Introduction
Irritable bowel syndrome (IBS) is a functional
gastrointestinal disorder with symptoms including abdominal pain,
frequent diarrhea, constipation and altered bowel habits (1). Based on the predominant symptoms, IBS
is classified as IBS-associated diarrhea (IBS-D),
constipation-predominant IBS (IBS-C) and IBS with alternating stool
pattern (IBS-A) (2). IBS is a
common clinical problem and has affected 8 to 20% of the general
population worldwide (3). To date,
great progress has been made in exploring the mechanism underlying
IBS.
One mechanism underlying the pathophysiology of IBS
is based on changes in immune activity. The imbalance of pro- and
anti-inflammatory cytokines contributes to the development of IBS
(4). An accumulation of the
pro-inflammatory cytokine tumor necrosis factor α (TNF-α) and a
decreased production of interleukin 10 (IL-10) have been reported
in IBS patients (4,5). Interleukin 8 (IL-8) is a modulator in
recruitment and activation of polymorphonuclear neutrophils. The
protein levels of IL-8 were found to be elevated during the
development of IBS (6). In
addition, proteasome-mediated protein degradation may be another
factor leading to IBS. Furthermore, increasing occludin degradation
was shown to contribute to the development of IBS (7). However, few studies have focused on
the association between systemic cytokine expression and tight
junction proteins in IBS.
The role of psychological factors in IBS is being
increasingly recognized. IBS patients commonly show psychological
symptoms, including anxiety, depression and fatigue (8,9). In
one study, patients with IBS with increased fatigue and depression
were shown to have an elevated mass of mast cells in the caecal
mucosa (10). A significant
association was made between the severity of psychological symptoms
and the cellularity of the lamina propria in patients with IBS. To
date, the association between cytokine expression and psychological
factors has not been clearly determined.
The present study investigated the expression of the
cytokines TNF-α, IL-8 and IL-10, as well as occludin in IBS-D
patients and healthy controls. In addition, patients with IBS-D
completed a self report questionnaire and psychological assessment,
which were used for the correlation of cytokine profiles with
occludin protein production as well as abdominal and psychological
symptoms in patients with IBS-D.
Materials and methods
Study population
The present study was approved by the Clinical
Research Ethics Committee of QiLu Hospital of Shandong University
in China and all participants or their parents signed informed
consent forms prior to enrolment in the study.
A total of 42 patients with IBS-D were recruited
from gastroenterology clinics at QiLu Hospital, including 15 males
(mean age, 36.4±6.9 years; range, 20–57 years and 27 females (mean
age, 38.1±5.9 years; range, 18–56 years) (Table I). All patients had IBS symptoms
corresponding to the Rome III criteria, including abdominal
discomfort or pain (11). Symptoms
were required to be present for the previous six months and persist
for at least three months. IBS patients undergoing more than three
bowel movements per day and loose stool forms were diagnosed with
IBS-D based on the Bristol Stool Form Scale (12). Patients with organic
gastrointestinal disorders and acute gastrointestinal infection and
organic disease (including diabetes mellitus, hepatic or renal
dysfunction, and thyroid disease) were excluded. The patient cohort
was selected following positive diagnosis using colonoscopy,
biopsy, abdominal ultrasonography and other clinical or
laboratorial tests, including blood and stool analyses followed by
assessment of erythrocyte sedimentation rate, C-reactive protein,
liver and renal function, thyroid function, fasting plasma glucose,
calcium and electrolytes.
 | Table IBasic characteristics of patients and
healthy controls. |
Table I
Basic characteristics of patients and
healthy controls.
| Gender | Patients
| Controls
|
|---|
| Male | Female | Male | Female |
|---|
| Number | 15 | 27 | 8 | 12 |
| Agea (years) | 36.4±6.9 | 38.1±5.9 | 37.9±7.8 | 35.1±8.1 |
| Range (years) | 20–57 | 18–56 | 21–59 | 19–60 |
In addition, 20 healthy asymptomatic controls were
recruited from the Department of Health Examination of QiLu
Hospital, including eight males (mean age, 37.9±7.8 years; range,
21–59 years) and 12 females (mean age, 35.1±8.1 years; range, 19–60
years) (Table I). The controls
were not on any medication for gastrointestinal disease and had a
normal body mass index.
Isolation and culture of peripheral blood
mononuclear cells
A amount of 5 ml peripheral blood were extracted
from the healthy individuals and IBS-D patients following fasting
for 12 h. Isolation of peripheral blood mononuclear cells (PBMCs)
was performed by density gradient centrifugation at 2,000 × g for
20 min. Blood was collected into sterile heparinized tubes and
mixed with 5 ml RPMI 1640 (Gibco-BRL, Invitrogen Life Technologies,
Carlsbad, CA, USA). The diluted blood was treated with 5 ml
Ficoll-Paque (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at
2,000 ×g for 20 min. The PBMCs were then washed twice with
phosphate-buffered saline (PBS) and RPMI 1640 media (Gibco-BRL).
The viability of PBMCs was detected by trypan blue (Gibco-BRL)
staining.
