Introduction
Aristolochic acid (AA) is contained in the Chinese
medicinal herbs asarum and aristolochia (1–3).
Numerous studies have demonstrated that AA can cause nephropathy
(4–6). In traditional Chinese medicine,
asarum and aristolochia are usual components of complex Chinese
remedies applied to treat arthritic pain, cough and
gastrointestinal symptoms (7–9).
Therefore, AA-induced nephropathy is widely discussed in China
(10,11). Previous studies showed that AA can
induce renal tubular cell death and fibrosis, leading to
nephropathy (12–14). As shown in numerous studies,
AA-induced tubular cell injury was associated with apoptosis
(12,15–17).
However, certain studies reported that AA induced cell death
through necrosis (18–20).
Apoptosis can be induced via a caspase-dependent or
caspase-independent pathway (21,22).
AA-induced renal tubular cell death was reported to proceed via the
caspase-dependent apoptotic pathway by numerous studies (13,23,24).
In general, AA mainly induces caspase-3 activation, resulting in
tubular cell apoptosis (25–27).
The present study further investigated the cytotoxic effects of AA
on renal tubular cells; dose- and time-dependency were examined and
apoptotic pathways were tested by using a caspase inhibitor.
Although AA-induced renal damage has been reported
in numerous studies (4–6), the mechanisms of AA-induced renal
tubular cell death have remained to be clarified. Previous studies
reported increases of oxidative stress in AA-treated renal tubular
cells and suggested that the increase of reactive oxygen species
(ROS) is a possible mechanism for AA-induced renal damage (16,27,28).
However, it has remained elusive which ROS is elevated by AA
treatment. O2− and H2O2
belong to ROS families which are common in cells (29,30).
Thus, O2− and H2O2
levels in renal cells following treatment with AA were examined in
the present study.
Based on the observation that the cytotoxicity of AA
is mediated via the induction of oxidative stress (16,27,28),
N-acetyl cysteine (NAC) and glutathione (GSH),
anti-oxidative agents, were studied to prevent cell death through
AA treatment (16). Vitamin C is a
common nutrient with anti-oxidative properties (31–33).
In the present study, it was hypothesized that vitamin C is able to
inhibit AA-induced cytotoxicity. Thus, AA-treated renal tubular
cells were co-treated with vitamin C, and its effects on cell
viability, caspase-3 activation and H2O2
levels were tested.
The present study indicated that co-administration
of vitamin C may be employed to reduce the nephrotoxic effects of
the Chinese medicinal herbs asarum and aristolochia.
Materials and methods
Materials
Luminol, lucigenin, aristolochic acid vitamin C,
tubulin antibody and Hoechst 33342 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). TGF-β was obtained from R&D
Systems (Minneapolis, MN, USA; cat. no. AB-100-NA). An MTT assay
kit was purchased from Bio-Basic Canada Inc. (Markham, OT, Canada).
Caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK) was
purchased from BD Pharmingen (Franklin Lakes, NJ, USA). Fetal
bovine serum, Dulbecco's modified Eagle's medium (DMEM),
non-essential amino acids, l-glutamine and
penicillin/streptomycin were obtained from Gibco-BRL (Invitrogen
Life Technologies, Carlsbad, CA, USA). Caspase-3 and cleaved
caspase-3 antibodies (cat. no. 9662) were purchased from Cell
Signaling Technology, Inc. (Beverly, MD, USA).
Cell culture
The rat kidney tubular epithelial cell line NRK-52E
was obtained from the Bioresource Collection and Research Center
(Shin Chu, Taiwan). NRK-52E cells were cultured in a DMEM medium
supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/ml
penicillin/streptomycin and 0.1 mM non-essential amino acids and
maintained at 37°C in a humidified atmosphere containing 5%
CO2.
Cell survival assay
The cell survival rate of NRK-52E cells was analyzed
using the MTT assay according to the manufacturer's instructions.
Briefly, NRK 52E cells were seeded into 96-well plates at a density
of 8×103 cells/well, and incubated for 24 h in 100
µl culture medium. The suitable concentration and optimum
exposure time of AAI were determined as 5, 10, 20 and 100 µM
at 6 h time intervals. At the 6 h time intervals, the control group
and experimental groups were examined with the MTT assay kit. Cells
were incubated with MTT solution at 37°C for 3 h and the survival
rate was then measured at 570 nm absorbance using a Multiskan™ FC
Microplate Photometer (Molecular Devices, Sunnyvale, CA, USA). The
cell survival rate was calculated using the following formula: A570
experimental group/A570 control group ×100% (34).
Observation of cell morphology and
apoptotic features
Cell morphology was observed under a phase-contrast
microscope. Apoptotic features were observed by Hoechst 33342 (cat.
no. 23491-52-3; Sigma-Aldrich) nuclear staining (35–37).
In brief, cells were treated with Hoechst 33342 (10 µg/ml)
for 10 min. DNA fragmentation and nuclear condensation were
observed under an Olympus DP71 fluorescence microscope (excitation,
352 nm; emission, 450 nm; Olympus Corporation, Tokyo, Japan).
Caspase inhibition assay
Z-VAD-FMK is a general caspase inhibitor. In the
present study, NRK-52E cells were pre-treated with 20 µM
Z-VAD-FMK prior to AA treatment (0, 5, 10, 20 or 100 µM).
The survival rates of NRK-52E cells were determined using an MTT
assay as described above (38).
Determination of
H2O2 and O2−
ratios
H2O2 and
O2− ratios were determined by using the
lucigenin-amplified chemiluminescence method (39,40).
In brief, the culture supernatant (200 µl) was treated with
0.2 mmol/l of luminol solution (100 µl) and subsequently
evaluated using a chemiluminescence analyzing system (CLA-FSI;
Tohoko Electronic Industrial Co., Ltd., Sendai, Japan) for the
determination of H2O2 levels. Samples (200
µl) were treated with 0.1 mmol/l lucigenin solution (500
µl) and were then measured using the CLA-FSI
chemiluminescence analyzing system for the determination of
O2− levels. The ratios of
H2O2 and O2− were
calculated as (experimental group counts/control group counts)
×100%.
Western blot analysis
Western blot analysis was performed as in a previous
study (34). Briefly, cells were
lysed with radioimmunoprecipitation assay buffer (cat. no. 20-188;
EMD Millipore, Billerica, MA, USA). After centrifugation at 16,000
× g for 10 min at 4°C, proteins were obtained and their
concentration was determined using the Bradford assay (cat. no.
23200; Thermo Fischer Scientific, Inc., Waltham, MA, USA). Equal
amounts of samples were separated by 13.3% SDS-PAGE (Bio-Rad Rad
Laboratories, Inc., Hercules, CA, USA), and then transferred onto
polyvinylidene difluoride membranes (EMD Millipore). The membranes
were blocked with 5% milk for 2 h at room temperature. After
washing with phosphate-buffered saline (PBS), samples were
incubated with the primary antibodies for 4 h overnight. Next,
samples were washed with PBS and treated with anti-rabbit-HRP
secondary antibodies (cat. no. NA934; Amersham Biosciences Inc.,
Beverly, MD, USA) for 1 h at room temperature. Finally, samples
were visualized by using Western Lightning Chemiluminescence
Reagent Plus (Perkin Elmer, Waltham, MA, USA) and quantified using
a densitometer (41,42).
