Introduction
Ponicidin is a natural ent-kaurane diterpenoid,
which is extracted from the traditional Chinese herb Isodon
adenolomus (1,2). Ponicidin has been shown to possess
antibacterial properties, as well as anti-inflammatory and
anti-viral regulatory functions (3). Recent studies have reported that
ponicidin exerts antitumor effects, and may function as a potential
cytotoxic drug for the treatment of hepatocellular cancer, lung
cancer, and monocytic leukemia (4–7).
Zhang et al (4) reported
that ponicidin exerts marked anti-proliferative effects on
hepato-cellular cancer cells by inducing apoptosis, via the
suppression of survivin and Bcl-2 expression, and the activation of
Bax expression (4). Liu et
al (7) reported the
anti-proliferative effects of ponicidin on leukemia cells in
vitro, via the down-regulation of survivin and Bcl-2
expression, thus inducing potent apoptosis. Furthermore, Zhao et
al detected the cytotoxic effects of ponicidin in lung cancer;
ponicidin was able to disrupt the mitochondrial membrane potential,
and trigger the activation of caspases-3, -8 and -9 (2). These previous reports suggest that
ponicidin may serve as a novel cytotoxic drug for the treatment of
various types of cancer.
Colorectal cancer is the third most frequently
diagnosed type of cancer, and the second leading cause of
cancer-associated mortality worldwide (8). Leucovorin and fluorouracil combined
with or without oxaliplatin are considered the first-line treatment
in advanced colorectal cancer (9).
However, after numerous rounds of treatment, the development of
chemoresistance is inevitable, and the treatment of metastatic
colorectal cancer remains to be improved (10). The usage of ponicidin for the
treatment of colorectal cancer has not yet been investigated. In
order to determine whether ponicidin has therapeutic potential in
colorectal cancer, the present study aimed to determine the effects
of various doses of ponicidin on cell proliferation and apoptosis
in vitro, using the HT29 colorectal cancer cell line. In
addition, the underlying molecular mechanisms of ponicidin in
cancer cells were investigated.
Materials and methods
Reagents and cell culture
Ponicidin was isolated from Isodon adenolomus
(Kunming Institute of Botany, Kunming, China) as described
previously (2). The HT29 human
colorectal cancer cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal calf
serum (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin and
100 μg/ml streptomycin (Sigma-Aldrich), in a humidified
incubator containing 5% CO2 at 37°C. In vitro
experiments were conducted in triplicate at 70% cell
confluence.
Cell proliferation assay
A total of 1×103 HT29 cells were seeded
in 96-well plates and treated with various doses of ponicidin (0,
10, 20 and 50 μg/ml), for various time-points (6, 12, 24 and
48 h). After incubation, the medium was removed and cell
proliferation was measured using the Cell Counting kit (CCK)-8
assay kit (Dojindo Molecular Technologies, Inc., Rockville, MD,
USA). CCK-8 solution (150 μl) was added to each well and
incubated for 2 h. Subsequently, the absorbance was measured at 450
nm using a microplate reader (HTX; Biotek, Beijing, China).
Cell cycle and apoptosis analyses
For the cell cycle analysis, flow cytometric
measurements of DNA content were detected in 70% ethanol-fixed HT29
cells using propidium iodide (PI). The fixed cells (106
cells/ml) were washed with phosphate-buffered saline (PBS), treated
with 200 μg/ml RNase A (Sigma-Aldrich) for 30 min at room
temperature, and stained with 50 μg/ml PI (Sigma-Aldrich).
Measurements were made using a flow cytometer (BD Influx; BD
Biosciences, Franklin Lakes, NJ, USA).
For the apoptosis assay, fluorescein isothiocyanate
(FITC)-conjugated Annexin-V and PI were used to detect apoptotic
cells. Trypsinized cells (106 cells/ml; Sigma-Aldrich)
were washed twice with PBS and resuspended in binding buffer
containing Annexin-V-FITC and PI (BD Biosciences). The cells were
incubated at room temperature for 15 min. The samples were analyzed
using a flow cytometer (BD Biosciences).
Hoechst 33342 staining
Hoechst 33342 staining was used to observe the
apoptotic morphology of the cells. HT29 cells (106
cells/ml), untreated or treated with ponicidin, were fixed with 4%
formaldehyde in PBS for 10 min. The cells were subsequently stained
with 10 μg/ml Hoechst 33342 (Yeasen Corporation, Shanghai,
China) for 1 h, and subjected to fluorescence microscopy (TE2000;
Nikon, Shanghai, China).