Cell culture and ELISA
PBMCs were resuspended to achieve a density of
1×106 cells/ml in RPMI 1640 medium with 10% fetal bovine
serum, 100 U/ml penicillin (Beyotime Institute of Biotechnology,
Haimen, China) and 100 μg/ml streptomycin (Beyotime
Institute of Biotechnology). PBMCs were cultured at 37°C in a
humidified 5% CO2 atmosphere for 24 h. Cell-free
supernatants were obtained following centrifugation at 3,000 ×g for
10 min (4°C) and stored at −80°C for further investigation. The
expression of TNF-α, IL-8 and IL-10 in PBMCs was measured using
ELISA kits (Peprotech, Rocky Hill, NJ, USA) according to the
manufacturer's instructions. Optical density (OD) was measured at a
wavelength of 450 nm and the reference wavelength was corrected to
590 nm, using an automated ELISA reader (ELx800; BioTek, Winooski,
VA, USA). The limit of sensitivity of the assays was 2 pg/ml.
Immunohistochemical analysis of
occluding
Tissue samples from the rectosigmoid colon were
obtained from all participants. Sections of colonic biopsies were
fixed in 4% paraformaldehyde (Damao Chemical Reagent Factory,
Tianjin, China) for 2 h at room temperature, embedded in optimum
cutting temperature (OCT) compound (Fisher Thermo Scientific,
Waltham, MA, USA) and subsequently frozen at -80°C. The sections of
frozen colonic tissues (4 μm) were mounted on glass slides
and air-dried for 30 min, then fixed with acetone (Big Alum
Chemical Reagent Factory) (10 min, 4°C) and washed in PBS. Sections
were treated with 3% H2O2 in methanol (Big
Alum Chemical Reagent Factory) for 15 min and non-specific binding
was blocked with 5% goat serum (Intergen, Purchase, NY, USA) for 4
h at room temperature. Then sections were incubated with rabbit
monoclonal anti-occludin antibody (1:100 dilution; Zymed,
Invitrogen Life Technologies) overnight at 4°C and horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G
antibody (1:5,000; ZSJQ-BIO, Beijing, China) for 2 h at room
temperature. The slides were washed in PBS and stained with
diaminobenzidene (DAB; Amresco LLC, Solon, OH, USA). The
microphotographs were examined under a microscope (BX-51; Olympus
Corp., Tokyo, Japan).
Western blot analysis
For western blot analysis, biopsy specimens from the
rectosigmoid colon were immediately stored at −80°C. Protein was
extracted from 100-mg colonic samples by homogenization in ice-cold
lysate buffer (20 mmol/l Tris-HC1, pH 7.5; 100 mmol/l NaCl; 0.5
mmol/l EDTA; 1 mmol/l PMSF; 1 mmol/l DTT; 2 mmol/l sodium
orthovanadate; 0.5% Nonider P40; and 0.1% protease inhibitor
cocktail; all from Sigma-Aldrich) for 30 min at 4°C and
centrifugation at 15,000 × g for 15 min at 4°C. The protein content
was determined by Pierce bicinchoninic acid (BCA) protein assay kit
(Fisher Thermo Scientific). Protein samples (25 mg) were separated
using 10% SDS-PAGE and transferred to nitrocellulose membranes
(Pall Corporation, Dreieich, Germany). Membranes were blocked in 5%
nonfat milk (BD Biosciences, San Jose, CA, USA) for 1 h at room
temperature. The membranes were incubated with rabbit monoclonal
anti-occludin (1:50 dilution; Zymed) and monoclonal anti-β-actin
(1:1,000; Kangtai Biological Products Co., Ltd., Shanghai, China)
overnight at 4°C. The membranes were then incubated with
HRP-conjugated goat anti-rabbit IgG antibody (1:5,000; Biokangtai)
for 1 h at room temperature. The expression intensity was
determined by calculating the ratio of integrated optical density
(IOD) between occludin and β-actin (Santa Cruz Biotechnology,
Dallas, TX, USA).
Symptom questionnaire
In order to evaluate the severity of the symptoms, a
self-validated report questionnaire based on the Rome III criteria
was developed for the IBS-D patients according to previous studies
(13,14). The questionnaire items mainly
focused on the intensity and frequency of abdominal symptoms,
including abdominal pain, incomplete emptying following bowel
movement, three or more bowel movements per day, loose, mushy or
watery stools, and urgency to move bowels. The intensity of
abdominal symptoms was graded according to the criteria as follows:
0, Absence of symptoms; 1, mild symptoms; 2, moderate symptoms,
which do not affect day-to-day activities; and 3, severe symptoms,
which impaired day-to-day activities. The frequency scoring
criterion was 0, no onset; 1, one to three times per week; 2, four
to six times per week; and 3, every day. The total scores ranging
from 0 to 15 were calculated as the sum of each item score of
abdominal symptoms.
