Introduction
Diabetes mellitus is a global disease (1), which affects >200,000,000
individuals. The majority of patients with diabetes have
non-insulin-dependent diabetes mellitus (NIDDM; type 2 diabetes),
and the number of patients with this disease is expected to double
by 2030 (2). The insulin
stimulatory activities of sulfonylureas (SUs), which are used to
treat patients with NIDDM, have been reported to decrease with time
due to the gradual destruction of pancreatic β cells. In addition,
SUs are prescribed with several restrictions due to their
side-effects, including hypoglycemia (3). Therefore, the production of an
alternative antidiabetic treatment, which exhibits low toxicity
following extended use is required.
Interest in the biological activities of compounds
from marine organisms has increased over previous years (4). Compounds derived from various marine
organisms have been investigated (5,6), a
number of which have resulted in the production of commercially
available drugs (3). Fucoidan
(Fig. 1) is an extract of the
seaweed, Fucus vesiculosus, which has been widely
investigated (7). Due to its
anti-oxidative, anticancer, and anti-inflammatory activities, it
has been suggested to be important in cancer and inflammation
(8). In addition, fucoidan has
been reported to be associated with insulin resistance (9); however, the effects of fucoidan on
insulin stimulation and pancreatic protection in spontaneous
diabetes have not been investigated. Insulin is a key regulator of
body glucose levels (10);
therefore, the present study aimed to determine whether fucoidan
can stimulate insulin secretion and protect pancreatic
function.
Under physiological conditions, the insulin
secretory response to glucose is augmented by several factors,
which act through various mechanisms (11). Cyclic adenosine monophosphate
(cAMP) is an important amplifier of insulin release (12). It is known that cAMP directly
inhibits adenosine triphosphate (ATP)-sensitive K+
channels, and promotes depolarization of the plasma membrane. In
addition, cAMP increases cytosolic Ca2+ levels via the
opening of L-type voltage-sensitive Ca2+ channels in the
plasma membrane and promoting Ca2+-induced
Ca2+ release from intracellular stores (13).
To the best of our knowledge, the present study is
the first to determine the effects of fucoidan on insulin
stimulation and pancreatic protection These data indicate that
fucoidan stimulates insulin secretion and provides pancreatic
protection via the cAMP signaling pathway in vitro and in
vivo. Furthermore, these results indicate that fucoidan may
prevent or reduce the development of spontaneous diabetes and
provide a potential novel therapeutic strategy for the treatment of
diabetes.
Materials and methods
Animals
The present study was perforned at the Animal
Experimental Center of Mudanjiang Medical University (Mudanjiang,
China). Animal care and experiments were performed in accordance
with the Animal Experiment Guidelines of Mudanjiang Medical
University, and ethical approval was obtained from Mudanjiang
Medical University. Male Goto-Kakizaki (GK) and Wistar rats (aged,
6 weeks) were obtained from CLEA Japan, Inc. (Tokyo, Japan). The
rats were divided into three groups (Wistar, GK and GK/fucoidan
group) and each group contained 8 rats The rats were housed in a
controlled environment, with a temperature of 24±1°C, and a 12 h
light/12 h dark cycle, with light turned on at 7 a.m. The rats were
provided with access to standard rat food and water ad
libitum, with or without fucoidan at the recommended
concentration of 75 mg/kg body weight (14) for 13 weeks, beginning at 6 weeks of
age.
Measurement of blood glucose levels
At 6-weeks-old, the GK rats were provided with
access to standard rat food and water, with or without 75 mg/kg
body weight fucoidan (Sigma-Aldrich, Shanghai, China) for 13 weeks.
The body weight of each rat was measured twice per week. Blood
samples (20 µl) were obtained from the tail vein every week.
The blood glucose levels were measured using a hexokinase method
(15), with a rat blood glucose
kit (Wako Pure Chemical Industries, Ltd., Tokyo, Japan).
Histopathological studies
For histopathological analysis, the rats were
sacrificed using 0.3ml/100g body weight chloral hydrate
(Sigma-Aldrich), and the pancreata from GK and Wistar rats were
fixed in 10% neutral buffered formalin and subsequently embedded in
paraffin. Sections (4 µm) of paraffin-embedded tissues were
stained with hematoxylin and eosin (HE; Sigma-Aldrich) solution, in
order to detect histopathological features, as described previously
(16). Briefly, the pancreata
sections were placed in distilled water, stained with alum
hematoxylin, rinsed under running tap water, differentiated with
0.3% acid alcohol, rinsed in running tap water again, and then
rinse in Scott's tap water substitute (Sigma-Aldrich) before
rinsing in tap water again. The pancreata were then stained with
eosin for 2 mins. An image of the cross-section yielding the
maximum diameter of the glomerulus was captured and converted into
a digital image by an examiner in a blinded-manner, using a light
microscope equipped with a camera (Olympus BX-50; Olympus
Corporation, Tokyo, Japan).
RIN-5F cell culture
The RIN-5F rat insulin-secreting cell line, derived
from rat pancreatic β cells, was purchased from American Type
Culture Collection (Manassas, VA, USA). The RIN-5F cells were
cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with
penicillin (100 U/ml), streptomycin (100 µg/ml) and 10%
fetal bovine serum (FBS; Sigma-Aldrich) at 37°C, in an atmosphere
containing 5% CO2. Fresh conditioned medium was added
every 3 days, and the subcultures were digested every 7–8 days
using 0.25% trypsin (Sigma-Aldrich).
Cytotoxicity analysis
The CellTiter 96® AQueous One Solution
Cell Proliferation Assay (Promega Corporation, Madison, WI, USA),
which has previously been reported as an effective cytoxicity assay
(17), was used in the present
study to determine the cytotoxicity of fucoidan. The RIN-5F cells
were seeded into 96-well plates at a density of 2×104
cells/well in conditioned RPMI 1640 medium and incubated at 37°C in
an atmosphere of 5% CO2 for 48 h. The cells were then
treated with various concentrations of fucoidan (0, 10,
1×102, 1×103, 1×104 and
1×105 µg/ml) for 24 h at 37°C, following which 20
µl CellTiter 96® AQueous One Solution Cell
Proliferation Assay solution was added to each well and the cells
were incubated for a further 1 h. The absorbance was measured at
490 nm using an MTP-800 microplate reader (Corona Electric Co.,
Ltd., Ibaraki, Japan).
