Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality worldwide. Since China
has a high incidence of hepatitis virus infection, the morbidity of
HCC is significantly higher than the world average. Each year China
has ~110,000 cases of mortality due to HCC (1–3).
Numerous factors contribute to the occurrence and development of
HCC, among which tumor immune tolerance, is of vital importance.
Regulatory T cells (Tregs) are the dominant cells inducing tumor
immune tolerance and thus have a significant role in the
development of HCC (4–6). HCC, with a high degree of malignancy,
is often in an advanced stage when it is initially diagnosed. The
majority of patients have no opportunity for the tumor to be
resected, and therefore, transcatheter arterial chemoembolization
(TACE) is the preferred method of treatment (7). Although this method has been widely
used in clinical treatment, whether it effects the expression of
Tregs in patients with HCC remains to be elucidated. The present
study investigated, from the perspective of tumor immunity, the
impact of TACE on Tregs and the cellular immune function of
patients with HCC prior to and following TACE.
Materials and methods
Patient characteristics
A total of 47 patients (40 males and 7 females;
average age, 51.7±12.3 years old) from West China Hospital
(Chengdu, China) and Affiliated Hospital of North Sichuan Medical
College (Nanchong, China) were collected between June 2008 and
September 2011, which were diagnosed as HCC, according to clinical
symptoms, imaging (CT or MRI) and AFP (an oncofetal protein of ~72
kDa, which is produced by normal gastrointestinal cells, yolk sac
cells and fetal hepatocytes immediately following birth), without
anticarcinogenic drug therapy, immunostimulants or
immunosuppressive agent therapy (8). Written, informed consent was obtained
from the patients and the study was approved by the Ethics
Committees of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China) and the West Hospital of Sichuan
University, (Chengdu, China).
Reagents and equipment
Mouse anti-human CD4 antibody (cat. no. EB11004873)
labeled with fluorescein isothiocyanate (FITC), mouse anti-human
CD25 antibody (cat. no. EB12025973) labeled with phycoerythrin
(PE), mouse anti-human CD8 antibody (cat. no. EB12043197) labeled
with PE-CY and the isotypic control antibody of mouse anti human
immunoglobulin G1 labeled with FITC, PE and PE-CY5 were purchased
from eBioscience, Inc. (San Diego, CA, USA). A flow cytometer
(P07900102; BD Biosciences, Franklin. Lakes, NJ, USA) and an ELISA
kit (America ADA Company, USA) were also used in the present
study.