Psychological assessment
The Zung self-rating anxiety scale (SAS) and the
self-rating depression scale (SDS) were applied to IBS-D patients
according to the previous studies with slight modifications
(15,16). For SDS or SAS rating,
questionnaires including 20 items were filled in by the patients to
rate their perceived moods and feelings. The scoring for each item
was graded according to the four quantitative terms: 1, a
percentage of the time; 2, some of the time; 3, a good part of the
time; 4, most of the time. The scoring for SAS or SDS was performed
using a multiple choice questionnaire with positive and negative
answering options. Initial scores were calculated as follows:
Scores obtained from the 20 items of the SDS or SAS questionnaires
were summarized and multiplied by 1.25. The final score was rounded
to the nearest whole score. A score <50 was considered to be
normal, a score of 50–74 was defined to indicate a mild disorder, a
score of 75–87 indicated a moderate disorder and a score >88
indicated a severe disorder. In the present study a score ≥50 on
the anxiety or depression scale was regarded to indicate
psychological distress.
Statistical analysis
Data were analyzed by SPSS 16.0 (SPSS Inc., Chicago,
IL, USA) and values were expressed as the mean ± standard
deviation. The independent-sample t-test was applied for assessing
the difference in cytokine expression, IOD of occludin, abdominal
symptoms scores and psychological scores between IBS-D patients and
normal controls. The correlation among the variables was evaluated
by Pearson correlation. P<0.05 was considered to indicate
statistically significant differences between values.
Results
Comparison of cytokine levels in the
IBS-D and control groups
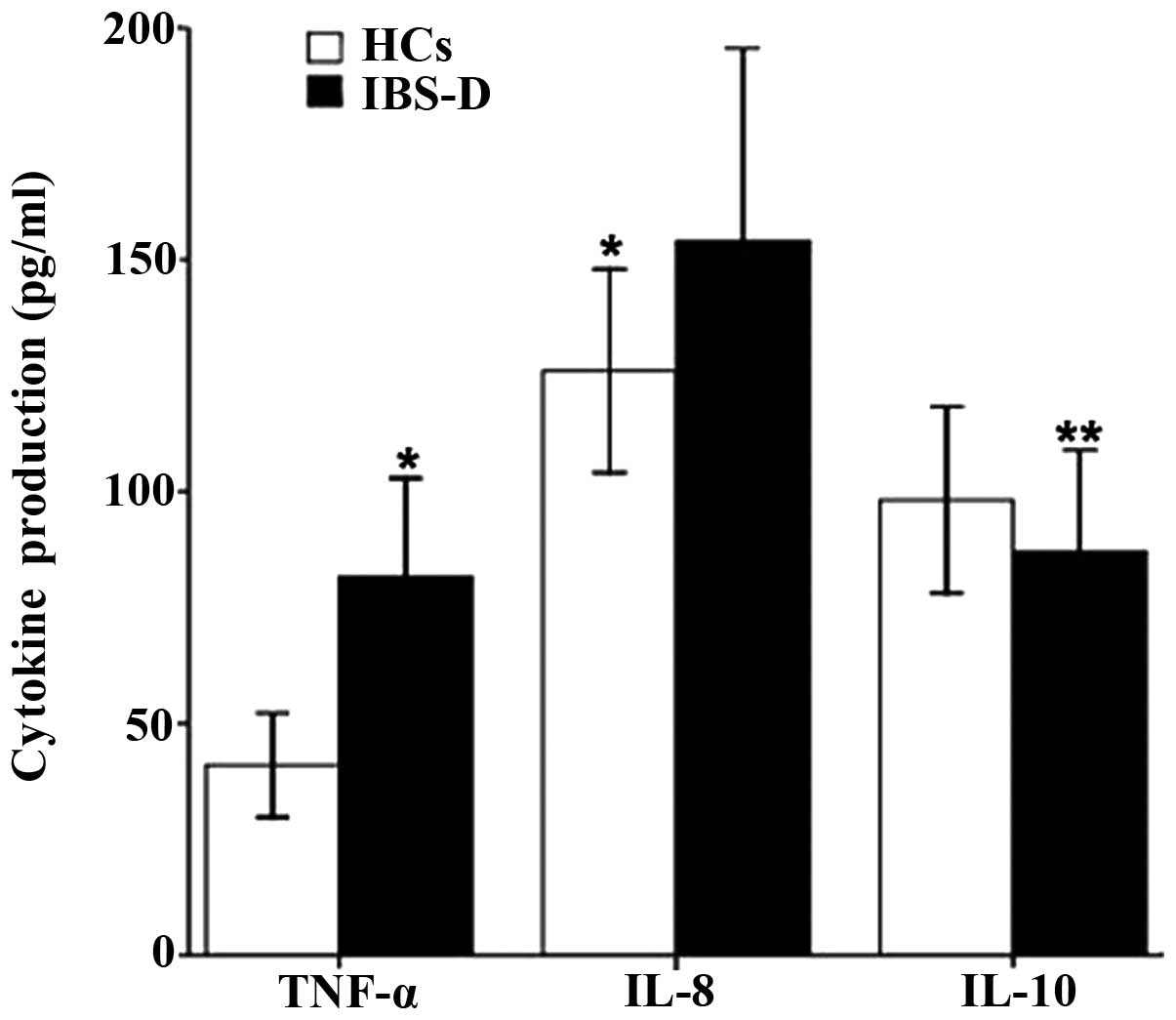
Overall, the TNF-α and IL-8 levels (81.51±22.18 vs.