Insulin secretion assay
In vivo, the GK rats at 6 weeks of age were
provided with access to standard rat food and water, with or
without fucoidan (75 mg/kg body weight), for 13 weeks. Blood
samples were obtained from the tail vein every week, in order to
perform an insulin secretion assay. In vitro, the RIN-5F
cells were seeded into 24-well plates at a density of
2×105 cells/well in RPMI 1640 medium, supplemented with
penicillin, streptomycin and 10% FBS, and incubated at 37°C in an
atmosphere of 5% CO2 for 24 h. The cells were then
treated with various doses of fucoidan (0, 10, 1×102,
1×103, 1×104 and 1×105
µg/ml) for 3 h; and with 1×104 µg/ml
fucoidan for various durations (3, 6, 12, 24 and 48 h), in a 20
mg/ml high glucose condition. Aliquots of the media were removed
from each of the wells and centrifuged (1,175 × g, for 5 min at
4°C). to remove the cells. The concentration of insulin in the
supernatants was determined using an enzyme immunoassay (EIA) kit
(Cayman Chemical, Ann Arbor, MI, USA). The absorbance was measured
at 490 nm using an MTP-800 microplate reader (Corona Electric Co.,
Ltd., Tokyo, Japan).
Treatment with glybenclamide
The RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI-1640 medium
supplemented with penicillin, streptomycin and 10% FBS at 37°C in
an atmosphere containing 5% CO2. After 24 h, the cells
were treated with 1×104 µg/ml fucoidan and
1×102 µM glybenclamide (Sigma Aldrich) in a 20
mg/ml high glucose condition. The concentration of insulin in the
supernatants was determined using the EIA kit and the absorbance
was measured by the MTP 800 microplate reader.
Treatment with amylin
The RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI-1640 medium
supplemented with penicillin, streptomycin and 10% FBS at 37°C in
an atmosphere containing 5% CO2. After 24 h incubation,
200 µM amylin (Sigma Aldrich) with either 1×104
µg/ml fucoidan or 1×102 µM glybenclamide
was added to the wells for 3 h in a 20 mg/ml high glucose
condition.
Measurement of intracellular cAMP
content
The RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI-1640 medium were
treated with 1×104 µg/ml fucoidan,
2×105 M phosphodiesterase inhibitor
[4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone; Sigma Aldrich],
2×105 M phosphodiesterase inhibitor + 1×104
µg/ml fucoidan, 5×105 M MDL 12330A hydrochloride
(Sigma Aldrich) or 5×105 M MDL 12330A hydrochloride +
1×104 µg/ml fucoidan, in a 20 mg/ml high glucose
condition. Intracellular cAMP content was measured using an enzyme
linked competitive immunoassay kit, containing a
cAMP-acetylcholinesterase conjugate and an anti-cAMP rabbit
antibody (Cyclic AMP EIA kit; Cayman Chemical), according to the
manufacturer's instructions. Briefly, the RIN-5F cells were
extracted in 0.05 M HCl for 20 min at 22°C. Following
centrifugation at 1,000 × g for 10 min at 22°C, the supernatant was
used to measure the cAMP content. The quantity of
cAMP-acetylcholinesterase-anti-cyclic AMP antibody complex was
measured with an enzyme assay using acetylcholine and 5,
5-dithio-bis (2-nitrobenzoic acid), termed Ellman's reagent,
contained within the Cyclic AMP EIA kit, as previously described
(18). The concentration of the
final reaction product, 5-thio-2-nitrobenzoic acid, was measured at
an absorbance of 420 nm using an MTP-800 Microplate Reader (Corona
Electric, Tokyo, Japan) and was converted to cAMP concentration
using the standard curve obtained with authentic cAMP solution in
the kit.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Each experiment was repeated at least three times.
Student's t-test was used to analyze the data and P<0.05 was
considered to indicate a statistically significant difference.
Results
Fucoidan reduces high blood glucose
levels in GK rats
At 6 weeks-old, the GK rats were provided with
access to standard rat food and water, with or without fucoidan (75
mg/kg body weight), for 13 weeks. Blood samples were obtained from
the tail vein every week. Body weight was significantly decreased
in the GK rats, compared with the control Wistar rats (Fig. 2A and B; P<0.01). Fucoidan had no
effect on body weight; however, the increased levels of blood
glucose levels in the GK rats were significantly reduced following
oral administration of fucoidan (Fig.
2C and D; P<0.01).