Statistical analysis
Student's t-test was utilized for the analysis of
data. Values are expressed as the mean ± standard error. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
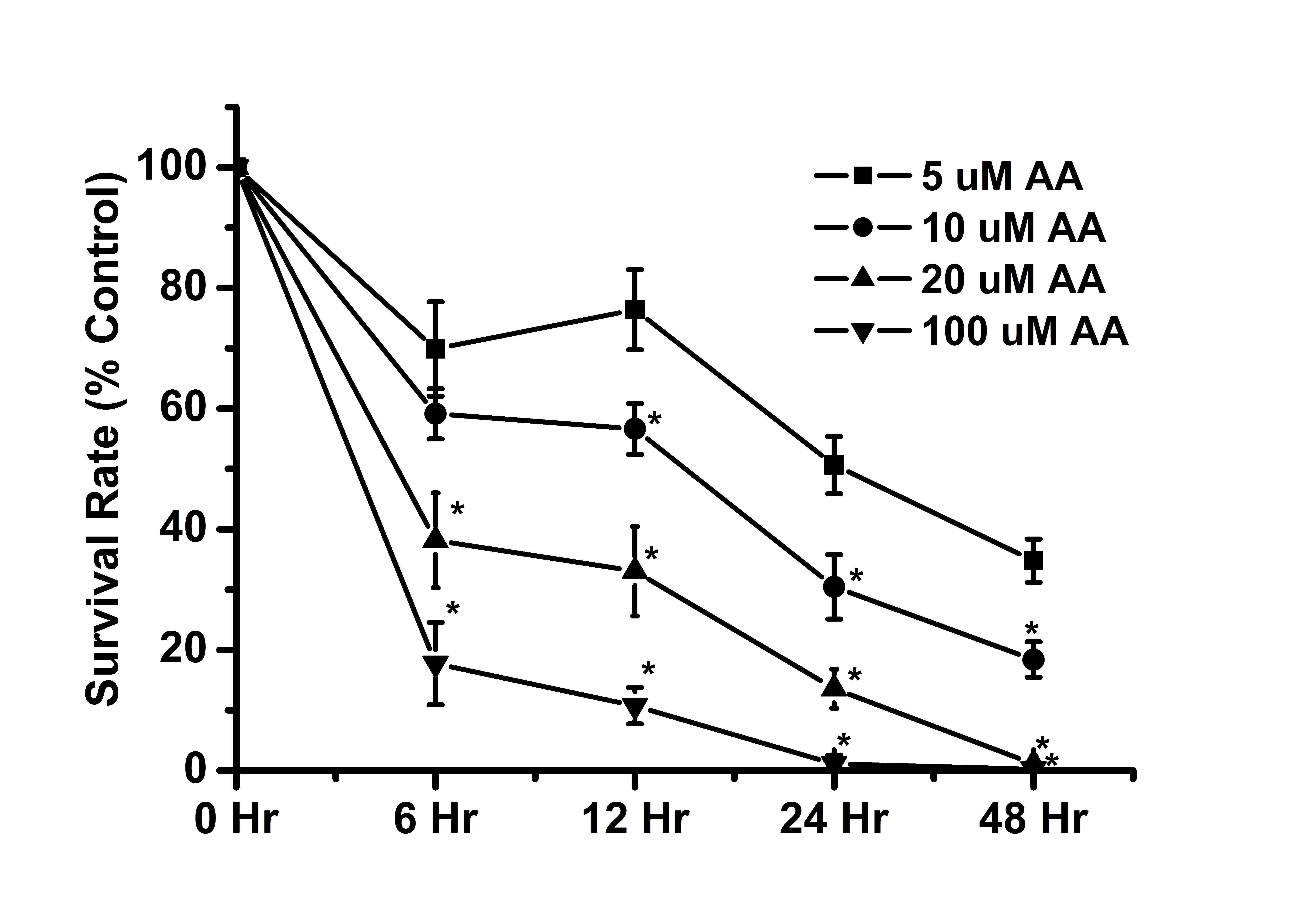
AA induces cytotoxicity in a dose- and
time-dependent manner
Previous studies have demonstrated that AA exerts
cytotoxic effects in renal tubular cells (16,43,44).
Similarly, the results of the present study also indicated that AA
can exert cytotoxic effects on renal tubular cells (Fig. 1). The cell survival rate was
<50% by the following treatments: 100 µM AA (6 h), 20
µM AA (6 h), 10 µM AA (24 h) and 5 µM AA (48
h). The results indicated that high-dose AA treatment exerted a
higher cytotoxic effect on renal tubular cells than low-dose AA
treatment. In addition, long durations of incubation had a greater
cytotoxic effect as compared with short incubations with AA. These
results suggested that AA induced cytotoxicity in a dose- and
time-dependent manner.
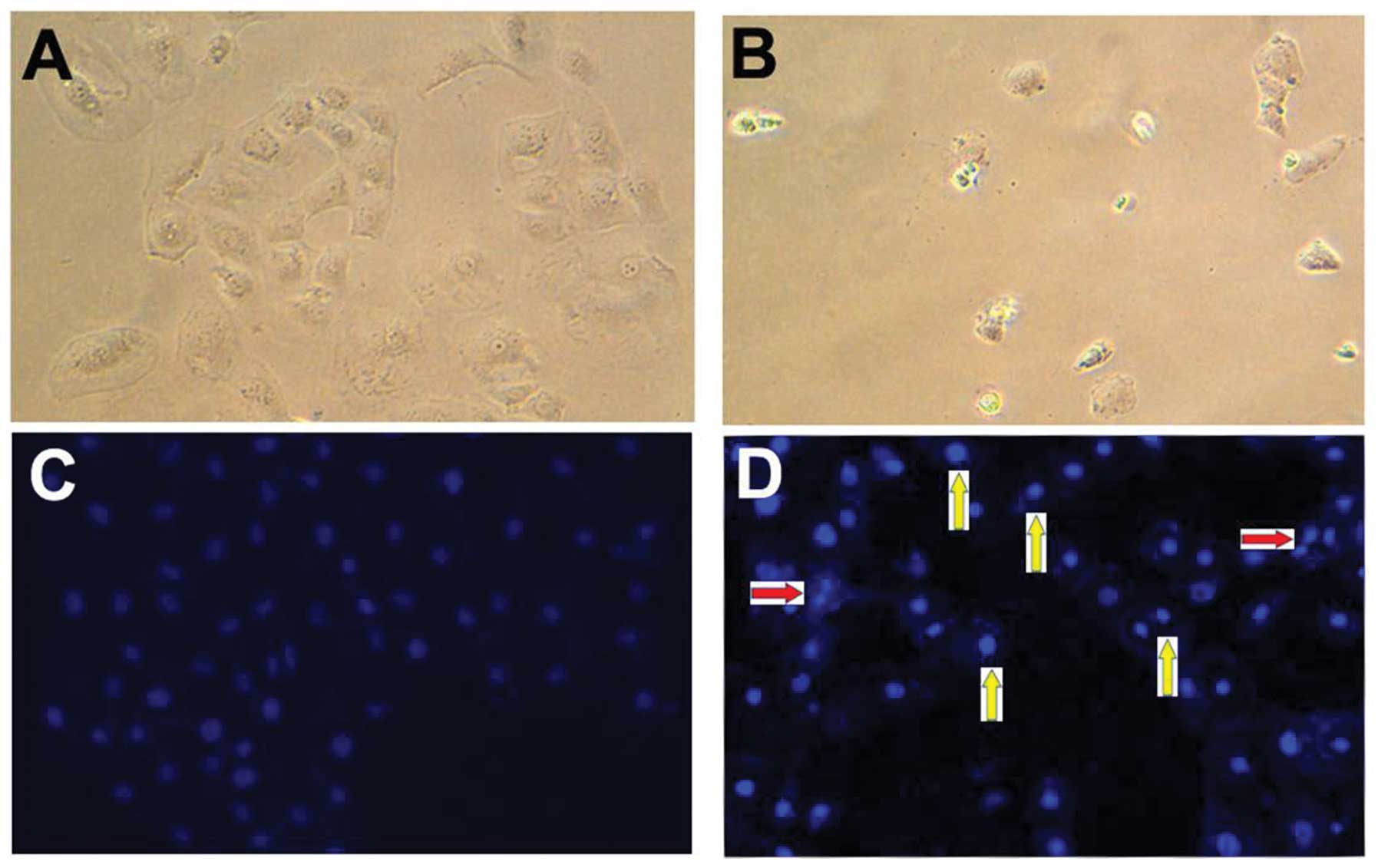
AA-induced cell death is associated with
the apoptotic pathway
Cell death can be associated with apoptotic or
necrotic pathways (45,46). In the present study, cell
morphology was observed under a phase-contrast and a fluorescent
microscope (Fig. 2). Compared with
control cells (Fig. 2A), shrunken
types were observed among AA-treated cells (Fig. 2B) under a phase-contrast
microscope. In addition, apoptotic characteristics, including
nuclear condensation and DNA fragmentation, were determined by
Hoechst nuclear staining (35,36).