TACE
A total of 3 ml venous blood was obtained from
patients with HCC and healthy individuals with an EDTA-K2
anticoagulants mining vessel (BD Bioscience) 1 day prior to TACE
and 1 month following TACE. For TACE, the Seldinger technique was
used percutaneously via the right femoral artery for superselective
catheter insertion into the right or left hepatic artery, which was
determined by the location of the tumor (9). Subsequently, injection of lipiodol
(Di Xin Chemical Co., Ltd., Wuhan, China) and chemotherapeutic drug
suspension was administered through the transcatheter, which
included lipiodol epirubicin (3–12 ml; Di Xin Chemical Co., Ltd.),
epirubicin 30–50 mg and 5-FU (Yezhou Sheng Technology Co., Ltd.,
Shanghai, China; 1.0 g). The dose of lipiodol and the
chemotherapeutic drug were determined according to the size of the
tumor and liver function condition. If the tumor diameter was >5
cm the lipiodol dose was 12 ml and the epirubicin dose was 40 mg.
If the tumor diameter was <5 cm the lipiodol dose was 6 ml and
the epirubicin dose was 40 mg. Gelatin sponge particles were used
to embolize the hepatic artery to reduce the rate of blood
flow.
Flow cytometry
The-cell surface expression levels of CD4, CD8 and
CD25 were evaluated using flow cytometry, followed by incubation
with FITC-conjugated anti-CD4 antibody, PE-conjugated anti-CD25
antibody and PE-CY5-conjugated anti-CD8 antibody at room
temperature. FITC-conjugated mouse IgG, PE-conjugated mouse IgG or
PE-CY5-conjugated mouse IgG (Becton Dickinson) were respectively
incubated with the cells as a control. After 10 min, red cells were
removed using lysis buffer, and were washed once with PBS. The
cells were resuspended in 0.5 ml PBS. Analysis was performed on the
results obtained from at least 10,000 cells, which were acquired on
a FACSCalibur (BD Bioscience). An ELISA assay was used to determine
the content of interleukin (IL)-35 in the peripheral blood,
according to the manufacturer's instructions.
Statistical analysis
The data are expressed as the mean ± standard
deviation and analyzed using the SPSS 16.0 software package (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Alteration of T cell subsets in the
peripheral blood of patients with HCC
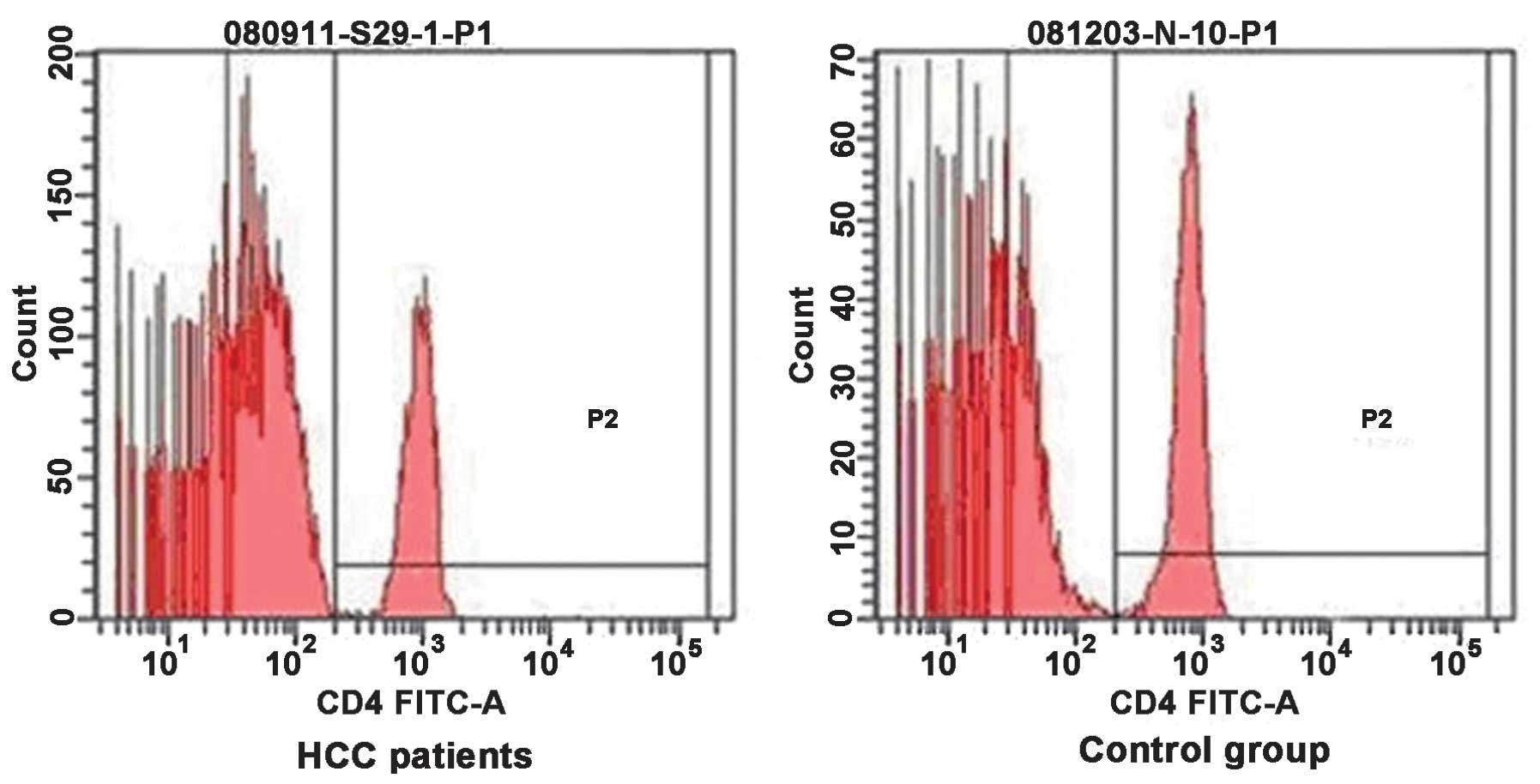
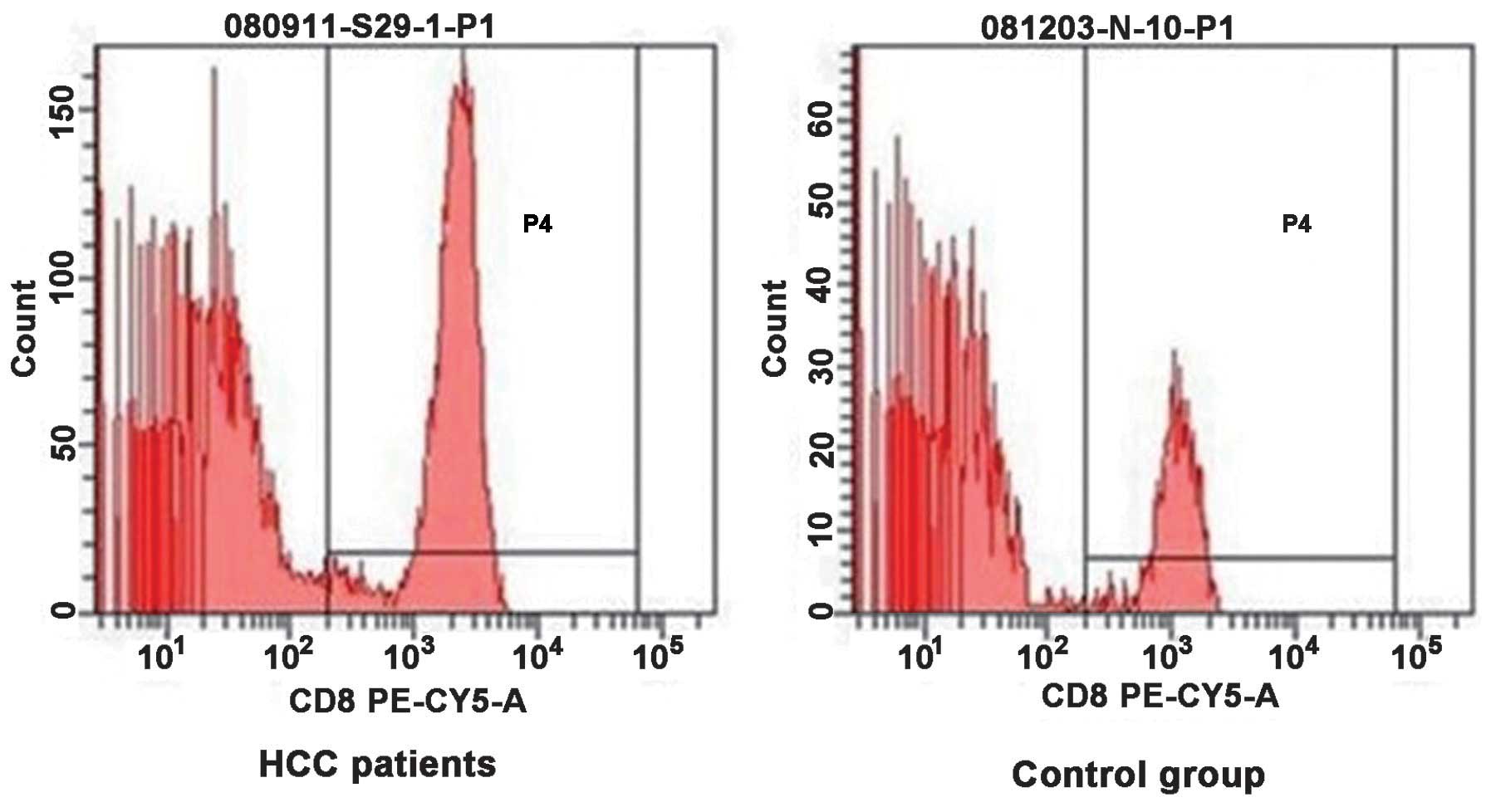
Flow cytometry of CD4+ and
CD8+ T cells in the peripheral blood of patients with
HCC and normal control individuals were compared in Figs. 1 and 2, respectively. The proportion of
CD4+ T cells in the PBMC of patients with HCC was lower
compared with that of the normal control group (26.71±5.57, vs.
