Introduction
Skin is an essential natural barrier to protect body
from outside physical, chemical, microbial and other infestation;
in addition, skin is a visual indicator of the body's aging process
(1). 90% of the ultraviolet (UV)
radiation reaching the surface of the earth is long-wavelength
radiation (UVA; 320–400 nm), which is 20 times higher than that of
medium-wavelength radiation (UVB; 280–320 nm), while
short-wavelength radiation (UVC; <290 nm) is completely absorbed
by the ozone shield (2). Under
physiological conditions, UVA penetrates the epidermis into the
dermis; therefore, it has been well-established that UVA
irradiation is responsible for photoaging and photocarcinogenesis
(3).
Pyrroloquinoline quinine (PQQ) is a non-covalently
bound redox co-factor of bacterial dehydrogenases, which was
initially isolated from cultures of methylotropic bacteria, and
does not rely on nicotinamide adenine dinucleotide phosphate (NADP)
and NAD as well as flavin adenine dinucleotide (FAD). It was later
reported that PQQ was abundant in various types of plant as well as
in milk, animals and humans. PQQ was therefore defined as a novel
type of vitamin (4), which was
found to have important roles in the promotion of growth, cell
proliferation and growth factor secretion (5). Evidence has suggested that PQQ is
also involved in redox processes in the mitochondrial respiratory
chain, scavenging of reactive oxygen species (ROS), attenuation of
oxidative stress in mitochondria (6) and the protection of neurons (7); in addition, PQQ was reported to
antagonize several types of oxidative stress-induced cell damage,
including reoxygenation injury in the heart, ethanol-induced liver
damage and hyperoxia-induced cognitive deficits (8). The present study aimed to determine
whether PQQ was involved in the protection of human dermal
fibroblasts (HDFs) from UVA-induced senescence.
In order to investigate the protective mechanisms of
PQQ against UVA-induced HDF aging, the present study assessed the
expression of two members of the sirtuin (SIRT) family: SIRT1 and
SIRT6. Nuclear and cytoplasm-localized SIRT1 has been reported to
have important roles in apoptosis, differentiation and oncogenic
transformation (9–14). SIRT1 was also found to deacetylate
transcription factors and co-activators, such as heat shock factor
1, which induces the transcription of molecular chaperones
associated with the pathogenesis of Parkinson's disease and
amyothrophic lateral sclerosis (15–20).
Furthermore, SIRT6 was shown to be crucial in the regulation of
mammalian longevity; therefore, it was hypothesized that SIRT6 may
have potential for the treatment of age-associated diseases
(21,22).
The present study aimed to determine whether PQQ
influences the damage of UVA irradiation, and how it functions in
the cell. It was hypothesized that PQQ protects human dermal
fibroblasts from senescence caused by UVA irradiation. An in
vitro cell-senescence model was constructed through the
exposure of PQQ-pre-treated HDFs to UVA, and the effect of PQQ on
protein and/or mRNA expression levels of the senescence marker
genes matrix metalloproteinase (MMP)1 and MMP3 as well as SIRT1,
SIRT6, nuclear factor erythroid 2-related factor 2 (Nrf2) and heme
oxygenase 1 (HO-1) were detected using polymerase chain reaction
(PCR) and western blot analyses. Furthermore, senescence-associated
β-galactosidase (SA-β-Gal) staining was used to determine the
senescence status of HDFs (8).
SA-β-Gal activity distinguishes senescent cells from those which
are quiescent or terminally differentiated, therefore acting as a
senescence biomarker.
Materials and methods
Cell culture
Primary HDFs were purchased from BioHermes Bio&
Medical Technology Co., Ltd (Wuxi, China). Fibroblasts were
subsequently cultured in a 10-cm dish in Dulbecco's modifed Eagle's
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 15% fetal bovine serum (FBS; Gibco Life
Technologies), penicillin (100 U/ml) and streptomycin (100 μg/ml;
Gibco Life Technologies). Cells were divided into aliquots and
seeded into a six-well plate (Corning Incorporated, Corning, NY,
USA), then incubated in an incubator (3111; Thermo Fisher
Scientific, Waltham, MA, USA) at 37°C with 5% CO2 in a
humidified atmosphere. For cell culture, the medium was replaced
every 2–3 days and fibroblasts were passaged at 80% confluence.
Cells were then seeded in a 6-well plate at a density of
1×106 cell/cm2 for use in subsequent
experiments.
UVA irradiation
At 24 h following the addition of PQQ
(Sigma-Aldrich, St. Louis, MO, USA) into the culture media, cells
in the six-well plate were exposed to 9 J/cm2 UVA
irradiation. Cells were washed with phosphate-buffered saline (PBS;
Gibco Life Technologies) and covered with a thin layer of PBS prior
to UVA exposure. The culture plate lid was removed, and the
six-well plate was placed on a brass block embedded on ice, in
order to reduce any evaporation, at a distance of 15 cm from the
UVA light source. As the UVA irradiation source, an Ultraviolet
phototherapy instrument (SS-04A; Shanghai SIGMA High-Tech Co., Ltd,
Shanghai, China) equipped with a 15-W ozone-free UVA lamp (CEL015
W; Philips, Groningen, Holland) was used. The incidence dose of UVA
was measured with a UVA/UVB-ultraviolet meter (Factory affiliated
to Beijing Normal University, Beijing, China). Following exposure
to UVA for varying lengths of time, PBS was replaced with culture
medium and cells were incubated under standard conditions for 72 h
prior to analysis.
