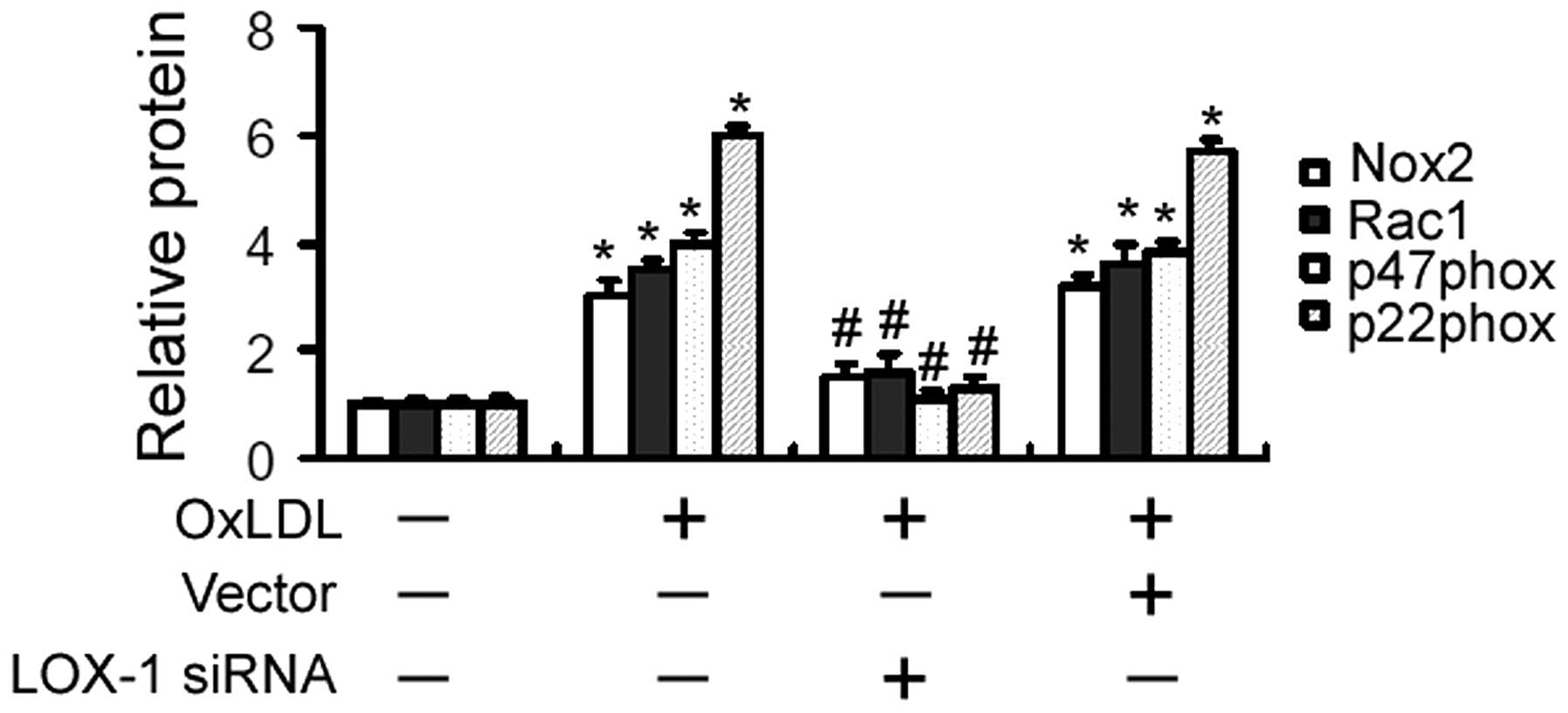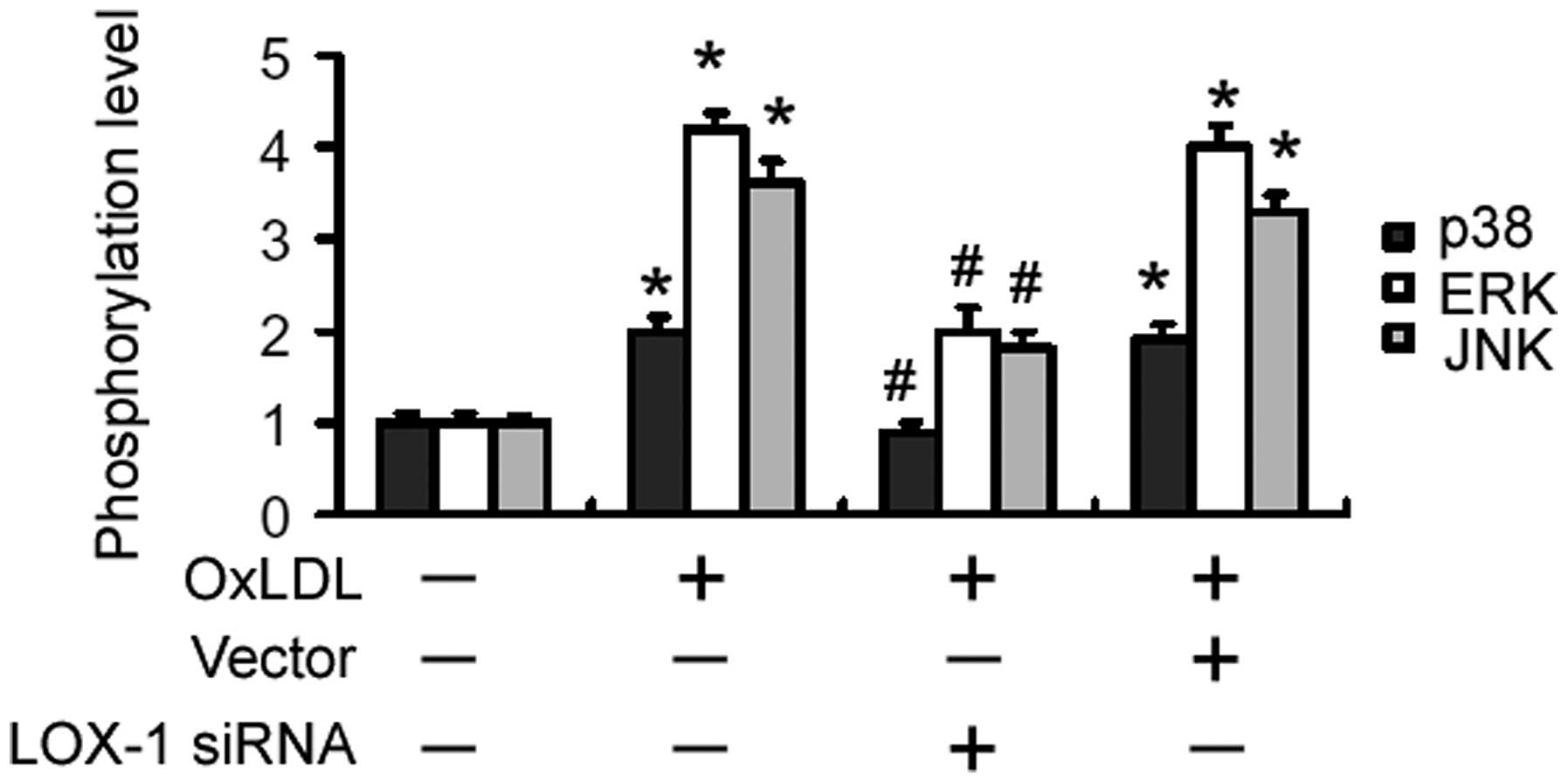Introduction
Atherosclerosis is a leading cause of mortality in
developed and developing countries, which is characterized by the
development of plaques in arteries that ultimately leads to
cardiovascular disease (1). One of
the early events of atherogenesis is the formation of foam cells,
which are macrophages with ingested oxidized low-density
lipoprotein (OxLDL) (2).
Compelling evidence indicates that oxidative stress, particularly
excessive production of reactive oxygen species (ROS), has a causal
role in atherosclerosis (3,4).
Lipid oxidation triggered by ROS can amplify foam cell formation
through oxLDL formation and uptake. Increased ROS can also promote
vasoconstriction, platelet aggregation and adhesion of neutrophils
to the endothelium, leading to vascular inflammation and
dysfunction in atherosclerosis. The proatherogenic activity of ROS
is mediated through activation of numerous signaling pathways,
including mitogen-activated protein kinases (MAPKs) (5). p38 MAPK has been implicated in the
development of atherosclerosis via promotion of cholesterol ester
accumulation in macrophages and foam cell formation (6). Despite extensive studies on the role
of ROS in atherosclerosis (3–5),
relatively little is known about the molecular mechanisms
underlying the regulation of ROS production.
Nicotinamide adenine dinucleotide phosphate (NADPH)
oxidases (NOX) are a family of enzymes that use NADPH as a
substrate to convert molecular oxygen to ROS (7). In phagocytic cells, NADPH oxidases
consist of membrane-associated cytochrome b558 comprising of the
catalytic gp91phox (or Nox2) and regulatory p22phox subunits, and
cytosolic components, including p47phox, p67phox, p40phox and a
small GTPase (Rac1 or Rac2). As a major source of ROS production by