34.74±2.86%; P<0.05), while the percentage of CD8+ T
cells was higher in the patients with HCC compared with the normal
control group (25.99±4.61, vs. 20.73±1.33%; P<0.05). The ratio
of CD4+ T cells to CD8+ T cells was lower
compared with the control group (1.03±0.14, vs. 1.68±0.16;
P<0.05). As shown in Table I,
the patients with HCC exhibited lower cellular immune function
compared with the healthy individuals.
 | Table IComparison of T cell subsets in
peripheral blood between patients with hepatocellular carcinoma and
controls (mean ± standard deviation). |
Table I
Comparison of T cell subsets in
peripheral blood between patients with hepatocellular carcinoma and
controls (mean ± standard deviation).
| Group | HCC patients
(n=47) | Healthy adults
(n=15) | t | P-value |
|---|
| CD4+
T/PBMC (%) | 26.71±5.57 | 34.74±2.86 | −5.343 | 0.000 |
| CD8+
T/PBMC (%) | 25.99±4.61 | 20.73±1.33 | 4.344 | 0.000 |
| CD4+
T/CD8+ T | 1.03±0.14 | 1.68±0.16 | −15.372 | 0.000 |
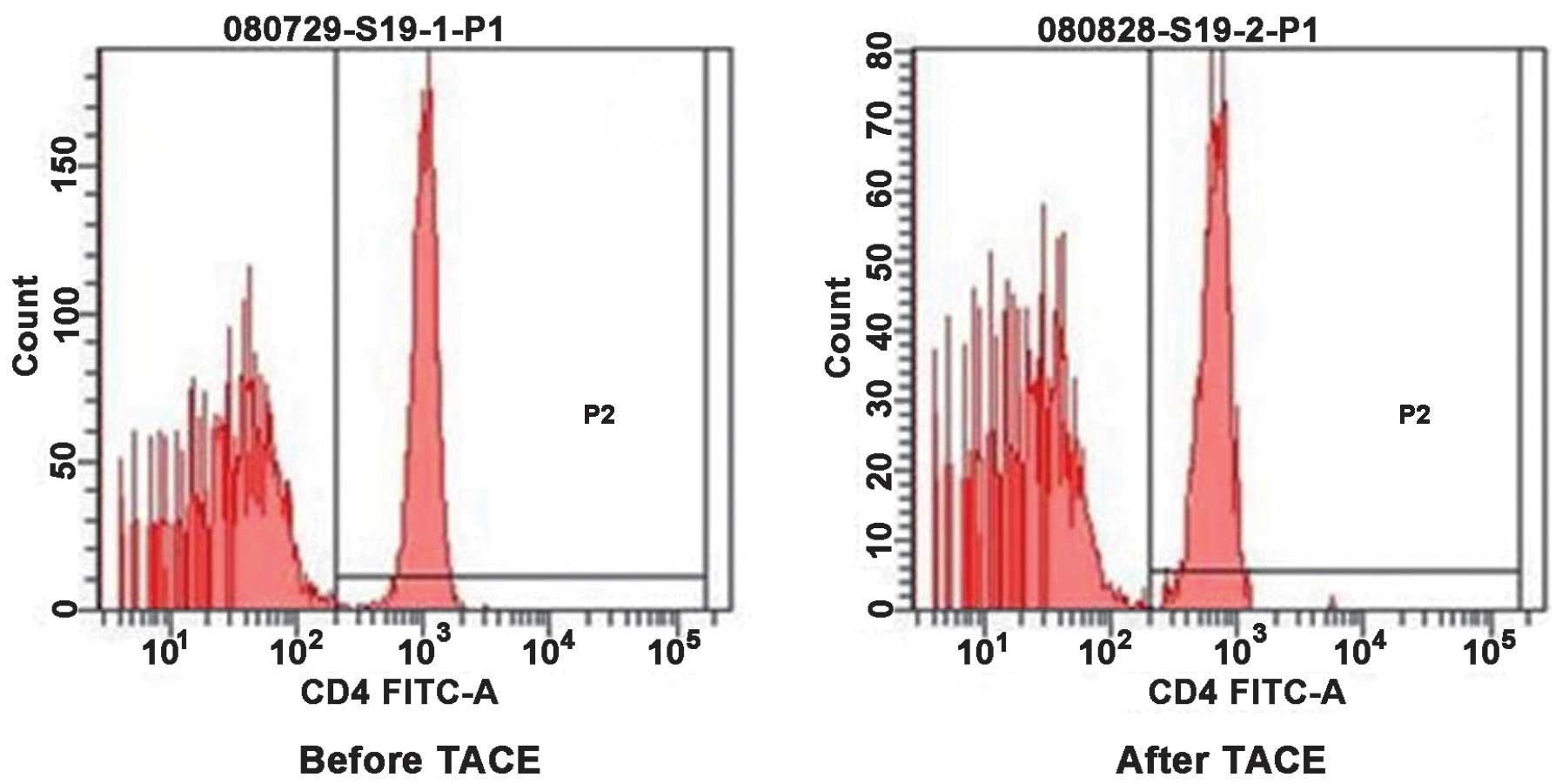
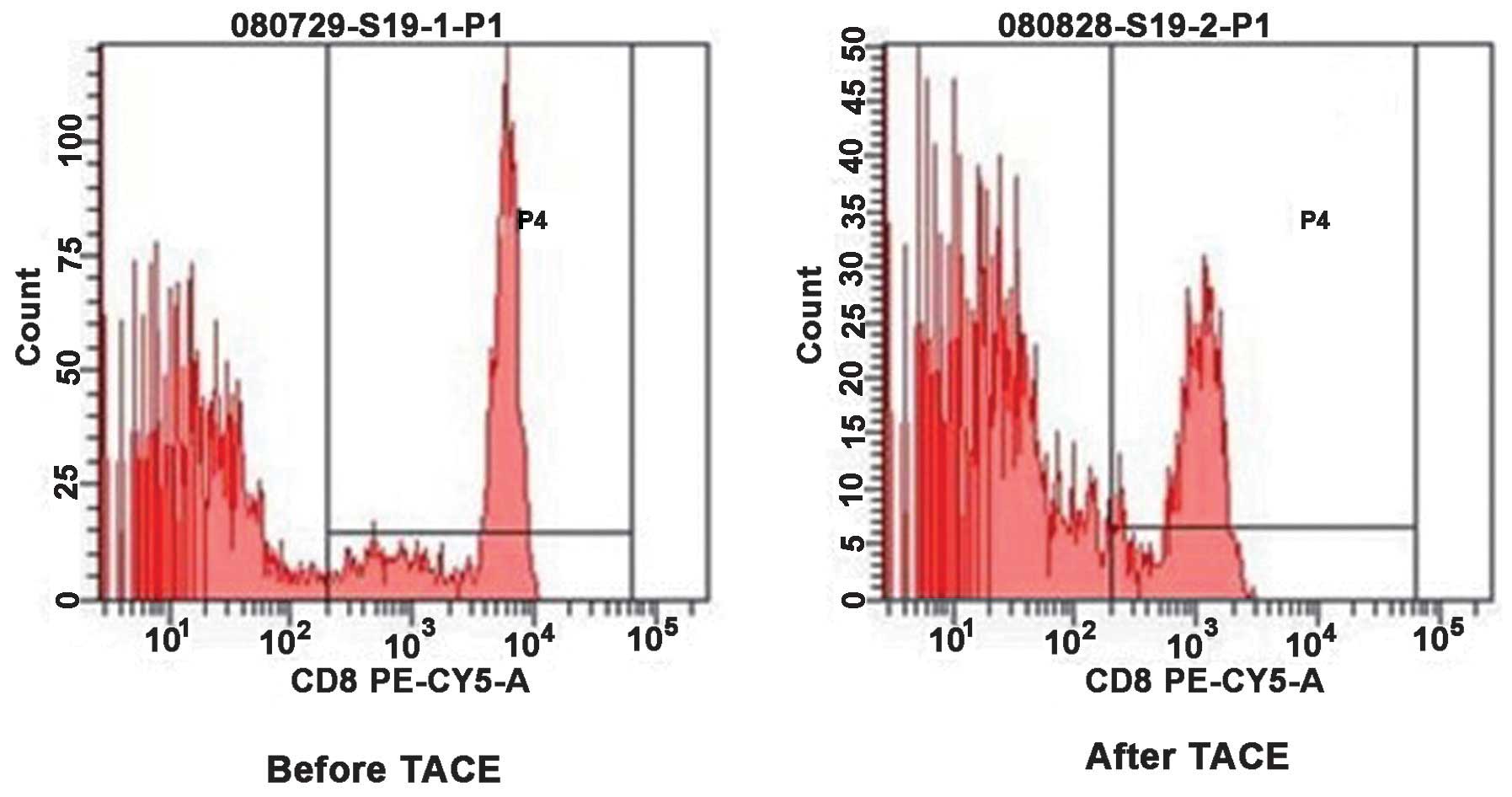
Change of T cell subsets in the
peripheral blood of patients with HCC prior to and following
TACE
As shown in Figs. 3
and 4, the present study assessed
the CD4+ T cells and CD8+ T cells in the
peripheral blood of patients with HCC prior to and following TACE
by flow cytometric analysis. The comparison between prior to and
following TACE is demonstrated in Table II. The proportion of