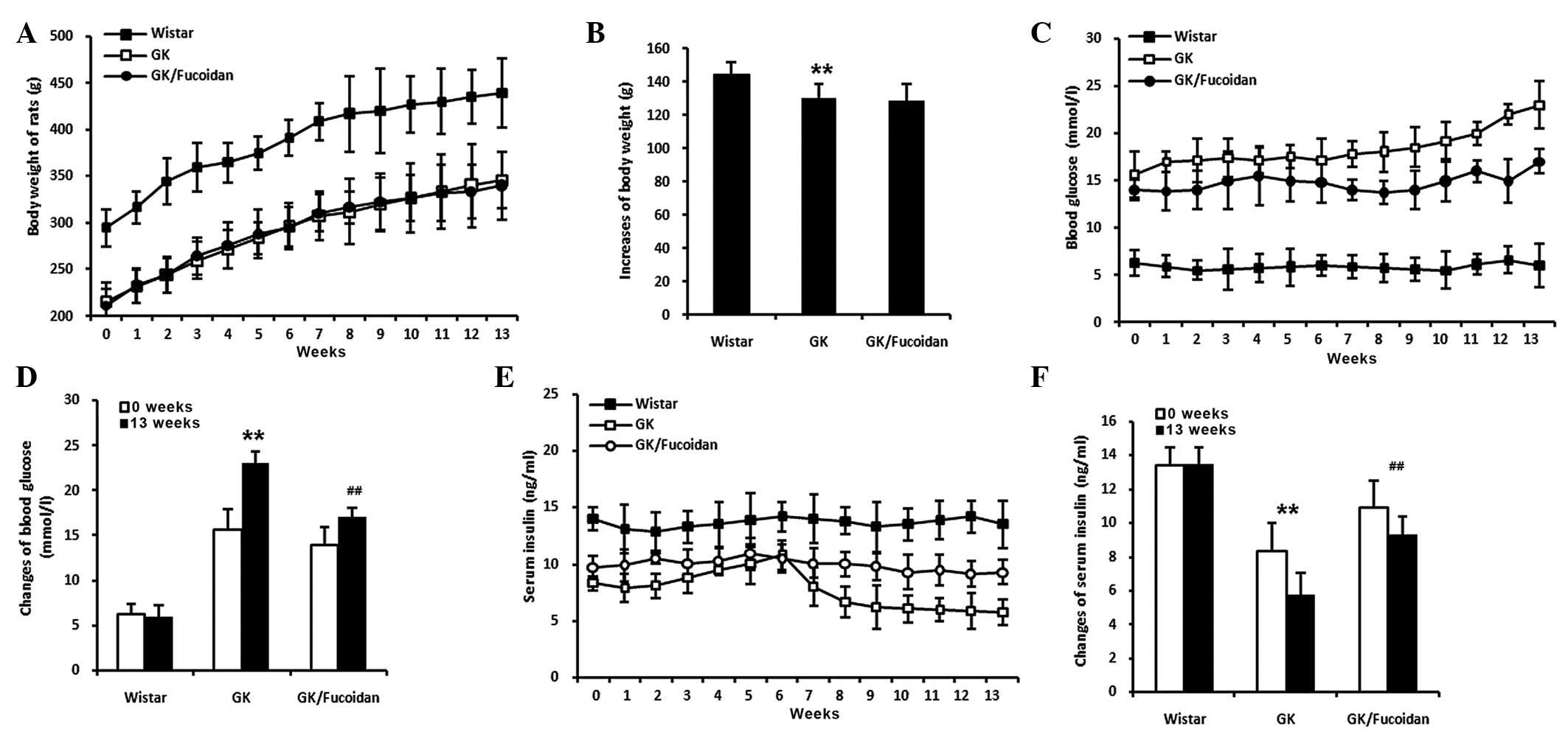 | Figure 2Effects of fucoidan on GK rats. At 6
weeks-old, the GK rats were provided with access to standard rat
food and water, with or without fucoidan (75 mg/kg body weight),
for 13 weeks. Blood samples were obtained from the tail vein every
week. (A) Body weight was significantly decreased in the GK rats,
compared with the control Wistar rats; however, fucoidan did not
have an affect body weight. (B) Quantification of the increases in
body weight after 13 weeks. (C) Blood glucose levels were
significantly increased in the GK rats, compared with the control
Wistar rats. The blood glucose levels were significantly reduced in
the GK rats treated with fucoidan. (D) Quantification of the
changes in blood glucose levels between 0 and 13 weeks. (E) Serum
insulin levels were significantly decreased in the GK rats,
compared with the control Wistar rats. The decreased serum insulin
levels were significantly recovered in the GK rats treated with
fucoidan. (F) Quantification of the changes in serum insulin levels
between 0 and 13 weeks. Data are expressed as the mean ± standard
deviation (n=8). **P<0.01, GK rats, vs. Wistar rats;
##P<0.01, GK rats treated with fucoidan, vs. GK rats.
GK, Goto-Kakizaki. |
Fucoidan recovers serum insulin levels in
GK rats
The GK rats were provided with access to standard
rat food and water, with or without fucoidan (75 mg/kg body
weight), for 13 weeks from 6 weeks of age. Blood samples were
obtained from the tail vein every week. The serum insulin levels
were significantly decreased in the GK rats, compared with the
control Wistar rats (Fig. 2E and
F; P<0.01). The decreased levels of serum insulin observed
were significantly recovered in the GK rats following oral
administration of fucoidan (P<0.01).
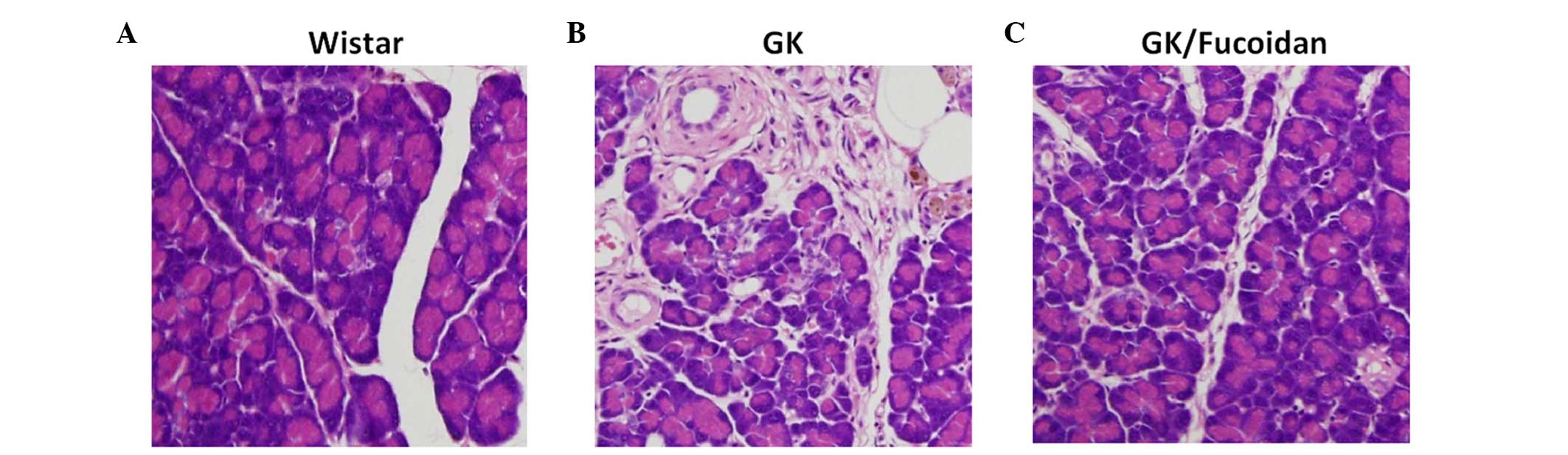
Fucoidan reduces histopathological
pancreatic changes in GK rats
The pancreata from the GK and Wistar rats were fixed
in 10% neutral buffered formalin and subsequently embedded in
paraffin. Sections (4 µm) of the paraffin-embedded tissues
were stained with HE solution, in order to detect histopathological
features. Islet atrophy, fibrosis of pancreatic ducts and blood
vessels, and macrophages containing brown coarsely granular
material were observed in the pancreata of the GK rats, compared
with the control Wistar rats (Fig.