vascular cells, NOX enzymes are important in atherosclerosis
(8). NOX enzymes have been
suggested as important therapeutic targets for the treatment of
cardiovascular diseases (9).
Lectin-like oxidized low-density lipoprotein
receptor-1 (LOX-1) is the main OxLDL receptor of endothelial cells
and is also expressed in macrophages and smooth muscle cells
(10). Under physiological
conditions, LOX-1 is almost undetectable. However, in response to
proatherogenic stimuli (e.g. exposure to OxLDL), LOX-1 is
upregulated and can be detected in atherosclerotic lesions
(11). Multiple lines of evidence
from animal studies suggest that LOX-1 has a central role in the
pathogenesis of atherosclerosis (12,13).
Deletion of LOX-1 has been found to attenuate atherogenesis in
low-density lipoprotein receptor (LDLR) knockout mice fed a
high-cholesterol diet (12). LOX-1
deficiency leads to a reduction in macrophage trafficking in the
aorta of LDLR knockout mice (13).
There is a close association between LOX-1 expression and ROS
generation in human vascular smooth muscle cells (14). However, the role of LOX-1 in
OxLDL-induced oxidative stress in macrophages remains to be
elucidated.
Therefore, in the present study, small interfering
RNA (siRNA) technology was employed to decrease the expression of
LOX-1 in macrophages and its effects on OxLDL-induced ROS
generation and NOX expression were examined. The in vivo
effects of reducing LOX-1 were also examined in a mouse model
(ApoE-/-) of high-fat diet-induced atherosclerosis.
Materials and methods
Antibodies
Rabbit anti-human LOX-1 polyclonal antibody (cat.
no. Ab60178), anti-human Nox2 polyclonal antibody (cat. no.
Ab80508), anti-human p22phox polyclonal antibody (cat. no.
Ab75941), goat anti-human p47phox polyclonal antibody (cat. no.
Ab795), and mouse anti-human Rac1 monoclonal antibody (cat. no.