Western blot analysis
Western blot analysis was performed according to a
standard method (8). In brief, 10
mg protein lysates were loaded onto the SDS-PAGE gel and
transferred onto the membrane. The blots were quantified by
Quantity One 1-D analysis software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to measure the density of the bands. The
following antibodies were used: Anti-p38 (cat. no. 8690; 1:1,000
dilution), anti-phosphorylated (p)-p38 (cat. no. 4511; 1:800
dilution), anti-AKT (cat. no. 9272S; 1:1,000 dilution), anti-p-AKT
(cat. no. 4058S; 1:1,000 dilution), anti-extracellular
signal-regulated kinases (ERK) (cat. no. 4376S; 1:1,000 dilution),
anti-p-ERK (cat. no. 4370S; 1:1,000 dilution) and anti-GAPDH (cat.
no. 5174; 1:1,500 dilution) purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA); anti-caspase 3 (cat. no.
ab4051; 1:400 dilution; Abcam, Cambridge, MA, USA); anti-Bcl-2
(cat. no. sc-492; 1:100 dilution) and anti-Bax (cat. no. sc-493;
1:150 dilution) purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Horseradish peroxidase (HRP)-anti-rabbit
immunoglobulin G (IgG) (cat. no. A0208; 1:1,000 dilution) and
HRP-anti-mouse IgG (cat. no. A0216; 1:1,000 dilution) were
purchased from Beyotime Institute of Biotechnology (Haimen,
