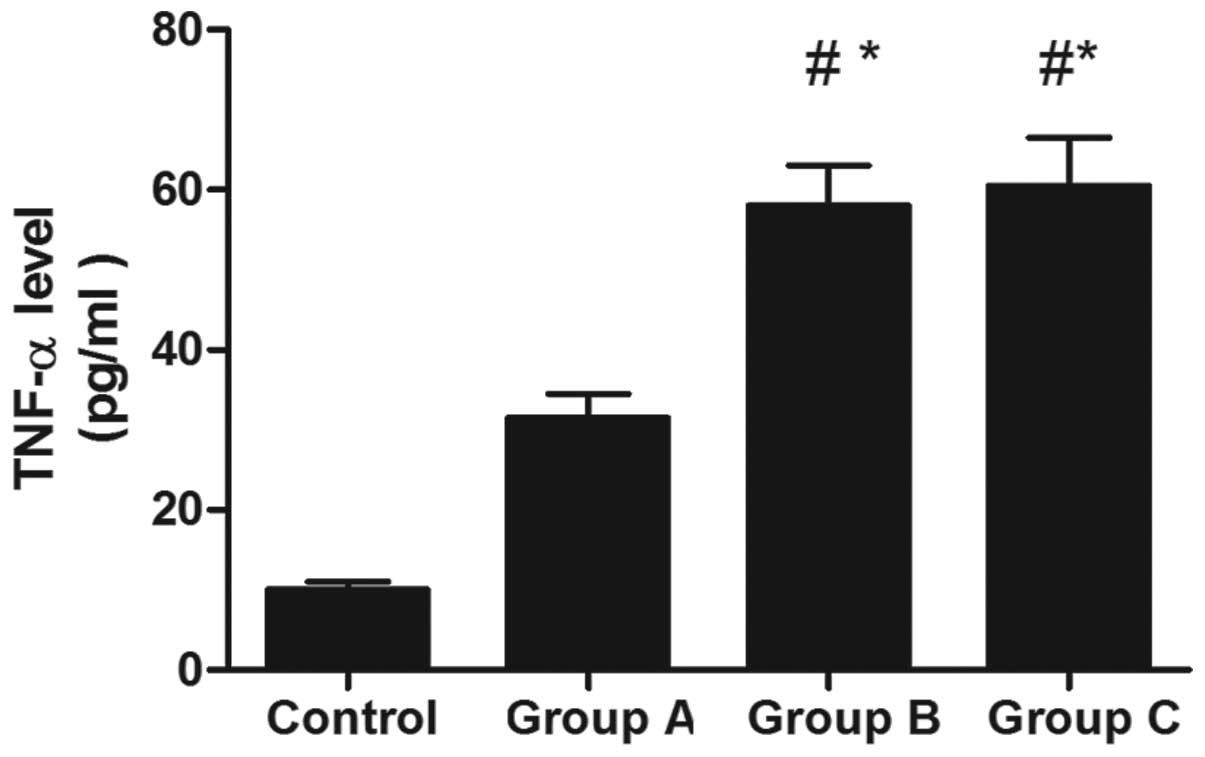
Levels of TNF-α in the serum following
lentivirus-mediated RNAi treatment
At 5 weeks subsequent to treatment with the
lentivirus-mediated RNAi, the level of TNF-α in the serum was
decreased significantly in group A, as compared with that in groups
B and C (Fig. 4).
TNF-α RNAi modulates the expression
levels of NF-κB, MMP-1, MMP-9, COX-1, COX-2 and SDF-1 in rotator
cuff disease model rats
The effects of TNF-α silencing on the expression
levels of NF-κB, MMP-1, MMP-9, COX-1, COX-2 and SDF-1 in
vivo were determined by injecting lentivirus-siTNF-α into the
rat subacromial bursa. At 5 weeks following injection, the animals
were sacrificed via the injection of 4% pentobarbital sodium 2
ml/kg. The tendons were dissected and homogenized to extract
proteins for western blot analysis, to determine the protein
expression levels of NF-κB, MMP-1, MMP-9, COX-1, COX-2 and SDF-1.