41.77±10.74 pg/ml, P<0.001; 152.21±38.10 vs. 126.19±24.12 pg/ml,
P=0.007) were significantly higher in IBS-D patients than those in
healthy controls (Fig. 1).
However, IBS-D patients showed decreased IL-10 production compared
with that in controls (87.11±22.46 vs. 98.54±16.43 pg/ml, P=0.047)
(Fig. 1).
Comparison of occludin expression between
IBS-D and control groups
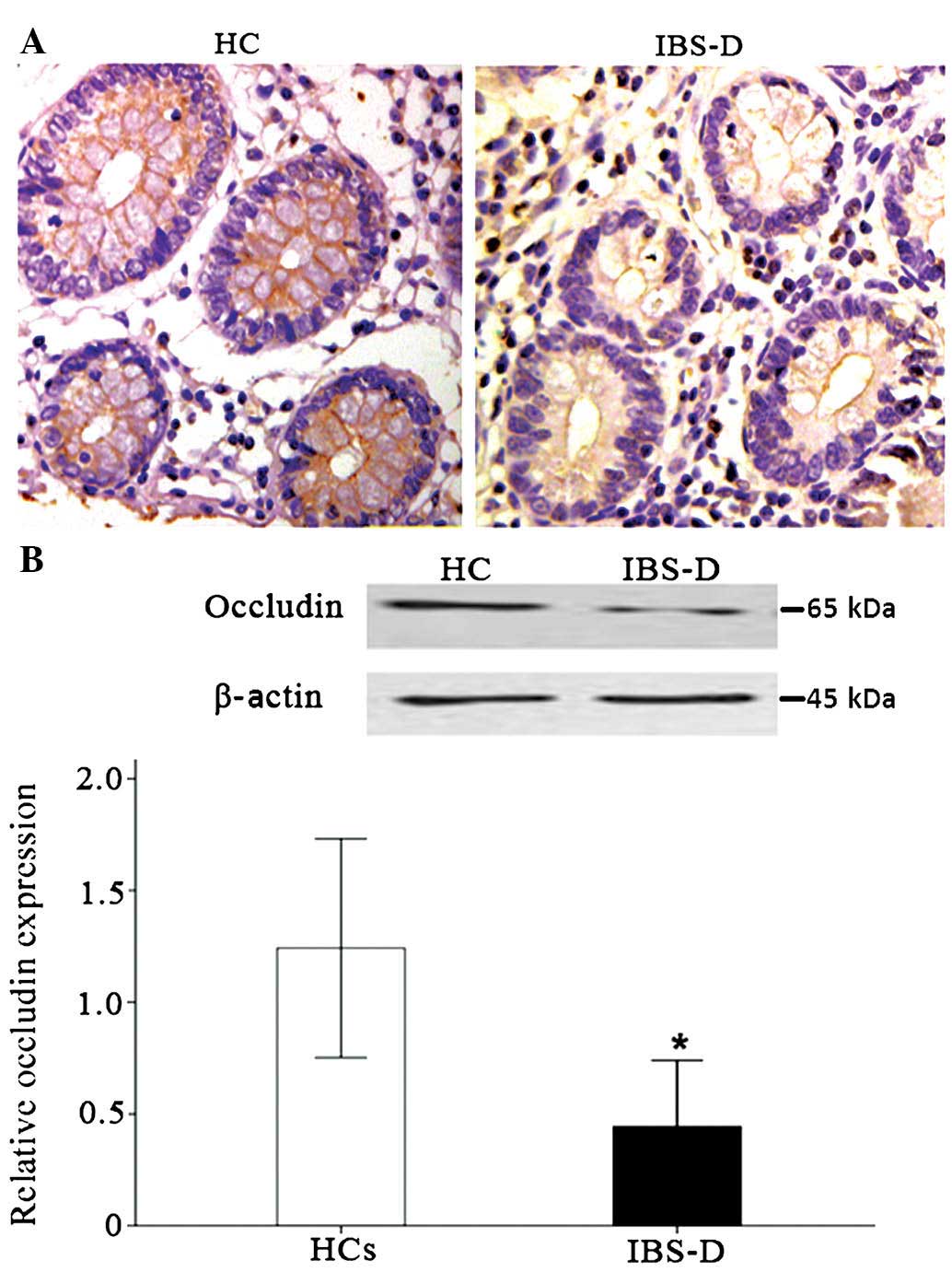
Immunohistochemical analysis of occludin is shown in
Fig. 2. Occludin was primarily
present on the surface of colonic epithelial cells. In healthy
controls, the staining of occludin was intense and continuous,
while faint and discontinuous staining was observed in the colon
tissue of IBS-D patients (Fig.
2A). Western blot analysis showed a protein band at ~65 kDa for
occludin. Relative occludin expression in the rectosigmoid colon
was lower in patients with IBS-D compared with that in healthy
controls (0.38±0.14 vs. 1.24±0.39, P<0.001) (Fig. 2B).