Ab33186) were purchased from Abcam (Cambridge, UK). Rabbit
anti-human β-actin polyclonal antibody (cat. no. 4967), anti-human
p38 polyclonal antibody (cat. no. 9212), anti-human phosphorylated
p38 (cat. no. 9211), rabbit anti-human extracellular
signal-regulated protein kinases 1 and 2 (ERK1/2) monoclonal
antibody (cat. no. 4965), rabbit anti-human phosphorylated ERK1/2
polyclonal antibody (cat. no. 9101), rabbit anti-human c-Jun
NH2-terminal kinase (JNK) polyclonal antibody (cat. no. 9252) and
rabbit anti-human phosphorylated JNK monoclonal antibody (cat. no.
4671) were purchased from Cell Signaling Technology, Inc. (Beverly,
MA, USA). Rabbit anti-human CD68 polyclonal antibody (cat. no.
sc-9139) was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). These primary antibodies were diluted 1:1,000 prior
to use.
Plasmid and adenovirus construction
Two shRNAs targeting mouse LOX-1 (GenBank
accession no. NM_138648.2) were designed, with target sequences as
follows: LOX-1-siRNA1, 5′-GTCAGTGACCCTTATTGTA-3′ and LOX-1-siRNA2,
5′-GTGGCCAGTTACTACAAAT-3′. The shRNA oligonucleotides were
separately inserted into the pGenesil-1 expression plasmid, which
contains the human/mouse U6 promoter and the reporter gene of green
fluorescence protein (GFP). The recombinant plasmids were sequenced
to confirm the identity of the inserts. LOX-1-shRNA was cloned into
an adenoviral shuttle vector and joined with the recombinant
adenovirus type 5 (rAd5) vector to construct rAd5-LOX-1-shRNA.
Cell culture, transfection and cell
treatment
Mouse RAW264.7 macrophages (ATCC, Manassas, VA, USA)
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10%
fetal bovine serum, penicillin (100 U/ml) and streptomycin (100
µg/ml) at 37°C in a 5% CO2 incubator.
At 60% confluency, cells were transfected with 1.0
µg LOX-1 shRNA or an empty vector using Lipofectamine 2000
(Invitrogen Life Technologies) and incubated for 24 h at 37°C.
shRNA transfection efficiency was estimated by measuring
GFP-positive cells using flow cytometry. The transfection
efficiency obtained in the present study was ~72%. LOX-1 mRNA
expression was determined using quantitative reverse transcription
quantitative polymerase chain reaction (RT-qPCR) analysis, as
described below.
Cells were assigned to one of the four treatment
groups: Untreated group, no treatment; OxLDL group, treatment with
OxLDL; control group, pre-transfection with the empty vector
followed by OxLDL exposure and the LOX-1 shRNA group,
pre-transfection with LOX-1 shRNA followed by OxLDL exposure. Since
LOX-1-siRNA2 demonstrated a higher knockdown efficiency than
LOX-1-siRNA1, the former was used in the following experiments.
Cells were pre-transfected with the empty vector or LOX-1 shRNA and
then exposed to OxLDL (50 mg/l) 24 h after transfection. Following
treatment, cells were subjected to oxidative stress and gene
expression analyses.
RNA isolation and RT-qPCR analysis
Following treatment, total RNA was extracted from
cells using TRIzol reagent (Invitrogen Life Technologies) according
to the manufacturer's instructions. Reverse transcription was
performed using the AMV First Strand cDNA Synthesis kit (Shanghai
Sangon Biological Engineering Technology & Services Co., Ltd.,
Shanghai, China). qPCR amplification was conducted on an Applied
Biosystems StepOnePlus Real-Time PCR System (Applied Biosystems,
Foster City, CA, USA). The sequences of the primers and the probe
for PCR amplification of LOX-1 were as follows: LOX-1, forward
5′-GCCTCCCAACGAGTTAGAAGAG-3′ and reverse 5′-CGGGACGTGGCCATTATATT-3′
and 5′-fluorescein-TGGCACTTGTCTGTCACTGGAGCCTGAT-3′ (probe). As an
internal quantitative control, β-actin was amplified in a parallel
reaction with the primers: β-actin, forward
5′-TCAGGTCATCACTATCGGCAAT-3′ and reverse
5′-GGATGTCAACGTCACACTTCATG-3′, and
5′-fluorescein-TCCAGCCTTCCTTCCTGGGTATGGAATC-3′ (probe). All assays
were performed in triplicate and the threshold cycle was calculated
as described previously (15). The
relative LOX-1 mRNA expression level was determined by
normalization to β-actin mRNA.
Western blot analysis
Following treatment, cells were resuspended in the
lysis buffer containing dithiothreitol and protease inhibitors and
lysed on ice. Cell lysates were centrifuged at 12,000 x g for 5 min
at 4°C. Protein content in the supernatants was determined by a BCA
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Equal quantities of lysate protein were separated by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto a polyvinylidene difluoride membrane. Following blocking
non-specific sites for 1 h at room temperature with 5% fat-free
milk, the membrane was incubated overnight at 4°C with individual
primary antibodies. Following washing, the membrane was incubated
with HRP-conjugated secondary antibody. Bands were developed by
using the diaminobenzidine (DAB) substrate (Sigma-Aldrich, St.
Louis, MO, USA). The intensity of signal bands was quantified by
densitometry. The relative protein expression level was determined
by normalization to β-actin.
Oxidative stress assessment
Following treatment, cells were harvested and
intracellular malondialdehyde (MDA) level and superoxide dismutase
(SOD) activity were measured using the malondialdehyde colorimetric
assay kit and the superoxide dismutase activity assay kit,
respectively. (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's instructions.