CD4+ T cells in the PBMCs of patients with HCC prior to
and following TACE was 26.71±5.57, vs. 30.52±4.19% (P<0.05) and
that of CD8+ T cells was 25.99±4.61, vs. 23.91±3.50%
(P<0.05). The ratio of CD4+/CD8+ T cells
was 1.03±0.14, vs. 1.29±0.14 (P<0.05). These results
demonstrated that the cellular immune function was partially
restored in patients with HCC following TACE treatment.
 | Table IIComparison of T cell subsets in the
peripheral blood of patients with hepatocellular carcinoma prior to
and following TACE (mean ± standard deviation). |
Table II
Comparison of T cell subsets in the
peripheral blood of patients with hepatocellular carcinoma prior to
and following TACE (mean ± standard deviation).
| Group | Prior to TACE
(n=47) | Following TACE
(n=43) | t | P-value |
|---|
| CD4+
T/PBMC (%) | 26.71±5.57 | 30.52±4.19 | −3.642 | 0.008 |
| CD8+
T/PBMC (%) | 25.99±4.61 | 23.91±3.50 | 2.397 | 0.019 |
| CD4+
T/CD8+ T | 1.03±0.14 | 1.29±0.14 | −8.805 | 0.000 |
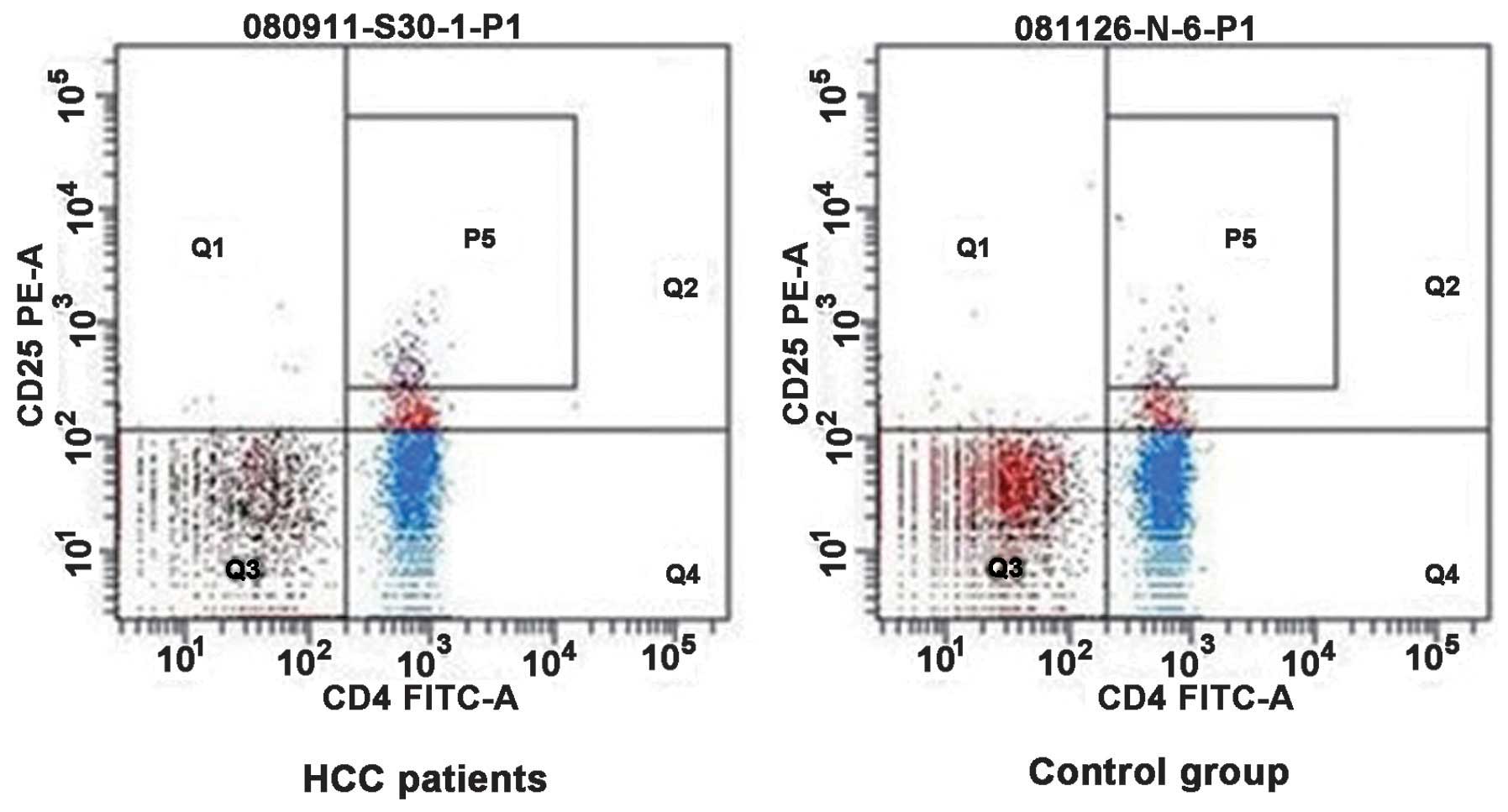
Treg proportion in the peripheral blood
of patients with HCC
CD4+ CD25+ Treg cells were
detected in the peripheral blood of patients with HCC and healthy
individuals by flow cytometric analysis. These results are
presented in Fig. 5. The detection
results of the two groups were analyzed in Table III. The percentage of
CD4+ CD25+ Treg cells in CD4+ T
cells isolated from the peripheral blood of patients with HCC was
higher compared with the normal control group (11.12±3.58, vs.
