Introduction
Adenoid cystic carcinoma (ACC) is characterised by a
prolonged clinical course, frequent local recurrence and perineural
invasion (1–3). The standard primary treatment
strategies for local and locoregional disease are surgery and/or
irradiation (3); however, the
long-term outcomes of these treatments are unfavourable. Various
drugs have been used in the field of targeted therapy against ACC;
however, the predominant effect of these drugs is disease
stabilisation. Therefore, novel strategies are required to improve
kill ACC cells with lower systemic toxicity, particularly for use
in combination therapy.
Apigenin is a natural phyto-oestrogen flavonoid,
which exerts various biological effects, including anti-oxidative,
anti-inflammatory and anticancer activities (4). Apigenin has been shown to inhibit
prostate (5), thyroid (6), breast (7), pancreatic (8), ovarian (9), and head and neck cancer (10), via the selective inhibition of
tumour cell proliferation. Numerous studies have investigated the
effects of apigenin; however, the underlying biomolecular mechanism
remains to be elucidated.
Apigenin has recently been reported to target the
hypoxic markers hypoxia-inducible factor-1α (HIF-1α), glucose
transporter-1 (GLUT-1), and vascular endothelial growth factor in
human pancreatic (8,11), ovarian (12), and lung carcinoma cell lines
(13). Similar to other malignant
tumours, ACC cells exhibit increased glucose uptake and
utilisation, as compared with their non-malignant counterparts, and
GLUT-1 is considered to have a key molecular regulatory role in
this process. In addition, GLUT-1 is correlated with the aggressive
biological behaviour of other types of human cancer (14,15).
Our previous study indicated that the radioresistance of laryngeal
carcinoma may be associated with increased expression of GLUT-1
mRNA and protein (16). In
addition, GLUT-1 antisense oligodeoxynucleotides were able to
enhance the radiosensitivity of laryngeal carcinoma, predominantly
via inhibition of GLUT-1 expression (16). Therefore, GLUT-1 is regarded as a
potential therapeutic target in certain types of cancer (17,18).
To the best of our knowledge, there are currently no studies
regarding the association between apigenin and GLUT-1 in ACC.
The present study aimed to investigate whether
apigenin inhibits the proliferation of ACC cells, and whether it
may suppress the expression of GLUT-1 in ACC cells.
Materials and methods
Cell culture
The ACC-2 human adenoid cystic carcinoma cell line
was purchased from the Cell Research Institute of the Chinese
Academy of Sciences (Shanghai, China). The ACC-2 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Life
Technologies, Grand Island, NY, USA) supplemented with 10%
heat-inactivated foetal bovine serum (Hyclone, GE Healthcare,
Logan, UT, USA), 2 mM L-glutamine, 100 U/ml penicillin, and 100
g/ml streptomycin (100biotech Co., Ltd., Hangzhou, China) at 37°C
in an atmosphere containing 5% CO2. The cells were
trypsinised and harvested after reaching 80–90% confluence.
Proliferation assay of ACC-2 cells using
the Cell Counting kit-8 (CCK-8) system
Cultured ACC-2 cells were trypsinised with 0.25%
trypsin (100biotech Co., Ltd). Cell proliferation was measured
using the CCK-8 system (Beyotime Institute of Biotechnology,
Nanjing, China), according to the manufacturer's instructions.
Briefly, 3×103 ACC-2 cells were seeded into 96-well
culture plates and treated with either DMEM, or 10, 40 or 160
µM apigenin (Selleckchem, Houston, TX, USA) The cells were
cultured in serum-free medium (100biotech Co., Ltd.) at 37°C for 1,
2, 3, 4 or 5 days. A total of 10 µl CCK-8 reagent was added
to each well, and incubated at 37°C for 3 h, and the absorbance was
measured at 450 nm. Optical density (OD) was calculated as follows:
OD = ODcell − ODblank.
Cell cycle analysis using flow
cytometry
A total of 3×103 ACC-2 cells were seeded
into 96-well culture plates and treated with either DMEM, or 10, 40
or 160 µM apigenin for 24, 48 or 72 h. The cells from each
group were trypsinised with 0.25% trypsin and rinsed in
phosphate-buffered saline (PBS). The cells were centrifuged at 800
× g for 5 min and resuspended in up to 500 µl PBS. The cells
were centrifuged at 2,000 × g for 5 min, collected and fixed in
ice-cold 70% ethanol for 24 h, prior to re-centrifugation. The
cells were then incubated with RNase (0.5 mg/ml) and stained with 1
ml of 50 µg/ml propidium iodide (PI; Sigma-Aldrich, St.
Louis, MO, USA) in the dark for 30 min at room temperature. The
cells were subsequently added to 5 µl Annexin V-fluorescein
isothiocyanate (FITC) and 5 µl PI, and incubated for a
further 15 min at 25°C. The cells were then added to 400 µl
binding buffer in the dark for 1 h. The FACScan analysis system (BD
Biosciences, Franklin Lakes, NJ, USA) was then used to collect flow
cytometry (FCM) data to determine changes in cell cycle
distribution. Each experiment was performed three times in
triplicate.