Compared with the control cells (Fig.
2C), nuclear condensation and DNA fragmentation were observed
in AA-treated cells under a fluorescent microscope (Fig. 2D). These observations indicated
that AA-induced cell death was associated with the apoptotic
pathway.
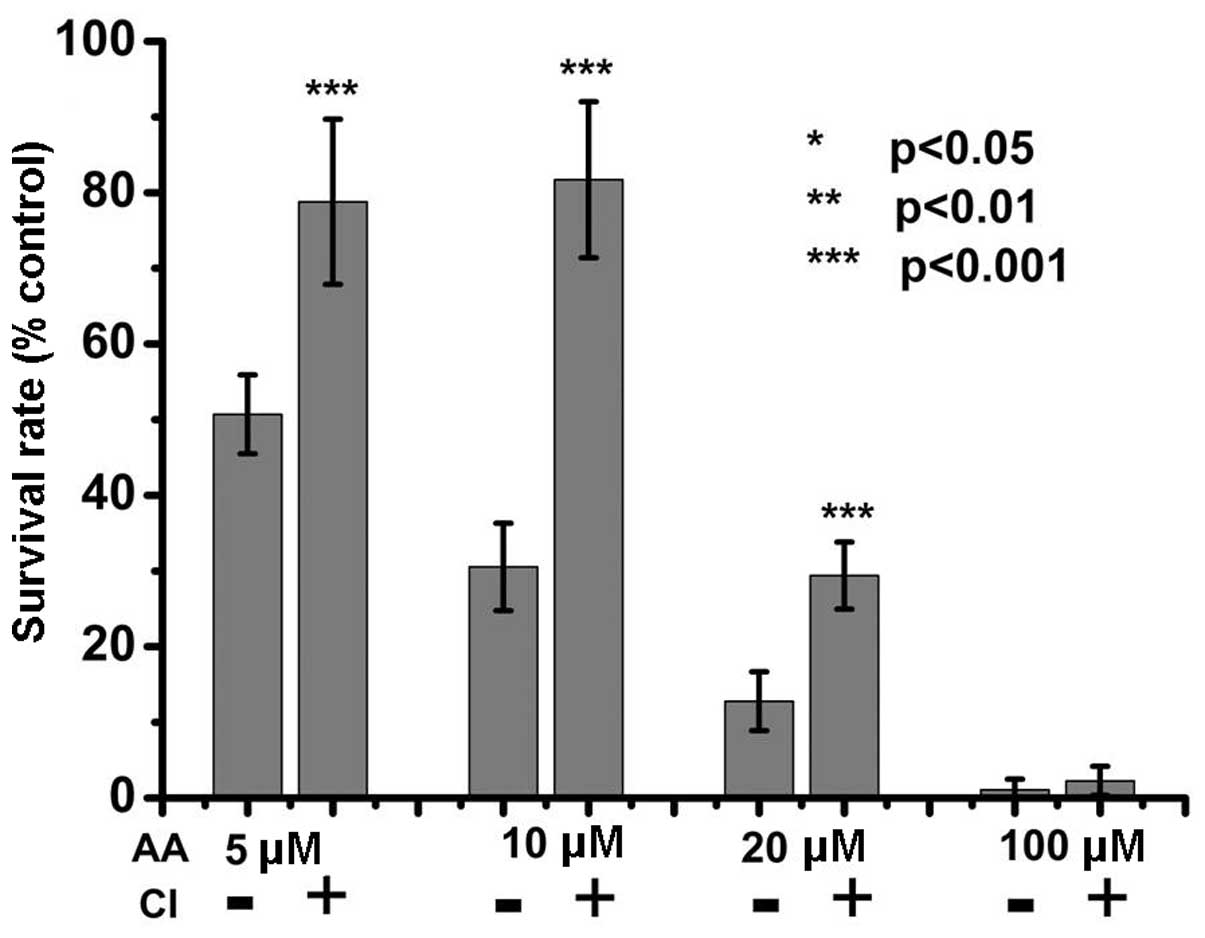
AA-induced apoptosis involves
caspase-dependent and -independent signaling
AA can activate caspase, resulting in cell
cytotoxicity (25–27). However, whether AA-induced
apoptosis is dependent on caspases has remained elusive. Therefore,
in the present study, caspase inhibitor was applied to cells prior
to AA treatment in order to investigate the association between
caspase activity and AA-induced cytotoxicity. The results showed
that blocking of caspase activity significantly inhibited the
cytotoxic effects of low doses of AA (5–20 µM) AA treatment
(Fig. 3); however, blocking of
caspase activity did not inhibit the cytotoxicity of AA at a high
dose (100 µM). These results indicated that caspase activity
is an important factor in low-dose AA-induced cytotoxicity. In
addition, caspase-independent apoptosis signaling, which remains to
be further investigated, is involved in the cytotoxic effects of
high doses of AA.