The expression levels of NF-κB, MMP-1, MMP-9, COX-1, COX-2 and
SDF-1 in group B were significantly increased compared with those
in group C and the control group, while they were significantly
decreased in group A compared with groups B and C (Fig. 5).
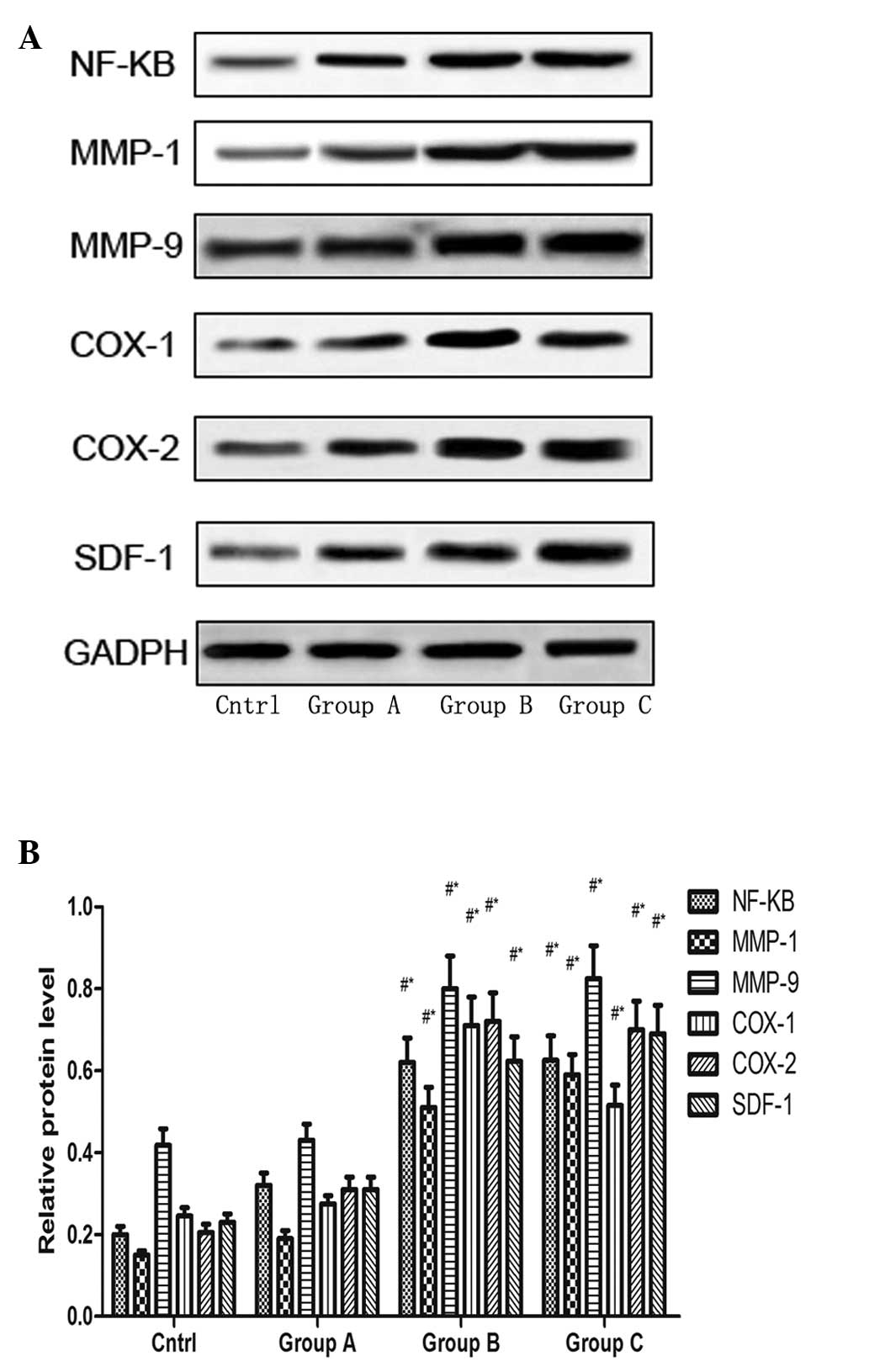 | Figure 5(A) Expression levels of NF-κB, MMP-1,
MMP-9, COX-1, COX-2 and SDF-1 following TNF-α expression silencing
in groups A, B and C. GAPDH indicates equal loading of protein. (B)
Densitometric analysis of the expression levels of NF-κB, MMP-1,
MMP-9, COX-1, COX-2 and SDF-1 in the different treatment groups.
