Introduction
Chemotherapy is one of the most important methods
for the clinical treatment of glioma. However, drug resistance of
glioma cells is gradually induced with the long-term application of
chemotherapeutic drugs, eventually resulting in treatment failure.
Multidrug resistance (MDR) is the main form of drug resistance in
glioma (1,2). The MDR gene mediates this process and
constitutes the classic pathway of drug resistance. The MDR gene
has now a the major prognostic factor for patients with glioma.
Therefore, the reversal of drug resistance is an urgent issue to be
addressed in the treatment of glioma and may improve treatment
outcomes.
With the advances in immunology, molecular biology
and oncology, adoptive immunotherapy has attracted large amounts of
attention as an important treatment method for tumors following
chemotherapy (3,4). Phytohemagglutinin (PHA) as a
polyclonal activator of cells can induce the transformation of
immature lymphocytes into lymphoblasts, followed by proliferation,
release of lymphokines and enhanced phagocytosis of macrophages
(5). Conventional cytokine-induced
killer cells (CIK) are a type of immunological effector cells with
potent killing ability. They are derived from human peripheral
blood mononuclear cells following stimulation with anti-CD3
monoclonal antibody, interleukin (IL)-1, IL-2 and interferon
(IFN)-γ under in vitro conditions, simulating the in
vivo physiological environment (6,7).
In the present study, CIK were generated by
stimulating peripheral blood mononuclear cells with PHA, IL-2 and
IFN-γ. The potency of these CIK against a cisplatin-resistant U87MG
glioma cell line (U87MG/DDP) was studied and the mechanisms of
action were investigated. CIK were cultured in order to provide not
only sufficient quantities of highly efficient killer cells for
future implementation of tumor biotherapy, but also a novel method
for the adoptive immunotherapy of glioma.
Materials and methods
Preparation and culturing of cells
Mononuclear cells were isolated from peripheral
blood obtained from healthy volunteers using density-gradient
centrifugation (speed, 800 × g; duration, 20 min) with lymphocyte
separation medium (density, 1.077±0.002). The mononuclear cells
were re-suspended in cell culture medium (Beyotime Institute of
Biotechnology, Shanghai, China) to a density of
4.0×106/ml. On day 0, the cells were co-stimulated with
PHA (25 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) and IFN-γ
(300 U/ml; R&D Systems, Inc., Minneapolis, MN, USA) for culture
in an incubator at 37°C with an atmosphere containing 5%
CO2 for 24 h. On day 2, IL-2 (1,000 U/ml; R&D
Systems, Inc.) was added. The medium was replaced with medium
containing IL-2 (1,000 U/ml) once every three days until the
effector cells were harvested on day 20. The U87MG cells and
U87MG/DDP cell lines were obtained from Tongpai Biological
Technology Co., Ltd. (Shanghai, China).
ELISA assay
A total of 5×105 CIK were harvested and
cytokine secretion by the CIK was detected by ELISA (R&D
Systems). For this, 3×105 cells were seeded into wells
of a six-well micro-plate and incubated overnight. Medium without
fetal calf serum was added and the secretion of IFN-γ, IL-2, IL-6
and IL-12 was measured 72 h later following the manufacturer's
instructions.
MTS assay
Cells in the logarithmic growth phase were seeded in
a 96-well microplate at 100 µl/well (5×104
cells/ml) and cultured overnight to allow for cell attachment.
Subsequently, 0, 0.1, 0.5, 1, 5, 10, 50 and 100 µM cisplatin
(Sigma-Aldrich) was added. The effector (CIK)/target (U87MG/DDP)
ratio (E/T ratio) was 10:1 and 20:1. After continuous culture for
72 h, the medium was removed. MTS (Promega Corporation, Madison,
WI, USA) was added in accordance with the manufacturer's
instructions and incubation was continued for 4 h. Finally, the
optical density (OD) at 490 nm wavelength was detected using a
microplate reader (Multiskan® Spectrum; Thermo
Scientific, Rockford, IL, USA). The inhibition rate of cisplatin on
the cells was calculated as follows: Inhibition rate =
(1−ODexperimental group/ODcontrol group)
x100%. Displaying the cisplatin concentration on the abscissa and
the inhibition rate on the ordinate, the curve was plotted and
fitted to obtain the IC50 value. The reversal fold (RF)
was calculated as RF = IC50 (CIK-treated
group)/IC50 (control group).
The cells were divided into the following treatment
groups: Parental group, U87MG cells; Group I, U87MG/DDP; Group II,
U87MG/DDP with 10:1 CIK; Group III, U87MG/DDP with 20:1 CIK. The
following assays were performed on 10:1 and 20:1 mixtures of glioma
and CIK cells.