Cell apoptosis analysis using flow
cytometry
A total of 3×103 ACC-2 cells were seeded
into 96-well culture plates and treated with either DMEM or 10, 40,
or 160 µM apigenin for 24, 48 or 72 h. The cells from each
group were trypsinised using 0.25% trypsin and rinsed in PBS. The
cells were centrifuged at 800 × g for 5 min and resuspended in up
to 500 µl PBS. The cells was centrifuged at 2,000 × g for 5
min, collected and fixed in ice-cold 70% ethanol for 24 h and
re-centrifuged. The cells were then incubated with 500 µl
binding buffer in the dark for 30 min at room temperature. The
cells were added to 5 µl Annexin V-FITC and 5 µl PI,
followed by incubation for 15 min at 25°C. The FACScan analysis
system was then used to collect FCM data on changes in cell
apoptosis. Each experiment was performed three times in
triplicate.
Determination of GLUT-1 mRNA expression
in ACC-2 cells by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR)
Briefly, 3×103 ACC-2 cells were seeded
into 96-well culture plates and treated with either DMEM, or 10, 40
or 160 µM apigenin for 24, 48, or 72 h. The ACC-2 cells were
homogenised in TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and total RNA was extracted,
according to the manufacturer's instructions. A total of l
µg RNA was added to M-MLV reverse transcriptase (100biotech
Co., Ltd.) in a 20 µl reaction volume, and the reaction mix
was pre-denatured at 65°C for 10 min. After the addition of 200 U
M-MLV, the samples were incubated at 42°C for 1 h and annealed at
70°C for 10 min. The newly synthesised cDNA was used as a template
for RT-qPCR. The 20 µl PCR reaction mix consisted of 10
µl 2X SYBR Green (Toboyo Co., Ltd., Tokyo, Japan), 1
µl cDNA template, 1 µl upstream and downstream
specific primers, and 8 µl deionised water. The PCR cycling
conditions were as follows: Pre-denaturation at 95°C for 2 min,
followed by 40 cycles at 95°C for 15 sec, 59°C for 20 sec and 72°C
for 20 sec and a final extension step at 72°C for 10 min.
Experiments were performed in triplicate and were repeated at least
twice independently. The primers used were as follows: Forward:
5′-CCGCAACGAGGAGAACCG-3′ and reverse: 5′-GTGACCTTCTTCTCCCGCATC-3′
for GLUT-1; and forward: 5′-TGTTGCCATCAATGACCCCTT-3′, and reverse:
5′-CTCCACGACGTACTCAGCG-3′ fir GAPDH. GAPDH was used as an internal
standard for data calibration. The lengths of the PCR products were
123 bp (GLUT-1) and 202 bp (GAPDH). Dissociation curve analysis was
conducted, and the 2−ΔΔCt quantification method was used
to calculate differential gene expression.
Determination of GLUT-1 protein levels in
ACC-2 cells by western blotting
The GLUT-1 and β-actin (control) protein expression
levels were detected in each group of ACC-2 cells by western
blotting. Following extraction of the GLUT-1 and β-actin proteins,
the protein concentration was determined using a bicinchoninic acid
protein quantitative kit (Wuhan Boster Biological Technology Co.
Ltd., Wuhan, China). Briefly, 80 µg protein was separated by
10% SDS-PAGE and transferred onto a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). Skimmed milk (2%) was used to block
the membrane at room temperature for 1 h. The membrane was then
incubated with the following primary antibodies: Rabbit anti-human
polyclonal GLUT-1 (1:1,000; cat. no. ab14683; Abcam, Cambridge, UK)
and mouse anti-human monoclonal β-actin (1:5,000; Abcam) at room
temperature for 3 h, followed by an incubation with donkey
anti-rabbit (1:5,000) and donkey anti-mouse (1:2,000) secondary
antibodies at room temperature for 1 h. The blots were visualized
using an enhanced chemiluminescence system (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and were exposed to X-ray
film. Protein expression was semi-quantitatively analysed using the
Kodak Gel Logic Analysis system (Kodak, Rochester, NY, USA).
Statistical analysis
Statistical analyses were performed using SPSS for
Windows, version 19.0 (IBM SPSS, Armonk, NY, USA). An independent
t-test was used for analysis. P<0.05 was deemed to indicate a
statistically significant difference.
