Introduction
Medicinal plants are valuable sources for the
discovery of novel lead compounds and novel chemical entities with
anti-cancer properties. Significant effort has been made to
identify compounds or herbs that kill tumors (1,2). The
search for anti-cancer agents in plant sources started in the 1950s
with the discovery and development of the vinca alkaloids
vinblastine and vincristine, and the isolation of the cytotoxic
podophyllotoxins (3).
Momordica cochinchinensis Spreng., a member
of the Cucurbitaceae family and an underutilized perennial
dioecious vegetable, has been highly valued for its nutritional and
medicinal qualities and wide range of uses. Its fruit and seeds
have been part of an indigenous diet, and the plant has been used
as a traditional medicine throughout East and Southeast Asia for a
long time (4,5). In the various Asian languages, the
fruit is called Gac in Vietnam, Fak kao in Thailand, Bhat kerala in
India, Mu Bie Zi in China and Mak kao in Laos (6). The ripened seeds with a palatable
taste, cooked to impart its red color, are traditionally used in
Vietnamese cuisine in the dish 'Xoi Gac' made for weddings and New
Year celebrations (4). The seeds
of the fruit are used in Traditional Chinese Medicine and have
various pharmacological actions against conditions including boils,
pyodermatitis, mastitis, tuberculous cervical lymphadenitis,
ringworm infections, freckles, sebaceous, hemorrhoids and
hemangiomas; furthermore, they have immune-stimulating,
anti-oxidant, anti-inflammatory and anti-cancer actions (6,7).
To date, the effects of Momordica
cochinchinensis on breast cancer cells have not been
investigated. In the present study, the effect of the ethyl acetate
extract of Momordica cochinchinensis (ESMC2) on the growth
of breast cancer cells was assessed. Furthermore, the underlying
mechanisms of the anti-proliferative action of ESMC2 on breast
cancer cells were assessed.
The modulation of the expression of cell-cycle
regulatory proteins following treatment with anti-proliferative and
apoptosis-inducing compound has been reported by numerous studies
(8–10). Cyclin (CCN)-dependent kinases
(Cdks), evolutionarily conserved proteins, are essential for cell
cycle control, and distinct pairs of cyclins and cdks regulate
progression in various stages of the cell cycle (11).
The ability of ESMC2 to inhibit cell proliferation
and cause cell cycle arrest, which is associated with apoptosis,
was assessed in the present study. Apoptosis, characterized by a
series of molecular signaling processes, is the major form of
programmed cell death and has critical roles in cancer. It can be
triggered by diverse intracellular signals that act upon the B-cell
lymphoma 2 (Bcl-2) protein family, which can be divided into two
groups: Suppressors of apoptosis [including Bcl-2, Bcl-extra large
protein and myeloid cell leukemia-1 (Mcl1)] and activators of
apoptosis [including Bcl-2-associated X protein (Bax), Bcl-2
homologous antagonist killer (Bak) and Bcl-2-associated death
promoter (Bad)] (12). In
addition, cell transformation often involves activation of
pro-survival pathways contributing to uncontrolled cell growth.
Cell survival is mediated by the phosphoinositide 3-kinase-mediated
activation of the anti-apoptotic kinase Akt (13,14).
Three major members of mitogen-activated protein kinases (MAPKs),
P38, c-Jun N-terminal kinase (JNK) and extracellular
signal-regulated kinase (Erk)1/2, have also been shown to regulate
apoptosis (15–17). Akt in turn phosphorylates and
inhibits the pro-apoptotic proteins. Therefore, Akt and Erk1/2 are
key junction points linking together signal transduction involved
in survival and proliferation (18). Suppression of p53 and constitutive
activation of nuclear factor kappa B (NF-κB) are considered as
potential targets for therapeutic intervention aimed at selective
elimination of cancer cells (19–21).
The present study examined the anti-tumor activities
of Momordica cochinchinensis Spreng. seeds in vitro
as well as in vivo. The chemical composition of ESMC2 was
also analyzed by gas chromatography-mass spectrometry (GC/MS). The
results of the present study scientifically validated the efficacy
of the traditional medicinal plant Momordica cochinchinensis
Spreng. and provided a basis for its application in anti-cancer
therapy.
Materials and methods
Materials
Cell culture mediums (L-15), trypsin, MTT, dimethyl
sulfoxide (DMSO), RNase, propidium iodide (PI),
Annexin-V-fluorescein isothiocyanate (FITC), Hoechst 33258 and
rhodamine 123 were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Protease inhibitor cocktail and phosphatase inhibitor
cocktail were purchased from Roche (Basel, Switzerland). Fetal
bovine serum (FBS) was purchased from Lanzhou National Hyclone
Bio-Engineering Co., Ltd. (Lanzhou, China). ERK1/2 rabbit
monoclonal antibody (mAb) [immunoglobulin (Ig)G; cat no. 4695],
phospho ERK1/2 rabbit mAb (IgG; cat no 4370), AKT rabbit mAb (IgG;
cat no. 4691), phospho AKT rabbit mAb (IgG; cat no 4060), phospho
p38 rabbit mAb (IgG; cat no. 4511) were obtained from Proteintech
Group (Chicago, IL, USA). Goat anti-mouse IgG-horseradish
peroxidase (HRP) (cat no. sc-2005) and goat anti-rabbit IgG-HRP
(cat no. sc-2004) were obtained from Santa Cruz Biotechnology
(Dallas, TX, USA). All high-performance liquid chromatography
(HPLC)-grade solvents were obtained from Fisher Thermo Scientific
(Pittsburgh, PA, USA). The HPLC-grade water was purified by a
MK-459 Millipore Milli-Q Plus ultrapure water system (Milford, MA,
USA).
Plant materials
Momordica cochinchinensis Spreng. seeds were
collected in Xi'an (China) in June 2011 and identified by Dr
Xiaofeng Niu, School of Pharmacy, Xi'an Jiaotong University (Xi'an,
China). A voucher specimen (no. 11119) is deposited at the
Institute of Materia Medica, School of Medicine, Xi'an Jiaotong
University, China (Xi'an, China).
Extraction method
The Soxhlet extraction method, one of the oldest
techniques of solid sample preparation for isolation of natural
products, has been the most used extraction technique worldwide for
a number of decades (22,23). The seeds of Momordica
cochinchinensis were ground into fine powder by a grinder
(FW100; Taisite Instrument Co., Ltd., Tianjin, China).
Subsequently, the Soxhlet extraction method using a Soxhlet
extractor (YMST-250S; Shanghai YuMing YiQi Co., Ltd., Shanghai,
China) with a series of solvents with an increasing polarity
gradient, including hexane, ethyl acetate, acetone, ethanol and
water, was applied. Firstly, 50 g Momordica cochinchinensis
powder was decocted in each solvent (500 ml) in the indicated
sequence (from heaxane to water) for 8 h each. The individual
extracts were filtered through a qualitative filter paper with
30–50 µM pore size (Hangzhou Special Paper Industry Co.,
Ltd., Hangzhou, China) in a funnel and collected. Finally, the
extracts were concentrated using a rotary evaporator (RE-52A;
Shanghai YaRong Biochemistry Instrument, Shanghai, China) and named
as ESMC1-5 according the order of extraction, respectively. The
weight of the extracted material of fraction 2 was 0.53 g with an
extraction yield of 1.06%. The dry powder was dissolved with DMSO
at 100 mg/ml and stored at 4°C. The stock solution was further
diluted with serum-free L15 medium immediately before use.
Identification and chemical analysis
using GC/MS
A GC/MS (GCMS-QP2010; Shimadzu, Kyoto, Japan) with a
Shimadzu AOC-20i auto-sampler system, and a Rtx-5 ms column (30 m ×
0.25 mm internal diameter; film thickness, 0.25 µm; Restek,
Bellefonte, PA, USA) were used. The column oven was programmed from
80 to 120°C at a rate of 10°C/min, then to 180°C at 5°C/min, then
to 240°C (5 min) at 10°C/min, and finally to 280°C (5 min) at
5°C/min. The inlet temperature was kept at 280°C. Helium carrier
gas was used at a constant flow rate of 1.18 ml/min. A sample of 1
µl was injected, and the split ratio of the injector was
10:1. MS conditions were as follows: Ionization energy, 70 eV; ion
source temperature, 200°C; and full-scan mode in the range of m/z
50-600 with 0.5 sec/scan velocity. The major components in this
sample were predicted by the National Institutes of Standard and
Technology (NIST) mass spectral library, V.8.0 (Shimadzu, Kyoto,
Japan).