Each column represents pooled data (n=4). The vertical bars
represent the mean ± standard error of the mean.
#P<0.05, compared with the control;
*P<0.05 compared with group A. Group A,
lentivirus-TNF-α-RNAi injection; group B, lentivirus-NC injection;
group C phosphate-buffered saline injection; NF, nuclear factor;
MMP, matrix metal-loproteinase; COX, cyclooxygenase; SDF, stromal
cell-derived factor; TNF, tumor necrosis factor. |
Discussion
As TNF-α is in the master regulator of the
inflammatory response in SAB, its expression level directly affects
the expression of the other inflammatory cytokines. Whether is it
possible to attenuate the increased generation of inflammatory
cytokines by inhibiting the expression of TNF-α, to limit the
progression of inflammation-induced injuries remains to be
elucidated To investigate this, the present study used a lentivirus
as a vector to silence TNF-α, which was then injected into the left
subacromial bursa in a carrageenan-induced rat SAB model. RT-qPCR
and western blot analyses revealed that the expression of TNF-α in
the muscle and bursal cavity surrounding the injection site was
decreased significantly 5 weeks after injection, and the
inflammatory response in the tendon was effectively downregulated,
attenuating the fibro-cartilaginous metaplasia in the rotator
cuff.
Voloshin et al (6) demonstrated that TNF-α is an important
factor, which mediates the occurrence of inflammation and pain. It
is a gene, which initiates transcription and is located upstream of
tissue metalloproteinase and COX. Kim et al (17) demonstrated that SAB cells secrete a
large quantity of SDF-1, ten times higher than that of normal
bursal cells. Yanagisawa et al (20) revealed that the increased level of
vascular endothelial growth factor in the subacromial bursa of
patients with rotator cuff tear is closely correlated with the
inflammatory response. In a study on changes in the gene expression
profile of SAB using the gene chip technique and
immunohistochemistry, Blaine et al (21) found that the expression levels of
IL-1α, IL-1β, IL-6 and TNF-α in inflammatory cytokines, MMP-1 and
MMP-9 in tissue metalloproteinase, and COX-1 and COX-2 in
cyclooxygenase increase markedly, and that SAB bursal cells are the
predominant source of secretion of these factors. It has been
observed that the expression of IL-1 is positively correlated with
bursal inflammation and shoulder joint pain in SAB (6). Numerous studies on arthropathies have
demonstrated that NF-κB is also an important transcription factor
involved in the expression of inflammatory cytokines (22). Schaffner et al (23) observed that
arginine-glycine-aspartic acid polypeptide induces synovial
fibroblasts to express MMP-1, indicating that the induction of
expression of MMP by the central cell binding domain fibronectin
may be mediated by α5β1. The NF-κB signaling transduction pathway
is the common intracellular signaling transduction pathway of
TNF-α, IL-lβ and other inflammatory mediators. NF-κB activation may
increase the transcription levels of IL-lB, IL-6, IL-8, TNF-α, MMP,
adhesion molecules and cyclooxygenase genes (22). It was revealed in the present study
that serum levels of TNF-α were increased in groups B and C. TNF-α
has inflammation-mediatory and immunoregulatory roles in the immune
response. Its effects include activating lymphocytes and releasing
other inflammatory cytokines, including IL-l and IL-6,
prostaglandin and metalloproteinase (24,25).