Results
Apigenin inhibits the growth of ACC-2
cells
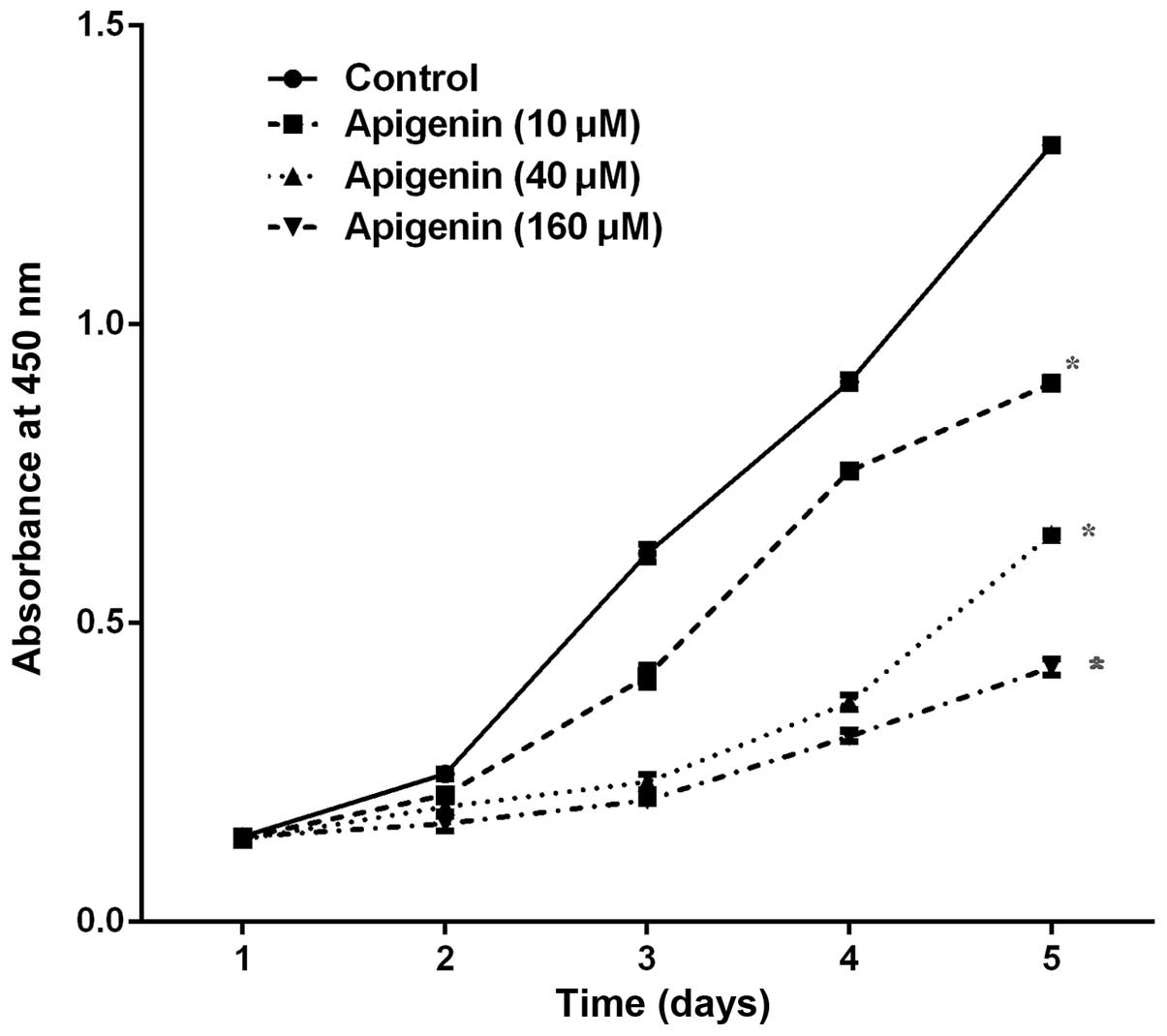
The CCK-8 assay demonstrated that various
concentrations (10–160 µM) and durations (1–5 days) of
apigenin treatment resulted in a dose- and time-dependent
inhibition of ACC-2 cell growth, as compared with the control
(P<0.05; Fig. 1).
Apigenin induces apoptosis and cell cycle
arrest in ACC-2 cells
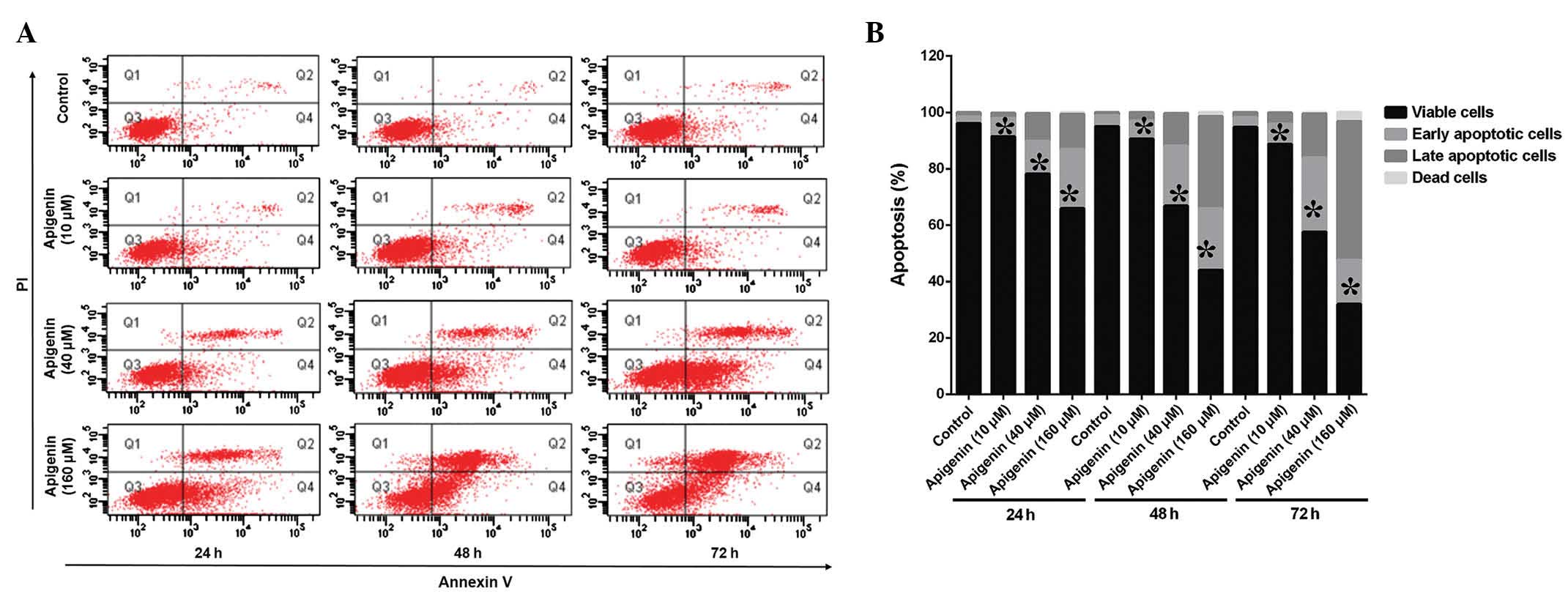
The ACC-2 cells were treated with various
concentrations (10–160 µM) of apigenin for 24, 48, and 72 h
and apoptotic cell death and cell cycle alterations were detected
using FCM (Fig. 2A). Treatment