China).
Statistical analysis
All experiments were performed in triplicate and the
results were presented as the mean ± standard deviation. Data were
analyzed using Student's t-test, and IBM SPSS 20 software (IBM
SPSS, Armonk, NY, USA) was used to conduct analyses.
Results
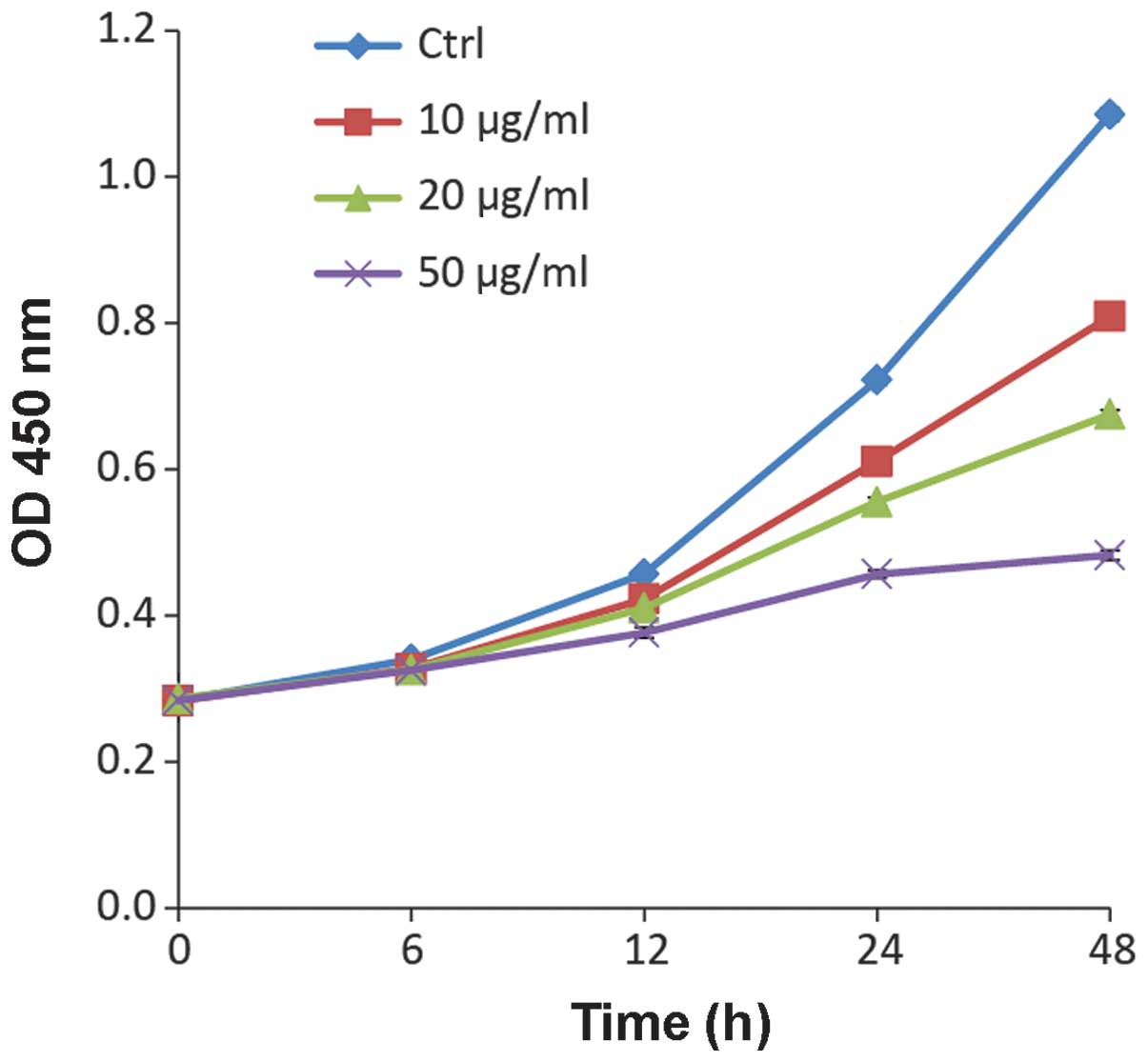
Ponicidin suppresses cell growth
To determine the suppression of cell growth by
ponicidin in colorectal cancer cells, HT29 cells were treated with
various doses of ponicidin for 0, 6, 12, 24 or 48 h. As shown in
Fig. 1, ponicidin exerted cell
growth inhibitory effects on HT29 cells in a dose-dependent manner.
The growth of the HT29 cells was markedly suppressed following 48 h
treatment with 50 μg/ml ponicidin, an ~4 fold reduction as
compared with the untreated control cells. The suppressive effects
of 10 and 20 μg/ml ponicidin were 1.5- and 2.1- fold,
respectively.
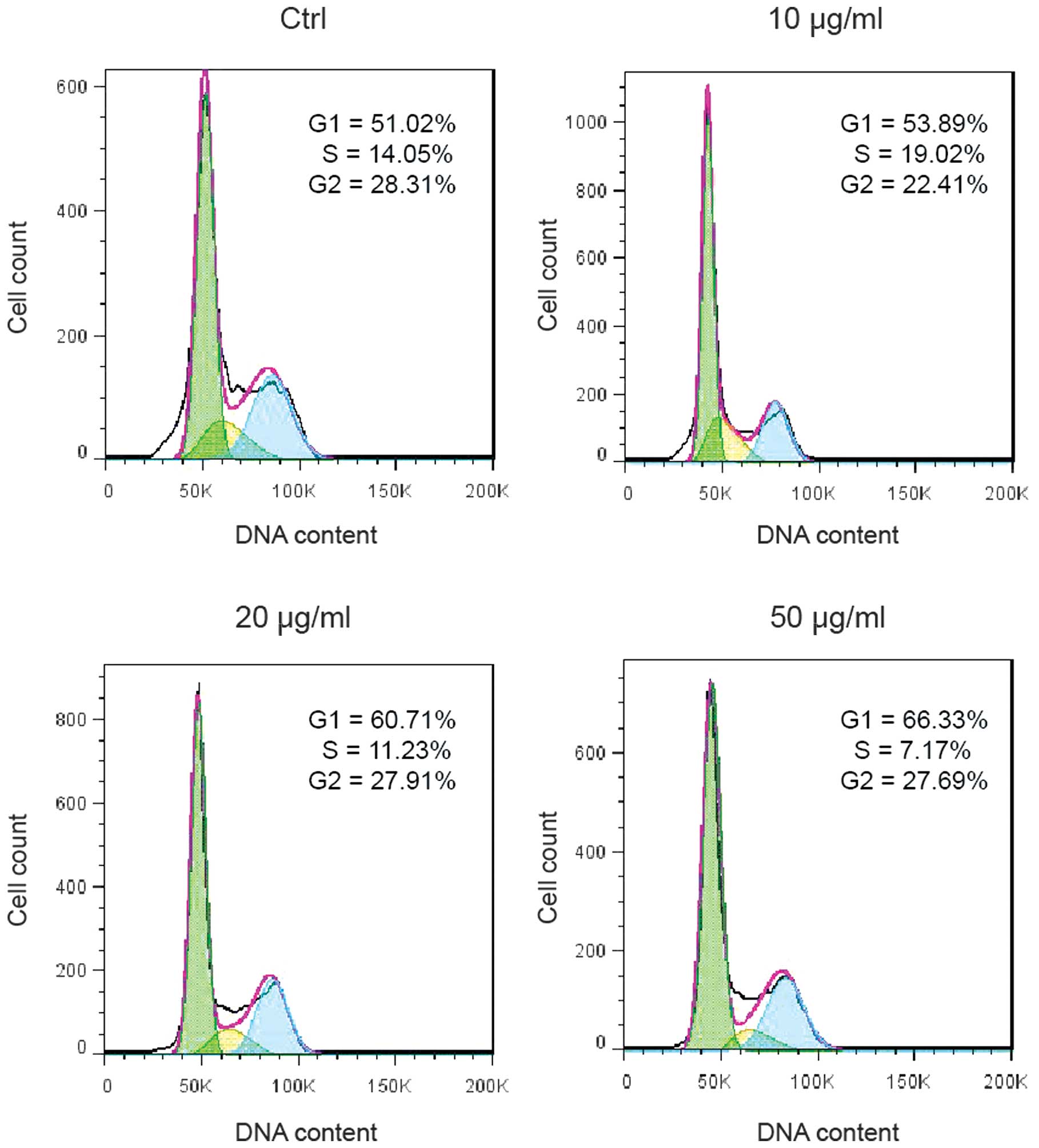
Ponicidin induces G1 cell cycle
arrest
In order to investigate the underlying mechanism of
ponicidin-induced cell growth suppression, cell cycle analysis was
performed on the HT29 cells treated with various doses of
ponicidin. Treatment with ponicidin suppressed DNA synthesis and