Total RNA isolation
UVA irradiation and PQQ treatment were performed as
described above. At 72 h post-irradiation, total RNA was extracted
from the cells using TRIzol reagent (Promega Corp., Madison, WI,
USA). RNA concentration and purity were determined using a
Nanodrop-2000 UV spectrophotometer (Thermo Fisher Scientific).
Ribosomal RNA band integrity was evaluated using conventional
denaturing agarose gel electrophoresis (23) using the SDS-PAGE gel quick
preparation kit (Beyotime Institute of Biotechnology, Shanghai,
China).
Reverse transcription quantitative PCR
(RT-qPCR)
Samples were normalized to total RNA content prior
to reference gene assessment and gene expression analysis. Equal
amounts of RNA (300 ng) from each sample were reverse-transcribed
using a PrimeScript™ RT Reagent kit with gDNA Eraser (Takara Bio,
Inc, Dalian, China) according to manufacturer's instructions. qPCR
was performed using SYBR green dye method (SYBR Premix Ex
Taq; Takara Bio Inc.) using an ABI700 Real-Time PCR detection
system (Applied Biosystems; Life Technologies, Thermo Fisher
Scientific). Three biological and two technical replicates per
biological sample were performed. The following standard cycling
conditions for qPCR runs were applied: 95°C for 3 min to activate
polymerase, 40 cycles of denaturation at 95°C for 15 sec and
annealing-extension at 60°C for 30 sec. Melting curve analysis was
performed following every run by defined heating up to 95°C to
assess the presence of unspecific PCR products. A negative control
was included in each assay run, using water instead of
template.
Specific primers for the RT-qPCR reaction were as
follows: MMP-1 forward, 5′-TTGGAGGGGATGCTCATT-3′ and reverse,
5′-ACACGCTTTGGGGTTTG-3′ with a product size of 103 bp; MMP3
forward, 5′-GCAGTTTGATCAGCCTATCC-3′ and reverse,
5′-TCCAGAGTGTCGGAGTCCAG-3′ with a product size of 138 bp; SIRT1
forward, 5′-TGACTGTGAAGCTGTACGAGGAG-3′ and reverse,
5′-GGAAGACCCAATAACAATGAGGA-3′ with a product size of 120 bp; SIRT6
forward, 5′-CCCACGGAGTCTGGACCAT-3′ and reverse,
5′-CTCTGCCAGTTTGTCCCTG-3′ with a product of 194 bp; β-actin
forward, 5′-TGGAATCTTGCTCTTATTTTCACA-3′ and reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′.
All primers were synthesized by Sangon Biotech, Co.,
Ltd. (Shanghai, China) and were used at 400 nM except for β-actin
at 300 nM. All PCR efficiencies were between 90 and 110%.
Expression data of reference genes were validated using geNorm 3.5
software (http://medgen.ugent.be/genorm/), as previously
described (24).
SA-β-Gal staining
SA-β-Gal activity was evaluated using a
β-galactosidase staining kit (Beyotime Institute of Biotechnology,
Haimen, China). Cells were washed with PBS and then fixed for 15
min at room temperature with fixative solution. The HDF cells were
then incubated at 37°C overnight. SA-β-Gal-positive staining is
expressed as a percentage of the total number of cells; cell
numbers were counted in four continuous visual fields using a
microscope (Olympus CX51; Olympus, Tokyo, Japan; total
magnification, x20).
Western blot analysis
Whole-cell lysate extracts were separated using 12%
SDS-PAGE, transferred to polyvinylidene difluoride membranes (Roche
Diagnostics, San Francisco, CA, USA), blocked with 5% bovine serum
albumin (Sigma-Aldrich) and detected using the following primary
antibodies: SIRT1 (mouse monoclonal IgG; cat. no. SC-74504; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), SIRT6 (rabbit
monoclonal IgG; cat. no. S4322; Santa Cruz Biotechnology, Inc.),
Nrf2 (rabbit monoclonal IgG; cat. no. SC-365949; BD Biosciences,
San Jose, CA, USA), HO-1 (mouse monoclonal IgG; cat. no. 610712; BD
Biosciences) and GAPDH (mouse monoclonal IgG; cat. no. G8795;
Sigma-Aldrich), at a dilution of 1:1,000 and at 4°C for 8 h.
Horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit
immunoglobulin G were used as secondary antibodies at a dilution of
1:5,000 (cat. no. A3682; Sigma-Aldrich) at room temperature for 2
h. GAPDH was used as an internal control. Blots were analyzed using
an Super Signal West Pico Enhanced Chemiluminisence western blot
analysis system (Thermo Fisher Scientific). Experiments were
performed three times under identical conditions.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism software (GraphPad Inc., La Jolla, CA, USA). Values
are presented as the mean ± standard deviation. The one-way
analysis of variance was used for comparisons involving more than
two groups. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
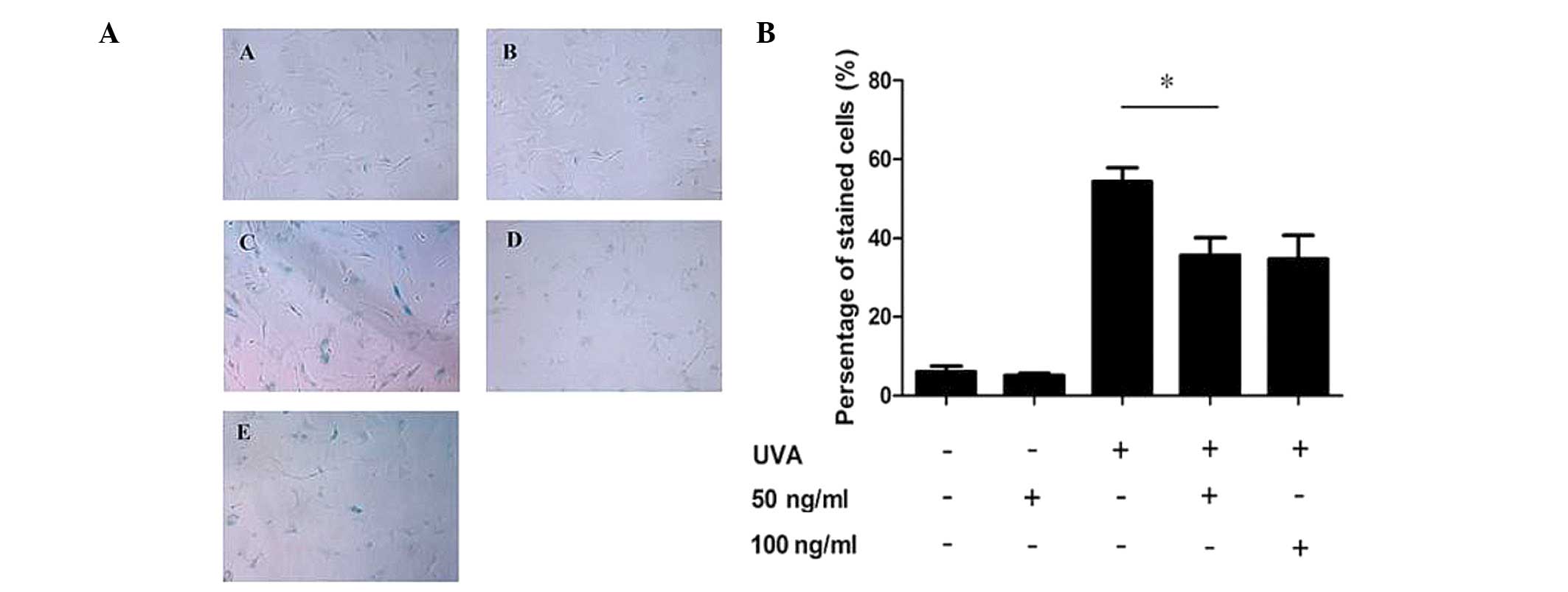
Low-dose PQQ reduces SA-β-Gal activity in
UVA-induced senescent HDFs
HDFs were pre-treated with various concentrations of
PQQ (50 and 100 ng/ml) and then subjected to UVA irradiation in
order to induce senescence, which was detected using an SA-β-Gal
staining kit. The results showed that the percentage of cells
stained by X-gal following 9 J/cm2 UVA irradiation was
markedly increased compared with that in the control group (53 and
8%, respectively; P<0.05), while 50 ng/ml PQQ attenuated the
ratio of positive staining compared with that of the UVA-only cells
(29 vs. 53%, respectively; P<0.01) (Fig. 1A and B). In addition, the
percentage of positively stained cells following treatment with 100
ng/ml PQQ and UVA were comparable to those of the cells treated
with 50 ng/ml PQQ and UVA (Fig. 1A and
B).