3B). Treatment with fucoidan markedly reduced these observed
histopathological changes in the pancreata of the GK rats (Fig. 3C).
Fucoidan exhibits no obvious cytotoxicity
towards RIN-5F cells
The RIN-5F cells were seeded into 96-well plates at
a density of 2×104 cells/well in conditioned RPMI 1640
medium at 37°C in an atmosphere containing 5% CO2 for 48
h. The RIN-5F cells were treated with various concentrations of
fucoidan (0, 10, 1×102, 1×103,
1×104, 1×105 µg/ml) for 24 h,
following which 20 µl CellTiter 96® AQueous One
Solution Cell Proliferation Assay solution was added to each well
and incubated for a further 1 h. The results of this assay revealed
that fucoidan did not exhibit obvious cytotoxicity on RIN-5F cells
(Fig. 4A).
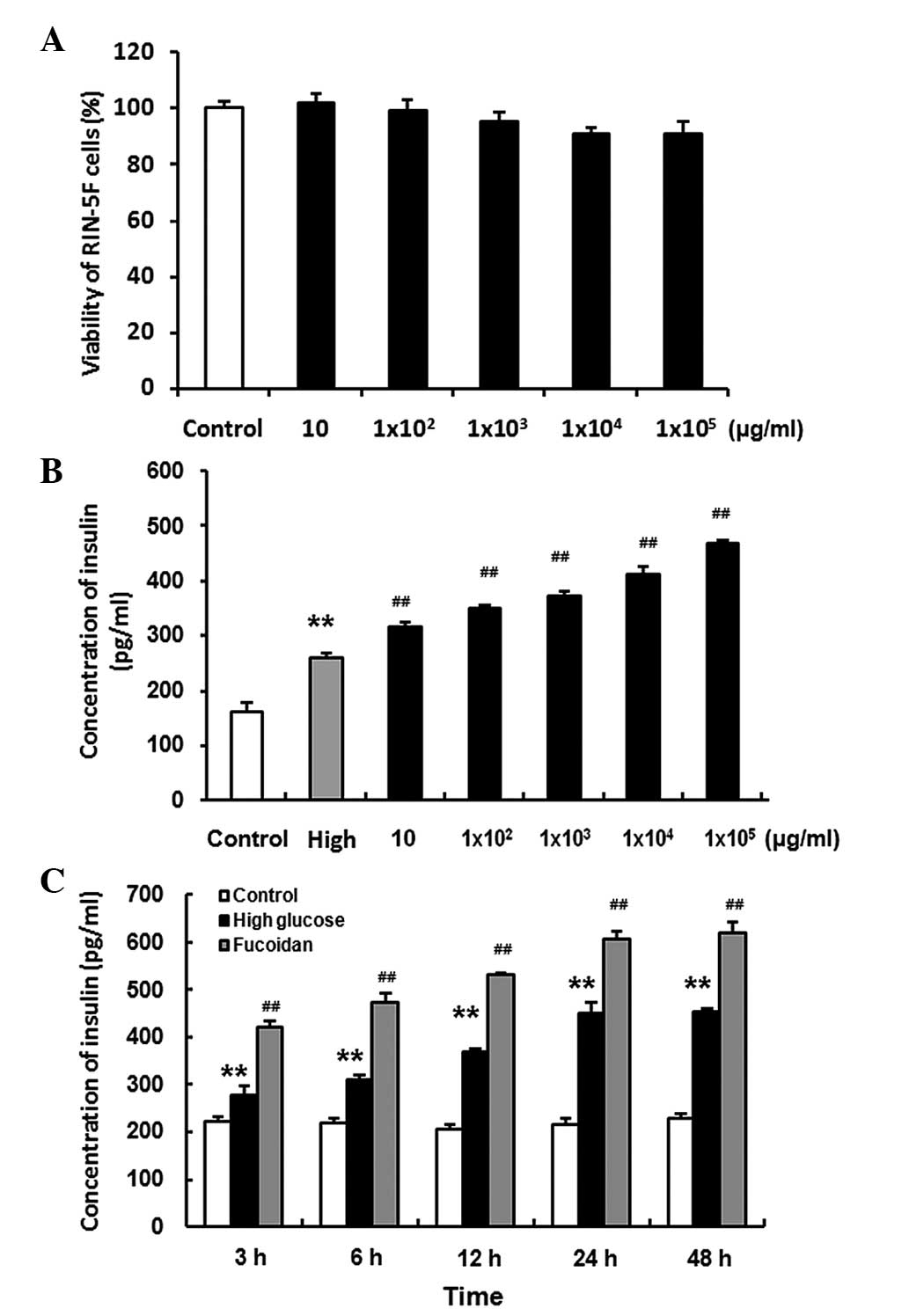 | Figure 4(A) RIN-5F rat insulin-secreting
cells were treated with various concentrations of fucoidan (0, 10,
1×102, 1×103, 1×104,
1×105 µg/ml). Fucoidan exhibited no cytotoxicity
towards the RIN-5F cells. (B and C) RIN-5F cells were treated with
various doses of fucoidan (0, 10, 1×102,
1×103, 1×104 and 1×105
µg/ml) for 3 h; and with 1×104 µg/ml
fucoidan for various time periods (3, 6, 12, 24 and 48 h,) in a 20
mg/ml high glucose condition. Treatment with fucoidan increased the
insulin secretion of RIN-5F cells in a dose-and time-dependent
manner. The concentrations of insulin were normalized with the
numbers of cells. Data are expressed as the mean ± standard
deviation (n=3). **P<0.01, high glucose, vs. control;
##P<0.01, high glucose + fucoidan, vs. high
glucose. |
Treatment with fucoidan increases the
insulin secretion of RIN-5F cells in a dose-and time-dependent
manner
RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI 1640 supplemented
with penicillin, streptomycin and 10% FBS at 37°C in an atmosphere
containing 5% CO2 for 24 h. The cells were then treated
with various doses of fucoidan (0, 10, 1×102,
1×103, 1×104 and 1×105
µg/ml) for 3 h (Fig. 4B);
and with 1×104 µg/ml fucoidan for 3, 6, 12, 24
and 48 h (Fig. 4C) in a 20 mg/ml
high glucose condition. The results revealed that fucoidan
increased insulin secretion in the RIN-5F cells in a dose- and
time-dependent manner (P<0.01).