with apigenin induced apoptosis in a dose- and time-dependent
manner, as compared with the control (Fig. 2B). The percentage of apoptotic
cells increased to 64.8% following treatment with 160 µM
apigenin. In addition, the results of the FCM demonstrated that the
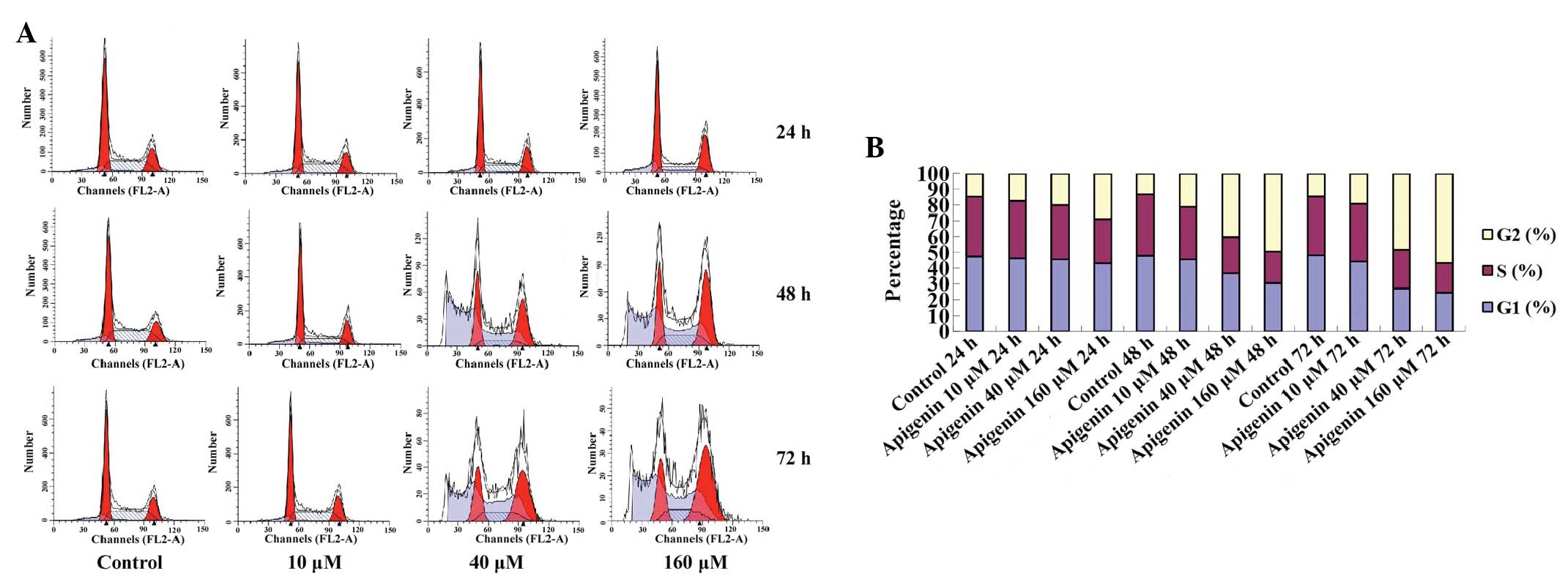
cell cycle progressed from G1 to G2 phase,
and was arrested at G2/M phase in a dose- and
time-dependent manner (Fig. 3A).
The G2/M-phase population of the control cells was
14.65%, and was markedly increased after 24, 48 and 72 h of
treatment with various concentrations of apigenin (Table I, Fig.