cell proliferation, as evidenced by a reduced percentage of S-phase
cells (19.02% in the 10 μg/ml group, 11.23% in the 20
μg/ml group, and 7.17% in the 50 μg/ml group, as
compared with 14.05% in the control group). G1 cell cycle arrest
was markedly increased following treatment with ponicidin (53.89%
in the 10 μg/ml group, 60.71% in the 20 μg/ml group,
and 66.33% in the 50 μg/ml group, as compared with 51.02% in
the control group) (Fig. 2).
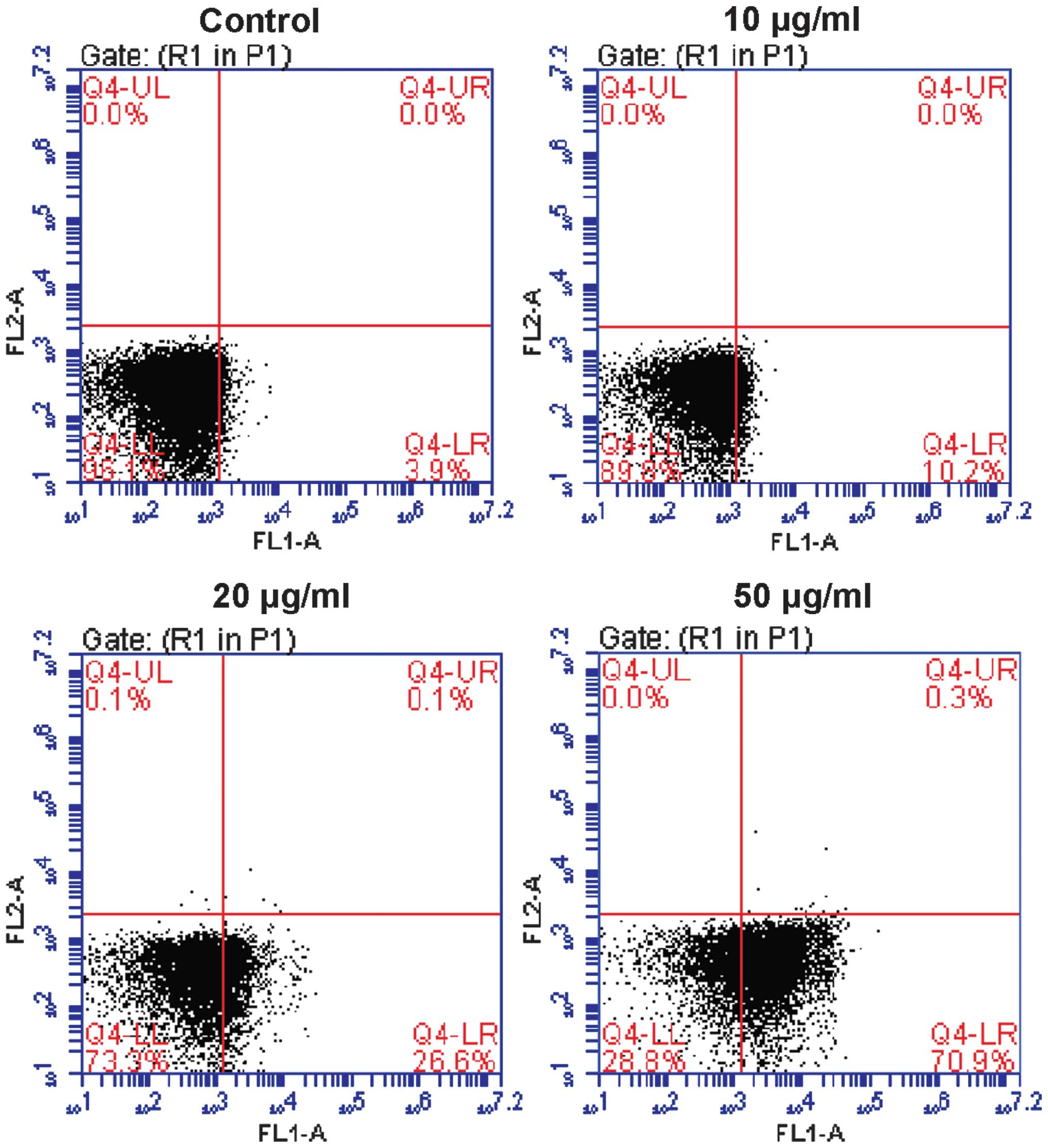
Ponicidin induces cell apoptosis
The present study also investigated whether
ponicidin-induced cell growth suppression was caused by the
induction of apoptosis. The HT29 cells were stained with PI and
analyzed by flow cytometry. Following treatment with ponicidin, the
apoptotic rate of the HT29 cells was increased to 10.2% in the 10
μg/ml group, 26.6% in the 20 μg/ml group, and 70.9%
in the 50 μg/ml group, as compared with 3.9% in the
untreated cells (Fig. 3). To
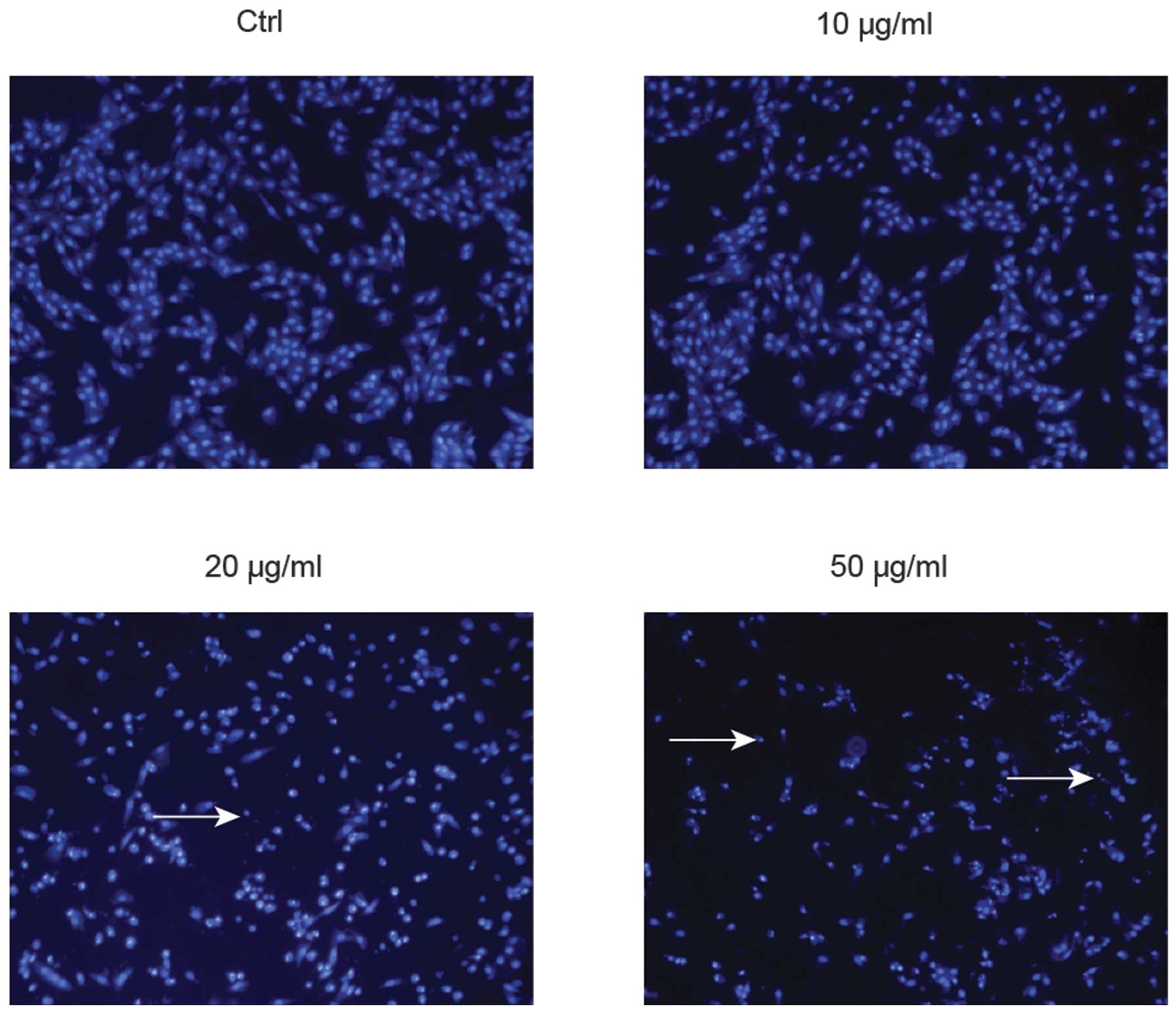
further validate the apoptotic effects of ponicidin, the number of
apoptotic bodies was determined in the treated cells (Fig. 4). Following treatment with
ponicidin, a marked increase in the number of apoptotic bodies was
detected in the HT29 cells. Treatment with 50 μg/ml