Correlation between cytokine production
and occludin expression in IBS-D patients
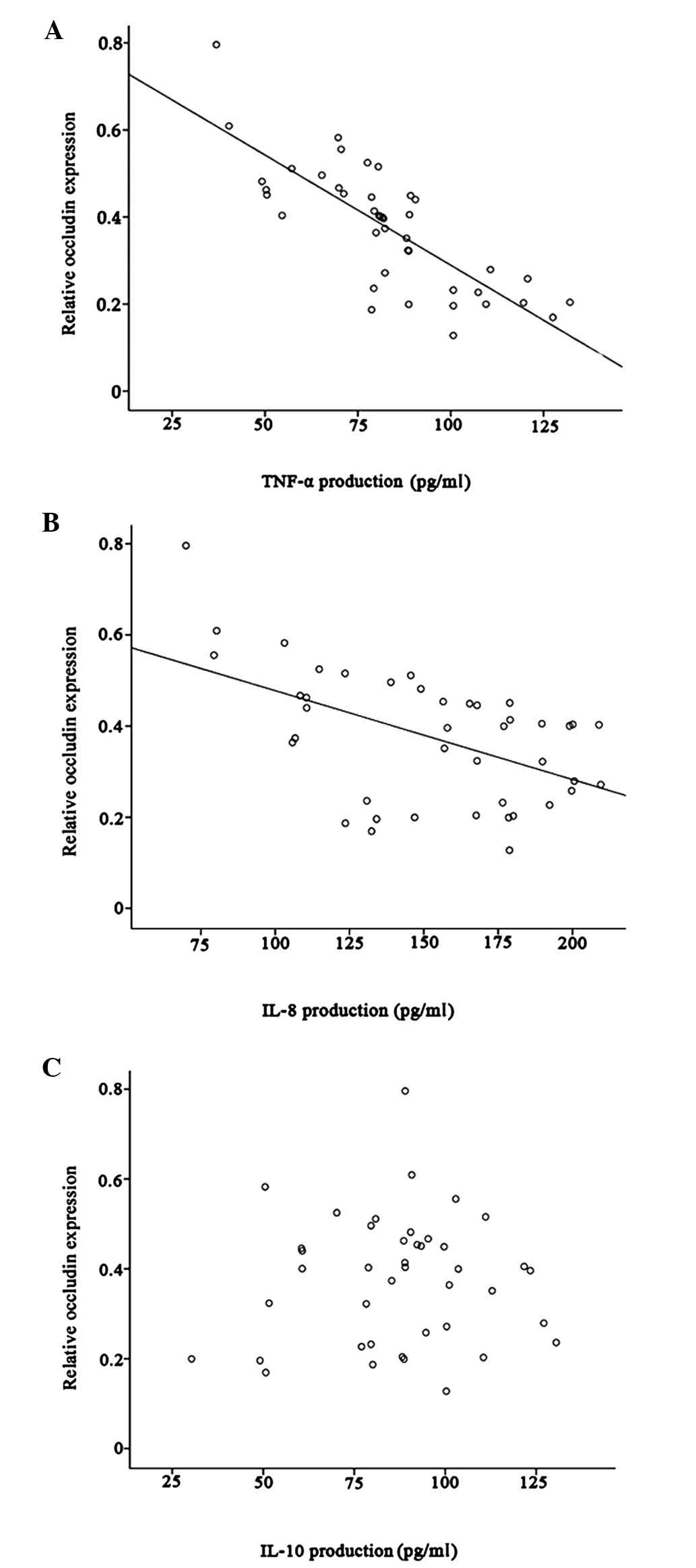
In patients with IBS-D, occluding expression was
negatively correlated with TNF-α and IL-8 production (r=−0.34,
P=0.028; r=−0.52, P<0.001, respectively) (Fig. 3A and B). However, there was no
correlation between occludin and IL-10 production (r=0.05, P=0.748)
(Fig. 3C).