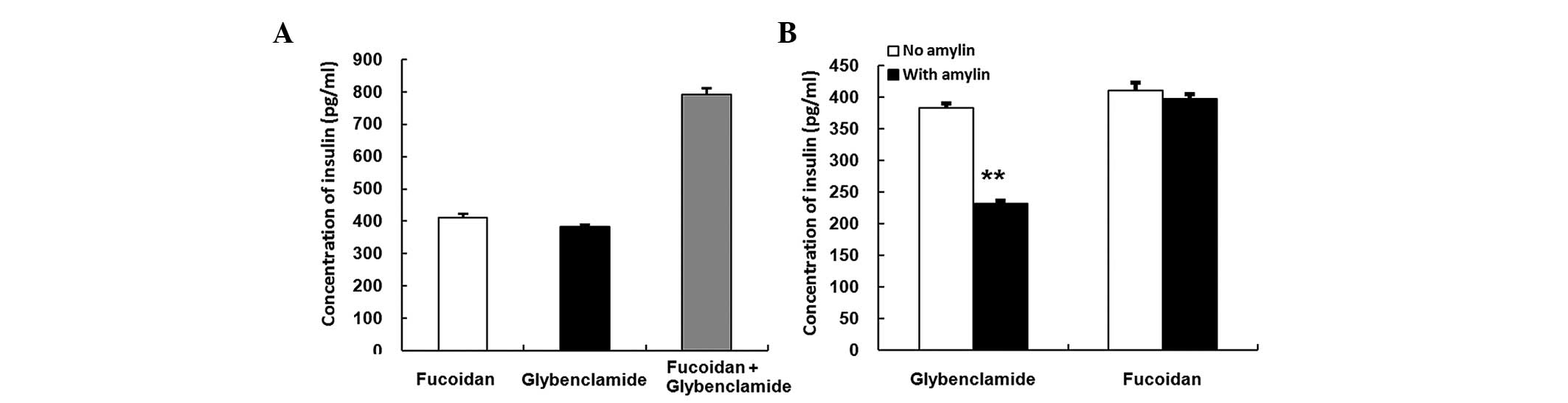
Stimulatory activities of fucoidan with
glybenclamide
The RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI 1640 medium
supplemented with penicillin, streptomycin and 10% FBS at 37°C in
an atmosphere containing 5% CO2. After 24 h, the cells
were treated with 1×104 µg/ml fucoidan and
1×102 µM glybenclamide, either alone or in
combination, in a 20 mg/ml high glucose condition for 3 h. The
results of the treatment suggested an additive effect of fucoidan
and glybenclamide (Fig. 5A).
Furthermore, the results indicate that the stimulatory effects of
fucoidan and glybenclamide arise as a result of different
mechanisms
Lack of inhibitory effect of amylin on
fucoidan
The RIN-5F cells were seeded in 24-well plates at a
density of 2×105 cells/well in RPMI 1640 medium
supplemented with penicillin, streptomycin and 10% FBS at 37°C in
an atmosphere containing 5% CO2. After24 h incubation,
200 µM amylin with either 1×104 µg/ml
fucoidan or 1×102 µM glybenclamide was added to
the wells for 3 h in a 20 mg/ml high glucose condition. Amylin
markedly inhibited the stimulatory activity of glybenclamide
(Fig. 5B, P<0.01); however, no
effect was observed on the stimulatory activity of fucoidan.
Involvement of the cAMP signaling
pathway
Following incubation of the RIN-5F cells in RPMI
1640 medium supplemented with penicillin, streptomycin and 10% FBS
at 37°C in an atmosphere containing 5% CO2 for 24 h, the
cells were treated with various doses of fucoidan (0, 10,
1×102, 1×103, 1×104 and
1×105 µg/ml) for 3 h (Fig. 6A); and with 1×104
µg/ml fucoidan for 3, 6, 12, 24 and 48 h (Fig. 6B) in a 20 mg/ml high glucose
condition. The concentration of cAMP was significantly increased in
the fucoidan-treated RIN-5F cells, and this occurred in a dose-and
time-dependent manner (P<0.01). Treatment with the
phosphodiesterase inhibitor, 2×10−5 M
4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone, which decreases the
degradation of cAMP, significantly increased the level of
fucoidan-induced insulin secretion. Conversely, treatment with
adenylyl cyclase inhibitor, 5×10−5 M MDL-12330A
hydrochloride, which decreases cAMP generation, significantly
decreased the level of fucoidan-induced insulin secretion
(P<0.01; Fig. 7A and B).