Cell culture and animals
Human breast cancer MDA-MB-231 cells were obtained
from the Shanghai Institute of Cell Biology of the Chinese Academy
of Sciences (Shanghai, China) and were cultured in L-15
supplemented with 10% (v/v) FBS and incubated at 37°C with 5%
CO2. Cells in the logarithmic growth phase of growth
were used for the experiments.
A total of 24 six-week-old female BALB/c-nu/nu nude
mice (body weight, 16–18 g) were purchased from the Shanghai
Institute of Experimental Animals of the Chinese Academy of
Sciences (Shanghai, China). Animals were maintained under specific
pathogen-free conditions at 25°C in 50–70% humidity with a 12-h
light/dark cycle and had access to sterile food and water.
Cell proliferation assay
The effect of ESMC2 on MDA-MB-231 cell proliferation
was evaluated by an MTT assay. Exponentially growing cells were
seeded into 96-well plates at a density of 2×104 cells
per well in medium. After 24 h of incubation at 37°C, cells were
treated with ESMC2 at various concentrations (8, 15, 30, 60, 120 or
250 µg/ml) for 48 h. Subsequently, 20 µl MTT (5
mg/ml) was added to each well and cells were incubated at 37°C for
4 h. After removal of the medium, 150 µl DMSO was added to
each well, and the optical density of the cells was determined with
a microplate reader (model 550; Bio-Rad Laboratories, Hercules, CA,
USA) at 490 nm. The cell viability was expressed in absorbance
units (24).
PI staining assay
Cell death was assessed using a PI staining assay.
MDA-MB-231 cells were trypsinized after treatment with ESMC2 at
various concentrations for 48 h and then cells were collected and
re-suspended in 1 ml phosphate-buffered saline (PBS). Cells were
stained with 0.5 ml staining solution (50 mg/ml PI, 100 mg/ml
RNase, 0.2% Triton X-100) and cells were incubated in 37°C for 30
min in the dark. Cell death was measured by flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) (25).
DNA morphological observation by Hoechst
staining assay
To visualize apoptotic cell death and nuclear
morphology, cells were stained with Hoechst 33258. The number of
apoptotic cells was measured by assessing the percentage of cells
displaying chromatin condensation within the total cell population.
Briefly, MDA-MB-231 cells were seeded in six-well plates (cat no.
3516; Corning-Costar, Corning, NY, USA) containing cover slips and
treated with ESMC2 at various concentrations. After 48 h of
treatment, cells were collected, washed with PBS and allowed to dry
on the slides. Genomic DNA was stained with Hoechst 33258 for 10
min at 37°C according to the manufacturer's instructions.
Approximately 300 nuclei were counted per sample under a
fluorescence microscope (Ti-U; Nikon, Tokyo, Japan) (26).
Analysis of cell apoptosis
MDA-MB-231 cells were treated with ESMC2 at various
concentrations for 48 h, and were then collected, washed and
re-suspended in PBS. The apoptotic cell death rate was examined
with Annexin V-FITC and PI double staining (5 µl Annexin
V-FITC, 10 µl PI for 15 min in the dark) according to the
manufacturer's instructions. The cell suspension was then analyzed
by flow cytometry (27).
Cell cycle analysis
For analysis of the cell cycle, MDA-MB-231 cells
were stained with PI as previously described (28). MDA-MB-231 cells were treated with
ESMC2 at the indicated concentrations. After 48 h of incubation,
cells were trypsinized, collected, washed in PBS, and subsequently
fixed in 70% ethanol. Following incubation with RNase (50
µg/ml) and PI (60 µg/ml) in the dark at room
temperature for 30 min, the cell suspension was analyzed by flow
cytometry (29).
Determination of the mitochondrial
transmembrane potential (ΔΨm)
The changes in the ΔΨm were determined by the
retention of the dye rhodamine 123. For fluorescence microscopic
imaging, MDA-MB-231 cells were seeded in a six-well chamber slide
at a density of 2×105 cells/well. After treatment with
30, 60 and 120 µg/ml ESMC2 for 48 h, cells were washed with
L15 medium twice. The wells were loaded with freshly-prepared
rhodamine 123 solution (1 mM) and further incubated at 37°C for 30
min. Following washing, rhodamine 123 fluorescence was measured by
flow cytometry with excitation and emission wavelengths of 488 and
530 nm (30).
Western blot analysis
MDA-MB-231 cells incubated with ESMC2 were analyzed
by western blot analysis. MDA-MB-231 cells treated with ESMC2 at
various concentrations for 48 h were lysed with lysis buffer
(C1053; Applygen, Beijing, China) containing protease inhibitor
cocktail and phosphate inhibitor cocktail on ice for 30 min. The
lysate was then collected, and centrifuged at 12,000 xg and 4°C for
10 min. The protein lysates were resolved by 10% SDS-PAGE and
separated proteins were transferred onto polyvinylidene difluoride
membranes and blocked with 5% skimmed milk for 2 h. The membranes
were then incubated with specific primary antibodies overnight at
4°C. The primary antibodies included anti-ERK1/2 (1:1,000
dilution), anti-phospho ERK1/2 (1:1,000 dilution), anti-AKT
(1:1,000 dilution), anti-phospho AKT (1:1,000 dilution), anti-p38
(1:1,000 dilution), anti-phospho p38 (1:1,000 dilution), anti-JNK
(1:1,000 dilution), anti-phospho JNK (1:1,000 dilution), anti-p53
(1:1,000 dilution), anti-Cyclin B1 (1:1,000 dilution), anti-Cyclin
D1 (1:1,000 dilution), anti-Cyclin E (1:1,000 dilution), anti-CDC2
(1:500 dilution), anti-Bcl-2 (1:1,000 dilution), anti-Bax (1:1,000
dilution), anti-Bak (1:1,000 dilution), anti-Mcl1 (1:1,000),
anti-Bad (1:1,500 dilution), anti-NF-κB (1:1,000 dilution) and
anti-GAPDH (1:1,000 dilution). Subsequently, membranes were
incubated with the relevant secondary antibodies (goat anti-mouse
IgG-HRP or goat anti-rabbit IgG-HRP) at 1:10,000 dilutions at room
temperature for 2 h in accordance with the manufacturer's
instructions. Finally, the blots were detected by using enhanced
chemiluminescence reagents (Immobilon® Western; Millipore Corp.,
Billerica, MA, USA) (31).
Subcutaneous xenograft model
Animal care was in accordance with institutional
guidelines The animal study was approved by the ethics committee of
Xi'an Jiaotong University, Xi'an, China. Prior to the xenograft
study, an acute toxicity test was performed on mice to determine
the 10% lethal dose (LD10) and LD50 of the
extracts of M. cochinchinensis. The LD10 (50
mg/kg) and 1/2 LD10 (25 mg/kg) were then used to
investigate the anti-tumor activity of M. cochinchinensis.
Solid tumor models were developed from MDA-MB-231 cell lines. A
total of 1×107 cells were suspended in 0.2 ml culture
medium without fetal bovine serum and injected subcutaneously into
the right axilla of the mice. Tumors were measured once every three
days and the tumor volume was calculated from caliper measurements
using the following formula: (short diameter)2 × (long
diameter)/2. When the tumor volume exceeded 100 mm3
(about one week after cell injection), the mice were randomly
divided into three groups: ESMC (25 or 50 mg/kg in normal saline;
n=8 per group), or vehicle control (normal saline; n=8). All these
groups were treated by intraperitoneal injection once per day.
Treatment started on the day of grouping and continued for 14 days.
All mice were sacrificed by cervical dislocation at the end of the
experiment, and the sub-cutaneous tumors were removed and weighed.
Tumor growth inhibition was calculated as: Inhibitory rate (%) =
(Mean final tumor weight of control group - mean final tumor weight
of treatment group) / mean final tumor weight of control group
×100%.