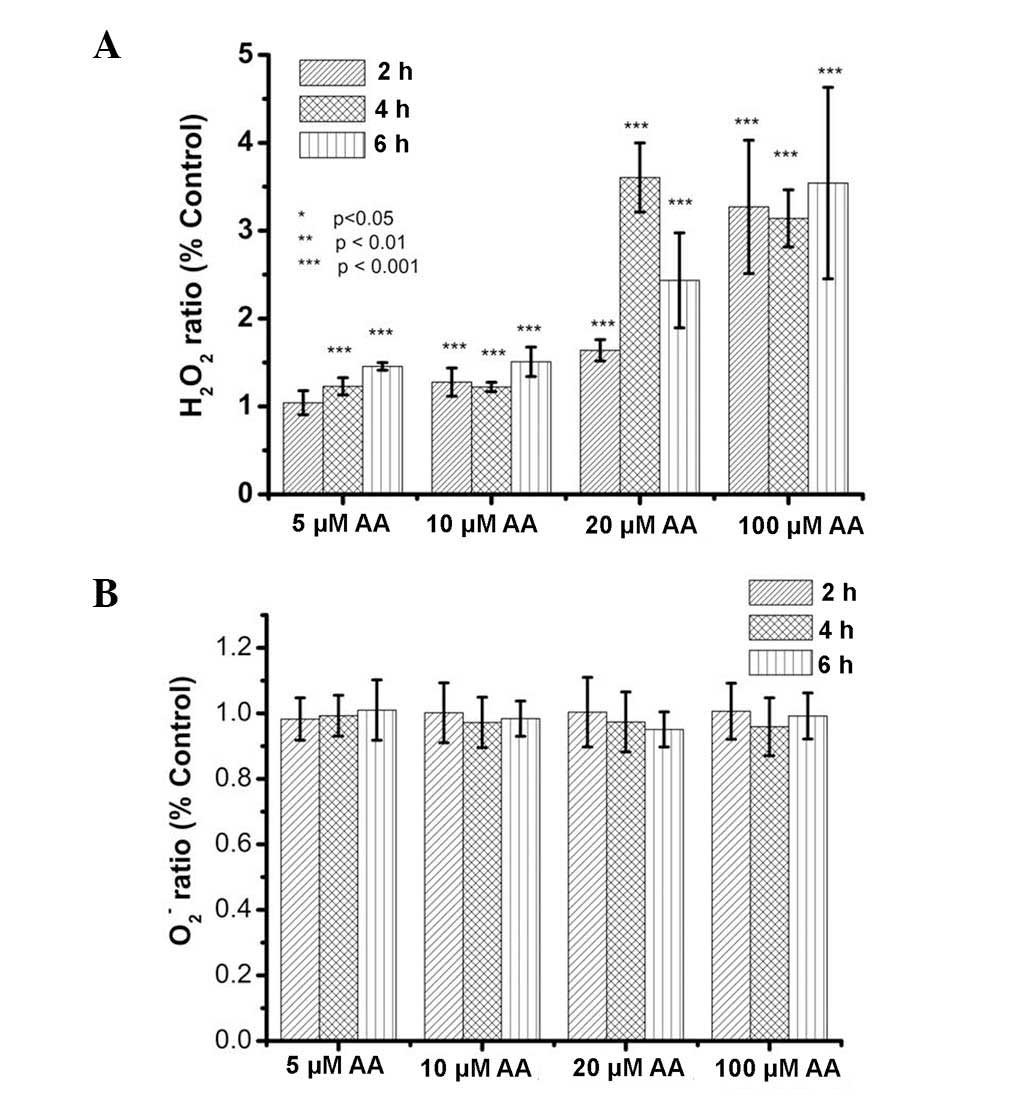
AA increases H2O2
but not O2− levels in kidney cells
H2O2 and
O2−, distinct species of the family of ROS,
are generally present in cells. Previous studies have demonstrated
that AA can induce increases in ROS in renal tubular cells
(16,27,28).
However, it has remained elusive which ROS are elevated by AA
treatment. Therefore, the present study examined
H2O2 and O2− levels in
kidney cells treated with AA by using the lucigenin-amplified
method (39,40). As shown in Fig. 4A, H2O2 levels
were elevated in renal tubular cells after AA treatment. The
results also indicated that AA induced increases in
H2O2 levels in a dose-dependent manner. By
contrast, the O2− ratio was not significantly
altered in renal cells following AA treatment (Fig. 4B). Therefore, the results of the
present study indicated that H2O2, but not
O2−, was among the ROS increased in kidney
cells by AA treatment.
Vitamin C reduces AA-induced increases in
H2O2 levels and inhibits AA-induced
cytotoxicity
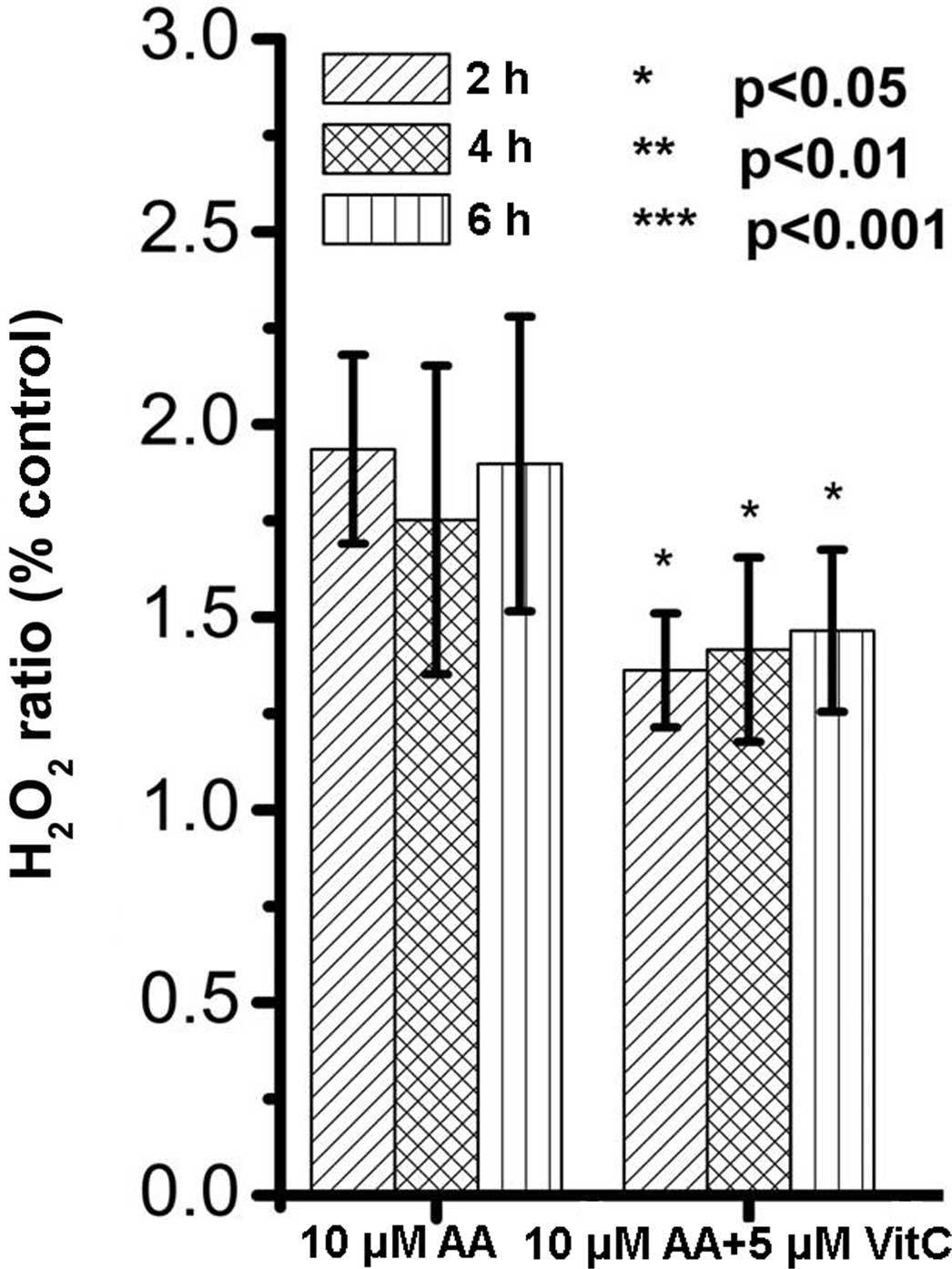
As AA treatment increased H2O2
levels (Fig. 4A), it was further
investigated whether H2O2 levels were
associated with AA-induced cytotoxicity in renal tubular cells.