ponicidin resulted in the most apoptotic bodies, and the highest
apoptotic rate in the HT29 cells. These results were concordant
with the findings regarding cell growth suppression.
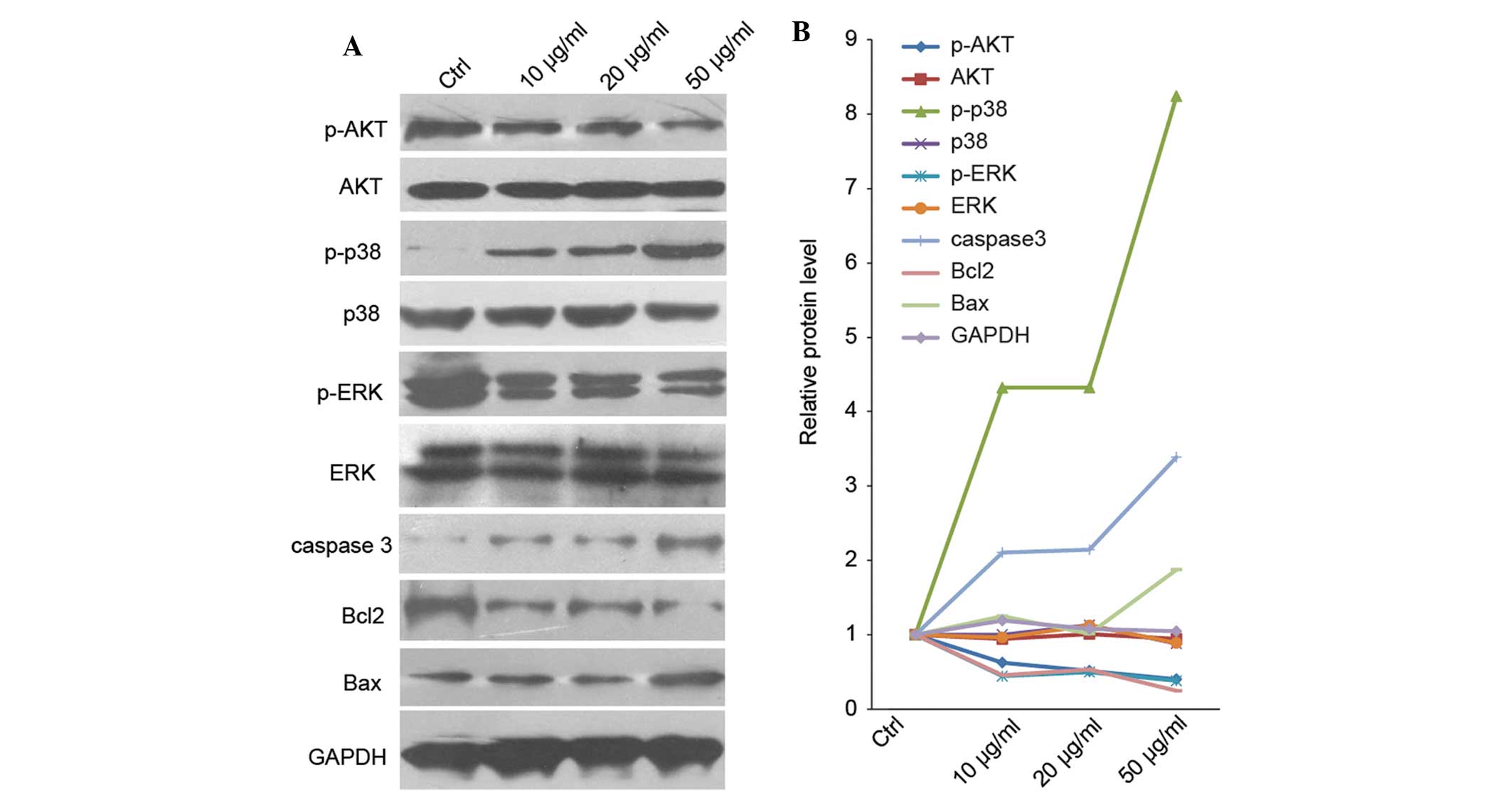
Ponicidin alters the expression levels of
apoptosis-associated proteins
In order to investigate the molecular mechanism
underlying ponicidin-induced apoptosis in HT29 cells,
apoptosis-associated protein expression was measured by western
blot analysis. As shown in Fig. 5,
the expression levels of p-p38 were markedly increased following
treatment with ponicidin (4.4-fold increase in the 10 μg/ml
group, 4.4-fold increase in the 20 μg/ml group and 8.3-fold
increase in the 50 μg/ml group), whereas total p38 protein
expression levels remained unchanged. Similarly, apoptotic markers,
caspase 3 and Bax were markedly upregulated in the treated cells;
however, p-AKT and p-ERK were downregulated in the treated cells.
These results indicate that proliferation signaling pathways were
suppressed following treatment with ponicidin (Fig. 5).
Discussion
Chinese herbal medicine is important in traditional
Chinese medical treatment. Recent studies have demonstrated that
Chinese herbal extracts may induce cancer cell death (11,12).