ROS levels were detected based on the oxidation of
2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) by peroxide to
produce the fluorescent product 2′,7′-dichlorofluorescein (DCF). In
brief, following treatment, cells were washed and incubated with
DCHF-DA (Beyotime Institute of Biotechnology, Haimen, China) for 30
min. Following washing, cells were applied to flow cytometry. The
fluorescence of DCF was measured at an excitation wavelength of 488
nm and an emission wavelength of 530 nm.
Animal experiments
In total, 45 ApoE-/- mice (8 weeks old, male) were
purchased from Peking University (Beijing, China). Mice were kept
on a 12-h light/dark cycle, with food and water freely available.
All animals were fed a high-fat diet (1% cholesterol, 10% pork lard
and 10% egg yolk) for 5 weeks. The animals were then randomly
divided into three groups: Control group (injection of
physiological saline), mock group (injection of mock adenovirus)
and the LOX-1-siRNA group (injection of the LOX-1-siRNA-expressing
adenovirus). The recombinant adenovirus was administered via tail
vein twice with a 10-day interval. Following this treatment, mice
were fed the high-fat diet for 4 weeks prior to sacrifice using
carbon dioxide anaesthesia. The animal experimental protocol was
approved by the Animal Care Committee of Shanxi Medical University
(Taiyuan, China).
At the end of the animal experiment, animals were
fasted for 12 h and blood samples were obtained from retro-orbital
bleeding. The serum levels of total cholesterol, triglycerides and
high-density lipoprotein cholesterol were measured using a total
cholesterol assay kit, a triglyceride colorimetric assay kit and a
high density lipoprotein cholesterol assay kit, respectively. These
commercial kits were obtained from Nanjing Jiancheng Bioengineering
Institute.
Mice were then perfused via the left ventricle with
phosphate-buffered saline followed by 4% paraformaldehyde. The
proximal aorta was carefully dissected and fixed overnight in 4%
paraformaldehyde prior to embedding in paraffin. Serial
5-µm-thick cryosections were prepared and stained with
Movat's pentachrome (Santa Cruz Biotechnology, Inc.) for lesion