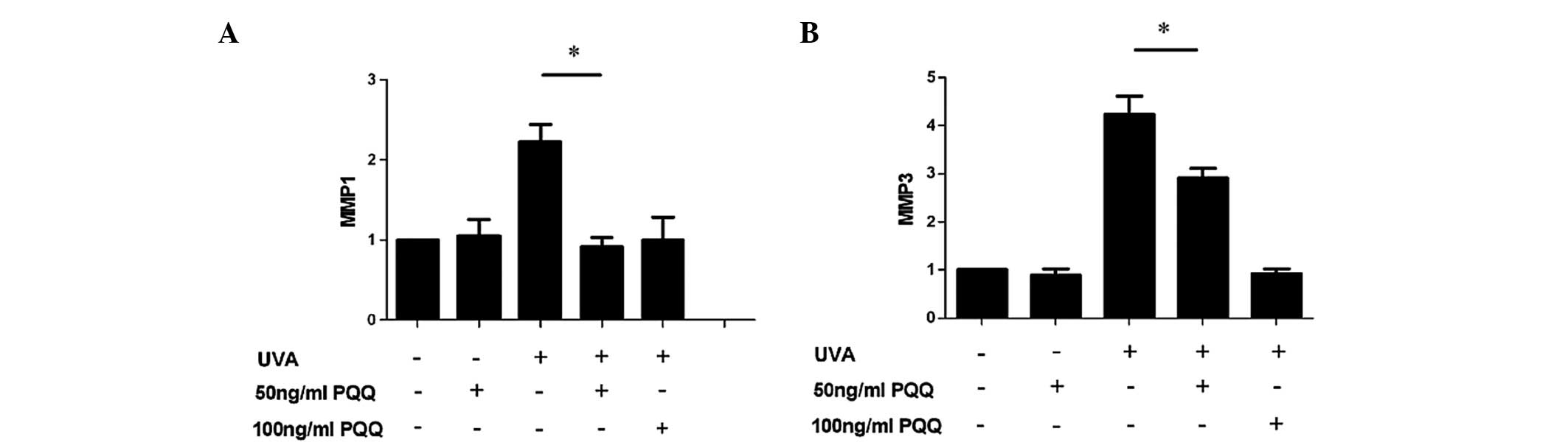
Low-dose PQQ reduces the expression of
senescence markers MMP1 and MMP3 in UVA-irradiated HDFs
RT-qPCR analysis showed that mRNA expression levels
of MMP1 and MMP3 were markedly reduced in UVA-irradiated HDFs
pre-treated with 50 or 100 ng/ml PQQ as compared with those in the
UVA-only group (P<0.05) (Fig.
2).
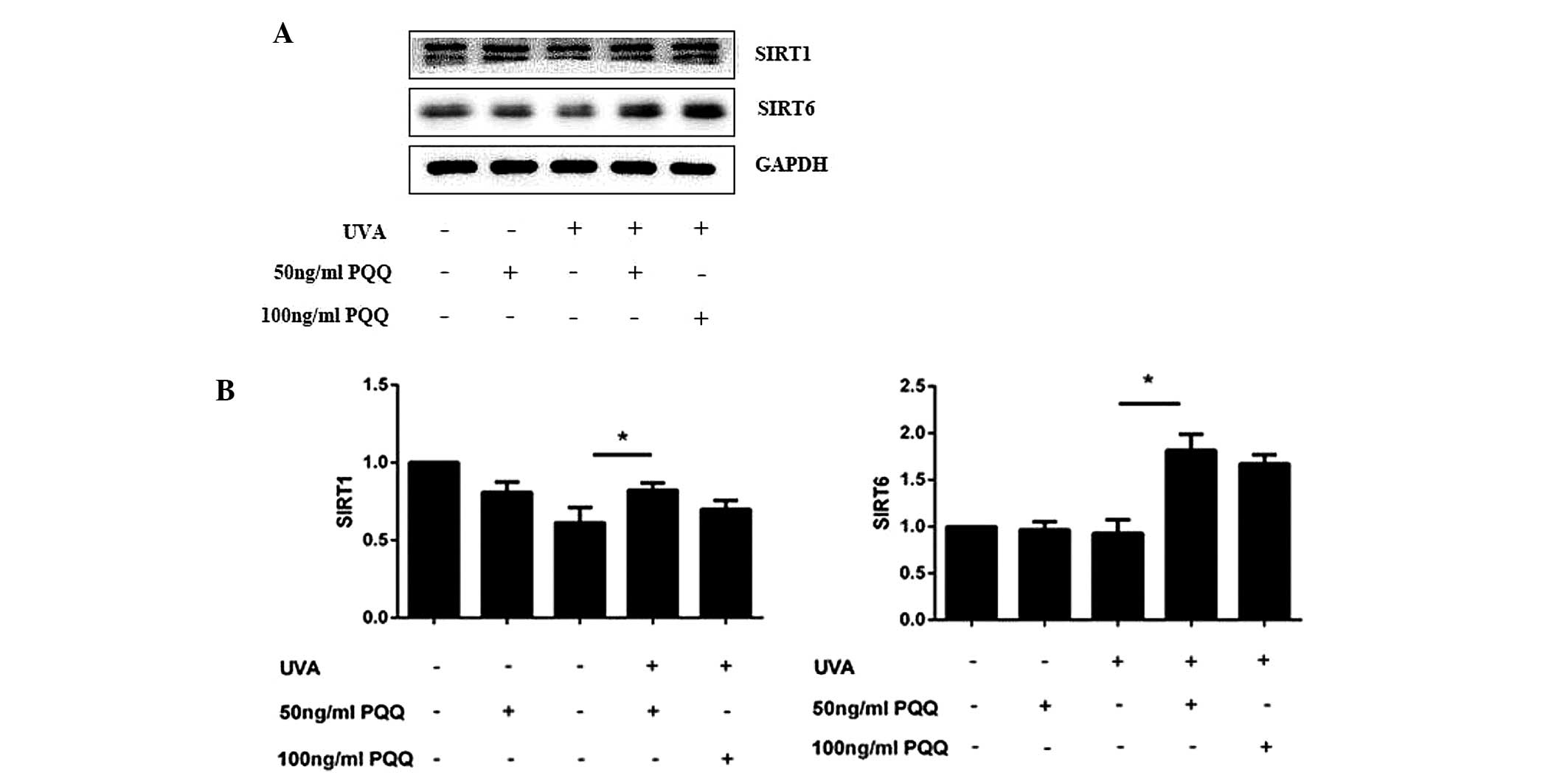
SIRT1 and SIRT6 expression levels are
increased in UVA-irradiated HDFs following pre-treatment with
PQQ
At 72 h following the addition of 50 ng/ml PQQ to
the culture media of UVA-irradiated HDFs, mRNA and protein
expression levels of SIRT1 and SIRT6 were found to be increased
(Fig. 3). In addition, notable
differences were observed in the protein levels of SIRT1 and SIRT6
at 72 h following UVA irradiation.