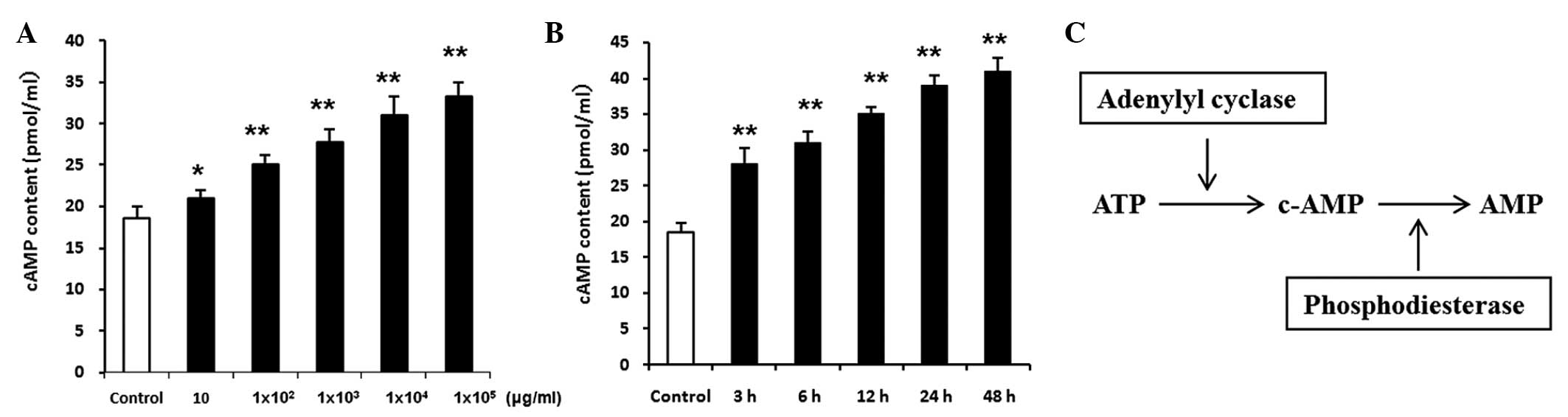 | Figure 6RIN-5F rat insulin-secreting cells
were treated with (A) various doses of fucoidan (0, 10,
1×102, 1×103, 1×104 and
1×105 µg/ml) for 3 h and with (B)
1×104 µg/ml fucoidan for various time periods (3,
6, 12, 24 and 48 h) in a 20 mg/ml high glucose condition. The
concentration of cAMP was significantly increased in the
fucoidan-treated RIN-5F cells, in a dose-and time-dependent manner.
(C) Generation and degradation of cAMP. Phosphodiesterase inhibitor
decreased the degradation of cAMP, whereas adenylyl cyclase
inhibitor decreased the generation of cAMP. The cAMP contents were
normalized with the numbers of cell. Data are expressed as the mean
± standard deviation (n=3). *P<0.05 and
**P<0.01, vs. control.. ATP, adenosine triphosphate;
cAMP, cyclic adenosine monophosphate. |
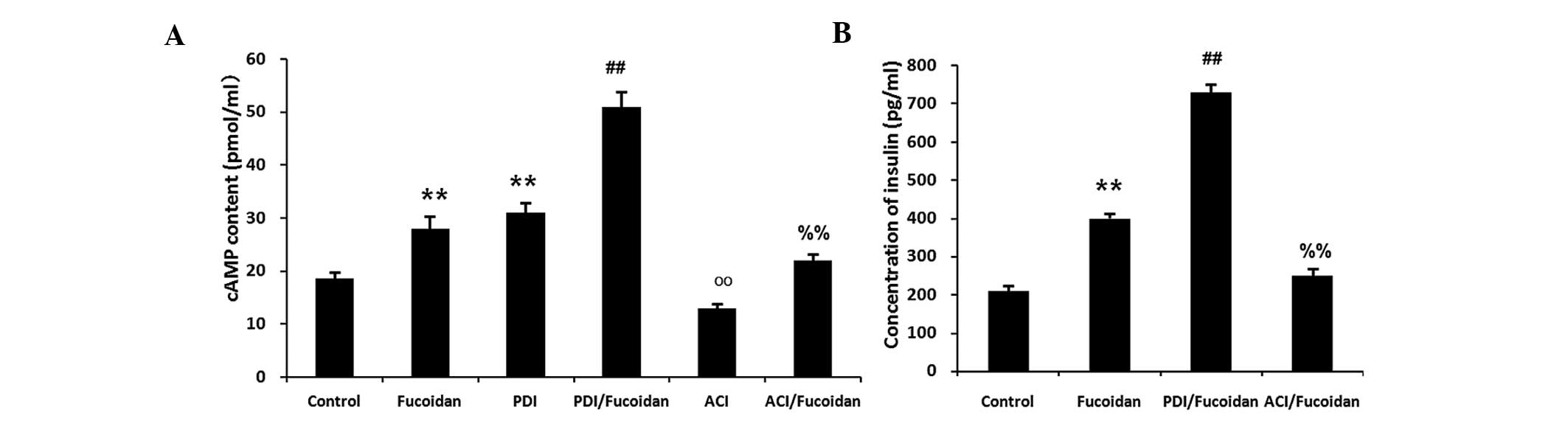 | Figure 7RIN-5F rat insulin-secreting cells
were treated with 1×104 µg/ml fucoidan,
2×10™5M PDI, PDI + fucoidan, ACI or ACI + fucoidan, in a
20 mg/ml high glucose condition. (A) Fucoidan and PDI increased the
concentration of cAMP, whereas ACI significantly decreased the
effects of fucoidan. (B) Treatment with a PDI significantly
increased the fucoidan-induced insulin secretion, whereas treatment
with an ACI significantly decreased the fucoidan-induced insulin
secretion. Data are expressed as the mean ± standard deviation
(n=3). **P<0.01, fucoidan or PDI, vs. control;
##P<0.01, PDI + fucoidan, vs. fucoidan; ooP<0.01,
ACI, vs. control; %%P<0.01, ACI + fucoidan, vs.
fucoidan. PDI, phosphodiesterase inhibitor; ACI, adenylyl cyclase
inhibitor; cAMP, cyclic adenosine monophosphate. |
Discussion
The present study is the first, to the best of our
knowledge, to demonstrate in vivo and in vitro, that
fucoidan, an extract of the seaweed Fucus vesiculosus,
reduced hyperglycemia and prevented or reduced the development of
spontaneous diabetes. Diabetes is a global disease (1), the incidence and prevalence of which
have reached epidemic proportions (19). The number of individuals affected
by diabetes mellitus is estimated to be >200,000,000. The
majority of patients with diabetes have NIDDM (2), and the number of patients with NIDDM
is expected to double by 2030 (20). The insulin stimulatory activities
of SUs have been reported to decrease with time, due to the gradual
destruction of pancreatic β cells, and SUs are prescribed with a
number of restrictions due to their associated side-effects,
including hypoglycemia (3).