area quantitation. The plaque area, external elastic membrane area,
plaque area/aortic area and fibrous cap area were measured using
Image Pro Plus version 6.0 (Media Cybernetics, Bethesda, MD, USA).
Additional cryosections from the proximal aorta were stained for
macrophages (anti-mouse CD68 antibody), using the standard
streptavidin-biotin-peroxidase complex technique. Sections were
incubated with biotinylated goat anti-mouse IgG and
peroxidase-labeled streptavidin. DAB was used as a substrate
chromogen solution for the development of peroxidase activity.
Omission of the primary antibody was included as one control to
determine staining specificity.
Statistical analysis
Data are expressed as the means ± standard
deviation. Statistical differences among multiple groups were
calculated using one-way analysis of variance followed by Tukey's
post hoc test. All statistical calculations were performed using
SPSS version 11 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
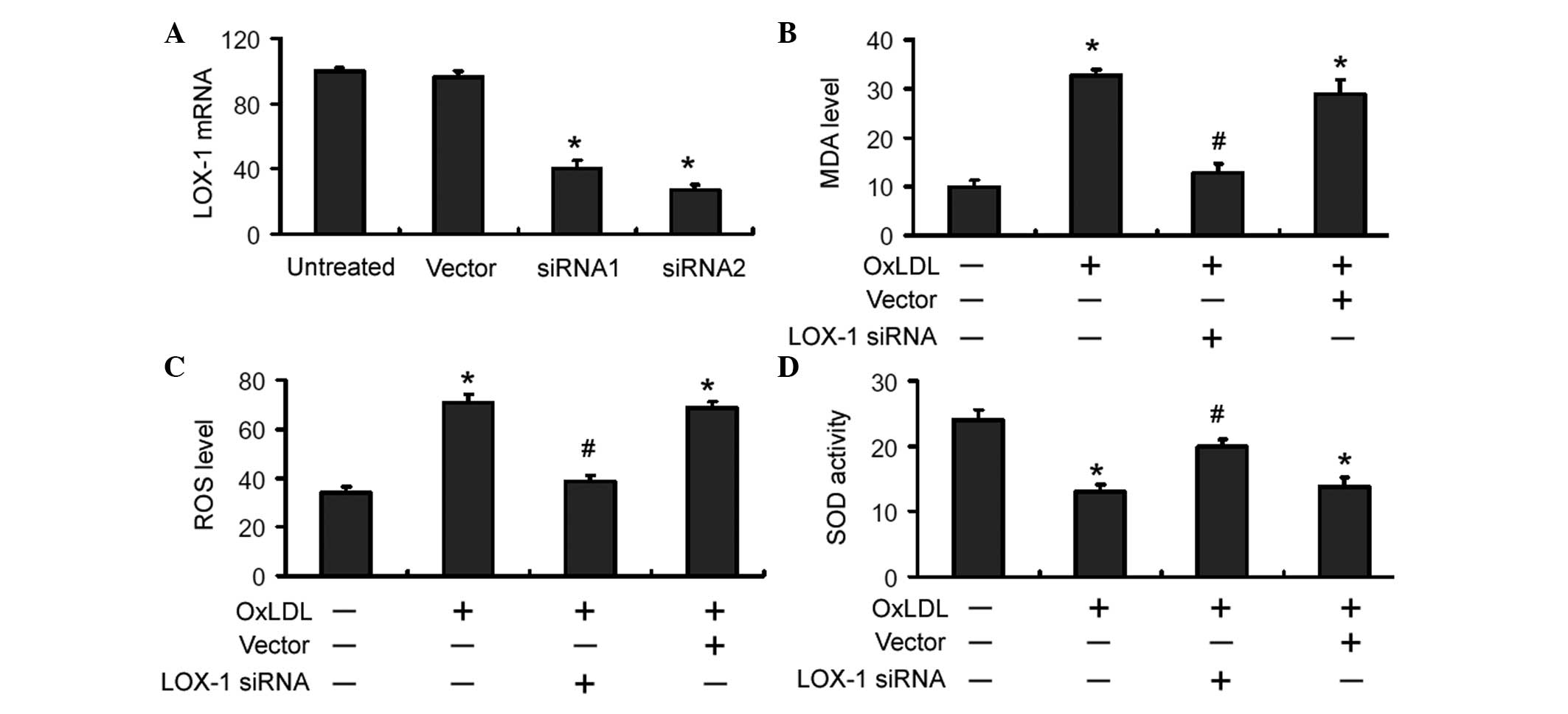
LOX-1 depletion attenuates OxLDL-induced
oxidative stress in RAW264.7 macrophages
To investigate the role of LOX-1 in OxLDL-induced
oxidative stress in macrophages, a LOX-1-siRNA-expressing plasmid
was constructed and delivered to RAW264.7 macrophages. As shown in
Fig. 1A, transient transfection of
LOX-1-targeting siRNA resulted in a significant reduction in the
mRNA level of LOX-1 in RAW264.7 macrophages. Compared with
untreated cells, OxLDL exposure led to a significant (P<0.05)
increase in the intracellular MDA and ROS levels and a significant
(P<0.05) decrease in the SOD activity (Fig. 1B-D). Notably, delivery of
LOX-1-targeting siRNA significantly (P<0.05) reversed the
alterations in oxidative stress parameters induced by OxLDL
(Fig. 1B-D).
LOX-1 silencing downregulates the
expression of Nox2, Rac1, p47phox and p22phox in OxLDL-treated
cells
OxLDL treatment led to a significant elevation in
the expression of Nox2, Rac1, p47phox and p22phox, as determined by
western blot analysis (Fig. 2).
The OxLDL-induced gene expression alterations were significantly
inhibited by pre-transfection of LOX-1-targeting siRNA (Fig. 2).
LOX-1 silencing interferes with