Mujumdar et al (13)
reported that the herbal extract triptolide resulted in the
downregulation of GRP78 protein expression in the cells, reduced
cancer cell survival, and facilitated cell death in human
pancreatic cancer cells and tissue in culture. Ponicidin is a
natural ent-kaurane diterpenoid compound that is extracted from the
traditional Chinese herb Isodon adenolomus (14,15).
Recent studies have reported the successful and large-scale
purification of ponicidin by liquid chromatography-tandem mass
spectrometry (16–19), thus indicating the practical usage
of ponicidin in therapeutic applications. Xu et al (20) identified the DNA binding and
cleavage properties of ponicidin; these properties may provide the
basis for the rational construction of novel, more efficient drugs
targeted to DNA, thus enabbling the development of effective
therapeutic agents for the target gene.
Notably, ponicidin has been shown to act as a
cytotoxic drug for hepatocellular cancer, lung cancer and
mono-cytic leukemia (1,7,12),
and therefore has high potential in translational research in
colorectal cancer. The present study examined the biological
function of ponicidin in the HT29 colorectal cancer cell line. The
results of the present study demonstrated that ponicidin was able
to significantly suppress the cell growth of HT29 cells by inducing
G1 cell cycle arrest and apoptosis. Treatment of HT29 cells with 50
μg/ml ponicidin resulted in a ~4 fold suppression of cell
growth, a ~15% increase in G1 cycle arrest, and a 67% increase in
cell death. Further investigation demonstrated that ponicidin
significantly upregulated p-p38, and suppressed the activation of
the AKT and MEK signaling pathways. The p38 signaling pathway is
activated by apoptotic stimuli, and induces apoptosis via its
downstream targets (14,21). Subsequent activation of apoptotic
marker genes, such as caspase 3 and Bax, was consistent with the
activation of the p38 signaling pathway. These results suggested
that ponicidin may act as an apoptotic stimulus and trigger
activation of the p38 signaling pathway in colorectal cancer cells.
Inducers of apoptosis have been applied in cancer therapy and
activation of apoptosis is a vital process by which cytotoxic drugs
destroy cancer cells (15,22). In conclusion, the results of
previous studies and of the present study suggest that ponicidin
may be used as a therapeutic cytotoxic drug for the treatment of
human cancers, including colorectal cancer.