Correlation between cytokine production
and abdominal symptoms score in patients with IBS-D
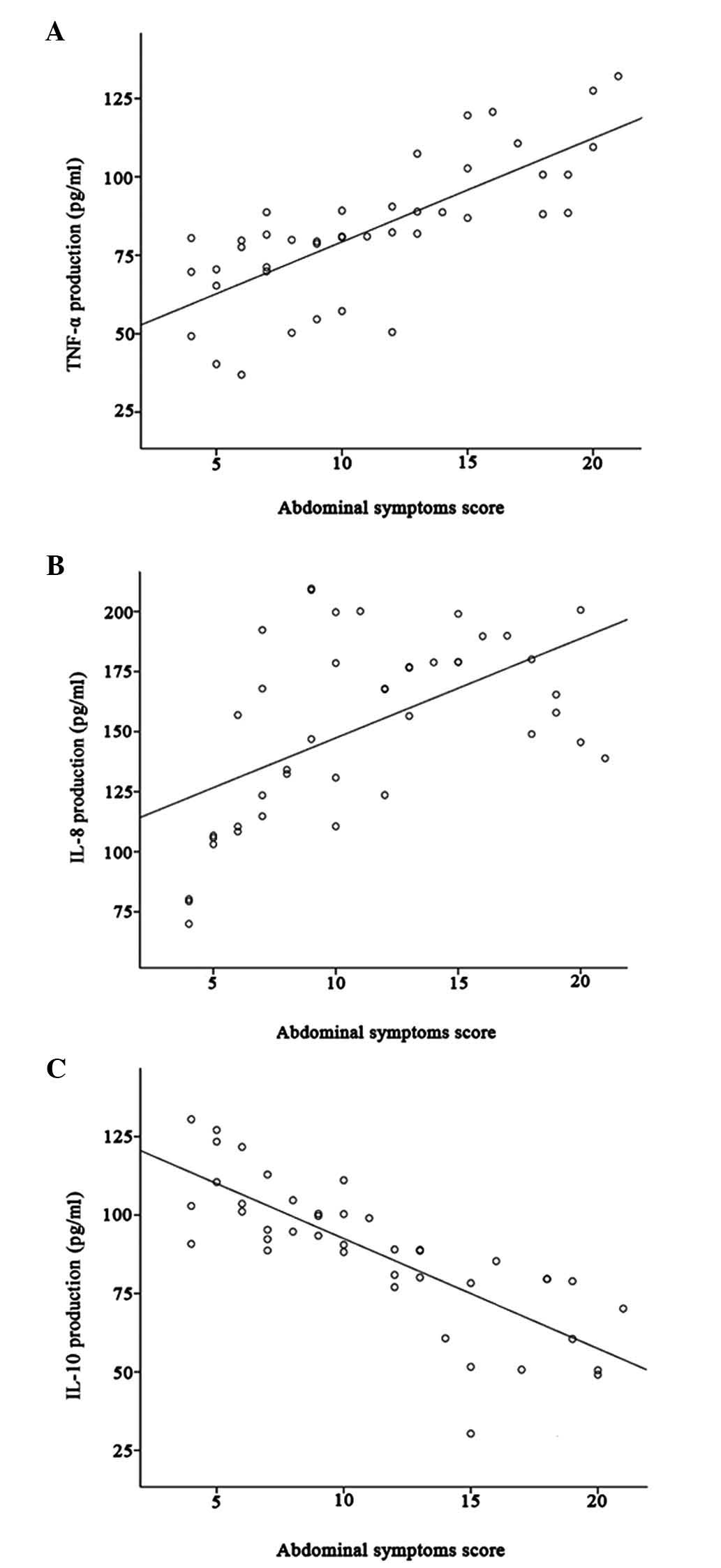
The mean abdominal symptoms score was 11.17±5.06 for
IBS-D patients and was positively correlated with TNF-α and IL-8
production (r=0.74, P<0.001; r=0.55, P<0.001, respectively);
however, it was negatively correlated with IL-10 production
(r=−0.80, P<0.001) (Fig.
4A-C).
Psychological distress and correlation
with cytokine levels in IBS-D patients
Among the 42 patients, 20 (66.7%) with an SDS score
≥50 were diagnosed with depression and 17 (40.4%) had an SAS score
≥50 and were considered to have anxiety. IBS-D patients with an SDS
score ≥50 had significantly higher TNF-α (P=0.004) and IL-8 levels
(P=0.008) than those with an SDS score <50. IBS-D patients with
an SAS score ≥50 showed significantly higher IL-8 expression
compared with those with an SAS score <50 (P=0.016). However,
there was no statistical difference in IL-10 expression between
patients with SAS or SDS score ≥50 and those with lower scores
(P=0.385; P=0.275) (Table
II).
 | Table IIAssociation of cytokine production in
peripheral blood mononuclear cells of patients with diarrheic
irritable bowel syndrome and anxiety as well as depression
scores. |
Table II
Association of cytokine production in
peripheral blood mononuclear cells of patients with diarrheic
irritable bowel syndrome and anxiety as well as depression
scores.
| Score | TNF-α (pg/ml) | IL-8 (pg/ml) | IL-10 (pg/ml) |
|---|
| SAS |
| ≥50 (n=17) | 86.1±23.4 | 169.3±30.7 | 82.6±25.7 |
| <50 (n=25) | 80.0±21.1 | 142.0±36.7b | 89.2±24.3 |
| SDS |
| ≥50 (n=20) | 93.3±22.0 | 168.5±35.2 | 83.5±25.3 |
| <50 (n=22) | 73.6±18.7a | 139.1±32.4a | 91.2±18.7 |
Discussion
IBS-D, a subgroup of IBS, is common in males and
females (17). Evidence showed
that immune activation was an important event in the development of
IBS (18). PBMCs, including
lymphocytes, leukocytes and macrophages, have key roles in immune
activation and have been used to investigate the specific cytokines
that can indicate the disease stages (19). In the present study, levels of the
pro-inflammatory cytokines TNF-α and IL-8 and those of the
anti-inflammatory cytokine IL-10 were assessed in PBMCs from IBS-D
patients. The results showed that TNF-α and IL-8 production were
significantly greater in PBMCs from IBS-D patients than those in
healthy controls, while IL-10 production was significantly
decreased in IBS-D patients. These findings were consistent with
the widely accepted mechanism of IBS, namely that the imbalance of
cytokines involved in inflammatory response results in persisting
inflammation (5). A previous study
showed that the frequency of the high-producer genotype for IL-10
was significantly lower in patients with IBS compared with that in
healthy ones, which may contribute to the progression of
inflammation in IBS (5).