As TNF-α also promotes angiogenesis and regulates adhesion
molecules, it is an important reactivator in the inflammatory
response (26).
In the present study, it was observed that the
protein expression of NF-κB was downregulated following TNF-α
interference, which further decreased the protein expression levels
of MMP-1, MMP-9, COX-1 and COX-2. This finding fully supported the
hypothesis of TNF-α as an important mediator of inflammation and
pain, as well as an upstream gene of tissue metalloproteinase and
cyclooxygenase. It is noteworthy that SDF-1 decreased with TNF-α
gene knockout, indicating that TNF-α also has a positive feedback
effect on SDF-1 in the SAB network, which was consistent with
previous studies (1). The
immunohistochemical staining results demonstrated that the
inflammation-induced decrease of fibronectin was attenuated
following TNF-α gene knockout, indicating the TNF-α gene knockout
effectively reduced inflammation-induced injuries.
The active TNF-α siRNA sequence was used to
construct a siRNA expression cassette, which was then incorporated
into a lentiviral vector system. A key advantage of lentiviral
vectors over other gene delivery systems is that they are able to
efficiently transduce post-mitotic cells, including subacromial
bursa cells, as lentiviral vectors enable the transduction of
non-dividing cells and can result in long-term gene expression in
subacromial bursa cells.
In conclusion, the present study succeeded in
knocking out the mRNA expression of TNF-α and downregulating the
protei expression levels of MMP-1, MMP-9, COX-1, COX-2 and SDF-1 in
the inflammatory network by injecting siRNA TNF-α into the
subacromial bursa of patients with SAB. This suggested that use of
the lentivirus-mediated RNAi technique to regulate the key target
in the chronic SAB inflammatory response circuit may prove to be an
effective approach for the clinical treatment of SAB or rotator
cuff disease in the future.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81171766),
and the Foundation of Science and Technology Commission of Shanghai
(grant no. 08QA1400400).
References
|
1
|
Blaine TA, Cote MA, Proto A, Mulcahey M,
Lee FY and Bigliani LU: Interleukin-1beta stimulates
stromal-derived factor-1alpha expression in human subacromial
bursa. J Orthop Res. 29:1695–1699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chard MD, Cawston TE, Riley GP, Gresham GA
and Hazleman BL: Rotator cuff degeneration and lateral
epicondylitis: a comparative histological study. Ann Rheum Dis.
53:30–34. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuda H, Hamada K and Yamanaka K:
Pathology and pathogenesis of bursal-side rotator cuff tears viewed
from en bloc histologic sections. Clin Orthop Relat Res. 254:75–80.
1990.PubMed/NCBI
|
|
4
|
Gotoh M, Hamada K, Yamakawa H, et al:
Interleukin-1-induced subacromial synovitis and shoulder pain in
rotator cuff diseases. Rheumatology (Oxford). 40:995–1001. 2001.
View Article : Google Scholar
|
|
5
|
Crofford LJ, Tan B, McCarthy CJ and Hla T:
Involvement of nuclear factor kappa B in the regulation of
cyclooxygenase-2 expression by interleukin-1 in rheumatoid
synoviocytes. Arthritis Rheum. 40:226–236. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voloshin I, Gelinas J, Maloney MD, O'Keefe
RJ, Bigliani LU and Blaine TA: Proinflammatory cytokines and
metalloproteases are expressed in the subacromial bursa in patients
with rotator cuff disease. Arthroscopy. 21:1076.e1–1076.e9. 2005.
View Article : Google Scholar
|
|
7
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song E, Lee SK, Wang J, et al: RNA
interference targeting Fas protects mice from fulminant hepatitis.
Nat Med. 9:347–351. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verma UN, Surabhi RM, Schmaltieg A,
Becerra C and Gaynor RB: Small interfering RNAs directed against
beta-catenin inhibit the in vitro and in vivo growth of colon
cancer cells. Clin Cancer Res. 9:1291–1300. 2003.PubMed/NCBI
|
|
10
|
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S
and Muramatsu T: A small interfering RNA targeting vascular
endothelial growth factor as cancer therapeutics. Cancer Res.