References
|
1
|
Zhang JX, Han QB, Zhao AH and Sun HD:
Diterpenoids from Isodon japonica. Fitoterapia. 74:435–438. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao W, Pu JX, Du X, Su J, Li XN, Yang JH,
Xue YB, Li Y, Xiao WL and Sun HD: Structure and cytotoxicity of
diterpenoids from Isodon adenolomus. J Nat Prod. 74:1213–1220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osawa K, Yasuda H, Maruyama T, Morita H,
Takeya K and Itokawa H: Antibacterial trichorabdal diterpenes from
Rabdosia trichocarpa. Phytochemistry. 36:1287–1291. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang JF, Liu PQ, Chen GH, Lu MQ, Cai CJ,
Yang Y and Li H: Ponicidin inhibits cell growth on hepatocellular
carcinoma cells by induction of apoptosis. Dig Liver Dis.
39:160–166. 2007. View Article : Google Scholar
|
|
5
|
Liu JJ, Huang RW, Lin DJ, Peng J, Zhang M,
Pan X, Hou M, Wu XY, Lin Q and Chen F: Ponicidin, an ent-kaurane
diterpenoid derived from a constituent of the herbal supplement
PC-SPES, Rabdosia rubescens, induces apoptosis by activation of
caspase-3 and mitochondrial events in lung cancer cells in vitro.
Cancer Invest. 24:136–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai N, He K, Zhou Z, Tsai ML, Zhang L,
Quan Z, Shao X, Pan MH and Ho CT: Ent-kaurane diterpenoids from
Rabdosia rubescens and their cytotoxic effects on human cancer cell
lines. Planta Med. 76:140–145. 2010. View Article : Google Scholar
|
|
7
|
Liu JJ, Zhang Y, Guang WB, Yang HZ, Lin DJ
and Xiao RZ: Ponicidin inhibits monocytic leukemia cell growth by
induction of apoptosis. Int J Mol Sci. 9:2265–2277. 2008.
View Article : Google Scholar
|
|
8
|
Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R,
Hu X, Ye X, Lu J, Fan F, Xia L, et al: miR-203 induces oxaliplatin
resistance in colorectal cancer cells by negatively regulating ATM
kinase. Mol Oncol. 8:83–92. 2014. View Article : Google Scholar :
|
|
9
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
10
|
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P,
van Buren G II, Samuel S, Kim MP, Lim SJ and Ellis LM:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ergil KV, Kramer EJ and Ng AT: Chinese
herbal medicines. West J Med. 176:275–279. 2002.PubMed/NCBI
|
|
12
|
Au AM, Ko R, Boo FO, Hsu R, Perez G and
Yang Z: Screening methods for drugs and heavy metals in Chinese
patent medicines. Bull Environ Contam Toxicol. 65:112–119. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mujumdar N, Banerjee S, Chen Z, Sangwan V,
Chugh R, Dudeja V, Yamamoto M, Vickers SM and Saluja AK: Triptolide
activates unfolded protein response leading to chronic ER stress in
pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol.
306:G1011–G1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kostenko S, Dumitriu G, Lægreid KJ and
Moens U: Physiological roles of
mitogen-activated-protein-kinase-activated p38-regulated/activated
protein kinase. World J Biol Chem. 2:73–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Chu Y, Du B, Wang L and Yu T:
LC-MS-MS for the determination of ponicidin in dog plasma. J
Chromatogr Sci. 52:211–217. 2014. View Article : Google Scholar
|
|
17
|
Ma B, Wang Y, Zhang Q, Liu Y, Li J, Xu Q
and Ying H: Simultaneous determination of oridonin, ponicidin and
rosmarinic acid from Herba Isodi Rubescentis extract by LC-MS-MS in
rat plasma. J Chromatogr Sci. 51:910–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Hou W, Song J, Liu H, Song S, Zhang
L and Shi Y: A simple and sensitive HPLC-UV method for the
determination of ponicidin in rat plasma and its application in a
pharmacokinetic study. Biomed Chromatogr. 25:362–366. 2011.
View Article : Google Scholar
|
|
19
|
Du Y, Liu P, Shi X, Jin Y, Wang Q, Zhang
X, Sheng X and Zhang L: A novel analysis method for diterpenoids in
rat plasma by liquid chromatography-electrospray ionization mass
spectrometry. Anal Biochem. 407:111–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Ke Y, Zhang Q, Qi X and Liu H: DNA
binding and cleavage properties of ponicidin. Tumori. 95:348–351.
2009.PubMed/NCBI
|
|
21
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perego P, Cossa G, Zuco V and Zunino F:
Modulation of cell sensitivity to antitumor agents by targeting
survival pathways. Biochem Pharmacol. 80:1459–1465. 2010.
View Article : Google Scholar : PubMed/NCBI
|