3B).
 | Table IDistribution of ACC-2 cells in the
cell cycle exposed to different concentration of apigenin over
time. |
Table I
Distribution of ACC-2 cells in the
cell cycle exposed to different concentration of apigenin over
time.
| Group | G1 (%) | S (%) | G2 (%) |
|---|
| 24 h | | | |
| Control | 47.42 | 37.93 | 14.65 |
| 10 µM
apigenin | 46.13 | 36.49 | 17.38 |
| 40 µM
apigenin | 45.42 | 34.54 | 20.04 |
| 160 µM
apigenin | 43.26 | 27.89 | 28.85 |
| 48 h | | | |
| Control | 47.89 | 38.74 | 13.37 |
| 10 µM
apigenin | 45.51 | 33.4 | 21.09 |
| 40 µM
apigenin | 36.81 | 22.91 | 40.28 |
| 160 µM
apigenin | 30.46 | 20.08 | 49.46 |
| 72 h | | | |
| Control | 48.05 | 37.46 | 14.49 |
| 10 µM
apigenin | 44.41 | 36.43 | 19.16 |
| 40 µM
apigenin | 27.32 | 24.41 | 48.27 |
| 160 µM
apigenin | 24.64 | 18.75 | 56.61 |
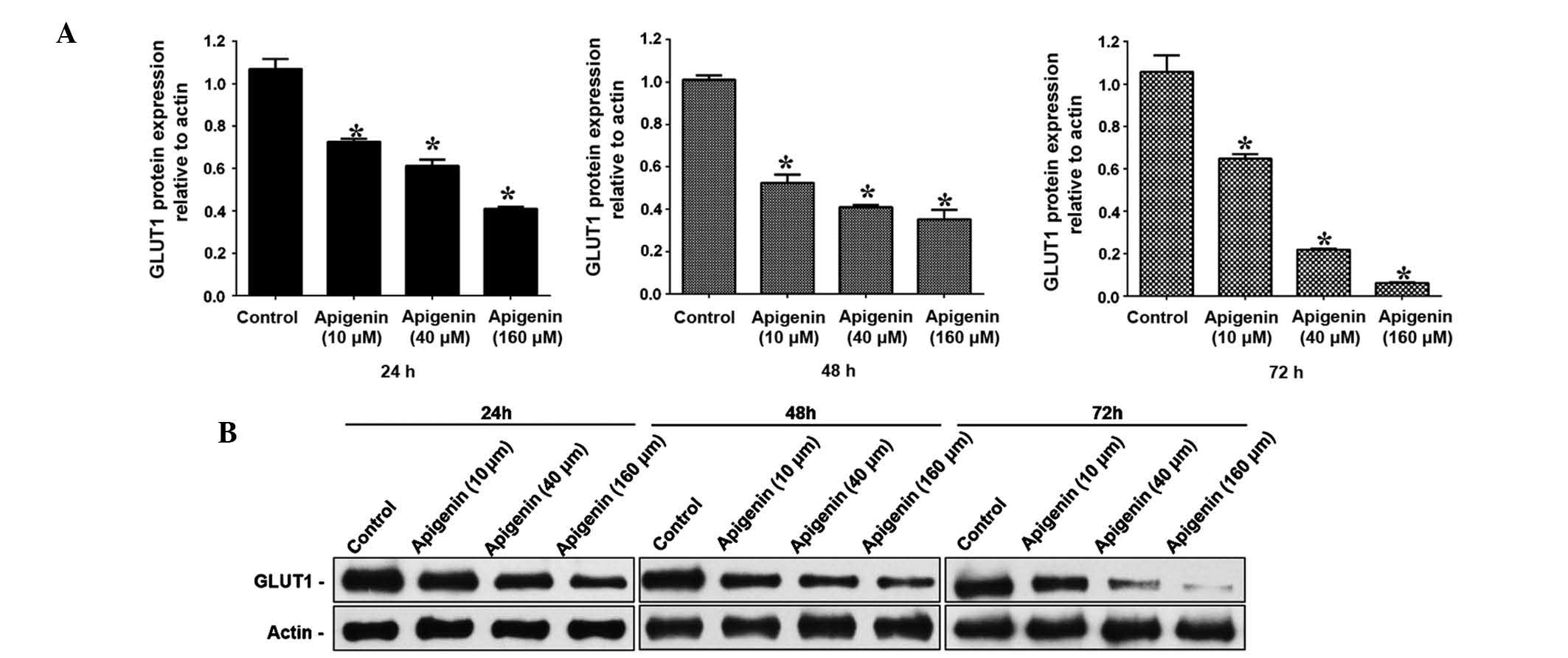
Apigenin downregulates the mRNA and
protein expression levels of GLUT-1 in ACC-2 cells
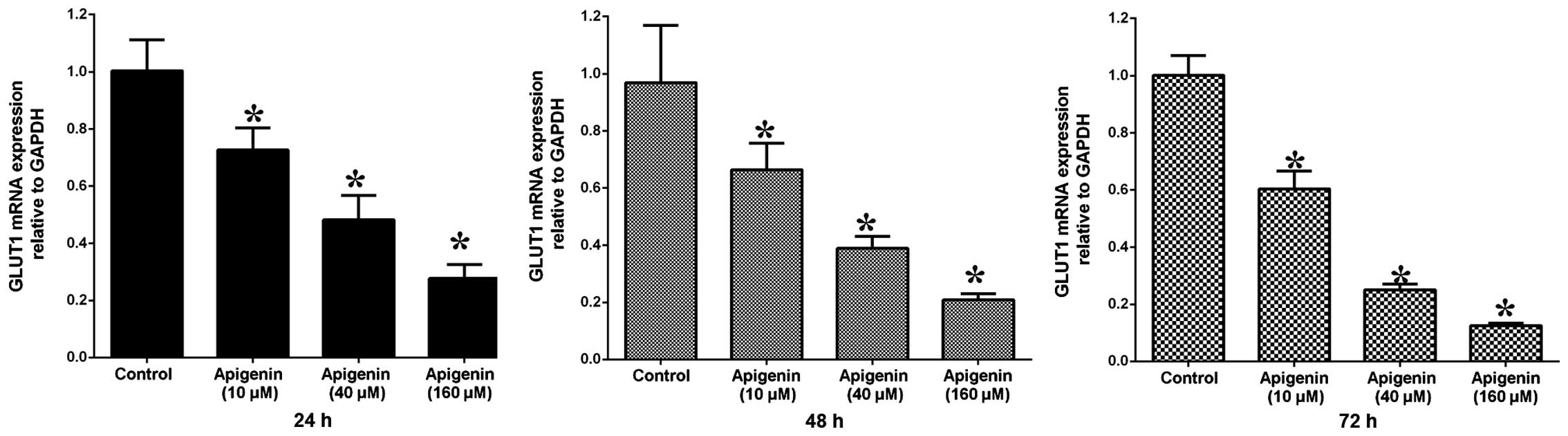
To determine the GLUT-1 mRNA and protein expression
levels in ACC-2 cells, RT-qPCR and western blotting were conducted,
respectively. GLUT-1 mRNA expression levels were significantly
reduced following treatment with increasing doses of apigenin
(P<0.05; Table II, Fig. 4). Following treatment with 10
µM apigenin, the mRNA expression levels of GLUT-1 did not
vary significantly with increasing treatment duration (P>0.05).
Conversely, following treatment with 40 and 160 µM apigenin,
the expression levels of GLUT-1 mRNA were significantly reduced
with increasing treatment duration (P<0.05; Table II, Fig. 4). Western blotting demonstrated
that the GLUT-1 protein expression levels were reduced following
apigenin treatment in a dose- and time-dependent manner (Fig. 5A and B).