Statistical analysis
All data were obtained from at least three
independent experiments and expressed as the mean ± standard error
of the mean. Comparisons between the different groups were
performed using Student's t-test. P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Identification of chemical components of
ESMC2
The major chemical components of ESMC2 according to
GC/MS analysis are listed in Table
I. Based on the similarity index of the major peaks with
entries of the NIST library, a total of 12 compounds were
identified in the ethyl acetate fraction.
 | Table ICompounds tentatively identified in
the ethyl acetate extract of seeds of Momordica
cochinchinensis (Lour.) Spreng. |
Table I
Compounds tentatively identified in
the ethyl acetate extract of seeds of Momordica
cochinchinensis (Lour.) Spreng.
| Peak number | Retention time
(min) | Percentage of the
peak | Molecular
formula | Similarity
indexa | Compound |
|---|
| 1 | 3.319 | 6.84 |
C6H12O2 | 97 | Hexanoic acid |
| 2 | 5.851 | 1.62 |
C10H18O | 93 | 2-Heptenal |
| 3 | 6.325 | 4.67 |
C9H14O | 97 | 2,4-Nonadienal |
| 4 | 7.391 | 2.33 |
C7H6N4O | 87 |
1,1′-Carbonyldiimidazole |
| 5 | 8.988 | 3.07 |
C11H18O2 | 73 |
1-Oxaspiro[4,4]nonan-4-one |
| 6 | 12.105 | 4.59 |
C8H14O | 84 |
5,5-Dimethyl-cyclohex-3-en-1-ol |
| 7 | 19.529 | 1.82 |
C20H32 | 84 |
(E,E)-7,11,15-Trimethyl-3-methylene-
1,6,10,14-tetraene |
| 8 | 20.121 | 7.47 |
C38H68O8 | 92 | l-(+)-Ascorbic acid
2,6-dihexadecanoate |
| 9 | 22.141 | 23.62 |
C18H34O2 | 91 | 6-Octadecenoic
acid |
| 10 | 22.381 | 37.46 |
C18H36O2 | 95 | Octadecanoic
acid |
| 11 | 27.371 | 3.34 |
C35H68O5 | 83 | Palmitin |
| 12 | 31.460 | 3.17 |
C21H42O4 | 85 | Octadecanoic acid
2,3-dihydroxypropyl ester |
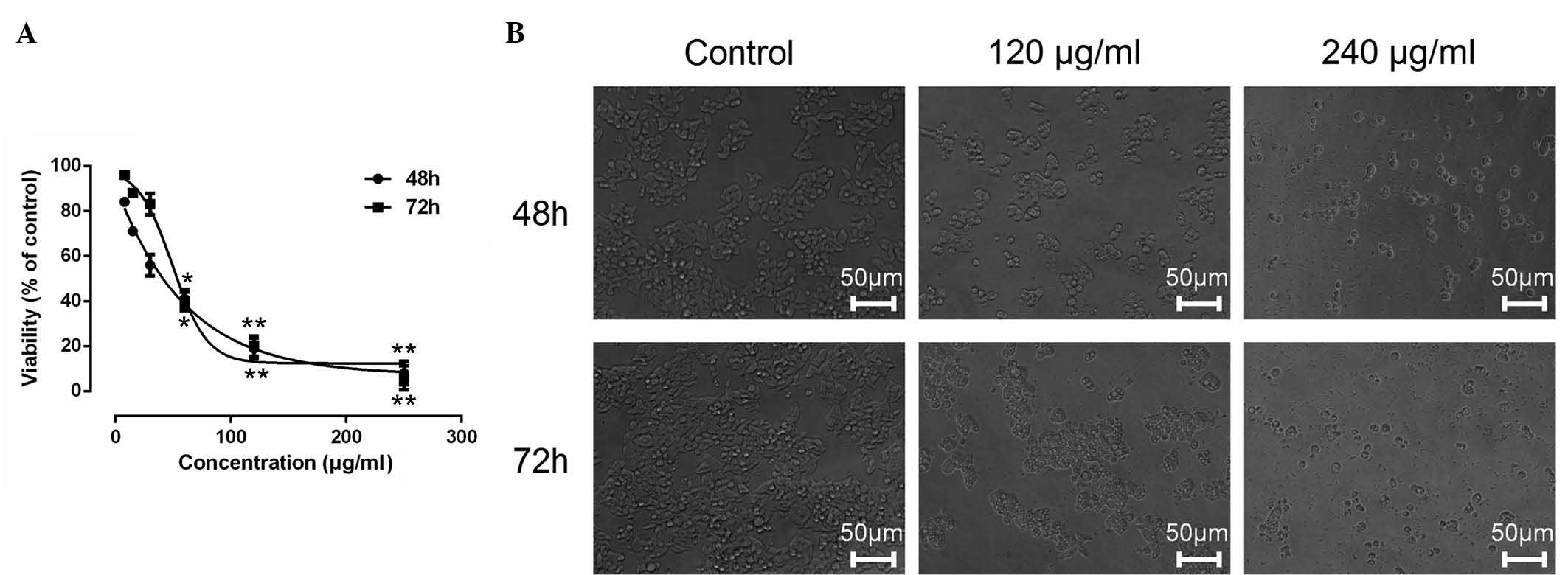
ESMC2 inhibits the growth of tumor
cells
The effects of ESMCs on the growth of tumor cells
were evaluated by MTT assay. ESMC2 showed significant
anti-proliferative effects on human breast cancer MDA-MB-231 cells
in a dose- and time-dependent manner, as shown in Fig. 1A. The 48-h 50% inhibitory
concentration (IC50) of ESMC2 on MDA-MB-231 was 35.04
µg/ml. By contrast, the other four extraction fractions
(ESMC1 and ESMC3-5) did not show any obvious inhibitory effects on
cancer cell growth (results not shown).
The cytotoxic effect of ESMC2 was also assessed by
observing ESMC2-induced morphological changes in MDA-MB-231 cells
under a phase-contrast microscope. The results revealed a
significant decrease in the number of cells after treatment with
ESMC2 (120 and 240 µg/ml) as compared with that in the
control group. Treatment with ESMC2 induced morphological changes
of MDA-MB-231 cells, which acquired a round and shrunken shape,
while retaining their polygonal structures (Fig. 1B).
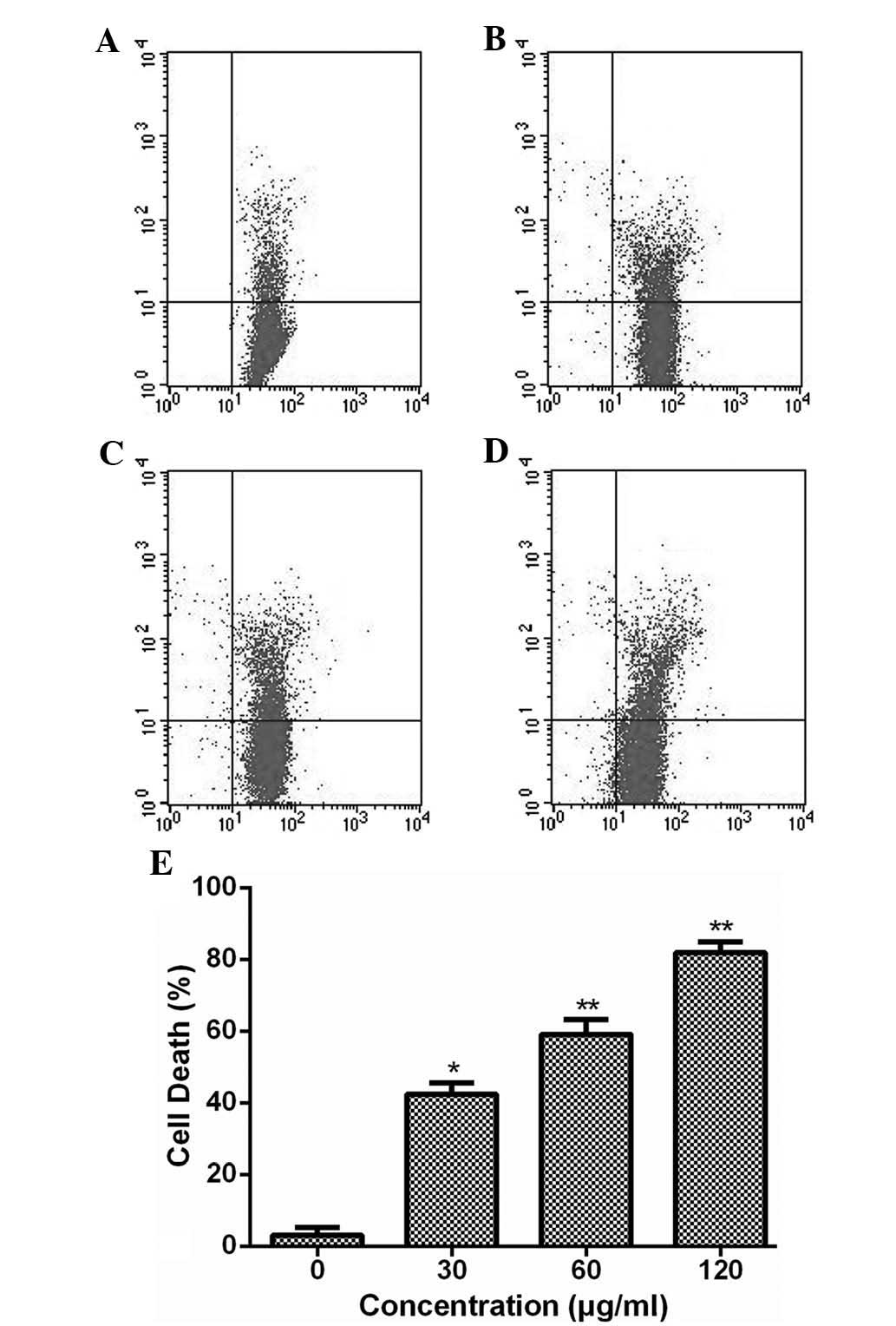
ESMC2 induces breast cancer cell
death
The ability of ESMC2 to induce cell death was
assessed using a PI staining assay. At each of the tested
concentrations (30–120 µg/ml), ESMC2 increased the PI
uptake, indicating that the rate of cell death was increased
(Fig. 2). In addition, it was
observed that ESMC2 induced cell death in MDA-MB-231 cells in a
dose-dependent manner. These results were consistent with those of
the MTT assay to assess cell growth inhibition and viability,
indicating that the anti-proliferative effect of ESMC2 on
MDA-MB-231 cells may be based on its ability to kill breast cancer
cells.