In order to further study the efficacy of PQQ,
western blot analysis was used to evaluate the effect of different
concentrations of PQQ on SIRT1 and SIRT1 protein expression in
UVA-irradiated HDFs. The results showed that 50 ng/ml and 100 ng/ml
PQQ had a significant effect on SIRT1 and SIRT6 levels. Therefore,
50 ng/ml PQQ was used for the subsequent evaluation of the effect
of PQQ on UVA-irradiation-induced HDF senescence at numerous
time-points (0, 1, 2, 3, 4, 5, 6 and 7 days). The results showed
that 50 ng/ml PQQ increased mRNA and protein expression of SIRT1
and SIRT6 within 72 h. 50 ng/ml PQQ was added at the 5th day
(Fig. 4).
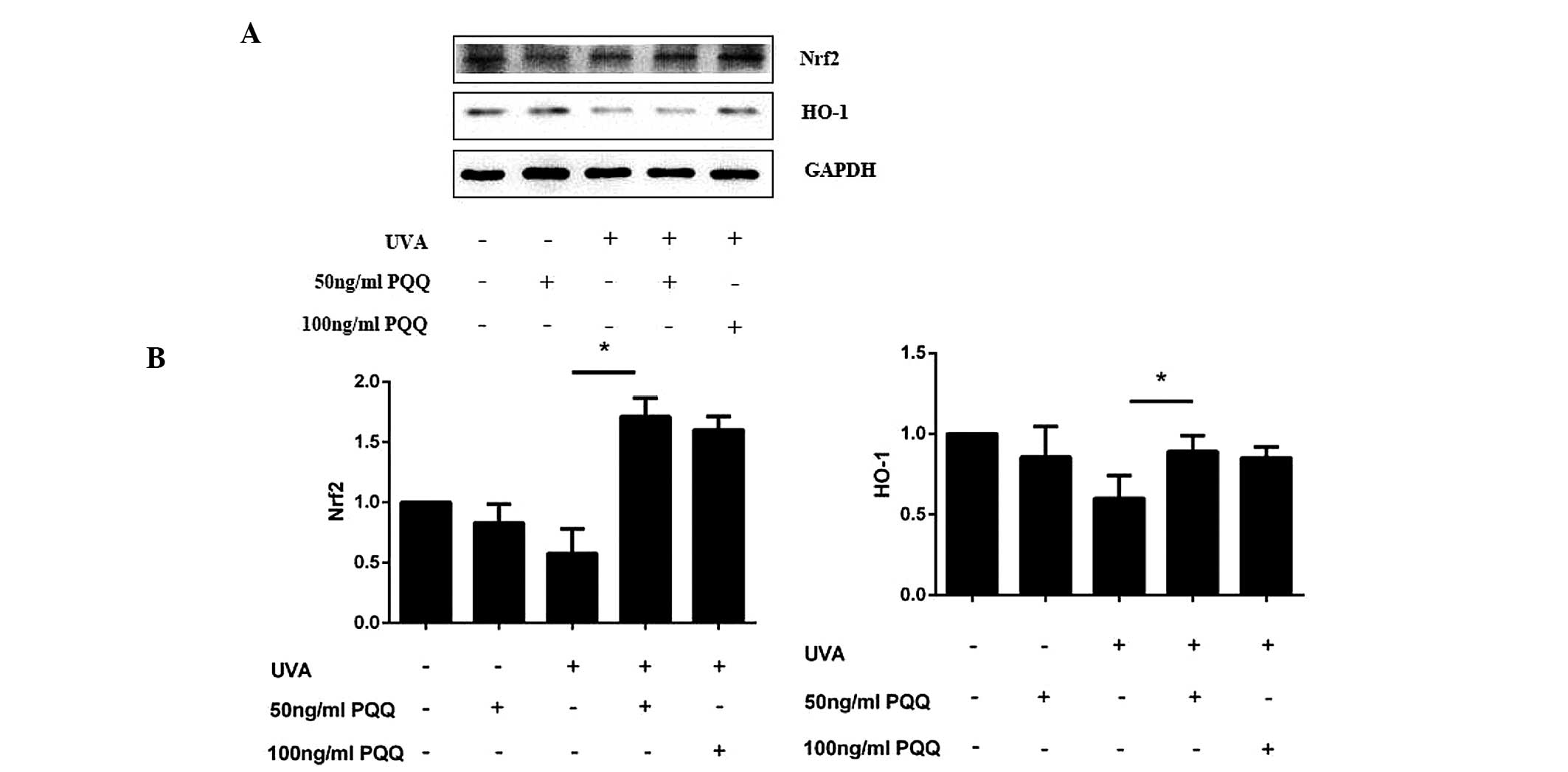