4.98±1.45%; P<0.05). The percentage of CD4+ CD25 high
T cells in CD4+ T cells (3.34±0.79, vs. 1.32±0.23%;
P<0.05) was markedly higher in patients with HCC. The present
study suggested that the tumor microenvironment may promote
proliferation of Treg.
 | Table IIIComparison of the percentage of
regulatory T cells in the peripheral blood of patients with HCC
compared with the control group (mean ± standard deviation). |
Table III
Comparison of the percentage of
regulatory T cells in the peripheral blood of patients with HCC
compared with the control group (mean ± standard deviation).
| Group | HCC patients
(n=47) | Healthy adult
(n=15) | t | P-value |
|---|
|
CD4+CD25+
T/CD4+ T (%) | 11.12±3.58 | 4.98±1.45 | 9.674 | 0.000 |
|
CD4+CD25highT/CD4+
T (%) | 3.34±0.79 | 1.32±0.23 | 6.452 | 0.000 |
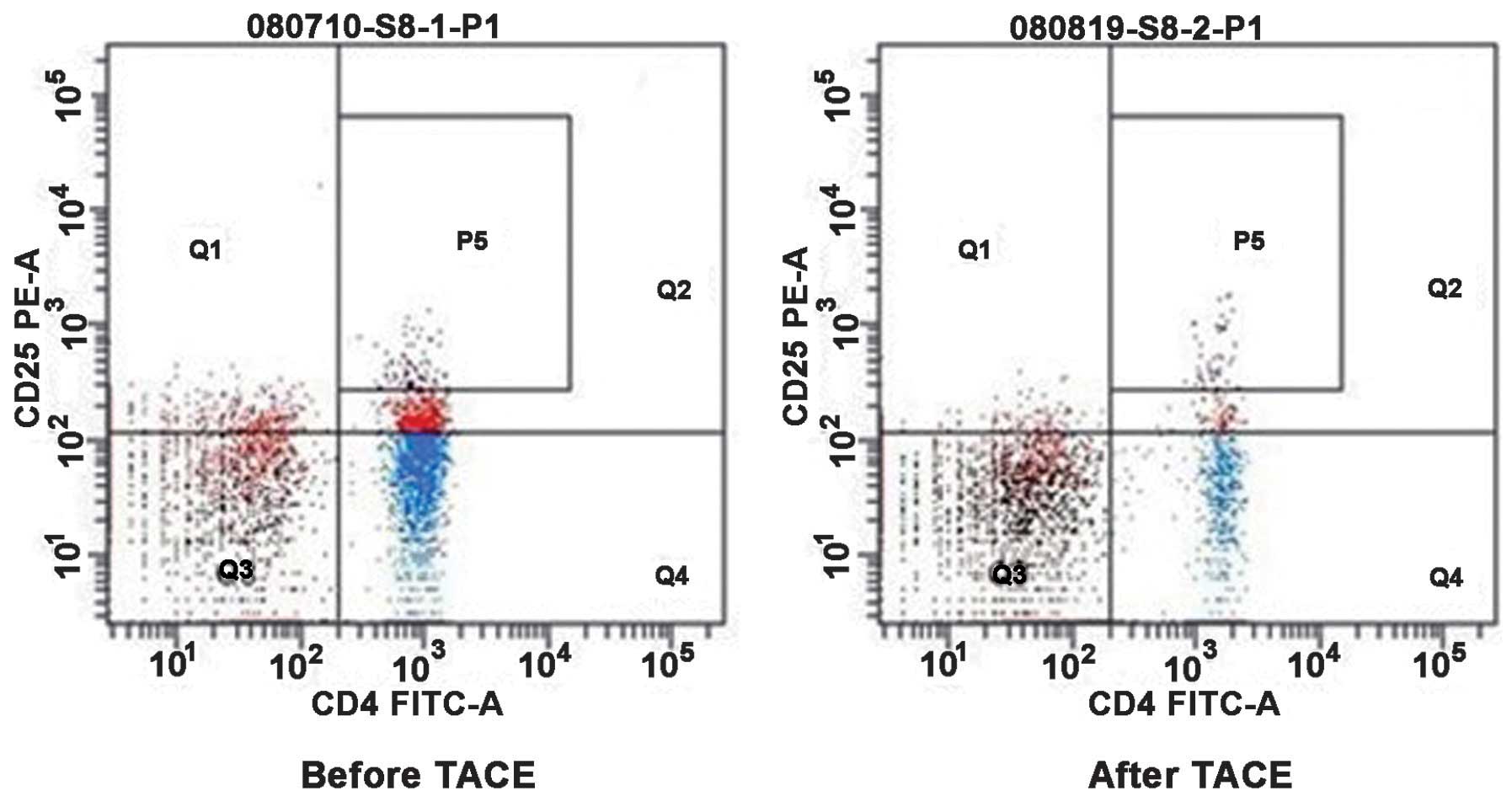
Influence of Tregs in the peripheral
blood of patients with HCC prior to and following TACE
Flow cytometry was performed to detect the
CD4+ CD25+ Treg cells, which were isolated in
the peripheral blood of patients with HCC prior to and following
TACE (Fig. 6). The percentage of
CD4+CD25+ Treg cells in CD4+ T
cells and CD4+CD25high T cells in the
peripheral blood of patients with HCC following TACE was reduced,
compared with prior to TACE (Table
IV). The percentage of CD4+ CD25+ T cells
decreased from 11.12±3.58% prior to TACE to 7.58±2.65% following
TACE (P<0.05), and the percentage of CD4+ CD25 high T
cells was reduced from 3.34±0.79% prior to TACE to 2.11±0.67%
following TACE (P<0.05). These results demonstrated that the
proportion of Treg cells in the patients with HCC following TACE
treatment decreased and its immunosuppressive function was
reduced.