Vitamin C, an anti-oxidative nutrient, was applied in AA-treated
renal tubular cells. The results showed that vitamin C was able to
attenuate the increases in H2O2 levels in
AA-treated renal cells (Fig. 5).
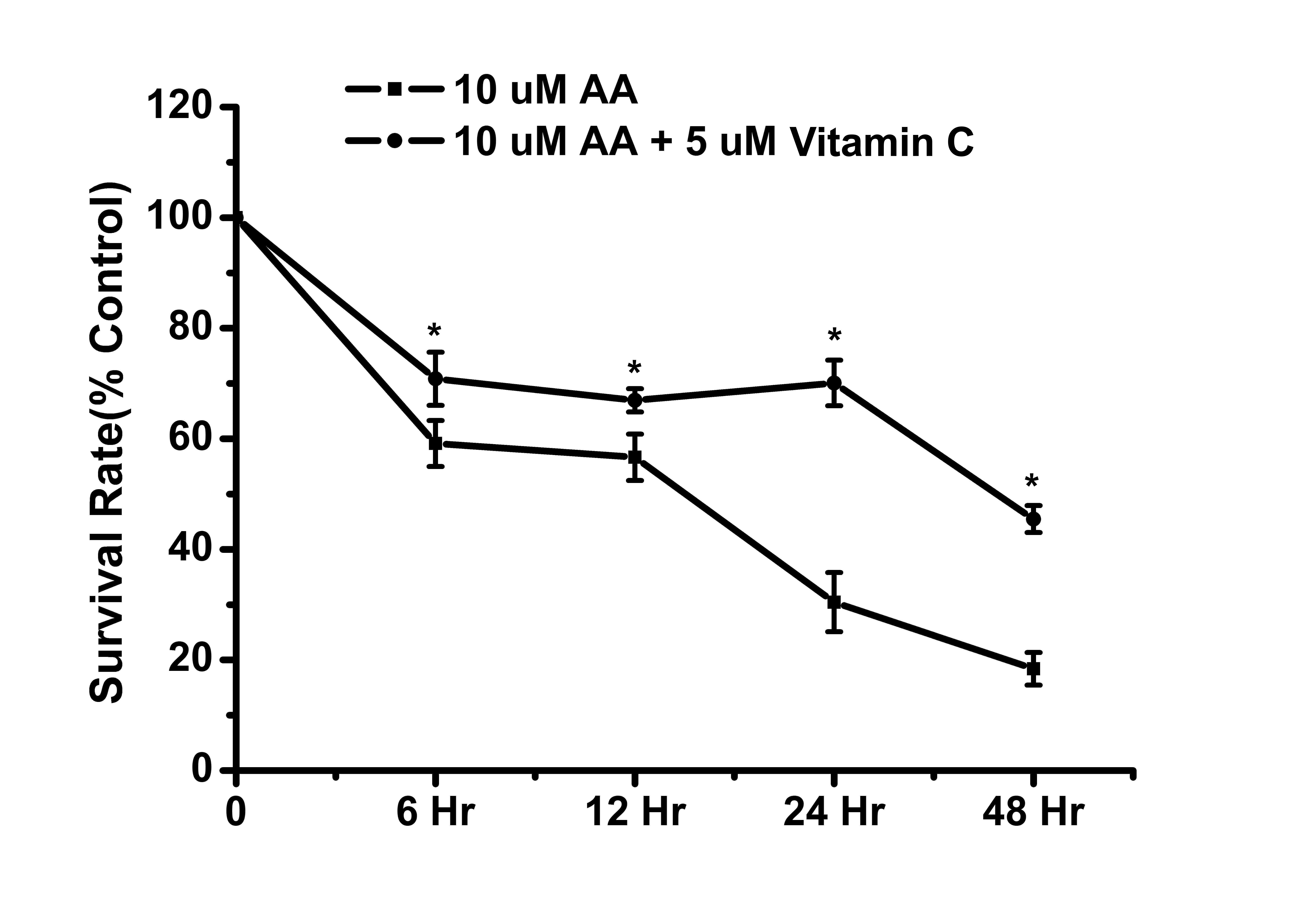
Furthermore, the cell survival rate of renal cells was assessed
following combined treatment with AA and vitamin C. The results
showed that vitamin C can inhibit AA-induced cytotoxicity in renal
tubular cells (Fig. 6). These
results suggested that H2O2 was an important
factor in AA-induced toxicity to renal tubular cells. In addition,
vitamin C effectively reduced H2O2 levels
alongside cytotoxicity in AA-treated cells.
Vitamin C decreases AA-induced caspase-3
activation to attenuate AA-induced cytotoxicity
The results of the present study demonstrated that
vitamin C decreased AA-induced increases in
H2O2 levels to inhibit AA-induced
cytotoxicity (Figs. 5 and 6). In addition, previous studies
(25–27) and the results of the present study
(Fig. 3) indicated that caspase-3
activity was an important factor associated with AA-induced
cytotoxicity. Therefore, the present study further investigated
whether vitamin C can decrease caspase-3 activity to attenuate
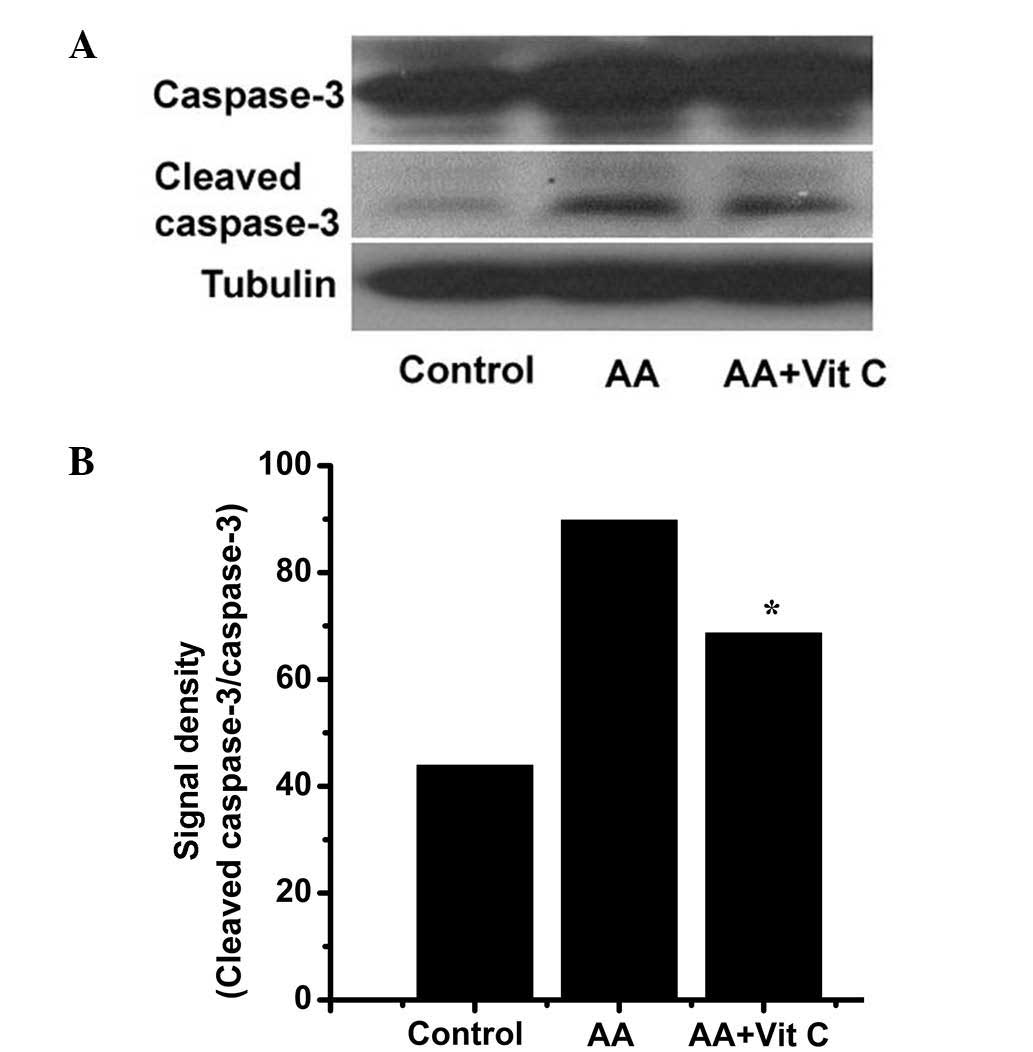
AA-induced cytotoxicity. For this, caspase-3 (inactivated
caspase-3) and cleaved caspase-3 (activated caspase-3) levels were
determined by western blot analysis (Fig. 7A) and the caspase-3 activity ratio
(cleaved caspase-3/caspase-3) was obtained by densitometric
analysis (Fig. 7B). Compared with
the control group, the caspase-3 activity ratio was obviously
increased in the AA-treated group (43.9 vs. 89.8%). Of note,
compared with that in the AA-treated group, the caspase-3 activity
ratio was decreased to 68.6% in the AA plus vitamin C-treated
group. These results indicated that vitamin C decreased AA-induced
caspase-3 activation. Regarding the association between AA-induced
cytotoxicity and vitamin C (Figs.