Interest regarding the biological activities of
compounds obtained from marine organisms has intensified in
previous years (4). Compounds
derived from various marine organisms have been investigated, a
number of which have been developed into commercially available
drugs (3). Fucoidan is a compound,
which possesses anti-oxidant, anticancer, and anti-inflammatory
activities, and has been widely investigated (7). Fucoidan can suppress various
inflammatory cytokines, including interleukin-1β, tumor necrosis
factor-α, interferon-γ and cyclooxygenase-2 (21). In addition, fucoidan has been
reported to be important role in cancer and inflammation (8), and to be associated with insulin
resistance (9). Therefore, the
present study aimed to investigate the effects of fucoidan on
insulin stimulation and pancreatic protection.
GK rats are non-obese rats, originally derived by
repeated inbreeding of glucose-intolerant Wistar rats (15), which exhibit spontaneous and
moderate NIDDM. Between 3 and 4 weeks of age, GK rats develop mild
hyperglycemia and hyperinsulinemia. Therefore, GK rats were
selected for use in the present study, and Wistar rats were used as
a control. In the in vivo investigation, the body weights
and levels of serum insulin decreased, and blood glucose levels
significantly increased in the GK rats, compared with the control
Wistar rats. Although fucoidan did not alter body weight, the
observed increase in blood glucose levels were reduced and the
decreased serum insulin levels were recovered in the GK rats
following oral administration of fucoidan for 13 weeks.
Histopathological analysis also demonstrated that islet atrophy,
fibrosis of pancreatic ducts and blood vessels, and macrophages
containing brown coarsely granular material were observed in the
pancreata of GK rats, compared with the control Wistar rats.
Treatment with fucoidan markedly reduced the histopathological
changes in the pancreatic tissues of the GK rats. In vitro,
dose- and time-dependent effects of fucoidan on insulin
concentration were determined in RIN-5F cells cultured in high
glucose conditions. Fucoidan was not observed to be cytotoxic
towards RIN-5F cells, and significantly stimulated insulin
secretion in a dose-and time-dependent manner.
Under physiological conditions, the insulin
secretory response to glucose is augmented by numerous factors,
which act through various mechanisms (11). It is well-known that insulin
secretion from pancreatic β cells is stimulated by SUs. SUs bind to
SU receptors, resulting in the closure of ATP sensitive
K+ channels in the pancreatic β cell plasma membrane,
which leads to membrane depolarization, rapid Ca2+
influx and insulin secretion (22). SUs act by direct interaction with
the secretory machinery. In addition, physiological insulin
secretion is regulated by numerous intrinsic factors (23), including free fatty acids, amino
acids and newly identified hormones, including glucagon-like
polypeptide-1 (24) and pituitary
adenylate cyclase activating polypeptide (25). Intracellular signal transduction,
including KATP channel-dependent and -independent
mechanisms, the ATP-gated P2X3 receptor mechanism (26), and the extracellular
calcium-sensing receptor mechanism, which includes the
mitogen-activated protein kinase cascade for insulin secretion,
have also been elucidated (27,28).
In the present study, glybenclamide was used as a positive control,
and the results suggested an additive effect of fucoidan and
glybenclamide. Amylin is an inhibitor of glybenclamide, which was
shown to markedly inhibit the stimulatory activity of
glybenclamide; however, it did not inhibit the stimulatory activity
of fucoidan, suggesting that the effects of fucoidan on insulin
secretion may be associated with a different mechanism, which
varies from the rapid Ca2+ influx caused by
glybenclamide.
cAMP is well documented to be an important amplifier
of insulin release (12). cAMP
directly inhibits KATP channels and promotes
depolarization of the plasma membrane. In addition, cAMP increases
cytosolic Ca2+ levels via opening of L-type
voltage-sensitive Ca2+ channels in the plasma membrane,
and promotion of Ca2+-induced Ca2+ release
from intracellular stores (13).
Elevation in cAMP levels has also been observed to stimulate
insulin exocytosis, independent of changes in intracellular
Ca2+ concentration (29). In order to better understand the
mechanisms underlying the stimulatory activity of fucoidan, the
concentration of cAMP was measured in RIN-5F cells in the present
study. The concentration of cAMP was significantly increased in the
fucoidan-treated RIN-5F cells, in a dose-and time-dependent manner.
The generation and degradation of cAMP is shown in Fig. 4C, where phosphodiesterase
inhibition decreased the degradation of cAMP and adenylyl cyclase
inhibition decreased the generation of cAMP. Treatment with the
phosphodiesterase inhibitor significantly increased
fucoidan-induced insulin secretion, whereas treatment with the
adenylyl cyclase inhibitor significantly decreased fucoidan-induced
insulin secretion. These results suggested that the cAMP signaling
pathway may be important in the process. However, the role of
fucoidan in the regulation of insulin secretion via the cAMP
signaling pathway under physiological conditions remains to be
fully elucidated. The complex process and mechanism requires
further investigation.
The present study is the first, to the best of our
knowledge, to determine the effects of fucoidan on insulin
stimulation and pancreatic protection via the cAMP signaling
pathway in vivo and in vitro. These results indicated
that fucoidan may prevent or reduce the development of spontaneous
diabetes, and may be considered a novel therapeutic strategy for
its treatment.
References
|
1
|
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong
H and Li D: Autologous transplantation of adipose-derived
mesenchymal stem cells ameliorates streptozotocin-induced diabetic
nephropathy in rats by inhibiting oxidative stress,
pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int
J Mol Med. 30:85–92. 2012.PubMed/NCBI
|
|
2
|
Tsur A, Harman-Bohem I, Buchs AE, Raz I
and Wainstein J: The guidelines for the diagnosis prevention and
treatment of type 2 diabetes mellitus - 2005. Harefuah.
145:583–586. 6302006.In Hebrew.
|
|
3
|
Genuth S: Management of the adult onset
diabetic with sulfonylurea drug failure. Endocrinol Metab Clin
North Am. 21:351–370. 1992.PubMed/NCBI
|
|
4
|
Onofrejová L, Vasícková J, Klejdus B,
Stratil P, Misurcová L, Krácmar S, Kopecký J and Vacek J: Bioactive
phenols in algae: The application of pressurized-liquid and
solid-phase extraction techniques. J Pharm Biomed Anal. 51:464–470.