64:3365–3370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aharinejad S, Paulus P, Sioud M, et al:
Colony-stimulating factor-1 blockade by antisense oligonucleotides
and small interfering RNAs suppresses growth of human mammary tumor
xenografts in mice. Cancer Res. 64:5378–5384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Yang H, Kong X, et al: Inhibition
of respiratory syncytial virus infection with intranasal siRNA
nanoparticles targeting the viral NS1 gene. Nat Med. 11:56–62.
2005. View
Article : Google Scholar
|
|
13
|
Schiffelers RM, Xu J, Storm G, Woodle MC
and Scaria PV: Effects of treatment with small interfering RNA on
joint inflammation in mice with collagen-induced arthritis.
Arthritis Rheum. 52:1314–1318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui J and Wang ZM: Value of bursectomy in
the surgical treatments for shoulder impingement syndrome. Zhong
Guo Gu Ke Za Zhi. 21:37–40. 2013.In Chinese.
|
|
15
|
Luo MC, Zhang DQ, Ma SW, et al: An
efficient intrathecal delivery of small interfering RNA to the
spinal cord and peripheral neurons. Mol Pain. 1:292005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hassani Z, Lemkine GF, Erbacher P, et al:
Lipid-mediated siRNA delivery down-regulates exogenous gene
expression in the mouse brain at picomolar levels. J Gene Med.
7:198–207. 2005. View
Article : Google Scholar
|
|
17
|
Kim EY, Hong YB, Lai Z, et al: Expression
and secretion of human glucocerebrosidase mediated by recombinant
lentivirus vectors in vitro and in vivo: implications for gene
therapy of Gaucher disease. Biochem Biophys Res Commun.
318:381–390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soslowsky LJ, Carpenter JE, DeBano CM,
Banerji I and Moalli MR: Development and use of an animal model for
investigations on rotator cuff disease. J Shoulder Elbow Surg.
5:383–392. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Figuiredo A, Cordeiro AL, Tomada N, Tomada
I, Rodrigues A, Gouveia A and Neves D: Real-time PCR study of Ang1,
Ang2, Tie-2, VEGF, and KDR expression in human erectile tissue
during aging. J Sex Med. 8:1341–1351. 2011. View Article : Google Scholar
|
|
20
|
Yanagisawa K, Hamada K, Gotoh M, et al:
Vascular endothelial growth factor (VEGF) expression in the
subacromial bursa is increased in patients with impingement
syndrome. J Orthop Res. 19:448–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blaine TA, Kim YS, Voloshin I, et al: The
molecular pathophysiology of subacromial bursitis in rotator cuff
disease. J Shoulder Elbow. 14(Suppl): 84–89. 2005. View Article : Google Scholar
|
|
22
|
Thiele K, Bierhaus A, Autschbach F, et al:
Cell specific effects of glucocorticoid treatment on the
NF-kappaBp65/IkappaBalpha system in patients with Crohn's disease.
Gut. 45:693–704. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaffner F1, Ray AM and Dontenwill M:
Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic
Target in Solid Tumors. Cancers (Basel). 5:27–47. 2013. View Article : Google Scholar
|
|
24
|
Ernandez T and Mayadas T: Immunoregulatory
role of TNFalpha in inflammatory kidney diseases. Kidney Int.
76:262–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas PS: Tumour necrosis factor-alpha:
The role of this multifunctional cytokine in asthma. Immunol Cell
Biol. 79:132–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foxwell BM, Bondeson J, Brennan F and
Feldmann M: Adenoviral transgene delivery provides an approach to
identifying important molecular processes in inflammation: evidence
for heterogenecity in the requirement for NFkappaB in tumour
necrosis factor production. Ann Rheum Dis. 59:i54–i59. 2000.
View Article : Google Scholar : PubMed/NCBI
|