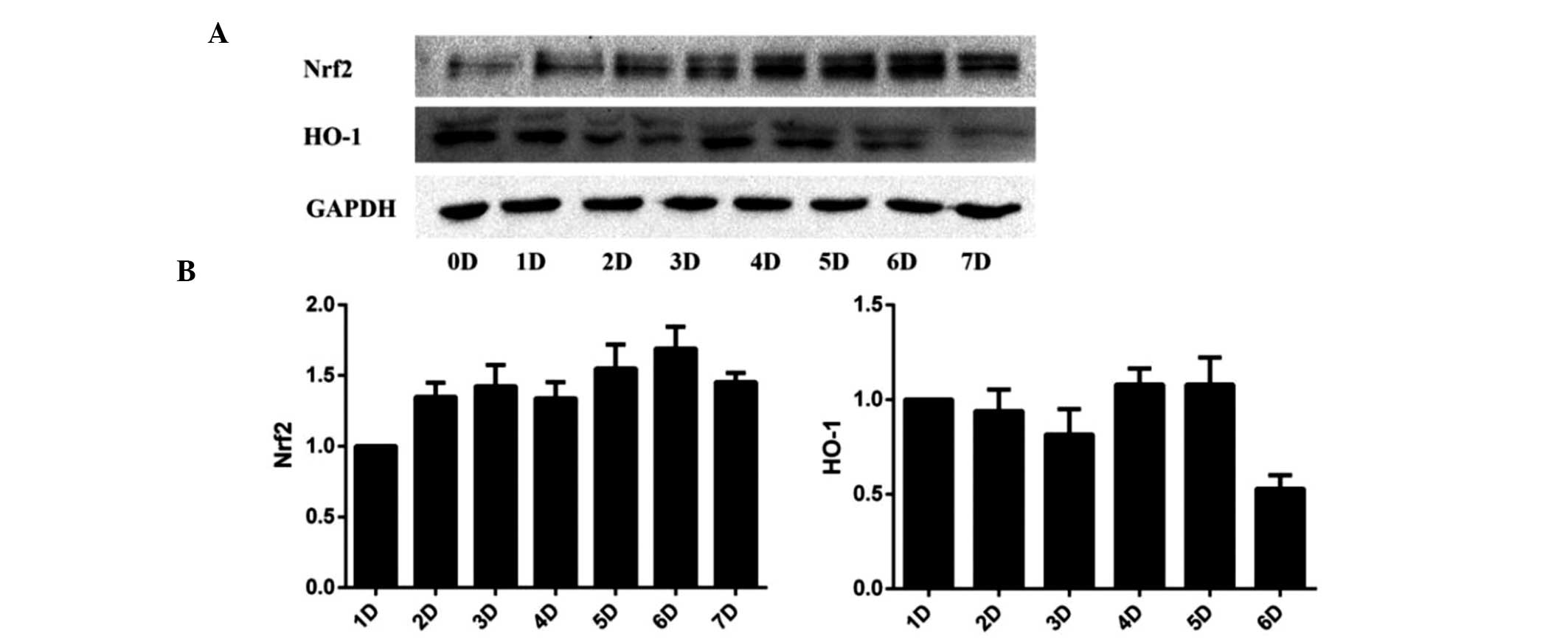
PQQ may activate the Nrf2/HO-1 pathway in
UVA-irradiated HDFs
As SIRT1/Nrf2/HO-1 is a classical anti-apoptotic
pathway (11,23,24),
the present study investigated whether this pathway was involved in
UVA-induced cell apoptosis. Altered expression levels of Nrf2 and
HO-1 mRNA expression levels were not found to be significant in
HDFs pre-treated with 50 ng/ml PQQ without UVA irradiation.
However, following UVA irradiation, PQQ was shown to alter the mRNA
and protein expression of Nrf2 and HO-1 (Fig. 5A and B; Fig. 6). Of note, 50 ng/ml PQQ achieved
the most significant effect on the expression of Nrf2 and HO-1
within 72 h.