2010. View Article : Google Scholar
|
|
5
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med
Rep. 11:3746–3752. 2015.PubMed/NCBI
|
|
6
|
Zhang P, Bi C, Schmitt SM, Li X, Fan Y,
Zhang N and Dou QP: Metal-based 2,3-indolinedione derivatives as
proteasome inhibitors and inducers of apoptosis in human cancer
cells. Int J Mol Med. 34:870–879. 2014.PubMed/NCBI
|
|
7
|
Myers SP, O'Connor J, Fitton JH, Brooks L,
Rolfe M, Connellan P, Wohlmuth H, Cheras PA and Morris C: A
combined Phase I and II open-label study on the immunomodulatory
effects of seaweed extract nutrient complex. Biologics. 5:45–60.
2011.PubMed/NCBI
|
|
8
|
Li C, Gao Y, Xing Y, Zhu H, Shen J and
Tian J: Fucoidan, a sulfated polysaccharide from brown algae,
against myocardial ischemia-reperfusion injury in rats via
regulating the inflammation response. Food Chem Toxicol.
49:2090–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hernández-Corona DM, Martínez-Abundis E
and González-Ortiz M: Effect of fucoidan administration on insulin
secretion and insulin resistance in overweight or obese adults. J
Med Food. 17:830–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eliza J, Daisy P, Ignacimuthu S and
Duraipandiyan V: Antidiabetic and antilipidemic effect of
eremanthin from Costus speciosus (Koen.)Sm., in STZ-induced
diabetic rats. Chem Biol Interact. 182:67–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henquin JC: The dual control of insulin
secretion by glucose involves triggering and amplifying pathways in
β-cells. Diabetes Res Clin Pract. 93(Suppl 1): S27–S31. 2011.
View Article : Google Scholar
|
|
12
|
Damdindorj B, Dezaki K, Kurashina T, Sone
H, Rita R, Kakei M and Yada T: Exogenous and endogenous ghrelin
counteracts GLP-1 action to stimulate cAMP signaling and insulin
secretion in islet β-cells. FEBS Lett. 586:2555–2562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furman B, Ong WK and Pyne NJ: Cyclic AMP
signaling in pancreatic islets. Adv Exp Med Biol. 654:281–304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meenakshi S, Umayaparvathi S, Saravanan R,
Manivasagam T and Balasubramanian T: Hepatoprotective effect of
fucoidan isolated from the seaweed Turbinaria decurrens in ethanol
intoxicated rats. Int J Biol Macromol. 67:367–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim CS, Sohn EJ, Kim YS, Jung DH, Jang DS,
Lee YM, Kim DH and Kim JS: Effects of KIOM-79 on hyperglycemia and
diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. J
Ethnopharmacol. 111:240–247. 2007. View Article : Google Scholar
|
|
16
|
Lan T, Shen X, Liu P, Liu W, Xu S, Xie X,
Jiang Q, Li W and Huang H: Berberine ameliorates renal injury in
diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P
signaling pathway. Arch Biochem Biophys. 502:112–120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Fujii I, Lin C, Ito K, Guan H,
Zhao J, Shinohara M and Matsukura M: The stimulatory activities of
polysaccharide compounds derived from algae extracts on insulin
secretion in vitro. Biol Pharm Bull. 31:921–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie P, Nishiura H, Semba U, Chen J, Zhao
R, Kuniyasu A and Yamamoto T: Inhibitory effects of C4a on
chemoattractant and secretagogue functions of the other
anaphylatoxins via Gi protein-adenylyl cyclase inhibition pathway
in mast cells. Int Immunopharmacol. 12:158–168. 2012. View Article : Google Scholar
|
|
19
|
Hall GM and Ruggier R: Diabetes mellitus
and anaesthesia. Curr Opin Anaesthesiol. 12:343–347. 1999.
View Article : Google Scholar
|
|
20
|
Parker JC: Troglitazone: The discovery and
development of a novel therapy for the treatment of Type 2 diabetes
mellitus. Adv Drug Deliv Rev. 54:1173–1197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukahori S, Yano H, Akiba J, Ogasawara S,
Momosaki S, Sanada S, Kuratomi K, Ishizaki Y, Moriya F, Yagi M and
Kojiro M: Fucoidan, a major component of brown seaweed, prohibits
the growth of human cancer cell lines in vitro. Mol Med Rep.
1:537–542. 2008.PubMed/NCBI
|
|
22
|
Samarasinghe S and Vokes T: Diabetes
insipidus. Expert Rev Anticancer Ther. 6(Suppl 9): S63–S74. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacques-Silva MC, Correa-Medina M, Cabrera
O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM,
Kenyon NS, Ricordi C, et al: ATP-gated P2X3 receptors constitute a
positive autocrine signal for insulin release in the human
pancreatic beta cell. Proc Natl Acad Sci USA. 107:6465–6470. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hölscher C: The incretin hormones
glucagonlike peptide 1 and glucose-dependent insulinotropic
polypeptide are neuroprotective in mouse models of Alzheimer's
disease. Alzheimers Dement. 10(1 Suppl): S47–S54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y, Luo T, Xu W, Ye Z and Hong A: A new
recombinant pituitary adenylate cyclase-activating peptide-derived
peptide efficiently promotes glucose uptake and glucose-dependent
insulin secretion. Acta Biochim Biophys Sin (Shanghai). 44:948–956.
2012. View Article : Google Scholar
|
|
26
|
Widenmaier SB, Ao Z, Kim SJ, Warnock G and
McIntosh CH: Suppression of p38 MAPK and JNK via Akt-mediated
inhibition of apoptosis signal-regulating kinase 1 constitutes a
core component of the beta-cell pro-survival effects of
glucose-dependent insulinotropic polypeptide. J Biol Chem.
284:30372–30382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chalon S, Vancassel S, Zimmer L,
Guilloteau D and Durand G: Polyunsaturated fatty acids and cerebral
function: Focus on monoaminergic neurotransmission. Lipids.
36:937–944. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fedor D and Kelley DS: Prevention of
insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin
Clin Nutr Metab Care. 12:138–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tengholm A: Cyclic AMP dynamics in the
pancreatic β-cell. Ups J Med Sci. 117:355–369. 2012. View Article : Google Scholar : PubMed/NCBI
|