5Figure 6–7), the results suggested that vitamin C
can attenuate AA-induced cytotoxicity, at least in part via
decreasing AA-induced H2O2 levels and
caspase-3 activity.
Discussion
Numerous studies have demonstrated that AA can
induce renal tubular cell death (4–6) and
kidney fibrosis (14,47), resulting in nephropathy. Regarding
AA-induced cell death, the apoptotic pathway was widely identified
in AA-treated cells (12,15–17);
however, necrosis was also reported by certain studies on
AA-treated cells (18–20). DNA fragmentation and nuclear
condensation were observed by fluorescent microscopic observation.
Therefore, the results of the present study indicated that
AA-induced cytotoxicity was mediated via the apoptotic pathway.
Previous studies have shown that AA can activate caspase-3 activity
leading to apoptosis (25–27). However, whether AA-induced
cytotoxicity depends on caspase-3 activation had remained elusive.
The results of the present study showed that AA induced caspase-3
activation in accordance with the results of previous studies
(25–27). Furthermore, the present study found
that the cytotoxicity of low doses of AA was attenuated by a
caspase inhibitor, whereas that of high doses of AA was not
inhibited. These data suggested that the cytotoxicity of AA at low
doses is mediated via caspase activation signaling, while the
cytotoxicity of AA at high doses involves caspase-dependent as well
as -independent signaling, which remains to be further elucidated
in the future.
Previous studies have demonstrated that AA induces
increases of ROS levels, resulting in renal injury (16,27,28).
However, these studies did not determine which type of ROS is
generated by AA treatment. O2− and
H2O2 are ROS which are common in cells, and
O2− can be converted into
H2O2 by superoxide dismutase (48,49).
Furthermore, H2O2 can be converted into
H2O by GSH and GSH peroxidase (50,51).
In the present study, O2− and
H2O2 levels were determined using the
lucigenin-amplified method (39,40).
The results showed that AA treatment induced increases of
H2O2 levels but not of
O2− levels. From these results it can
therefore be deduced that AA may be able to decrease GSH levels or
GSH peroxidase activities. This would also explain why NAC, which
is required for GSH synthesis, or GSH pre-treatment can inhibit
AA-induced cell death (16,27).
A major function of SOD is converting O2 to
H2O2. The present study demonstrated that AA
is not able to influence O2 levels. These results
suggested that AA may not be associated with SOD activity.
Because AA is a component of various Traditional
Chinese Medicinal plants (7,52–56),
it is important to discover means of preventing AA-induced renal
damage. Though studies have developed a monoclonal antibody against
AA-induced cytotoxicity (3,57,58),
most approaches are directed against oxidative stress to suppress
AA-induced cytotoxicity. Based on the above findings, elucidating
whether vitamin C inhibits AA-induced cytotoxicity in animal models
will require further study. To date, anti-oxidant agents including
Tiron, NAC, GSH, bone morphogenetic protein 7 and darbepoetin have
been studied for their ability to reduce AA-induced cytotoxicity
(13,27,59–61).
However, these substances are not easily available. Vitamin C has
anti-oxidative activities and is contained in numerous vegetables
and fruits (31–33). The present study therefore assessed
whether vitamin C can suppress AA-induced cytotoxicity to kidney
cells. The results demonstrated that vitamin C markedly attenuated
increases of H2O2 levels, caspase-3
activation and cytotoxicity following AA treatment. These results
therefore suggested that supplementation of vitamin C may be
beneficial for reducing AA-induced renal damage when using
Traditional Chinese Medicines.
In conclusion, the present study demonstrated that:
1) AA activates caspase-3 activity and induces increases of
H2O2 levels, resulting in renal tubular cell
death; 2) AA-induced cell death is mediated via caspase-dependent
and -independent pathways, depending on the dose; and 3) Vitamin C
can decrease AA-induced increases in H2O2
levels and caspase-3 activity to attenuate AA-induced
cytotoxicity.
Acknowledgments
The present study was supported by Ministry of
Science and Technology (grant no. MOST-103-2320-B-039) and the
National Health Research Institutes (grant no.
NHRI-EX102-10245BI).
References
|
1
|
Schaneberg BT and Khan IA: Analysis of
products suspected of containing Aristolochia or Asarum species. J
Ethnopharmacol. 94:245–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaneberg BT, Applequist WL and Khan IA:
Determination of aristolochic acid I and II in North American
species of Asarum and Aristolochia. Pharmazie. 57:686–689.
2002.PubMed/NCBI
|
|
3
|
Li XW, Morinaga O, Tian M, Uto T, Yu J,
Shang MY, Wang X, Cai SQ and Shoyama Y: Development of an Eastern
blotting technique for the visual detection of aristolochic acids
in Aristolochia and Asarum species by using a monoclonal antibody
against aristolochic acids I and II. Phytochem Anal. 24:645–653.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng C, Xie X, Wu M, Li C, Gao M, Liu M,
Qi X and Ren J: Tanshinone I protects mice from aristolochic acid
I-induced kidney injury by induction of CYP1A. Environ Toxicol
Pharmacol. 36:850–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grollman AP: Aristolochic acid
nephropathy: Harbinger of a global iatrogenic disease. Environ Mol
Mutagen. 54:1–7. 2013. View Article : Google Scholar
|
|
6
|
Yang L, Su T, Li XM, Wang X, Cai SQ, Meng
LQ, Zou WZ and Wang HY: Aristolochic acid nephropathy: Variation in
presentation and prognosis. Nephrol Dial Transplant. 27:292–298.