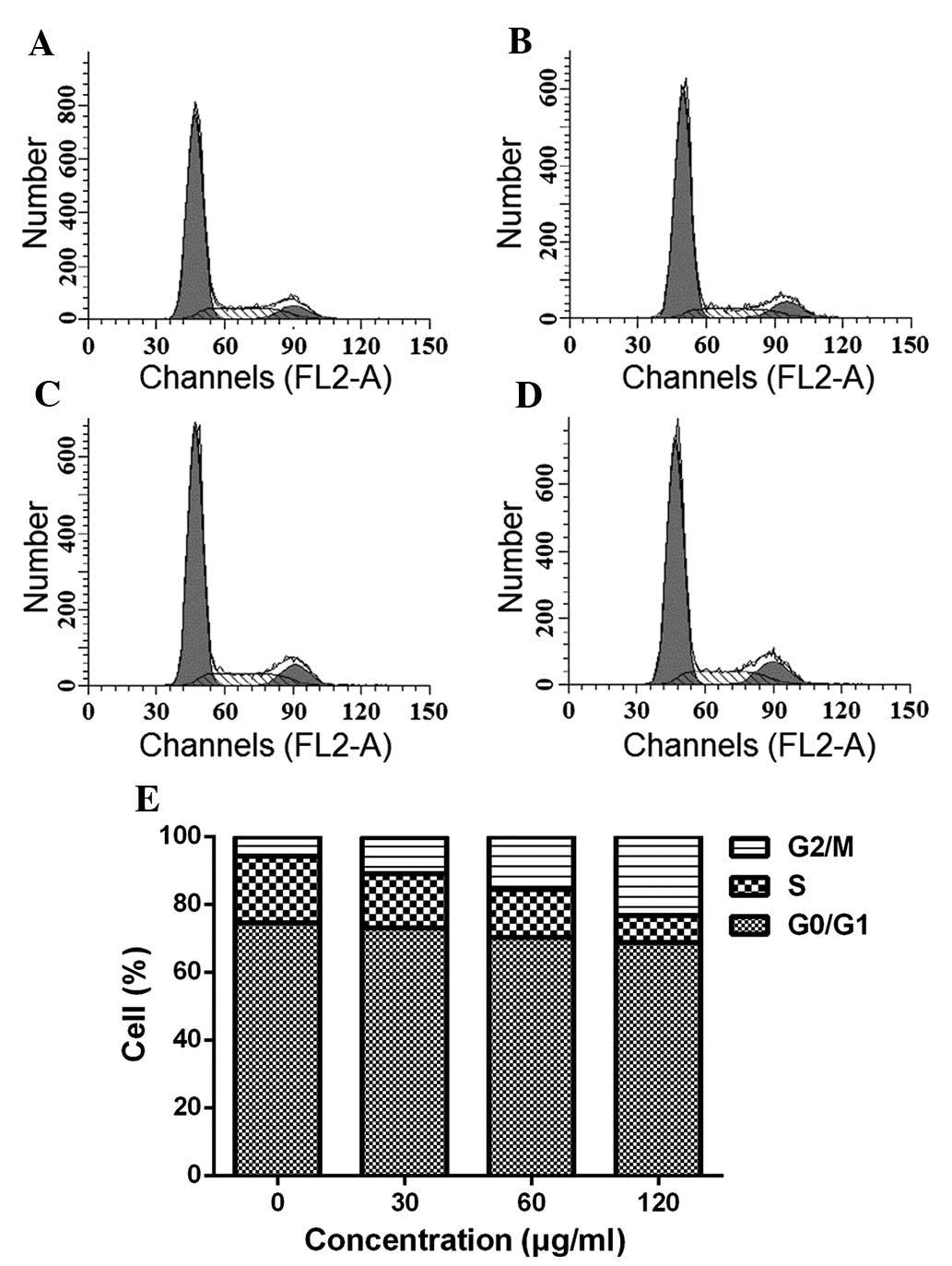
ESMC2 causes cell cycle arrest in G2
phase
To gain further insight into the underlying
mechanisms of the cytotoxic effects of ESMC on MDA-MB-231 cells,
the present study next investigated the effect of the preparation
on cell cycle progression. Cells treated with 30, 60 and 120
µg/ml ESMC2 for 48 h displayed a G2-phase arrest. As shown
in Fig. 3, the percentage of cells
accumulated in G2 phase was 5.66, 10.67, 15.63 and 23.20% following
treatment with 0, 30, 60 and 120 µg/ml ESMC2, respectively.
The accumulation of cells in S phase was maximal in the control
group and declined following treatment with ESMC2 in a
dose-dependent manner. The S-phase population was 19.71, 16.11,
14.17 and 7.97% following treatment with 0, 30, 60 and 120
µg/ml ESMC2 for 48 h, and these decreases were associated
with a concomitant increase in apoptosis and accumulation of cells
in G2 phase. This indicated that the ESMC2-mediated death of
MDA-MB-231 cells may have been initiated, at least in part, by the
induction of G2-phase arrest.
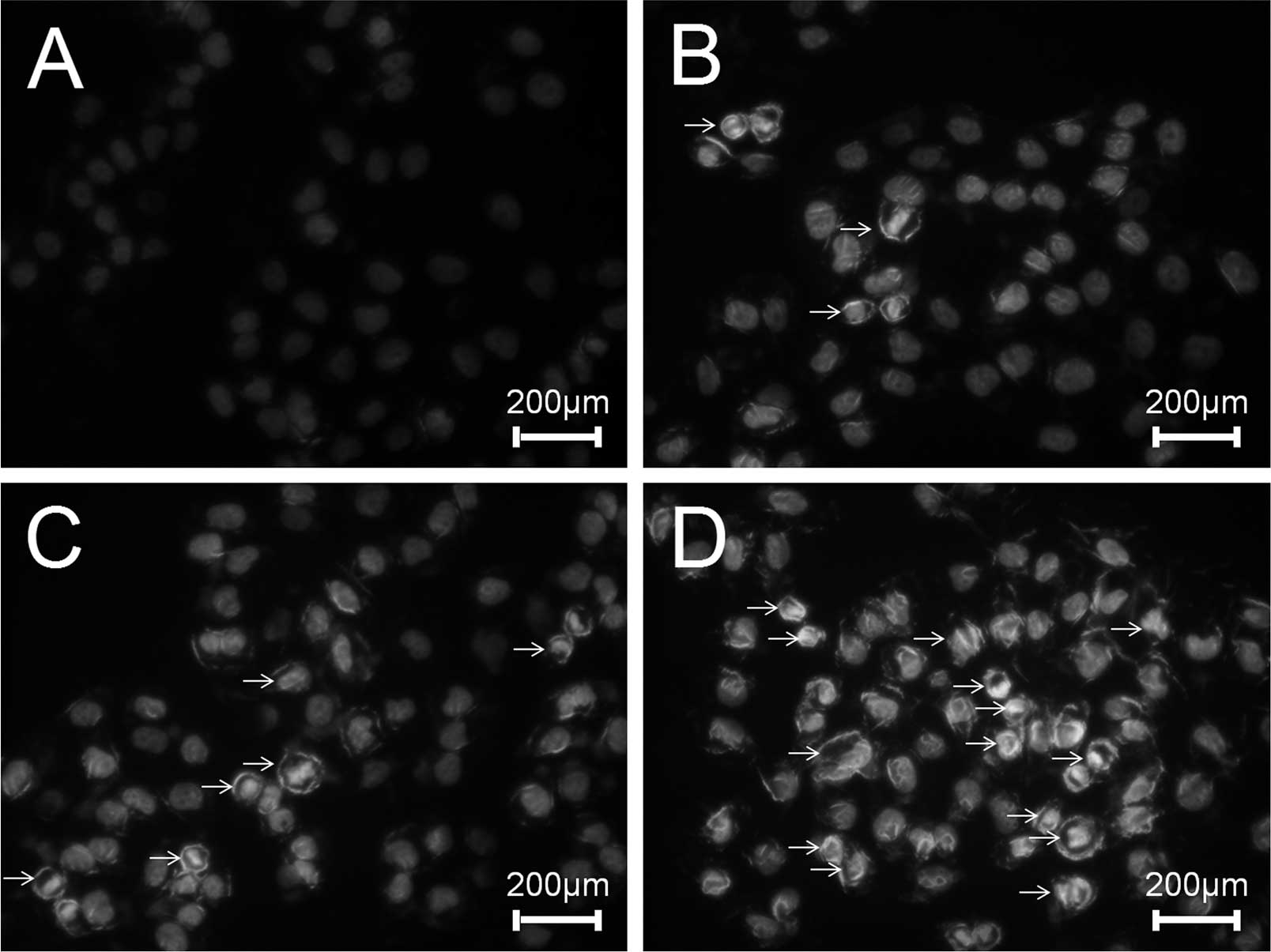
ESMC2 increases apoptosis of MDA-MB-231
cells
To investigate the effects of ESMC2 on the induction
of apoptosis, MDA-MB-231 cells were treated with ESMC2, followed by
assessment of DNA fragmentation, an important characteristic of
apoptosis, which can be easily distinguished by Hoechst staining.