Although a previous study reported that the
increasing activity of faecal serine protease from patients with
HBS-D was able to induce a marked internalization of tight junction
proteins (20), the present study
showed that occludin was restricted to the surface of colonic
epithelial cells in HCs and IBS-D patients without intensive
internalization. The present study also observed the dysfunction of
the tight junction protein occludin in IBS-D patients by
immunohistochemistry and western blot analysis. The
immunohistochemical staining of occludin was faint and
discontinuous in IBS-D patients and occludin protein was
significantly decreased compared with that in healthy controls.
Recent evidence also suggested that occudin expression was
decreased in IBS-D patients and the levels of occudin were
negatively correlated with the intensity of symptoms (21). Occludin is one of the tight
junction proteins, and an important component of intestinal mucosa.
Patients with occludin disruption also present with impaired
intestinal permeability and increased stool frequency (22). In the present study, reduced
occludin levels indicated that the tight junction was disrupted in
IBS-D patients. Therefore, although the occludin distribution
appeared normal under colonoscopy and conventional histology, the
intestinal mucosal barrier of the IBS-D patients had become
disrupted.
It is well documented that IBS patients show an
increased number of immunocompetent cells in the mucosal immune
response (23). Thus, it was
hypothesized that the increased mucosal immunocytes, along with
elevated levels of systemic cytokines, including TNF-α and IL-8,
contributed to mucosal barrier disruption. The findings of the
present study indicated that occludin expression was negatively
correlated with TNF-α and IL-8 production, but was not correlated
with IL-10 production. In addition, the present study indicated
that the mean symptom score of IBS-D patients was positively
correlated with TNF-α and IL-8 production, but was negatively
correlated with IL-10 production. The peripheral inflammation is
characterized by elevated pain sensitivity, which is mediated by
the release of inflammatory cytokines and growth factors (24). TNF-α was reported to contribute to
inflammatory sensory hypersensitivity (24). Therefore, elevated levels of
cytokines may be sufficient to sensitize sensory nerve terminals,
which may have resulted in a decreased threshold for visceral
sensitivity in IBS patients and explain the high abdominal symptoms
score in patients with IBS-D.
In addition, the present study also found
significant associations between psychological distress and
cytokine production. IBS patients with psychological distress
trended to show higher production of pro-inflammatory cytokines
(TNF-α and IL-8) and lower levels of the anti-inflammatory cytokine
IL-10. Similar results were reported in a recent study that
indicated that the levels of pro-inflammatory cytokine IL-1β were
significantly higher in IBS-D patients with anxiety and depression
and that the IL-10 levels were significantly lower compared with
those in IBS-D patients without anxiety and depression ones
(25). These results suggested
that psychological distress may cause changes in pro-inflammatory
and anti-inflammatory cytokine expression in IBS-D patients, which
may contribute to the occurrence or aggravation of IBS.
Psychiatric and psychological factors are closely
associated with the mechanism of IBS. The effect of psychological
factors on the symptoms and outcome of IBS is becoming increasingly
recognized. Studies have indicated that psychiatric and
psychological factors are independent risks for developing IBS
(26). Psychological stress can
contribute to the initiation and reactivation of experimental
gastrointestinal inflammation and increase the intestinal
permeability (27,28). Piche et al (10) also found that the number of IBS
mast cells was significantly correlated with the Fatigue Impact
Scale score and the Beck Depression Inventory score. The results of
the present study demonstrated that psychological factors may alter
immunological function and lead to the increased secretion of
pro-inflammatory cytokines.
However, it must be pointed out that the present
study has certain limitations: Due to the small cohort size used in
the present study, further studies using a large number of samples
are required to confirm the results. Furthermore, different
subgroups of IBS and additional cytokines should be studied to
better elucidate the pathological mechanisms of IBS.
In conclusion, the present study demonstrated that
the balance of cytokine production was impaired in IBS-D patients.
The imbalanced cytokines expression evoked colonic epithelial
barrier dysfunction and may be associated with abdominal symptoms
and psychological disorders.
Acknowledgments
This study was funded by a grant from the National
Science Foundation of China (NSFC; no. 30971336). The authors
appreciate the considerable assistance from the Gastroenterology
Kinetic Laboratory and the Key Laboratory of Cardiovascular
Remodeling and Function Research at Qilu Hospital, Shandong
University.
References
|
1
|
Schmulson MW and Chang L: Diagnostic
approach to the patient with irritable bowel syndrome. Am J Med.
107:20S–26S. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holten KB, Wetherington A and Bankston L:
Diagnosing the patient with abdominal pain and altered bowel
habits: is it irritable bowel syndrome? Am Fam Physician.
67:2157–2164. 2003.PubMed/NCBI
|
|
3
|
Andresen V and Camilleri M: Irritable
bowel syndrome: recent and novel therapeutic approaches. Drugs.