 | Table IVComparison of the percentage of
regulatory T cells in the peripheral blood of patients with
hepatocellular carcinoma prior to and following TACE (mean ±
standard deviation). |
Table IV
Comparison of the percentage of
regulatory T cells in the peripheral blood of patients with
hepatocellular carcinoma prior to and following TACE (mean ±
standard deviation).
| Group | Prior to TACE
(n=47) | Following TACE
(n=43) | t | P-value |
|---|
| CD4+
CD25+T/CD4+ T (%) | 11.12±3.58 | 7.58±2.65 | 7.883 | 0.000 |
| CD4+
CD25highT/CD4+ T (%) | 3.34±0.79 | 2.11±0.67 | 5.302 | 0.000 |
IL-35 in the peripheral blood of patients
with HCC
The quantity of IL-35 in the peripheral blood of
patients with HCC prior to and following TACE, and the healthy
individuals was detected by an ELISA. The quantities were
369.66±95.53, 352.28±107.50 and 316.24±89.21 pg/ml, respectively.
The analysis revealed no statistically significant difference
between the groups (P>0.05).
Change of AFP in the peripheral blood of
patients with HCC prior to and following TACE
Among the 43 patients diagnosed with HCC who were
followed-up during the entire investigation, 32 patients (76.2% of
total) were positive for AFP (AFP >20 ng/l). Following TACE (1
month), the quantity of AFP was decreased to 827±981 ng/l, which
was markedly lower than prior to TACE (1,647±1,649 ng/l;
P<0.05). These results indicated that TACE is effective for the
treatment of patients with HCC.
Correlation of index
The change in the percentage of Treg cells in
CD4+ T cells and the quantity of IL-35 demonstrated a
positive correlation with the change in AFP (P<0.05), with
correlation coefficients of 0.401 and 0.227, respectively. However,
the number of Tregs and the concentration of IL-35 revealed no
significant correlation (P>0.05).
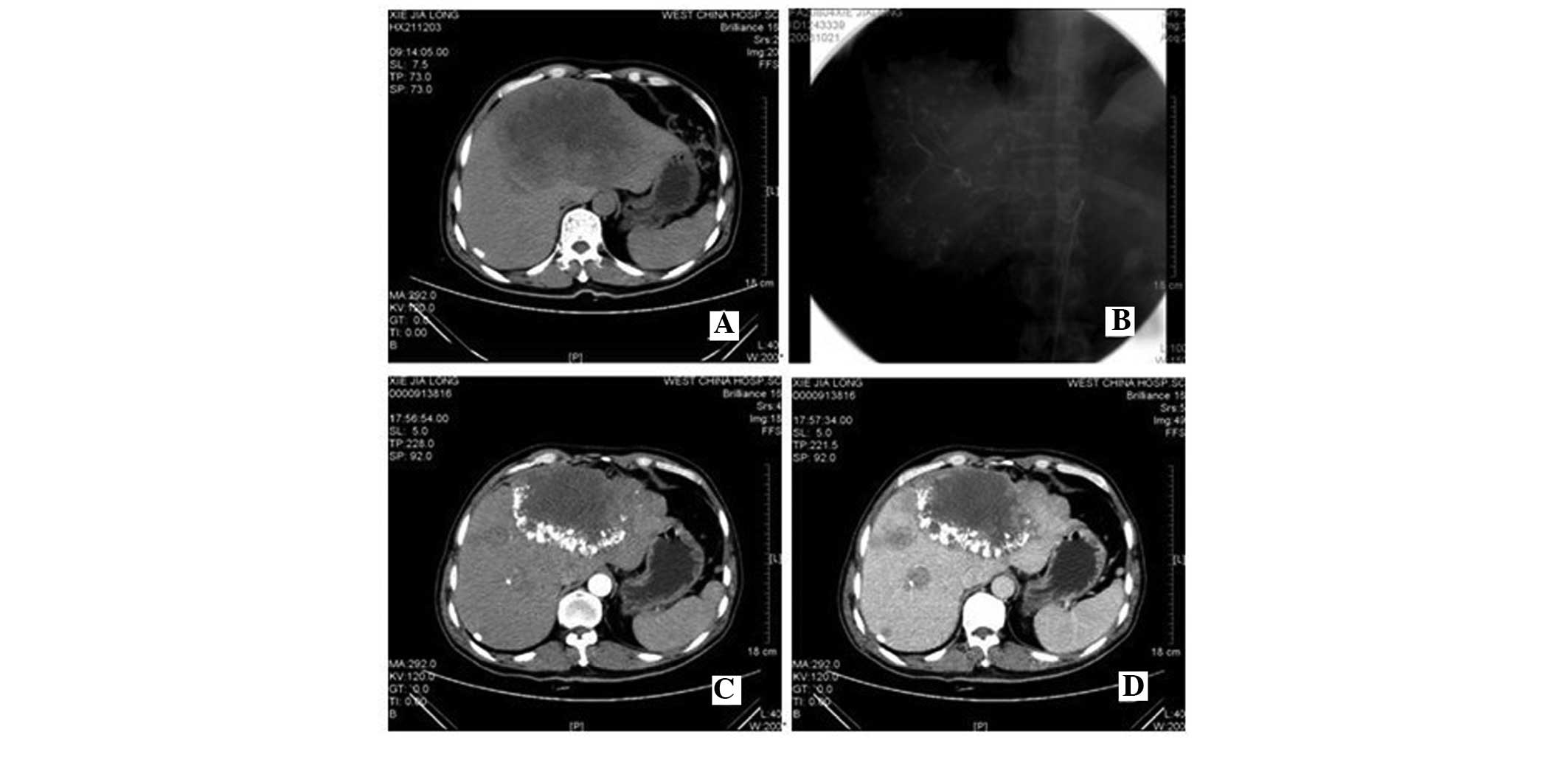
CT images of patients with HCC prior to
and following TACE
The CT images of patients with HCC following TACE (1
month) were assessed and they displayed clear Lipiodol deposition
in the tumor region (Fig. 7). A
total of 30 patients with HCC underwent CT examination prior to and
following TACE and follow-up results from those CT graphs were
obtained. The tumors were significantly reduced compared with that
prior to TACE in 17/30 (56.67%) patients. The tumor in 10/30
(33.33%) patients remained unchanged prior to and following TACE.