 | Table IImRNA expression levels of GLUT-1 in
ACC-2 cells treated with different concentrations of apigenin over
time. |
Table II
mRNA expression levels of GLUT-1 in
ACC-2 cells treated with different concentrations of apigenin over
time.
| Group | GLUT-1 mRNA
|
|---|
| 24 h | 48 h | 72 h |
|---|
| Control | 1.00±0.11 | 1.00±0.20 | 1.00±0.07 |
| Apigenin | | | |
| 10 µM | 0.72±0.08 | 0.69±0.09 | 0.60±0.06 |
| 40 µM | 0.48±0.09 | 0.40±0.04 | 0.25±0.02 |
| 160 µM | 0.28±0.05 | 0.22±0.02 | 0.13±0.01 |
Discussion
At present, patients with ACC receive comprehensive
treatment, including extensive local resection; medical neck
dissection when cervical lymph node metastases are present; and
treatment combined with postoperative radiotherapy and/or
chemotherapy in cases of advanced ACC; however, the results are
usually unfavourable. In addition, patients with ACC are usually
insensitive to chemo-radiotherapy, which results in high recurrence
rates and distant metastasis at an early stage (3). Therefore, identification of novel
molecular targets is of great importance.
Apigenin has previously been reported to exert its
anticancer effect via various mechanisms (5–11).
It has been reported that apigenin may suppress human cancer by
inhibiting the expression of GLUT-1 (8,11).
To the best of our knowledge, there are currently no studies
regarding the interaction between apigenin and GLUT-1 in ACC. The
results of the present study demonstrated that apigenin inhibits
the proliferation of ACC-2 cells in a dose-and time-dependent
manner, this finding is concordant with the results of previous
studies regarding other types of cancer (13,19,20).
In other cell lines, apigenin has also been shown to induce
apoptosis and cell cycle arrest (13,19,20).