In accordance with the abovementioned results, treatment with 30,
60 and 120 µg/ml of ESMC2 for 48 h led to a significant
nuclear condensation or nuclear fragmentation (Fig. 4).
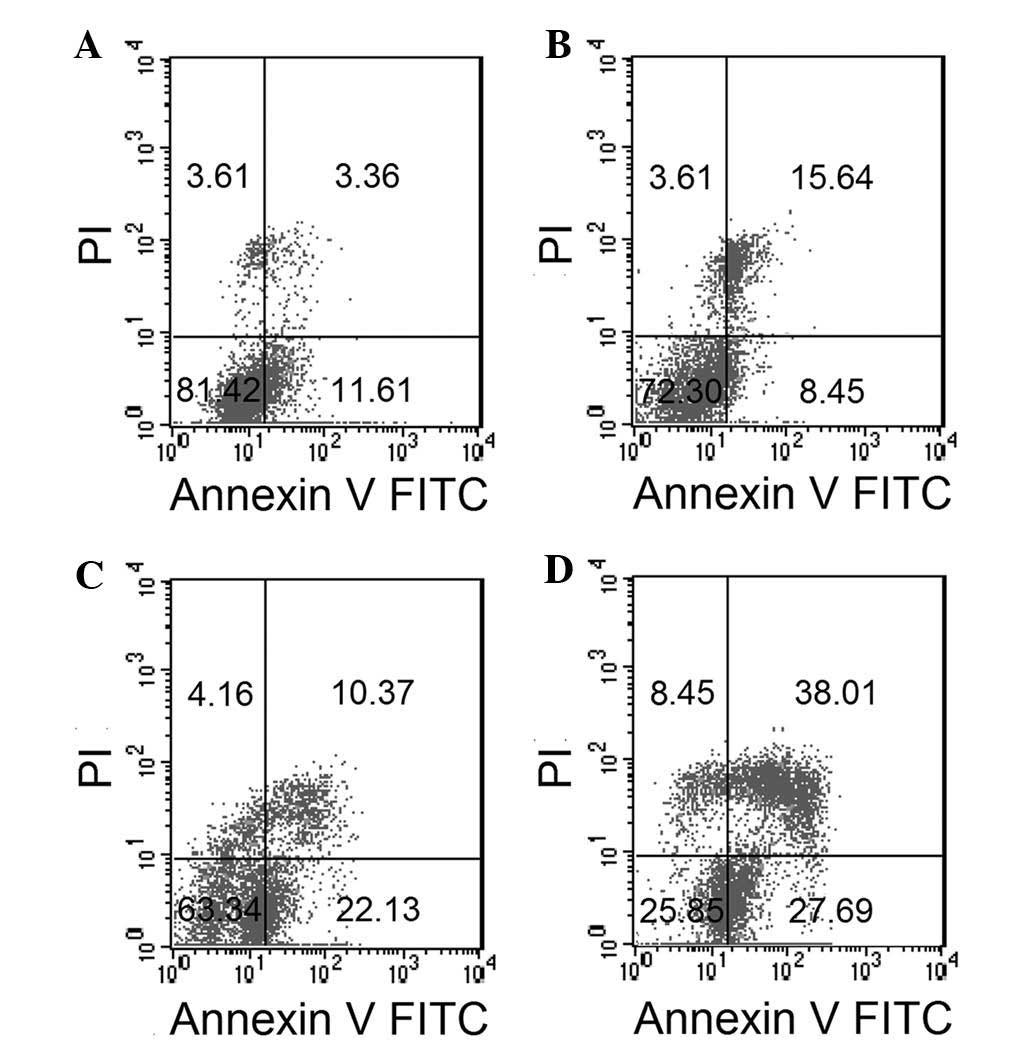
To confirm the effect of ESMC2 on cell apoptosis and
to distinguish between apoptosis and necrosis, treated cells were
stained with Annexin V-FITC and PI and analyzed by flow cytometry.
A dose-dependent increase in the percentage of apoptotic cells
(Annexin V-positive, PI-positive) was observed. The percentage of
apoptotic MDA-MB-231 cells resulting from treatment with 0, 30, 60
and 120 µg/ml ESMC2 was 14.97, 24.09, 32.50 and 65.70%,
respectively. In parallel with this, the rate of early apoptosis
and necrosis, shown in the lower right and upper left quadrants,
respectively, increased in a dose-dependent manner (Fig. 5).
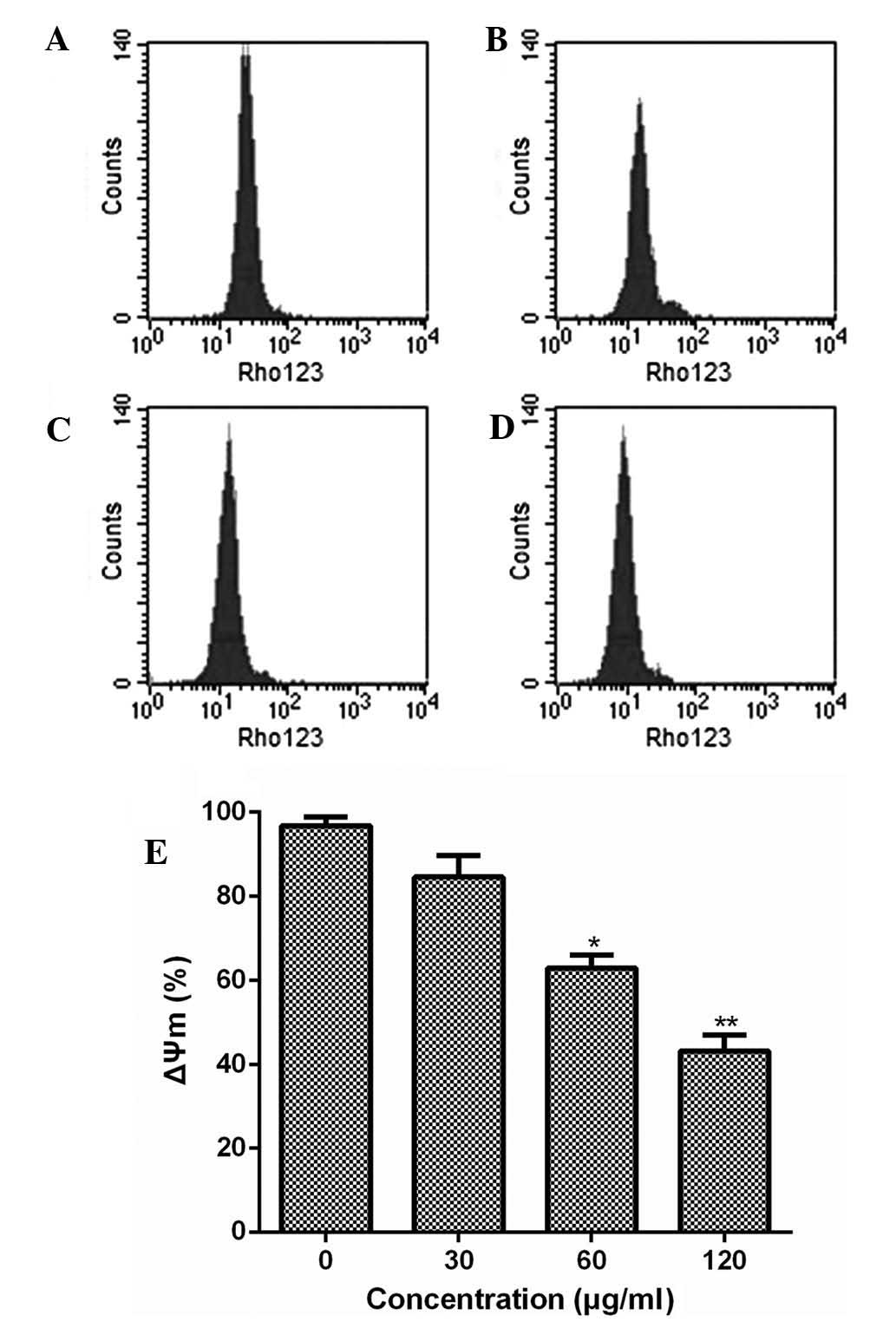
Furthermore, the present study examined the effect
of ESMC2 treatment on the mitochondrial transmembrane potential
using rhodamine 123 staining followed by flow cytometric analysis.