The tumor in 3/30 (10%) patients were larger than prior to
TACE.
Discussion
HCC has a 5-year survival rate of <5% and is the
fifth most common type of cancer worldwide (10). There are at least 1 million novel
cases per year (10). When HCC
occurs, there are two antitumor mechanisms, cellular immunity and
humoral immunity. The former exhibits a dominant role in the
antitumor immune response. T lymphocytes, particularly, are key in
immune regulation and immune surveillance during this whole
process. T lymphocytes are divided into two major subsets,
CD4+ and CD8+, and the constant ratio of
CD4+/CD8+ cells maintains the balance of the
cellular immune response. If the ratio is significantly lower, the
host immune function is weakened and this weakened antitumor effect
is conducive to tumorigenesis and development (11). Several previous studies have
substantiated that the immune function of patients with cancer are
suppressed, which is predominantly displayed as the decrease of
CD4+, ratio of CD4+/CD8+ and
natural killer (NK) cells; however, the changes in CD8+
occur at different degrees. The present study demonstrated that the
proportion of CD4+ cells and the ratio of
CD4+/CD8+ was markedly decreased, while the
proportion of CD8+ was significantly increased in the
PBMCs of the patients with HCC. These results suggested that the
cellular immune function of the patients with HCC was weakened or
inhibited.
It is important to understand how the quality and
magnitude of the adaptive immune response to nonself-antigens is
controlled, so as to avoid damage to the host. Tregs have an
indispensable role in maintaining immunological unresponsiveness to
self-antigens and in suppressing excessive immune responses
deleterious to the host (12).
Tregs, as major subsets of the T lymphocyte lineage in the healthy
adult, is not only associated with tumor development, but is also
important for the treatment and prognosis of the tumor (13). Immunological self tolerance is
maintained, at least in part, by Treg cells, which actively and
dominantly control potentially hazardous self-reactive T cells in
the periphery (14). Since the
majority of tumor antigens are self-antigens, the host usually
maintains immunological unresponsiveness to the early primary
tumor. There is a certain association between the formation of this
tumor immune tolerance and Tregs. Once Tregs are activated they can
suppress CD4+ and CD8+ T cells in a
non-antigen-specific manner, thereby inhibiting these effector
lymphocyte antitumor responses. Previous studies have demonstrated
that the proportion of Tregs in the peripheral blood of patients
with cancer is higher compared with that in healthy individuals.
Tregs can inhibit the proliferation, differentiation and function
of B cells, T helper cells and cytotoxic T cells by the secretion
of inhibitory cytokines, including IL-4, IL-10 and transforming
growth factor (TGF)-β, and can subsequently directly suppress the
proliferation and activity of CD4+ and CD8+ T
lymphocytes, NK cells and other immune cells, which performs an
immunosuppressive effect, leading to tumor immune tolerance
(15–17). Other previous studies revealed that
the quantity of Treg cells is increased in the peripheral blood and
tumor tissue of patients with HCC (18,19).
Among 35 peripheral blood specimens of patients with HCC, the
results confirmed that the proportion of
CD4+CD25+ T cells and
CD4+CD25high T cells in the total
CD4+ T cells was significantly higher compared with that
of healthy adults. The number of Treg cells in the tumor
microenvironment may be correlated with clinical
tumor-node-metastasis stage in HCC (20), which suggested that there may be an
immune mediation network in the tumor microenvironment and that
Tregs are important in this network. At the early stages of tumor
development, Treg cells assist tumor cell escape of the
surveillance of immune cells and enable them to proliferate.
Additionally, factors, including APC (macrophage cells) (21) and myeloid suppressor cells
(CD14+-HLA-DR−/low) (22) in tumor tissue, as well as TGF-β1
(23) and SDF-1 (24), can also stimulate and increase the
concentration of Treg cells in the tumor microenvironment. This
increased level of Treg cells continues to assist tumor cells in
escaping immune attack, accelerate growth and deteriorate, by
inhibiting NK cells and CD8+ cytotoxic T cells (15,24,25).
Tregs and HCC reinforce each other to create a vicious cycle. If
the tumor could be destroyed or excised, this cycle would be
inhibited. Currently, there are several therapeutic methods,
including surgical resection, liver transplantation, radiofrequency
ablation, TACE and systemic chemotherapy, which prevent HCC
metastasis and recurrence. Immunotherapy is an important strategy
for the treatment of HCC, which may maximize the killing of tumor
cells without harming normal cells.
The crucial problem with HCC immunotherapy being
implemented is the low efficiency of the immune response induced.
It is precisely a phenomenon, in which the Treg cells are a major
factor for inhibiting the specific tumor immune response in
patients with HCC patients (26,27).
Therefore, the induction of Treg cells may be reduced by destroying
the tumor microenvironment to increase the efficacy of antitumor
immune and HCC immunotherapy.
TACE is the preferred method of treatment for
unresectable advanced HCC. Although TACE has been widely used
clinically, it remains unclear if it may improve the immune status
of patients with HCC and affect the quantity and function of Tregs.
The present study demonstrated that the tumor volume of patients
with HCC was substantially reduced following TACE treatment. In
addition, the number of Treg cells in the peripheral blood
decreased significantly, the proportion of CD4+ T cells
and the ratio of CD4+/CD8+ T cells increased
by varying degrees. The Treg cells exhibited a positive correlation
with the change of AFP prior to and following TACE. These results
indicated that TACE improved the patients immune status and
weakened the immune tolerance to increase the antitumor effects by
reducing tumor burden and decreasing Tregs in the peripheral
blood.
Previous studies demonstrated that Treg cells in
patients with HCC performed tumor immune evasion by inhibiting NK
cells and CD8+ cytotoxic T cells (15,24,25).
Treg cells in the tumor tissue can suppress the immunologic
effector T cells, including T cells, NK cells and dendritic cells,
by a variety of mechanisms. Collison et al (28) revealed IL-35 as a novel
immunosuppressive cytokine, which is important in the process of
native Treg immunosuppression. Whether the Treg cells in patients
with HCC perform their suppression via IL-35 remains to be
elucidated. The results from the present study demonstrated that
the level of IL-35 in the peripheral blood is not significantly
different between patients with HCC and healthy adults. The level
of IL-35 revealed no significant decrease prior to and following
TACE. The change in Tregs and the level of IL-35 revealed no
significant correlation. Therefore, the present study cannot infer
that the Treg cells in patients with HCC perform immune suppression
via IL-35 in vivo.