Discussion
In a recent study, PQQ was reported to act as a
growth factor which contributed to mammalian growth and stimulated
epithelial cells through the activation of epidermal growth factor
receptor (25). PQQ has also been
reported to protect primary cultured hippocampal neurons against
glutamate-induced cell apoptosis, the mechanism of which was found
to proceed via scavenging ROS and activating phosphatidylinositol
3-kinase/Akt/glycogen sythase kinase 3β/Nrf2 signaling pathways
(26). In addition, PQQ was
reported to attenuate oxidative stress-induced cell damage in the
heart, liver and brain (27). In
the present study, a photoaging model was constructed in order to
study the protective effects of PQQ on UVA-induced HDF senescence
as well as the mechanisms by which this proceeds. The results
demonstrated that low-dose PQQ protected HDFs from senescence
following UVA irradiation, which may be associated with the
anti-apoptotic Nrf2/HO-1 and SIRT1 pathways.
Fibroblasts are the primary cellular components of
the human dermis and reflect the senescence status of skin at the
cellular level (23).
Long-wavelength UV radiation penetrates the epidermis to reach the
dermis, where it exacerbates the photoaging of skin (21). In the present study, it was
demonstrated that exposure of HDF to UVA under non-toxic conditions
increased the mRNA and protein levels of MMP1 and MMP3. In
addition, the expression of SA-β-Gal, a biomarker of fibroblasts
in vivo and in vitro (8), was significantly upregulated in
UVA-irradiated HDFs. However, PQQ reduced the expression of
SA-β-Gal as well as mRNA expression levels of MMP1 and MMP3. These
findings suggested that PQQ has a protective effect on cultured
HDFs against UVA radiation.
Sirtuins (Sir) are class III deacetylases, a family
of proteins that require NAD as a co-factor; therefore, they are
controlled by cellular [NAD]/[NADH] ratios and respond to changes
in the cellular metabolism (8),
which is essential for lifespan extension by calorie restriction in
yeast, worms and flies (28).
Seven Sir2 homologs (SIRT1–7) have been identified in mammals and
have important roles in the regulation of oxidative stress, DNA
damage and metabolism, therefore making them good candidates as
lifespan regulators (29). PQQ has
been shown to activate cyclic adenosine monophosphate response
factors, including Nrf1, Nrf2 and mitochondrial transcription
factors to enhance mitochondriogenesis (30–33).
The present study demonstrated that mRNA and protein expression of
SIRT1, SIRT6 and Nrf2 were increased in a group of HDFs treated
with 50 ng/ml PQQ and UVA radiation; of note, improvements were
observed within 72 h. In addition, decreased mRNA and protein
expression of SIRT1, SIRT6, Nrf2 and HO-1 following UVA treatment
were partially recovered when treated with 50 ng/ml PQQ. This
observation indicated that Nrf2/HO-1 signaling may have an
important role in the protective effect of PQQ against UVA
irradiation-induced senescence in HDFs. As the increased expression
of SIRT1, Nrf2 and HO-1 showed significant consistency, it was
suggested that the SIRT1/Nrf2/HO-1 pathway was activated following
PQQ treatment in UVA-irradiated HDFs.
In conclusion, the results of the present study
demonstrated that PQQ protected HDFs from UVA irradiation in
vitro, as shown by the upregulation of SIRT1 and SIRT6
following treatment with PQQ. In addition, the Nrf2/HO-1 signaling
pathway was activated. The present study provided experimental
evidence for the use of PQQ as a drug to prevent skin cell
senescence and aging.
Acknowledgments
The present study was supported by the Nanjing
Medical Science and Technology Development Fund Project (grant no.
2011NJMU242).
References
|
1
|
Kohl E, Steinbauer J, Landthaler M and
Szeimies RM: Skin aging. J Eur Acad Dermatol. 25:873–884. 2011.
View Article : Google Scholar
|
|
2
|
He YY, Council SE, Feng L and Chignell CF:
UVA-induced cell cycle progression is mediated by a disintegrin and
metal-loprotease/epidermal growth factor receptor/AKT/Cyclin D1
pathways in keratinocytes. Cancer Res. 68:3752–3758. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krutmann J: Ultraviolet A
radiation-induced biological effects in human skin: relevance for
photo aging and photodermatosis. J Dermatol Sci. 23(Suppl 1):
S22–S26. 2000. View Article : Google Scholar
|
|
4
|
Salisbury SA, Forrest HS, Cruse WB and
Kennard O: A novel coenzyme from bacterial primary alcohol
dehydrogenases. Nature. 280:843–844. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felton LM and Anthony C: Biochemistry:
role of PQQ as a mammalian enzyme cofactor? Nature. 433:E10–E12.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsushitak K, Toyama H, Yamada M, et al:
Quinoproteins: structure function and biotechnologies applications.