Treatment of MDA-MB-231 cells with ESMC2 caused a decrease in
rhodamine 123 fluorescence intensity from 96.42% in untreated cells
to 84.13, 62.98 and 43.30% in cells treated with 30, 60 and 120
µg/ml of ESMC2, respectively (Fig. 6). This loss of the mitochondrial
membrane potential further demonstrated that ESMC2 caused apoptosis
in breast cancer cells.
ESMC2 increases the expression of
apoptosis- and cell cycle- associated proteins and the activation
of MAPK/JNK signaling molecules
Multiple signal transduction pathways are involved
in the regulation of cell cycle progression and the induction of
apoptosis. The results of the present study showed that ESMC2 was
able to induce G2-phase arrest; therefore, its effect on the
expression of cell cycle-regulating proteins was assessed.
MDA-MB-231 cells treated with ESMC2 (30, 60 and 120 µg/ml)
for 48 h were subjected to western blot analysis. Treatment with
ESMC2 led to a significant increase in CDC2, Cyclin B1 and cyclin E
expression, and a subsequent decrease in Cyclin D1 expression in a
dose-dependent manner (Fig. 7A).
Next, the expression of Bcl-2 family proteins in MDA-MB-231 cells
treated with increasing concentrations of ESMC2 (30, 60 and 120
µg/ml) for 48 h was examined by western blot analysis.
Treatment with ESMC2 led to a marked decrease in Bcl-2 and Mcl-1
expression and a significant upregulation of Bax, Bak and Bad
expression (Fig. 7B). To further
explore the underlying mechanisms of ESMC2-induced apoptosis, the
effect of ESMC2 on the MAPK pathway was assessed by determining the
levels of total as well as phosphorylated Erk1/2, JNK, P38 and Akt.
According to western blot analysis, treatment with ESMC2 led to a
significant decrease in the expression of phospho-Erk1/2,
phospho-JNK, phospho-P38 and phospho-Akt, while total protein
levels remained constant (Fig.
7C). The results suggested that the protein phosphorylation,
and thereby their activation, was downregulated by ESMC2 in a
dose-dependent manner. Further western blot experiments showed that
ESMC2 was able to upregulate p53 (Fig.
7D) and downregulate NF-κB (Fig.
7E) expression.
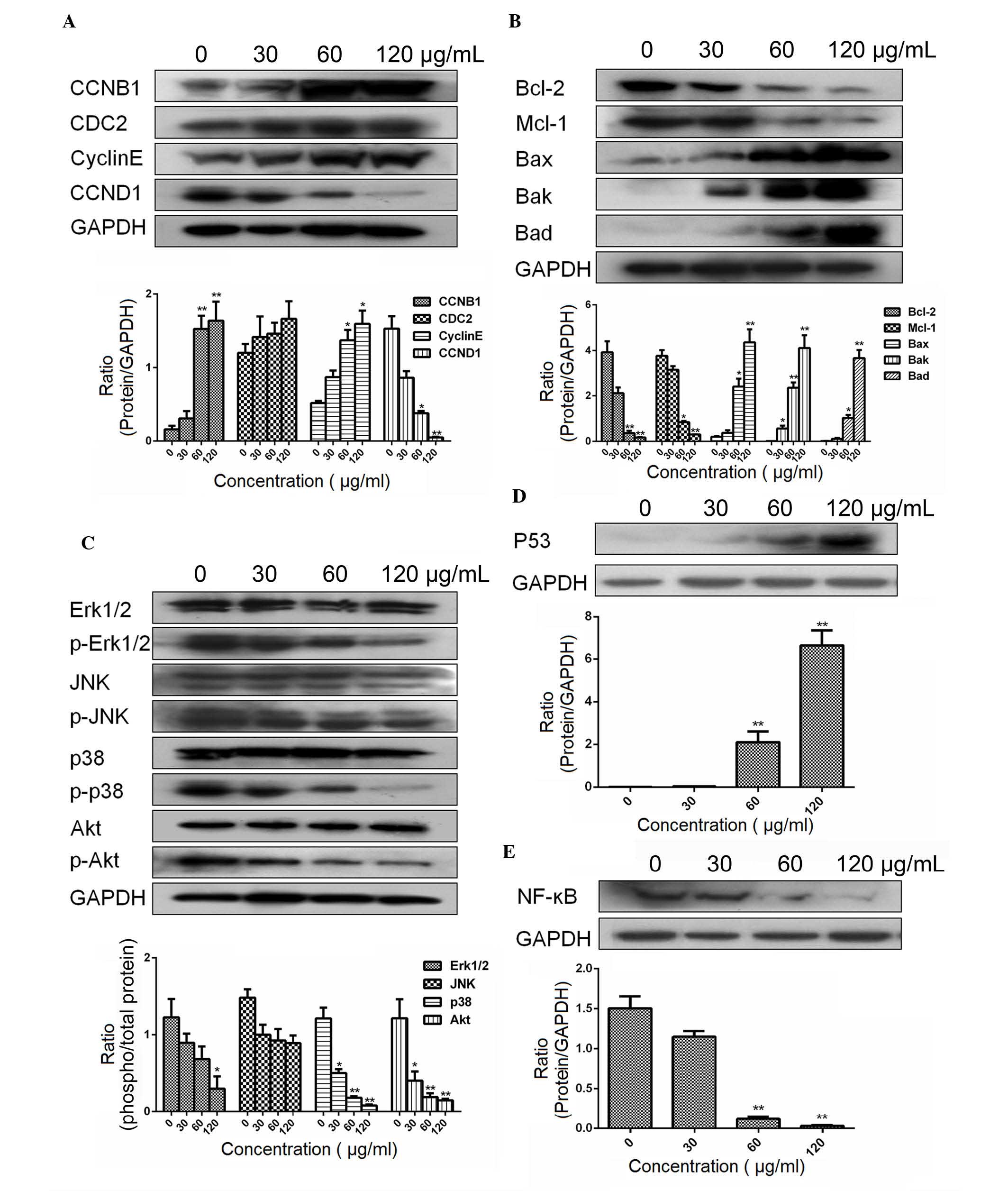 | Figure 7Effects of ESMC2 on protein
expression in MDA-MB-231 cells assessed by western blot analysis.
(A) Effects of ESMC2 on the expression of the cell cycle-associated
proteins Cyclin D1, Cyclin B1, cyclin E and CDC2. Treatment with
ESMC2 (0, 30, 60 and 120 µg/ml for 48 h) increased the
expression levels of Cyclin B1, cyclin E and CDC2, and
down-regulated the expression of Cyclin D1 in a dose-dependent
manner. (B) Effects of ESMC2 on Bcl-2 family proteins. The
expression of Bax, Bak and Bad was upregulated, while the
expression of Bcl-2 and Mcl-1 was downregulated. (C) Effects of
ESMC2 on the levels of total Erk1/2 and phospho-Erk1/2, total JNK
and phospho-JNK, total P38 and phospho-P38, as well as total Akt
and phospho-Akt. Treatment of MDA-MB-231 cells with ESMC2 led to a
significant decrease in the expression of phospho-Erk1/2,
phospho-P38 and phospho-Akt in a dose-dependent manner, while the
respective total protein levels remained constant. (D) Effects of
ESMC2 on the expression of P53. (E) Effects of ESMC2 on the
expression of NF-κB. GAPDH was used as a loading control. Lanes in
blots (from left to right): Cells treated with 0, 30, 60 and 120
µg/ml ESMC2. Values are expressed as the mean ± standard
error of the mean of at least three independent experiments.