Acknowledgments
This study was financially supported by a grant from
the National Natural Science Foundation of China (no's 30700773;
81070378 and 81270561).
References
|
1
|
El-Serag HB and Rudolph KL: Hepato
cellular carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kew MC: Epidemiology of chronic hepatitis
B virus infection, hepatocellular carcinoma, and hepatitis B
virus-induced hepatocellular carcinoma. Pathol Biol (Paris).
58:273–277. 2010. View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pedroza-Gonzalez A, Verhoef C, Ijzer mans
JN, Peppelenbosch MF, Kwekkeboom J, Verheij J, Janssen HL and
Sprengers D: Activated tumor-infiltrating CD4+
regulatory T cells restrain antitumor immunityin patients with
primary or metastatic liver cancer. Hepatology. 57:183–194. 2013.
View Article : Google Scholar
|
|
5
|
Yang ZQ, Yang ZY, Zhang LD, Ping-Bie, Wang
SG, Ma KS, Li XW and Dong JH: Increased liver-infiltrating
CD8+ Foxp3+ regulatory T cells are associated
with tumor stage in hepato-cellular carcinoma patients. Hum
Immunol. 71:1180–1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yi Y and He HW: The functional impairment
of HCC-infiltrating γδ T cells, partially mediated by regulatory T
cells in a TGFβ-and IL-10-dependent manner. J Hepatol. 58:977–983.
2013. View Article : Google Scholar
|
|
7
|
Han KH and Kudo M: Asian consensus
workshop report: Expert consensus guideline for the management of
intermediate and advanced hepatocellular carcinoma in Asia.
Oncology. 81(Suppl 1): 158–164. 2011. View Article : Google Scholar
|
|
8
|
Smith JA, Francis TI, Edington GM and
Williams AO: Immunofluorescent localisation of human alpha
feto-protein in fetal and neonatal livers and cultured cells from
hepatocellular carcinoma. Br J Cancer. 25:343–349. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heger N, Bayindir S, Steckenmesser R and
Schirmer H: Percutaneous catheter-arteriographies: Seldinger
technique. Minn Med. 53:1093–1097. 1970.PubMed/NCBI
|
|
10
|
Motola-Kuba D, Zamora-Valdés D, Uribe M
and Méndez-Sánchez N: Hepatocellular carcinoma. An overview. Ann
Hepatol. 5:16–24. 2006.PubMed/NCBI
|
|
11
|
Horiguchi S, Petersson M, Nakazawa T,
Kanda M, Zea AH, Ochoa AC and Kiessling R: Primary chemically
induced tumors induce profound immunosuppression concomitant with
apoptosis and alterations in signal transduction in T cells and NK
cells. Cancer Res. 59:2950–2956. 1999.PubMed/NCBI
|
|
12
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke X, Wang J, Li L, Chen IH, Wang H and
Yang XF: Roles of CD4+ CD25 (high) FOXP3+
Tregs in lymphomas and tumors are complex. Front Biosci.
13:3986–4001. 2008.PubMed/NCBI
|
|
14
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on self tolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View
Article : Google Scholar
|
|
15
|
Jiang S and Lechler RI: CD4+
CD25+ regulatory T-cell therapy for allergy, autoimmune
disease and transplant rejection. Inflamm Allergy Drug Targets.
5:239–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu J, Xu D, Liu Z, Shi M, et al: Increased
regulatory T cells correlate with CD8 T-cell impairment and poor
survival in hepatocellular carcinoma patients. Gastroenterology.
132:2328–2339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghiringhelli F, Ménard C, Terme M, et al:
CD4+ CD25+ regulatory T cells inhibit natural
killer cell functions in a transforming growth
factor-beta-dependent manner. J Exp Med. 202:1075–1085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pandiyan P, Zheng L, Ishihara S, Reed J
and Lenardo MJ: CD4+ CD25+Foxp3+
regulatory T cells induce cytokine deprivation-mediated apoptosis
of effector CD4+ T cells. Nat Immunol. 8:1353–1362.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ormandy LA, Hillemann T, Wedemeyer H,
Manns MP, Greten TF and Korangy F: Increased populations of
regulatory T cells in peripheral blood of patients with
hepatocellular carcinoma. Cancer Res. 65:2457–2464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XH, Yamagiwa S, Ichida T, et al:
Increase of CD4+ CD25+ regulatory T-cells in
the liver of patients with hepatocellular carcinoma. J Hepatol.
45:254–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen X, Li N, Li H, Zhang T, Wang F and Li
Q: Increased prevalence of regulatory T cells in the tumor
microenvironment and its correlation with TNM stage of
hepatocellular carcinoma. J Cancer Res Clin Oncol. 136:1745–1754.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Ding T, Pan W, Zhu LY, Li L and
Zheng L: Increased intratumoral regulatory T cells are related to
intratumoral macrophages and poor prognosis in hepatocellular
carcinoma patients. Int J Cancer. 125:1640–1648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoechst B, Ormandy LA, Ballmaier M, et al:
A new population of myeloid-derived suppressor cells in
hepatocellular carcinoma patients induces CD4(+) CD25(+) Foxp3(+) T
cells. Gastroenterology. 135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshizawa K, Abe H, Kubo Y, et al:
Expansion of CD4(+)CD25(+) FoxP3(+) regulatory T cells in hepatitis
C virus-related chronic hepatitis, cirrhosis and hepatocellular
carcinoma. Hepatol Res. 40:179–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai L, Zhang Z, Zhou L, et al: Functional
impairment in circulating and intrahepatic NK cells and relative
mechanism in hepatocellular carcinoma patients. Clin Immunol.
129:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Unitt E, Rushbrook SM, Marshall A, et al:
Compromised lymphocytes infiltrate hepatocellular carcinoma: the
role of T-regulatory cells. Hepatology. 41:722–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HH, Mei MH, Fei R, et al: The
frequency, phenotypes and functions of CD4+
CD25+ regulatory T cells in hepatocellular carcinoma
patients. Zhonghua Gan Zang Bing Za Zhi. 15:266–272. 2007.In
Chinese. PubMed/NCBI
|
|
28
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
et al: The inhibitory cytokine IL-35 contributes to regulatory
T-cell function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|