OxLDL-induced activation of MAPK signaling pathways
Compared with untreated cells, OxLDL-treated cells
demonstrated a rapid increase in the phosphorylation levels of p38,
JNK and ERK1/2 up to 60 min after treatment, followed by a gradual
decrease to basal levels (Fig. 3).
When cells were pre-transfected with LOX-1-targeting siRNA,
OxLDL-induced activation of MAPKs was significantly impaired
(Fig. 3).
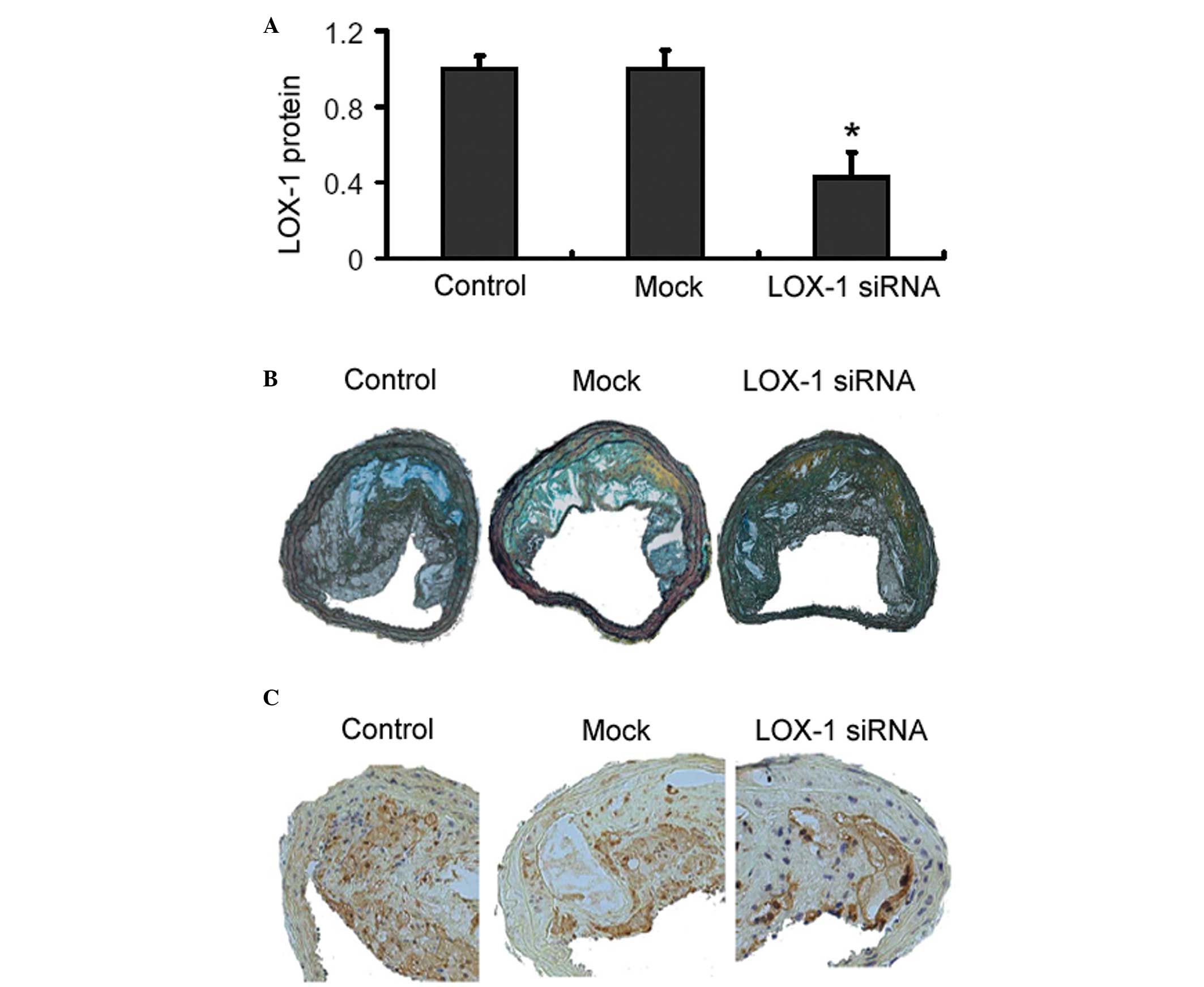
Effects of adenoviral delivery of LOX-1
siRNA on body weight, plasma lipid levels and atherosclerotic
lesions in ApoE-/- mice
Body weight and plasma lipid levels obtained at the
end of the animal experiment were not statistically different among
the experimental groups (data not shown). Western blot analysis
revealed that adenoviral delivery of LOX-1 siRNA significantly
decreased the expression of LOX-1 in the aorta of ApoE-/- mice
(Fig. 4A). Movat staining revealed
that there were no significant differences in the plaque area,
external elastic membrane area and plaque area/aortic area among
the animal groups investigated (P>0.05 for each comparison;
Fig. 4B and Table I). Notably, LOX-1 siRNA-treated
mice exhibited a significant increase in the size of the fibrous
cap, compared with the control mice (5.41±0.46 vs. 4.81±0.34,
P<0.05; Fig. 4B and Table I). The delivery of LOX-1 siRNA
markedly decreased the macrophage content of lesions, compared with
the control group (Fig. 4C).
 | Table IMovat pentachrome staining of aortic
lesions from ApoE-/- mice. |
Table I
Movat pentachrome staining of aortic
lesions from ApoE-/- mice.
| Group (n=15) | Fibrous cap
(µm) | Plaque area
(×103 µm2) | Plaque area/aortic
area (%) |
|---|
| Control | 4.78±0.25 | 189.72±7.21 | 0.68±0.04 |
| Mock | 4.81±0.34 | 180.04±8.32 | 0.69±0.03 |
| LOX-1-siRNA | 5.41±0.46a | 169.62±6.14 | 0.64±0.07 |
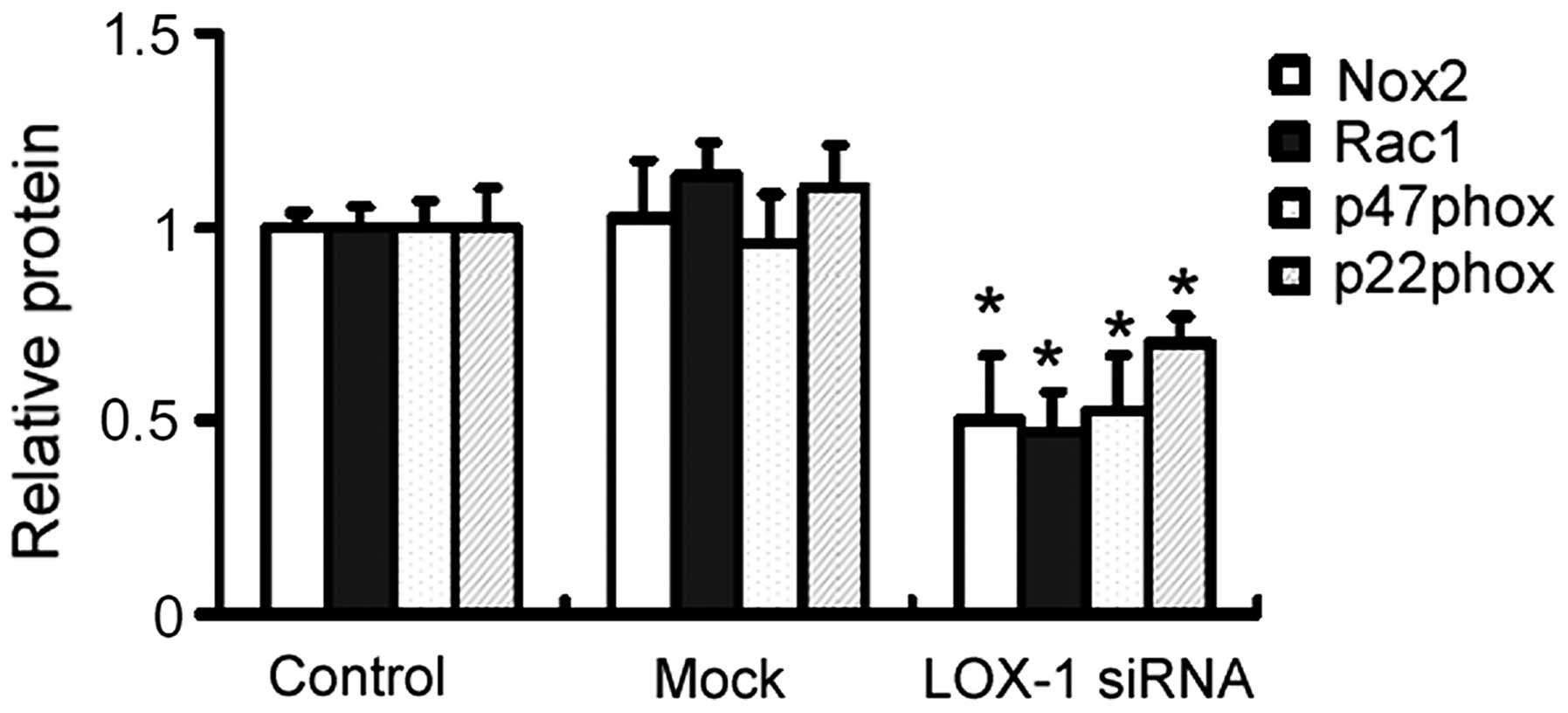
Effects of LOX-1 silencing on the
expression of Nox2, Rac1, p47phox and p22phox in aortic
plaques
Western blot analysis demonstrated that the protein
expression levels of NOX1, Rac1, p47phox and p22phox in aortic
lesions were significantly lower in the LOX-1 siRNA group compared
with the control group (P<0.05 for each comparison; Fig. 5).