*P<0.05, **P<0.01 vs. the control
group. CDC2, cyclin-dependent kinase 1; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; Bad, Bcl-2-associated death
promoter; Bak, Bcl-2 homologous antagonist killer; Mcl-1, myeloid
cell leukemia 1; Erk, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated; NF-κB, nuclear factor
kappa B; ESMC2, ethyl acetate extracts of the seeds of Momordica
cochinchinensis. |
Effect of ESMC2 on the growth of
MDA-MB-231 cells in athymic mice
The anti-tumor properties of ESMC2 were evaluated
using an MDA-MB-231 cell xenograft model. ESMC2 inhibited tumor
growth in MDA-MB-231-xenografted athymic mice in a dose-dependent
manner (Table II). At the end of
the study, the tumors in the group treated with ESMC (25 or 50
mg/kg) were smaller and lighter compared with those in the
vehicle-treated control group. The tumor growth inhibition was
67.61 and 81.82% respectively. Of note, mice receiving ESMC2 showed
no apparent weight loss during the experiment, suggesting that
ESMC2 at the concentrations used is non-toxic to athymic mice.
 | Table IIEffect of ESMC2 on ZR-75-30 cells
xenografted in athymic mice. |
Table II
Effect of ESMC2 on ZR-75-30 cells
xenografted in athymic mice.
| Group | Body weight (g)
| Ratio of body
growth (%) | Tumor size
(mm3)
| Tumor weight
(g) | Tumor growth
inhibition (%) |
|---|
| Start | End | Start | End |
|---|
| Vehicle
control | 20.22±1.11 | 22.63±2.45 | 11.92 | 107.80±24.03 | 223.00±42.31 | 0.443±0.266 | – |
| ESMC (25
mg/kg) | 20.18±0.981 | 22.50±1.00 | 11.50 | 104.00±17.94 | 144.25±64.99 | 0.143±0.098 | 67.61% |
| ESMC (50
mg/kg) | 20.00±1.16 | 22.20±0.739 | 11.00 | 100.25±25.86 | 129.75±36.43 | 0.080±0.054a | 81.82% |
Discussion
Preliminary experiments by our group have indicated
that extracts of Momordica cochinchinensis Spreng. exerted
an anti-cancer effect. In order to identify the active components,
Momordica cochinchinensis was extracted with solvents
covering a range of polarities (increasing polarity gradient of
hexane, ethyl acetate, acetone, ethanol and water) via the Soxhlet
extraction method. This method has the advantage that each
extracted fraction contains individual compounds. The inhibitory
effects of ESMCs on breast cancer cells was investigated using an
MTT assay. Among the various fractions, ESMC2, which was isolated
from Momordica cochinchinensis by ethyl acetate extraction,
showed significant anti-proliferative effects on cancer cells.
Therefore, the present study further investigated the effects of
ESMC2 on MDA-MB-231 cells as well as its possible mechanisms of
action.
Cell cycle arrest and apoptosis represent two
effective mechanisms involved in the induction of cell death
(32). The cell cycle of somatic
cells is tightly regulated by an intricate network of positive and
negative signals. Much is known about the molecules involved in
cell cycle control and the regulation of checkpoints to mediate
cell cycle progression (33). When
MDA-MB-231 cells in the exponential growth phase were treated with
ESMC2, G2-phase arrest was observed, alongside the induction of
apoptosis. In the cell cycle, the G2 checkpoint control mechanism
ensures that the cell is ready to enter the M (mitosis) phase and
divide. The S phase is associated with DNA synthesis and has a
crucial role in the progression of the cell cycle. The G2 phase is
therefore a key stage in the cell cycle and the target of numerous
drugs (34). Positive factors
regulating cell cycle checkpoints include CDKs and cyclins; a
variety of cyclins, which are generated through the use of
alternate transcription initiation sites, exhibit distinct
expression and degradation patterns, contributing to the temporal
coordination of each mitotic event (11). In the present study, ESMC2
inhibited the proliferation of MDA-MB-231 cells, partly as a result
of the accumulation of cells in G2 phase of the cell cycle. The
S-phase population decreased, whereas the G2-phase population
increased in the treated group compared with that in the control
group. The observed G2 arrest was followed by cell growth
suppression.
To identify the mechanisms leading to cell cycle
arrest, series of signaling proteins were assessed using western
blot analysis. These included G2/mitotic-specific cyclin-B1
(encoded by the CCNB1 gene in humans), cyclin B1, a regulatory
protein involved in mitosis (35),
and cyclin E, which binds to the G1-phase-associated protein Cdk2,
which is required for the transition from G1 to S phase of the cell
cycle and regulates cell division (36). CDC2 remains at a constant level
during the cell cycle (37).
Furthermore, G0/G1-phase-regulating protein cyclin-D1, encoded by
the CCND1 gene in humans, was determined (38). In the present study, treatment with
ESMC2 increased the protein expression of Cyclin B1, cyclin E and
CDC2, which explains the accumulation of cells in G2 phase of the
cell cycle in MDA-MB-231 cells following ESMC2 treatment. The
decrease in the number of cells in S phase following ESMC2
treatment was likely to be associated with the concomitant increase
in apoptosis and accumulation of cells in the G2 phase.
Numerous apoptotic stimuli induce cell cycle arrest
prior to cell death, thereby affecting the cell cycle and the
apoptotic machinery (39). In the
present study, apoptotic cells were distinguished by Hoechst
staining, which was more intense in the ESMC2-treated groups than
that in the control group. In the Annexin-V/PI assay, MDA-MB-231
cells were exposed to ESMC2 at a range of concentrations for 48 h,
leading to apoptosis in a dose-dependent manner. Bcl-2 family
proteins are regulators of apoptosis (12). The Bcl-2 family of homologous
proteins represents a critical checkpoint within most apoptotic
pathways, acting upstream of signaling in response to irreversible
damage to cellular constituents and including anti-apoptotic as
well as pro-apoptotic proteins. The balance between these two
classes of proteins is critical for predicting (at least in part)
how cells respond to apoptotic or survival signals. In contrast to
inactive Bax, which is monomeric and located in the cytosol or
loosely associated with membranes, Bcl-2 is an integral membrane
protein localized to the mitochondria. It is conceivable that the
signal for Bax activation emanates from the mitochondria (12). The present study showed that
ESMC2-induced apoptosis was associated with the upregulation of the
expression of Bax, Bak and Bad, and the downregulation of the
expression of Bcl-2 and Mcl-1. These results support the notion
that ESMC2 induces apoptosis by affecting the expression of Bcl-2
family proteins. This leads to the collapse of the mitochondrial
membrane potential, as detected by rhodamine 123 staining, and
ultimately results in apoptosis.
P53 is considered a 'guardian of the genome' and has
a pivotal role in the regulation of cell cycle progression,
checkpoint activation, apoptosis and repair of DNA damage.
Manipulation of p53-mediated pathways is, therefore, an ongoing
focus for the development of effective anti-cancer agents (19). NF-κB signaling has critical roles
in numerous physiological and pathological processes. One function
of NF-κB is to promote cell survival via the induction of proteins
that inhibit components of the apoptotic machinery in normal and
cancerous cells. In addition, continuous activation of NF-κB
promotes cell proliferation, which is involved in the pathogenesis
of numerous human cancers (20).
The results of the present study showed that treatment with ESMC2
led to the upregulation of p53 and downregulation of NF-κB
expression.
The MAPKs are important in the regulation of
apoptosis. It has been originally shown that ERKs are important for
cell survival, whereas p38/MAPKs are stress-responsive signaling
molecules and are thus involved in apoptosis (15). Cell survival is regulated by
phosphoinositide 3-kinase-mediated activation of the anti-apoptotic
kinase Akt. Akt in turn phosphorylates and inhibits the
pro-apoptotic proteins. Akt and Erk1/2 are key junction points
linking together signal transduction involved in survival and
proliferation (15). In the
present study, ESMC2 was shown to restrain protein phosphorylation
and accordingly promote apoptosis, finally leading to the
inhibition of MDA-MB-231-cell survival and proliferation.