Appl Microboil Biot. 58:13–22. 2002. View Article : Google Scholar
|
|
7
|
Liu CL, Siesjö BK and Hu BR: Pathogenesis
of hippocampal neuronal death after hypoxia-ischemia changes during
brain development. Neuroscience. 127:113–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimri GP, Lee X, Basile G, et al: A
biomarker that identifies senescent human cells in culture and in
aging skin in vivo. Cell Biology. 92:9363–9367. 1995.
|
|
9
|
Ohwada K, Takeda H, Yamazaki M, Isogai H,
Nakano M, Shimomura M, Fukui K and Urano S: Pyrroloquinoline
quinone (PQQ) prevents cognitive deficit caused by oxidative stress
in rats. J Clin Biochem Nutr. 42:29–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Shen M, Ding M, Shen D and Ding
F: The neuropro-tective action of pyrroloquinoline against
glutamate-induced apoptosis in hippocampal neurons is mediated
through the activation of PI3K/Akt pathway. Toxicol Appl Pharm.
252:62–72. 2011. View Article : Google Scholar
|
|
11
|
Kanfi Y, Naiman S, Amir G, et al: The
sirtuin SIRT6 regulates lifespan in male mice. Nature. 483:218–221.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hekimi S and Guarente L: Genetics and the
specificity of the aging process. Science. 299:1351–1354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howitz KT, Bitterman KJ, Cohen HY, et al:
Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Landry J, Sutton A, Tafrov ST, et al: The
silencing protein SIR2 and its homologs are NAD-dependent protein
deacetylases. Proc Natl Acad Sci USA. 97:5807–5811. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugino T, Maruyama M, Tanno M, et al:
Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth
factor-induced neurite outgrowth in PC12 cells. FEBS Lett.
584:2821–2826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hisahara S, Chiba S, Matsumoto H, et al:
Histone deacetylase SIRT1 modulates neuronal differentiation by its
nuclear translocation. Proc Natl Acad Sci USA. 105:15599–15604.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michán S, Li Y, Chou MM, et al: SIRT1 is
essential for normal cognitive function and synaptic plasticity. J
Neurosci. 30:9695–9707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Q, Yan T, Ge X, Sun C, Shi X and Zhai
Q: Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol.
213:88–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Byles V, Chmilewski LK, Wang J, et al:
Aberrant cytoplasm localization and protein stability of SIRT1 is
regulated by PI3K/IGF-1R signaling in human cancer cells. Int J
Biol Sci. 6:599–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herskovits AZ and Guarente L: Sirtuin
deacelylase in neurode-generative disease of aging. Cell Res.
23:746–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrmann G, Brenneisen P, Wlaschek M, et
al: Psoralen photo-activation promotes morphological and functional
changes in fibroblasts in vitro reminiscent of cellular senescence.
J Cell Sci. 111:759–767. 1998.
|
|
22
|
Brugè F, Venditti E, Tiano L, Littarru GP
and Damiani E: Reference gene validation for qPCR on normoxia- and
hypoxia-cultured human dermal fibroblasts exposed to UVA: is
β-actin a reliable normalize for photoaging studies? J Biotechnol.
156:153–162. 2011. View Article : Google Scholar
|
|
23
|
Kawahara TL, Michishita E, Adler AS, et
al: SIRT6 links histone H3 lysine 9 deacetylation and organismal
life span. Cell. 136:62–74. 2008. View Article : Google Scholar
|
|
24
|
Herrmann G, Wlaschek M, Lange TS, et al:
UVA irradiation stimulates the synthesis of various
matrix-metalloproteinases (MMPs) in cultured human fibroblasts. Exp
Dermatol. 2:92–97. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masek T, Vopalensky V, Suchomelova P and
Pospisek M: Denaturing RNA electrophoresis in TAE agrose gels. Anal
Biochem. 336:46–50. 2005. View Article : Google Scholar
|
|
26
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric average of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar
|
|
27
|
Li H, Wu S, Shi N, Lian S and Lin W:
Nrf2/HO-1 pathway activation by manganese is associated with
reactive oxygen species and ubiquitin-proteasome pathway, not MAPKs
signaling. J Appl Toxicol. 31:690–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura K, Takada M, Ishii T, et al:
Pyrroloquinoline quinine stimulates epithelial cell proliferation
by activating epidermal growth factor receptor through redox
cycling. Free Radical Bio Med. 53:1239–1251. 2012. View Article : Google Scholar
|
|
29
|
Zhang Q, Ding M, Cao Z, et al:
Pyrroloquinoline quinine protects rat brain cortex against acute
glutamate-induced neurotoxicity. Neutovhrm Res. 38:1661–1671.
2013.
|
|
30
|
Li HH, He B, Peng H and Liu SQ: Effects of
pyrroloquinoline quinine on proliferation and expression of c-fos,
c-jun, CREB and PCNA in cultured Schwann cells. Zhonghua Zheng Xing
Wai Ke Za Zhi. 27:298–303. 2011.In Chinese. PubMed/NCBI
|
|
31
|
Blander G and Guarente L: The Sir family
of protein deaceylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar
|
|
32
|
Lin SJ, Defossez PA and Guarente L:
Requirement of NAD and SIR2 for life-span extension by calorie
restriction in Sacharomyces cerevisiae. Science. 289:2126–2128.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frye RA: Characterization of five human
cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins
(sirtuins) metabolize NAD and may have protein
ADP-ribosyltransferase activity. Biochem Biophys Res Commun.
260:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|