Discussion
Oxidative stress is widely accepted as a critical
factor contributing to atherosclerosis (16). A previous study demonstrated that
oxygen-free radicals are capable of oxidizing LDL-cholesterol,
promoting the formation of foam cells and ultimately leading to the
development of atherosclerosis (17). ROS are highly reactive molecules
and can affect several biological aspects of atherosclerosis,
including endothelial cell, vascular smooth cell and macrophage
function and survival as well as lipoprotein metabolism (18). Therefore, it is of significance to
determine the mechanisms regulating the production of ROS. Our data
revealed that OxLDL exposure induced oxidative stress in
macrophages, as evidenced by increased MDA and ROS levels. SOD is a
member of the antioxidant family and capable of scavenging cellular
ROS, thus representing an important defensive mechanism against
oxidative stress (19). It was
found that OxLDL treatment led to a significant decrease in the
activity of SOD in macrophages, further confirming the induction of
oxidative stress. OxLDL-induced macrophage oxidative stress has
also been described in a previous study, where OxLDL enhanced ROS
formation through the heparin-binding epidermal growth factor-like
growth factor-dependent pathway in J774a.1 macrophages (20). LOX-1 is the main OxLDL receptor of
endothelial cells (10).
Endothelial overexpression of LOX-1 has been found to increase
plaque formation and promote atherosclerosis in vivo
(21). Upregulation of LOX-1
contributes to palmitic acid-induced uptake of OxLDL in macrophage
cells (22). The present data
indicated the involvement of LOX-1 in OxLDL-induced oxidative
stress in macrophages, as targeting LOX-1 significantly decreased
MDA and ROS levels and increased SOD activity in OxLDL-treated
macrophages. Since NOX enzymes are a major source of ROS in
numerous cell types, the effect of LOX-1 silencing on the
expression of NOX enzymes was examined. LOX-1 depletion
significantly inhibited the induction of Nox2, Rac1, p47phox and
p22phox by OxLDL. Taken together, these results suggest that OxLDL
induces macrophage oxidative stress via LOX-1-dependent
upregulation of NOX enzymes. To the best of our knowledge, the
present study is the first to demonstrate the role of LOX-1 in
OxLDL-induced oxidative stress in macrophages. In agreement with
the present findings, LOX-1 has been reported to be involved in
OxLDL-induced oxidative DNA damage in endothelial cells (23).
Activation of MAPKs is implicated in the development
of atherosclerosis (6). It has
been documented that MAPK-mediated signaling pathways are involved
in Chlamydia pneumoniae-induced macrophage-derived foam cell
formation (24). Excessive ROS
production is responsible for activation of MAPKs in
atherosclerosis (5).
Pharmacological inhibition of ROS was reported to inactivate p38
MAPK and stress-activated protein kinase signaling, consequently
impairing inflammatory responses to lipopolysaccharide in
macrophages (25). The present
study revealed that accompanying reduction in ROS production,
OxLDL-induced phosphorylation of MAPKs was significantly inhibited
in LOX-1-depleted macrophages. These findings indicate that
prevention of ROS generation due to LOX-1 deficiency adversely
affects the activation of intracellular signaling pathways,
including MAPKs, which provides a molecular explanation for reduced
atherosclerosis in LOX-1-depleted mice (12).
Using a mouse model of high-fat diet-induced
atherosclerosis, it was found that LOX-1 depletion led to a
significant increase in the size of the fibrous cap. However, the
plaque area, external elastic membrane area and plaque area/aortic
area were not significantly altered by LOX-1 silencing. Notably,
LOX-1 depletion significantly decreased the macrophage content in
plaque lesions, coupled with reduced expression of NOX1, Rac1,
p47phox and p22phox. These results suggest that LOX-1 silencing
protects against atherosclerosis, at least partially, through
prevention of oxidative stress via attenuation of macrophage
accumulation and downregulation of NOX enzymes. LOX-1 has been
demonstrated to induce macrophage migration in atherosclerosis
(13), which provides an
explanation for our findings of reduced macrophage accumulation in
aortic lesions of LOX-1 siRNA-treated mice.
In conclusion, to the best of our knowledge, the
present study provides the first evidence that LOX-1 is required
for OxLDL-induced oxidative stress in macrophages, which in part,
involves the regulation of NOX enzymes. In vivo animal
experiments further demonstrate that LOX-1 depletion attenuates
high-fat diet-induced atherosclerosis largely through prevention of
macrophage accumulation and downregulation of NOX enzymes. These
findings suggest that LOX-1 is a promising target for the
prevention of atherosclerosis.