In T24 bladder cancer cells, Zhu et al (21) demonstrated that treatment with
apigenin resulted in increases in apoptosis and
G2/M-phase arrest, with an almost 2.6-fold increase, in
a dose-dependent manner. In a lung adenocarcinoma cell line, Bruno
et al (19) demonstrated
that apigenin significantly decreased cell proliferation and
augmented cell death and apoptosis. In addition, apigenin has been
shown to inhibit growth, induce apoptosis, and promote
G2/M phase cell cycle arrest in head and neck squamous
cell carcinoma cells (10). In the
present study, apigenin induced ACC-2 cell apoptosis, and
G2/M-phase arrest. The percentage of apoptotic cells
increased to 64.8% following treatment with 160 µM apigenin,
and the percentage of cells in G2/M phase
dose-dependently increased from 17.38 to 28.85% after 24 h, from
21.09 to 49.46% after 48 h, and from 19.16 to 56.61%, an almost
2.7-fold increase, after 72 h. In addition, the percentage of cells
in the G2/M phase increased in a time-dependent manner
from 17.38 to 56.61%, an almost 3.3-fold increase. The results of
the present study, as well as those of previous studies, suggested
that apigenin-induced cell growth inhibition may be due to cell
cycle arrest (21). In addition,
apigenin has been shown to cause G0/G1-phase
arrest, resulting in death and apoptosis of human prostate cancer
cells (20) and cervical carcinoma
cells (22). These results
suggested that apigenin-induced cell cycle arrest may be caused by
various molecular regulatory mechanisms, and the distinct
characteristics of cancer cell lines.
The anticancer mechanism of apigenin remains unclear
(23). Apigenin has recently been
reported to target hypoxic markers, and GLUT-1 is an intrinsic
marker of malignant tumour hypoxia (24). GLUT-1 overexpression enables the
transport of increased levels of glucose to fulfil the high
metabolic rate and rapid growth requirements of malignant cells. In
our previous study, it was demonstrated that overexpression of
GLUT-1 may have a role in the development of recurrence of ACC-2
cells (2). In addition, GLUT-1 has
been suggested as a potential therapeutic target in other types of
cancer (14,15). In certain human cancer cell lines,
apigenin has been shown to decrease the expression of GLUT-1, and
exert an anticancer effect (5–12).
The present study demonstrated that apigenin-induced cell apoptosis
may be due to decreased expression of GLUT-1 in ACC-2 cells.
Corresponding with the inhibition of proliferation of ACC-2 cells
and increased cell apoptosis, the expression levels of GLUT-1 were
significantly decreased following treatment with apigenin in a
dose- and time-dependent manner. The relative mRNA expression
levels of GLUT-1 were decreased by ~5.5-fold from 0.72 to 0.13, and
the relative protein expression levels of GLUT-1 decreased by
~14.6-fold from 0.73 to 0.05. The mechanism underlying these
changes may involve inhibition of glucose absorption by ACC-2
cells. In human pancreatic cancer cells, Melstrom et al
(8) demonstrated that 100
µM apigenin inhibited cell growth and lowered GLUT-1 mRNA
expression. The same study also reported that apigenin was able to
inhibit GLUT-1 expression at the transcriptional and translational
levels in a dose- and time-dependent manner in human pancreatic
cancer. However, the mechanism by which apigenin inhibits GLUT-1
expression is currently unknown. Melstrom et al (8) reported that apigenin may inhibit the
phosphoinositide 3-kinase (PI3K)/Akt pathway in order to reduce
GLUT-1 expression, thus inhibiting the absorption of glucose by
pancreatic cancer cells, resulting in apoptosis. However, it was
also detected that overexpression of phosphorylated-Akt did not
completely attenuate the effects of apigenin on GLUT-1, indicating
that the PI3K/Akt pathway is not solely responsible for the
downregulation of GLUT-1 in pancreatic cancer cells treated with
apigenin (8). Therefore, the
mechanism by which apigenin inhibits GLUT-1 expression requires
further investigation. Our future studies aim to investigate
whether apigenin inhibits GLUT-1 expression via the PI3K/Akt-HIF
axis in ACC.
In conclusion, the present study demonstrated that
apigenin inhibits proliferation and induces cell apoptosis, and
G2/M-phase arrest in ACC-2 cells, possibly due to
decreased GLUT-1 expression.
Acknowledgments
The present study was supported by the Health
Department of Zhejiang Province, China (grant no. 2012KYB206), and
the National Natural Science Foundation of China (grants nos.
81172562 and 81372903).
References
|
1
|
Kim B: Palliative radiotherapy in a
patient with pulmonary adenoid cystic carcinoma. Cancer Res Treat.
39:185–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang J, Bao YY, Zhou SH, Luo XM, Yao HT,
He JF and Wang QY: Recurrent prognostic factors and expression of
GLUT-1, PI3K and p-Akt in adenoid cystic carcinomas of the head and
neck: Clinicopathological features and biomarkers of adenoid cystic
carcinoma. Oncol Lett. 4:1234–1240. 2012.PubMed/NCBI
|
|
3
|
Papaspyrou G, Hoch S, Rinaldo A, Rodrigo
JP, Takes RP, van Herpen C, Werner JA and Ferlito A: Chemotherapy
and targeted therapy in adenoid cystic carcinoma of the head and
neck: A review. Head Neck. 33:905–911. 2011. View Article : Google Scholar
|
|
4
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemo-prevention: Progress potential and promise
(review). Int J Oncol. 30:233–245. 2007.
|
|
5
|
Oishi M, Iizumi Y, Taniguchi T, Goi W,
Miki T and Sakai T: Apigenin sensitizes prostate cancer cells to
Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS
One. 8:e559222013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: Apigenin induces c-Myc-mediated apoptosis in FRO
anaplastic thyroid carcinoma cells. Mol Cell Endocrinol.