2012. View Article : Google Scholar
|
|
7
|
Tsai DM, Kang JJ, Lee SS, Wang SY, Tsai
IL, Chen GY, Liao HW, Wei-Chu L, Kuo CH and Tseng YJ: Metabolomic
analysis of complex chinese remedies: Examples of induced
nephrotoxicity in the mouse from a series of remedies containing
aristolochic Acid. Evid Based Complement Alternat Med.
2013:2637572013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arlt VM, Stiborova M and Schmeiser HH:
Aristolochic acid as a probable human cancer hazard in herbal
remedies: A review. Mutagenesis. 17:265–277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heinrich M, Chan J, Wanke S, Neinhuis C
and Simmonds MS: Local uses of Aristolochia species and content of
nephrotoxic aristolochic acid 1 and 2 - a global assessment based
on bibliographic sources. J Ethnopharmacol. 125:108–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Zhang L, Wang W and Wang H; China
National Survey of Chronic Kidney Disease Working Group:
Association between aristolochic acid and CKD: A cross-sectional
survey in China. Am J Kidney Dis. 61:918–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Broe ME: Chinese herbs nephropathy and
Balkan endemic nephropathy: Toward a single entity, aristolochic
acid nephropathy. Kidney Int. 81:513–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pozdzik AA, Salmon IJ, Debelle FD,
Decaestecker C, Van den Branden C, Verbeelen D, Deschodt-Lanckman
MM, Vanherweghem JL and Nortier JL: Aristolochic acid induces
proximal tubule apoptosis and epithelial to mesenchymal
transformation. Kidney Int. 73:595–607. 2008. View Article : Google Scholar
|
|
13
|
Wang Z, Zhao J, Zhang J, Wei J, Zhang J
and Huang Y: Protective effect of BMP-7 against aristolochic
acid-induced renal tubular epithelial cell injury. Toxicol Lett.
198:348–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin TC, Lee TC, Hsu SL and Yang CS: The
molecular mechanism of leptin secretion and expression induced by
aristolochic acid in kidney fibroblast. PloS One. 6:e166542011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Dou Y, Zheng X, Tan Y, Cheng J, Li
L, Du Y, Zhu D and Lou Y: Cysteinyl leukotrienes synthesis is
involved in aristolochic acid I-induced apoptosis in renal proximal
tubular epithelial cells. Toxicology. 287:38–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Wang Y, Jin J, Guan C, Li M, Xi C,
Ouyang Z, Chen M, Qiu Y, Huang M, et al: Endoplasmic reticulum
stress mediates aristolochic acid I-induced apoptosis in human
renal proximal tubular epithelial cells. Toxicol In Vitro.
26:663–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W and Zhang J: Protective effect of
erythropoietin against aristolochic acid-induced apoptosis in renal
tubular epithelial cells. Eur J Pharmacol. 588:135–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baudoux TE, Pozdzik AA, Arlt VM, De Prez
EG, Antoine MH, Quellard N, Goujon JM and Nortier JL: Probenecid
prevents acute tubular necrosis in a mouse model of aristolochic
acid nephropathy. Kidney Int. 82:1105–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao J, Yang XD, Wang XY, Qu L, Liu G and
Li XM: Differential changes of intrarenal oxygenation in rat models
of acute tubular necrosis caused by aristolochic acid and
gentamicin. Chinese Medical Journal. 90:1208–1212. 2010.In
Chinese.
|
|
20
|
Yang L, Li X and Wang H: Possible
mechanisms explaining the tendency towards interstitial fibrosis in
aristolochic acid-induced acute tubular necrosis. Nephrol Dial
Transplant. 22:445–456. 2007. View Article : Google Scholar
|
|
21
|
Zhou Y, Bi Y, Yang C, Yang J, Jiang Y,
Meng F, Yu B, Khan M, Ma T and Yang H: Magnolol induces apoptosis
in MCF-7 human breast cancer cells through G2/M phase arrest and
caspase-independent pathway. Pharmazie. 68:755–762. 2013.PubMed/NCBI
|
|
22
|
An FF, Liu YC, Zhang WW and Liang L:
Dihydroartemisinine enhances dictamnine-induced apoptosis via a
caspase dependent pathway in human lung adenocarcinoma A549 cells.
Asian Pac J Cancer Prev. 14:5895–5900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Fu W, Wang H, Liang Y, Wang Y, Yao
W, Chen W, Li Q, Ying PH, Shi X, et al: Renal microvascular injury
in chronic aristolochic acid nephropathy and protective effects of
Cozaar. Ren Fail. 34:60–67. 2012. View Article : Google Scholar
|
|
24
|
Zhang L, Li J, Jiang Z, Sun L, Mei X, Yong
B and Zhang L: Inhibition of aquaporin-1 expression by RNAi
protects against aristolochic acid I-induced apoptosis in human
proximal tubular epithelial (HK-2) cells. Biochem Biophys Res
Commun. 405:68–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J and Zhang L, Jiang Z, Shu B, Li F,
Bao Q and Zhang L: Toxicities of aristolochic acid I and
aristololactam I in cultured renal epithelial cells. Toxicol In
Vitro. 24:1092–1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi X, Cai Y, Gong L, Liu L, Chen F, Xiao
Y, Wu X, Li Y, Xue X and Ren J: Role of mitochondrial permeability
transition in human renal tubular epithelial cell death induced by
aristolochic acid. Toxicol Appl Pharmacol. 222:105–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu FY, Wu TS, Chen TW and Liu BH:
Aristolochic acid I induced oxidative DNA damage associated with
glutathione depletion and ERK1/2 activation in human cells. Toxicol
In Vitro. 25:810–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YY, Chung JG, Wu HC, Bau DT, Wu KY,
Kao ST, Hsiang CY, Ho TY and Chiang SY: Aristolochic acid
suppresses DNA repair and triggers oxidative DNA damage in human
kidney proximal tubular cells. Oncol Rep. 24:141–153.
2010.PubMed/NCBI
|
|
29
|
Yao CW, Piao MJ, Kim KC, Zheng J, Cha JW
and Hyun JW: 6′-o-galloylpaeoniflorin protects human keratinocytes
against oxidative stress-induced cell damage. Biomol Ther (Seoul).
21:349–357. 2013. View Article : Google Scholar
|
|
30
|
Sen S, Kawahara B, Fry NL, Farias-Eisner
R, Zhang D, Mascharak PK and Chaudhuri G: A light-activated NO
donor attenuates anchorage independent growth of cancer cells:
Important role of a cross talk between NO and other reactive oxygen
species. Arch Biochem Biophys. 540:33–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kataoka T, Nishiyama Y, Yamato K, Teraoka
J, Morii Y, Sakoda A, Ishimori Y, Taguchi T and Yamaoka K:
Comparative study on the inhibitory effects of antioxidant vitamins
and radon on carbon tetrachloride-induced hepatopathy. J Radiat Res
(Tokyo). 53:830–839. 2012. View Article : Google Scholar :
|
|
32
|
Al-Rejaie SS, Abuohashish HM, Alkhamees
OA, Aleisa AM and Alroujayee AS: Gender difference following high
cholesterol diet induced renal injury and the protective role of
rutin and ascorbic acid combination in Wistar albino rats. Lipids
Health Dis. 11:412012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taniguchi M, A rai N, Kohno K, Ushio S and
Fukuda S: Anti-oxidative and anti-aging activities of
2-O-α-glucopyranosyl-L-ascorbic acid on human dermal fibroblasts.