Acknowledgments
The present study was supported by grants from the
Scientific and Technological Project of Shanxi Province of China
(no. 20100311098-4), the Doctoral Foundation of The Second Hospital
of Shanxi Medical University of China (no. 20100404), the Youth
Foundation of Shanxi Medical University of China (no. 02201421) and
the Youth Foundation of Health and Family Planning Commission of
Shanxi Province of China (no. 2014041).
References
|
1
|
Robinson JG and Gidding SS: Curing
atherosclerosis should be the next major cardiovascular prevention
goal. J Am Coll Cardiol. 63:2779–2785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenyo IM and Gafencu AV: The involvement
of the monocytes/macrophages in chronic inflammation associated
with atherosclerosis. Immunobiology. 218:1376–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stocker R and Keaney JF Jr: Role of
oxidative modifications in atherosclerosis. Physiol Rev.
84:1381–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar :
|
|
5
|
Griendling KK, Sorescu D, Lassègue B and
Ushio-Fukai M: Modulation of protein kinase activity and gene
expression by reactive oxygen species and their role in vascular
physiology and pathophysiology. Arterioscler Thromb Vasc Biol.
20:2175–2183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mei S, Gu H, Ward A, Yang X, Guo H, He K,
Liu Z and Cao W: p38 mitogen-activated protein kinase (MAPK)
promotes cholesterol ester accumulation in macrophages through
inhibition of macroautophagy. J Biol Chem. 287:11761–11768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abe J and Woo CH: NADPH oxidase in
vascular injury: A new insight about its regulation and role in T
cells. Circ Res. 104:147–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drummond GR, Selemidis S, Griendling KK
and Sobey CG: Combating oxidative stress in vascular disease: NADPH
oxidases as therapeutic targets. Nat Rev Drug Discov. 10:453–471.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm.
2013:1527862013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao S and Geng YJ: LOX-1: A male
hormone-regulated scavenger receptor for atherosclerosis. Vascul
Pharmacol. 59:138–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehta JL, Sanada N, Hu CP, Chen J,
Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, et
al: Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice
fed high cholesterol diet. Circ Res. 100:1634–1642. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding Z, Mizeracki AM, Hu C and Mehta JL:
LOX-1 deletion and macrophage trafficking in atherosclerosis.
Biochem Biophys Res Commun. 440:210–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai Y, Mercanti F, Dai D, Wang X, Ding Z,
Pothineni NV and Mehta JL: LOX-1, a bridge between GLP-1R and
mitochondrial ROS generation in human vascular smooth muscle cells.
Biochem Biophys Res Commun. 437:62–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Appay V, Bosio A, Lokan S, Wiencek Y,
Biervert C, Küsters D, Devevre E, Speiser D, Romero P, Rufer N and
Leyvraz S: Sensitive gene expression profiling of human T cell
subsets reveals parallel post-thymic differentiation for CD4+ and
CD8+ lineages. J Immunol. 179:7406–7414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tinkel J, Hassanain H and Khouri SJ:
Cardiovascular antioxidant therapy: A review of supplements,
pharmacotherapies and mechanisms. Cardiol Rev. 20:77–83.
2012.PubMed/NCBI
|
|
17
|
Prasad K and Kalra J: Experimental
atherosclerosis and oxygen free radicals. Angiology. 40:835–843.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding Z, Liu S, Wang X, Khaidakov M, Dai Y
and Mehta JL: Oxidant stress in mitochondrial DNA damage, autophagy
and inflammation in atherosclerosis. Sci Rep. 3:10772013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: Role in redox signaling, vascular function and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao P, Wang XM, Qian DH, Qin ZX, Jin J, Xu
Q, Yuan QY, Li XJ and Si LY: Induction of oxidative stress by
oxidized LDL via meprinα-activated epidermal growth factor receptor
in macrophages. Cardiovasc Res. 97:533–543. 2013. View Article : Google Scholar
|
|
21
|
Akhmedov A, Rozenberg I, Paneni F, Camici
GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A,
Lohmann C, et al: Endothelial overexpression of LOX-1 increases
plaque formation and promotes atherosclerosis in vivo. Eur Heart J.
35:2839–2848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishiyama J, Taguchi R, Yamamoto A and
Murakami K: Palmitic acid enhances lectin-like oxidized LDL
receptor (LOX-1) expression and promotes uptake of oxidized LDL in
macrophage cells. Atherosclerosis. 209:118–124. 2010. View Article : Google Scholar
|
|
23
|
Thum T and Borlak J: LOX-1 receptor
blockade abrogates oxLDL-induced oxidative DNA damage and prevents
activation of the transcriptional repressor Oct-1 in human coronary
arterial endothelium. J Biol Chem. 283:19456–19464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng B, Wu X, Sun S, Wu Q, Mei C, Xu Q,
Wu J and He P: MAPK-PPARα/γ signal transduction pathways are
involved in Chlamydia pneumoniae-induced macrophage-derived foam
cell formation. Microb Pathog. 69–70:1–8. 2014. View Article : Google Scholar
|
|
25
|
Tumurkhuu G, Koide N, Dagvadorj J, Hassan
F, Islam S, Naiki Y, Mori I, Yoshida T and Yokochi T: MnTBAP, a
synthetic metalloporphyrin, inhibits production of tumor necrosis
factor-alpha in lipopolysaccharide-stimulated RAW 264.7 macrophages
cells via inhibiting oxidative stress-mediating p38 and SAPK/JNK
signaling. FEMS Immunol Med Microbiol. 49:304–311. 2007. View Article : Google Scholar : PubMed/NCBI
|