369:130–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao X, Liu B, Cao W, Zhang W, Zhang F,
Zhao H, Meng R, Zhang L, Niu R, Hao X and Zhang B: Autophagy
inhibition enhances apigenin-induced apoptosis in human breast
cancer cells. Chin J Cancer Res. 25:212–222. 2013.PubMed/NCBI
|
|
8
|
Melstrom LG, Salabat MR, Ding XZ, Milam
BM, Strouch M, Pelling JC and Bentrem DJ: Apigenin inhibits the
GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt
pathway in human pancreatic cancer cells. Pancreas. 37:426–431.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He J, Xu Q, Wang M, Li C, Qian X, Shi Z,
Liu LZ and Jiang BH: Oral administration of apigenin inhibits
metastasis through AKT/P70S6K1/MMP-9 pathway in orthotopic ovarian
tumor model. Int J Mol Sci. 13:7271–7282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan LP, Chou TH, Ding HY, Chen PR, Chiang
FY, Kuo PL and Liang CH: Apigenin induces apoptosis via tumor
necrosis factor receptor- and Bcl-2-mediated pathway and enhances
susceptibility of head and neck squamous cell carcinoma to
5-fluorouracil and cisplatin. Biochim Biophys Acta. 1820:1081–1091.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melstrom LG, Salabat MR, Ding XZ, Strouch
MJ, Grippo PJ, Mirzoeva S, Pelling JC and Bentrem DJ: Apigenin
down-regulates the hypoxia response genes: HIF-1α, GLUT-1, and VEGF
in human pancreatic cancer cells. J Surg Res. 167:173–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X
and Jiang BH: Apigenin inhibits tumor angiogenesis through
decreasing HIF-1alpha and VEGF expression. Carcinogenesis.
28:858–864. 2007. View Article : Google Scholar
|
|
13
|
Liu LZ, Fang J, Zhou Q, Hu X, Shi X and
Jiang BH: Apigenin inhibits expression of vascular endothelial
growth factor and angiogenesis in human lung cancer cells:
Implication of chemoprevention of lung cancer. Mol Pharmacol.
68:635–643. 2005.PubMed/NCBI
|
|
14
|
Li LF, Zhou SH, Zhao K, Wang SQ, Wu QL,
Fan J, Cheng KJ and Ling L: Clinical significance of FDG
single-photon emission computed tomography: Computed tomography in
the diagnosis of head and neck cancers and study of its mechanism.
Cancer Biother Radiopharm. 23:701–714. 2008. View Article : Google Scholar
|
|
15
|
Wu XH, Chen SP, Mao JY, Ji XX, Yao HT and
Zhou SH: Expression and significance of hypoxia-inducible factor-1α
and glucose transporter-1 in laryngeal carcinoma. Oncol Lett.
5:261–266. 2013.
|
|
16
|
Yan SX, Luo XM, Zhou SH, Bao YY, Fan J, Lu
ZJ, Liao XB, Huang YP, Wu TT and Wang QY: Effect of antisense
oligodeoxynucleotides glucose transporter-1 on enhancement of
radiosensitivity of laryngeal carcinoma. Int J Med Sci.
10:1375–1386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu YY, Bao YY, Zhou SH and Fan J: Effect
on the expression of MMP-2, MT-MMP in laryngeal carcinoma Hep-2
cell line by antisense glucose transporter-1. Arch Med Res.
43:395–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu TQ, Fan J, Zhou L and Zheng SS:
Effects of suppressing glucose transporter-1 by an antisense
oligodeoxynucleotide on the growth of human hepatocellular
carcinoma cells. Hepatobiliary Pancreat Dis Int. 10:72–77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruno A, Siena L, Gerbino S, Ferraro M,
Chanez P, Giammanco M, Gjomarkaj M and Pace E: Apigenin affects
leptin/leptin receptor pathway and induces cell apoptosis in lung
adenocarcinoma cell line. Eur J Cancer. 47:2042–2051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Mao Y, Chen H, Lin Y, Hu Z, Wu J,
Xu X, Xu X, Qin J and Xie L: Apigenin promotes apoptosis, inhibits
invasion and induces cell cycle arrest of T24 human bladder cancer
cells. Cancer Cell Int. 13:542013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng PW, Chiang LC and Lin CC: Apigenin
induced apoptosis through p53-dependent pathway in human cervical
carcinoma cells. Life Sci. 76:1367–1379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao YY, Zhou SH, Fan J and Wang QY:
Anticancer mechanism of apigenin and the implications of GLUT-1
expression in head and neck cancers. Future Oncol. 9:1353–1364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JI, Choi KU, Lee IS, Choi YJ, Kim WT,
Shin DH, Kim K, Lee JH, Kim JY and Sol MY: Expression of hypoxic
markers and their prognostic significance in soft tissue sarcoma.
Oncol Lett. 9:1699–1706. 2015.PubMed/NCBI
|