Xenotransplant models were used to investigate
whether the anti-proliferative effects of ESMC2 evidenced in
vitro may be utilized to achieve growth inhibition of solid
tumors in vivo. Tumor cells at the periphery of the solid
tumor are characterized by their continuous and active growth. As
the MDA-MB-231 cell line was most sensitive to the ESMC2 fraction
in vitro, the anti-tumor effects of ESMC2 were demonstrated
in the well-established MDA-MB-231 human breast cancer cell
xenograft model. A significant growth delay of the sub-cutaneously
xenotransplanted tumors was observed in the athymic mice treated
with ESMC2 compared with that in the untreated control group. The
final tumor volume and weight of the xenografts were reduced by
ESMC2 in a dose-dependent manner. At the same time, no body weight
loss was observed in ESMC2-treated groups compared with that in the
control group over the entire experimental period. All these
results indicated that ESMC2 exerted potent growth inhibitory and
anti-tumor effects in the human xenografts with no signs of
toxicity.
In conclusion, the present study was the first, to
the best of our knowledge, to provide evidence that the ethyl
acetate extracts of Momordica cochinchinensis inhibited the
proliferation of MDA-MB-231 cells, and induced cell cycle arrest
and apoptosis in MDA-MB-231 cells. Analysis of plant extracts using
GC/MS has been performed previously (40). The results of the present study
demonstrated that ESMC2 contains various bioactive components that
may have been responsible for the inhibition of tumor cell growth
and induction of apoptosis in the cancer cell line. Therefore,
these results warrant further pharmacological investigation of
Momordica cochinchinensis seeds with detailed phytochemical
analysis. Momordica cochinchinensis is a promising herbal
medicine for cancer prevention and treatment, and the results of
the present study will encourage future studies to increase the
knowledge on the anti-cancer potential of this food plant. A future
goal of our group is to identify the active components responsible
for the anti-cancer effects of this herb.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81227802, 81302800
and 81370088), the National Science Foundation for Post-doctoral
Scientists of China (grant no. 2013M532062), the Project of Shaanxi
Star of Science and Technology (grant no. 2012KJXX-06) and the
Ministry of Education's new century excellent talents supporting
plan (grant no. NCET-13-0467).
References
|
1
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li M, Hou X-F, Zhang J, Wang S-C, Fu Q and
He L-C: Applications of HPLC/MS in the analysis of traditional
Chinese medicines. J Pharm Anal. 1:81–91. 2011. View Article : Google Scholar
|
|
3
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parks SE, Murray CT, Gale DL, Al-Khawaldeh
B and Spohr LJ: Propagation and production of Gac (Momordica
cochinchinensis Spreng), a greenhouse case study. Experimental
Agriculture. 49:234–243. 2012. View Article : Google Scholar
|
|
5
|
Tsoi AY, Ng TB and Fong WP:
Immunomodulatory activity of a chymotrypsin inhibitor from
Momordica cochinchinensis seeds. J Pept Sci. 12:605–611. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubola J and Siriamornpun S:
Phytochemicals and antioxidant activity of different fruit
fractions (peel, pulp, aril and seed) of Thai gac (Momordica
cochinchinensis Spreng). Food Chem. 127:1138–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong RC, Fong WP and Ng TB: Multiple
trypsin inhibitors from Momordica cochinchinensis seeds, the
Chinese drug mubiezhi. Peptides. 25:163–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang F, Steelman LS, Shelton JG, Lee JT,
Navolanic PM, Blalock WL, Franklin R and McCubrey JA: Regulation of
cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway
(Review). Int J Oncol. 22:469–480. 2003.PubMed/NCBI
|
|
9
|
Harbour JW and Dean DC: Rb function in
cell-cycle regulation and apoptosis. Nat Cell Biol. 2:E65–E67.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao Y, Zhang YW, Sun LG, Liu B, Bao YL,
Lin H, Zhang Y, Zheng LH, Sun Y, Yu CL, et al: Juglanthraquinone C,
a novel natural compound derived from Juglans mandshurica Maxim,
induces S phase arrest and apoptosis in HepG2 cells. Apoptosis.
17:832–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB
activation, up-regulates the expression of p53 and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XH, Zhou GY, Ren T, Li H, Zhang Y,
Yin D, Qian H and Li Q: β-Arrestin prevents cell apoptosis through
pro-apoptotic ERK1/2 and p38 MAPKs and anti-apoptotic Akt pathways.
Apoptosis. 17:1019–1026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zachary I: VEGF signalling: integration
and multi-tasking in endothelial cell biology. Biochem Soc Trans.
31:1171–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen HM and Tergaonkar V: NFkappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy. Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luque de Castro MD and Garcia-Ayuso LE:
Soxhlet extraction of solid materials: an outdated technique with a
promising innovative future. Anal Chim Acta. 369:1–10. 1998.
View Article : Google Scholar
|
|
23
|
Luque de Castro MD and Priego-Capote F:
Soxhlet extraction: Past and present panacea. J Chromatogr A.
1217:2383–2389. 2010. View Article : Google Scholar
|
|
24
|
Zheng L, He X, Ma W, Dai B, Zhan Y and
Zhang Y: Ta1722, an anti-angiogenesis inhibitor targeted on VEGFR-2
against human hepatoma. Biomed Pharmacother. 66:499–505. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li DD, Guo JF, Huang JJ, Wang LL, Deng R,
Liu JN, Feng GK, Xiao DJ, Deng SZ, Zhang XS, et al: Rhabdastrellic
acid-A induced autophagy-associated cell death through blocking Akt
pathway in human cancer cells. PLoS One. 5:e121762010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang J, Dai B, Wang N and He L:
Anti-proliferative and apoptotic effects of the novel taspine
derivative tas41 in the Caco-2 cell line. Environ Toxicol
Pharmacol. 31:406–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Zhu J, Guo L, Shi X, Liu Y and Yang
X: Differential effects of polyphenols-enriched extracts from
hawthorn fruit peels and fleshes on cell cycle and apoptosis in
human MCF-7 breast carcinoma cells. Food Chem. 141:1008–1018. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhan Y, Zhang Y, Liu C, et al: A novel
taspine derivative, HMQ1611, inhibits breast cancer cell growth via
estrogen receptor α and EGF receptor signaling pathways. Cancer
Prev Res (Phila). 5:864–873. 2012. View Article : Google Scholar
|
|
29
|
Berdowska I, Zielinski B, Fecka I,
Kulbacka J, Saczko J and Gamian A: Cytotoxic impact of phenolics
from Lamiaceae species on human breast cancer cells. Food
Chemistry. 141:1313–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Y, Luo W, Zheng L, Li M and Zhang Y:
Construction of recombinant FGFR1 containing full-length gene and
its potential application. Plasmid. 64:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Zheng L, Zhang J, Dai B, Wang N,
Chen Y and He L: Antitumor activity of taspine by modulating the
EGFR signaling pathway of Erk1/2 and Akt in vitro and in vivo.
Planta Med. 77:1774–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farhana L, Dawson M, Rishi AK, Zhang Y,
Van Buren E, Trivedi C, Reichert U, Fang G, Kirschner MW and
Fontana JA: Cyclin B and E2F-1 expression in prostate carcinoma
cells treated with the novel retinoid CD437 are regulated by the
ubiquitin-mediated pathway. Cancer Res. 62:3842–3849.
2002.PubMed/NCBI
|
|
36
|
Lu HF, Chen YS, Yang JS, Chen JC, Lu KW,
Chiu TH, Liu KC, Yeh CC, Chen GW, Lin HJ, et al: Gypenosides
induced G0/G1 arrest via inhibition of cyclin E and induction of
apoptosis via activation of caspases-3 and -9 in human lung cancer
A-549 cells. In Vivo. 22:215–222. 2008.PubMed/NCBI
|
|
37
|
Tao W, Zhang S, Turenchalk GS, Stewart RA,
St John MA, Chen W and Xu T: Human homologue of the Drosophila
melanogaster lats tumour suppressor modulates CDC2 activity. Nat
Genet. 21:177–181. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Q, Fu HJ, Sun F, Zhang H, Tie Y, Zhu
J, Xing R, Sun Z and Zheng X: miR-16 family induces cell cycle
arrest by regulating multiple cell cycle genes. Nucleic Acids Res.
36:5391–5404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Ann Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar
|
|
40
|
Jonsson P, Gullberg J, Nordström A, Kusano
M, Kowalczyk M, Sjöström M and Moritz T: A strategy for identifying
differences in large series of metabolomic samples analyzed by
GC/MS. Anal Chem. 76:1738–1745. 2004. View Article : Google Scholar : PubMed/NCBI
|