Eur J Pharmacol. 674:126–131. 2012. View Article : Google Scholar
|
|
34
|
Yu YL, Su KJ, Chen CJ, Wei CW, Lin CJ,
Yiang GT, Lin SZ, Harn HJ and Chen YL: Synergistic anti-tumor
activity of isochaihulactone and paclitaxel on human lung cancer
cells. J Cell Physiol. 227:213–222. 2012. View Article : Google Scholar
|
|
35
|
Yiang GT, Chen YH, Chou PL, Chang WJ, Wei
CW and Yu YL: The NS3 protease and helicase domains of Japanese
encephalitis virus trigger cell death via caspase-dependent and
-independent pathways. Mol Med Rep. 7:826–830. 2013.PubMed/NCBI
|
|
36
|
Yiang GT, Yu YL, Chou PL, Tsai HF, Chen
LA, Chen YH, Su KJ, Wang JJ, Bau DT and Wei CW: The cytotoxic
protein can induce autophagocytosis in addition to apoptosis in
MCF-7 human breast cancer cells. In Vivo. 26:403–409.
2012.PubMed/NCBI
|
|
37
|
Yiang GT, Chou PL, Tsai HF, Chen LA, Chang
WJ, Yu YL and Wei CW: Immunotherapy for SV40 T/t antigen-induced
breast cancer by recombinant adeno-associated virus serotype 2
carrying interleukin-15 in mice. Int J Mol Med. 29:809–814.
2012.PubMed/NCBI
|
|
38
|
Wu CS, Yen CJ, Chou RH, Li ST, Huang WC,
Ren CT, Wu CY and Yu YL: Cancer-associated carbohydrate antigens as
potential biomarkers for hepatocellular carcinoma. PloS One.
7:e394662012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen KH, Li PC, Lin WH, Chien CT and Low
BH: Depression by a green tea extract of alcohol-induced oxidative
stress and lipogenesis in rat liver. Biosci Biotechnol Biochem.
75:1668–1676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin BR, Yu CJ, Chen WC, Lee HS, Chang HM,
Lee YC, Chien CT and Chen CF: Green tea extract supplement reduces
D-galactosamine-induced acute liver injury by inhibition of
apoptotic and proinflammatory signaling. J Biomed Sci. 16:352009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu YL, Wei CW, Chen YL, Chen MH and Yiang
GT: Immunotherapy of breast cancer by single delivery with
rAAV2-mediated interleukin-15 expression. Int J Oncol. 36:365–370.
2010.PubMed/NCBI
|
|
42
|
Yiang GT, Harn HJ, Yu YL, Hu SC, Hung YT,
Hsieh CJ, Lin SZ and Wei CW: Immunotherapy: rAAV2 expressing
interleukin-15 inhibits HeLa cell tumor growth in mice. J Biomed
Sci. 16:472009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang CC, Wu CT, Chen LP, Hung KY, Liu SH
and Chiang CK: Autophagy induction promotes aristolochic
acid-I-induced renal injury in vivo and in vitro. Toxicology.
312:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng Y, Yang X, Wang J, Fan J, Kong Q and
Yu X: Aristolochic acid I induced autophagy extenuates cell
apoptosis via ERK 1/2 pathway in renal tubular epithelial cells.
PloS one. 7:e303122012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q
and Wang S: Induction of necrotic cell death by oxidative stress in
retinal pigment epithelial cells. Cell Death Dis. 4:e9652013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Suzuki-Karasaki M, Ochiai T and
Suzuki-Karasaki Y: Crosstalk between mitochondrial ROS and
depolarization in the potentiation of TRAIL-induced apoptosis in
human tumor cells. Int J Oncol. 44:616–628. 2014.
|
|
47
|
Fragiadaki M, Witherden AS, Kaneko T,
Sonnylal S, Pusey CD, Bou-Gharios G and Mason RM: Interstitial
fibrosis is associated with increased COL1A2 transcription in
AA-injured renal tubular epithelial cells in vivo. Matrix Biol.
30:396–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shearer J: Dioxygen and superoxide
stability of metallopeptide based mimics of nickel containing
superoxide dismutase: The influence of amine/amidate vs.
bis-amidate ligation. J Inorg Biochem. 129:145–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Foresman EL and Miller FJ Jr:
Extracellular but not cytosolic superoxide dismutase protects
against oxidant-mediated endothelial dysfunction. Redox Biol.
1:292–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hatem E, Berthonaud V, Dardalhon M,
Lagniel G, Baudouin-Cornu P, Huang ME, Labarre J and Chédin S:
Glutathione is essential to preserve nuclear function and cell
survival under oxidative stress. Free Radic Biol Med. 67:103–114.
2014. View Article : Google Scholar
|
|
51
|
Sullivan-Gunn MJ and Lewandowski PA:
Elevated hydrogen peroxide and decreased catalase and glutathione
peroxidase protection are associated with aging sarcopenia. BMC
Geriatr. 13:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin HH, Chou SA, Yang HY, Hwang YH, Kuo
CH, Kao TW, Lo TC and Chen PC: Association of blood lead and
mercury with estimated GFR in herbalists after the ban of herbs
containing aristolochic acids in Taiwan. Occup Environ Med.
70:545–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma B, Li N and Lin G: Importance of
metabolic activation study to the safe use of Chinese herbal
medicines. Curr Drug Metab. 13:652–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai JN, Tang JL and Wang JD: Observational
studies on evaluating the safety and adverse effects of traditional
Chinese medicine. Evid Based Complement Alternat Med.
2013:6978932013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang HY, Wang JD, Lo TC and Chen PC:
Occupational kidney disease among Chinese herbalists exposed to
herbs containing aristolochic acids. Occup Environ Med. 68:286–290.
2011. View Article : Google Scholar
|
|
56
|
Eaton L: Traditional Chinese practitioner
breaches Medicine Act. BMJ. 340(feb19 1): c10282010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shang MY, Tian M, Tanaka H, Li XW, Cai SQ
and Shoyama Y: Quality control of traditional chinese medicine by
monoclonal antibody method. Curr Drug Discov Technol. 8:60–65.
2011. View Article : Google Scholar
|
|
58
|
Tian M, Tanaka H, Shang MY, Karashima S,
Chao Z, Wang X, Cai SQ and Shoyama Y: Production, characterization
of a monoclonal antibody against aristolochic acid-II and
development of its assay system. Am J Chin Med. 36:425–436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hamano Y, Aoki T, Shirai R, Hatano M,
Kimura R, Ogawa M, Yokosuka O and Ueda S: Low-dose darbepoetin
alpha attenuates progression of a mouse model of aristolochic acid
nephropathy through early tubular protection. Nephron Exp Nephrol.
114:e69–e81. 2010. View Article : Google Scholar
|
|
60
|
Hsing CH, Chou W, Wang JJ, Chen HW and Yeh
CH: Propofol increases bone morphogenetic protein-7 and decreases
oxidative stress in sepsis-induced acute kidney injury. Nephrol
Dial Transplant. 26:1162–1172. 2011. View Article : Google Scholar
|
|
61
|
Parissis JT, Kourea K, Andreadou I,
Ikonomidis I, Markantonis S, Ioannidis K, Paraskevaidis I,
Iliodromitis E, Filippatos G and Kremastinos DT: Effects of
Darbepoetin Alfa on plasma mediators of oxidative and nitrosative
stress in anemic patients with chronic heart failure secondary to
ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol.
103:1134–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|