66:1073–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Veek PP, van den Berg M, de Kroon
YE, Verspaget HW and Masclee AA: Role of tumor necrosis
factor-alpha and interleukin-10 gene polymorphisms in irritable
bowel syndrome. Am J Gastroenterol. 100:2510–2516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonsalkorale WM, Perrey C, Pravica V,
Whorwell PJ and Hutchinson IV: Interleukin 10 genotypes in
irritable bowel syndrome: evidence for an inflammatory component?
Gut. 52:91–93. 2003. View Article : Google Scholar
|
|
6
|
Nielsen OH, Rüdiger N, Gaustadnes M and
Horn T: Intestinal interleukin-8 concentration and gene expression
in inflammatory bowel disease. Scand J Gastroenterol. 32:1028–1034.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coëffier M, Gloro R, Boukhettala N, et al:
Increased proteasome-mediated degradation of occludin in irritable
bowel syndrome. Am J Gastroenterol. 105:1181–1188. 2010. View Article : Google Scholar
|
|
8
|
Simrén M, Abrahamsson H, Svedlund J and
Björnsson E: Quality of life in patients with irritable bowel
syndrome seen in referral centers versus primary care: the impact
of gender and predominant bowel pattern. Scand J Gastroenterol.
36:545–552. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piche T, Huet PM, Gelsi E, et al: Fatigue
in irritable bowel syndrome: characterization and putative role of
leptin. Eur J Gastroenterol Hepatol. 19:237–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piche T, Saint-Paul MC, Dainese R, et al:
Mast cells and cellularity of the colonic mucosa correlated with
fatigue and depression in irritable bowel syndrome. Gut.
57:468–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drossman DA and Dumitrascu DL: Rome III:
New standard for functional gastrointestinal disorders. J
Gastrointestin Liver Dis. 15:237–241. 2006.PubMed/NCBI
|
|
12
|
Lewis SJ and Heaton KW: Stool form scale
as a useful guide to intestinal transit time. Scand J
Gastroenterol. 32:920–924. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Endo Y, Yoshizawa M, Fukudo S, Sasaki M
and Hongo M: Panic disorder in irritable bowel syndrome. Jpn J
Psychosom Med. 40:339–346. 2000.
|
|
14
|
Liebregts T, Adam B, Bredack C, et al:
Immune activation in patients with irritable bowel syndrome.
Gastroenterology. 132:913–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zung WW: A rating instrument for anxiety
disorders. Psychosomatics. 12:371–379. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zung WW: A self-rating depression scale.
Arch Gen Psychiatry. 12:63–70. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang L, Ameen VZ, Dukes GE, McSorley DJ,
Carter EG and Mayer EA: A dose-ranging, phase II study of the
efficacy and safety of alosetron in men with diarrhea-predominant
IBS. Am J Gastroenterol. 100:115–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohman L and Simrén M: Pathogenesis of IBS:
role of inflammation, immunity and neuroimmune interactions. Nat
Rev Gastroenterol Hepatol. 7:163–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura M, Saito H, Kasanuki J, Tamura Y
and Yoshida S: Cytokine production in patients with inflammatory
bowel disease. Gut. 33:933–937. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gecse K, Roka R, Ferrier L, et al:
Increased faecal serine protease activity in diarrhoeic IBS
patients: a colonic lumenal factor impairing colonic permeability
and sensitivity. Gut. 57:591–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertiaux-Vandaele N, Youmba SB, Belmonte
L, et al: The expression and the cellular distribution of the tight
junction proteins are altered in irritable bowel syndrome patients
with differences according to the disease subtype. Am J
Gastroenterol. 106:2165–2173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunlop SP, Hebden J, Campbell E, et al:
Abnormal intestinal permeability in subgroups of
diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol.
101:1288–1294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chadwick VS, Chen W, Shu D, et al:
Activation of the mucosal immune system in irritable bowel
syndrome. Gastroenterology. 122:1778–1783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woolf C, Allchorne A, Safieh-Garabedian B
and Poole S: Cytokines, nerve growth factor and inflammatory
hyperalgesia: the contribution of tumour necrosis factor alpha. Br
J Pharmacol. 121:417–424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao J: Correlation between
anxiety-depression status and cytokines in diarrhea-predominant
irritable bowel syndrome. Exp Ther Med. 6:93–96. 2013.PubMed/NCBI
|
|
26
|
Nicholl BI, Halder SL, Macfarlane GJ, et
al: Psychosocial risk markers for new onset irritable bowel
syndrome - results of a large prospective population-based study.
Pain. 137:147–155. 2008. View Article : Google Scholar :
|
|
27
|
Qiu BS, Vallance BA, Blennerhassett PA and
Collins SM: The role of CD4+ lymphocytes in the susceptibility of
mice to stress-induced reactivation of experimental colitis. Nat
Med. 5:1178–1182. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiliaan AJ, Saunders PR, Bijlsma PB, et
al: Stress stimulates transepithelial macromolecular uptake in rat
jejunum. Am J Physiol. 275(5 Pt 1): G1037–G1044. 1998.PubMed/NCBI
|