Flow cytometry
After treatment with cisplatin and CIK for 72 h, the
tumor cells were collected and stained with fluorescein
isothiocyanate (FITC)-conjugated Annexin V/propidium iodide (PI)
away from light for 15 min. Apoptosis was then detected by flow
cytometry (BD FACSAria™; BD Biosciences, Franklin Lakes, NJ, USA).
The apoptotic rate was calculated using FACSDiva software version
8.0 (BD Biosciences).
After treatment with CIK for 72 h, the tumor cells
were collected and cultured with phycoerythrin-P-glycoprotein
(P-gp) or rhodamine (Rh)-123 antibody away from light for 30 min.
P-gp and Rh-123 expression in these cells was detected using flow
cytometry and the P-gp- or Rh-123-positive rate was calculated
using FACSDiva software.
For cell cycle analysis, cells were treatment with
CIK for 72 h and the tumor cells were collected and incubated with
PI away from light for 30 min. The cell cycle was determined by
flow cytometry and calculated using ModFit3.0 software (Verity
Software House, Topsham, ME, USA).
Western blot analysis
After treatment with CIK for 72 h, the tumor cells
were collected and lysed using lysis buffer (Beyotime Institute of
Biotechnology) to extract the total proteins. The concentration was
measured using a BCA Protein assay kit (Beyotime Institute of
Biotechnology). Proteins (100 µg) were separated using 12% SDS-PAGE
and transferred onto a polyvinylidene difluoride membrane. After
blocking in 5% skimmed milk powder at room temperature for 1 h, the
membrane was incubated with MDR1 [cat. no. sc-55510; mouse
immunoglobulin (Ig)G], MDR-associated protein (MRP)1 (cat. no.
sc-365635; mouse IgG1), B-cell lymphoma 2 (Bcl-2; cat. no. sc-7382;
mouse IgG1), survivin (cat. no. sc-374616; mouse IgG1), glutathione
S-transferase (GST)-π (cat. no. sc-374171; mouse IgG1), nuclear
factor-κB (cat. no. sc-292436; rabbit IgG), total-AKT (cat. no.
sc-5298; mouse IgG1) and phosphorylated (p)-Akt (cat. no.
sc-293125; mouse IgG1) primary antibodies at 4°C overnight
(dilution, 1:2,000; all obtained from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). β-actin (cat. no. sc-8432; mouse IgG1)
served as an internal control (dilution, 1:5,000; Santa Cruz
Biotechnology, Inc.). After washing the primary antibodies off with
10% phosphate-buffered saline (three washes), the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G at room temperature for 1 h. After washing, the
immunoreactive bands were developed by enhanced chemiluminescence
(EMD Millipore, Billerica, MA, USA) and the film was obtained from
SiDaTe (Suzhou, China). The relative expression was represented as
the ratio of target protein and β-actin bands.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) assay
After treatment with CIK for 72 h, the tumor cells
were collected to extract the total RNA from each group using the
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's instructions. The cDNA was obtained
through reverse transcription using a real-time PCR kit (Tiangen
Biotech Co., Ltd., Beijing, China) and an ABI7500 Real-Time PCR
system (Applied Biosystems Life Technologies, Foster City, CA,
USA). After denaturation at 94°C for 5 min, 35 cycles of
amplification were performed under the following conditions: 95°C
for 15 sec, 65°C for 40 sec and 72°C for 90 sec; followed by
extension at 72°C for 5 min after these cycles. The oligonucleotide
sequences (Sangon Biotech Co., Ltd., Shanghai, China) were as
follows: MDR1 forward, 5′-AAAAAGATCAACTCGTACCACTC-3′ and reverse,
5′-GCACAAAATACACCAACAA-3′; MRP1 forward, 5′-GTGGAATTCCGGAACTAC-3′
and reverse, 5′-CGGAGGTCGTGCAGGCCG-3′; GST-π forward,
5′-CTGGAAGGAGGAGGTGGTG-3′ and reverse, 5′-GACGCAGGATGGTATTGGAC-3′;
Survivin forward, 5′-CGAGGCTGGCTTCATCCACT-3′ and reverse
5′-ACGGCGCACTTTCTTCGCA-3′; Bcl-2 forward, 5′-GGCTGGGATGCCTTTGTG-3′
and reverse, 5′-GCCAGGAGAAATCAAACAGAGG-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The PCR products were quantified
using the 2−ΔΔCt method.
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 21.0 software (International Business Machines,
Armonk, NY, USA) was used for analysis. One-way analysis of
variance was used for comparison and P<0.05 indicated a
statistically significant difference.
Results
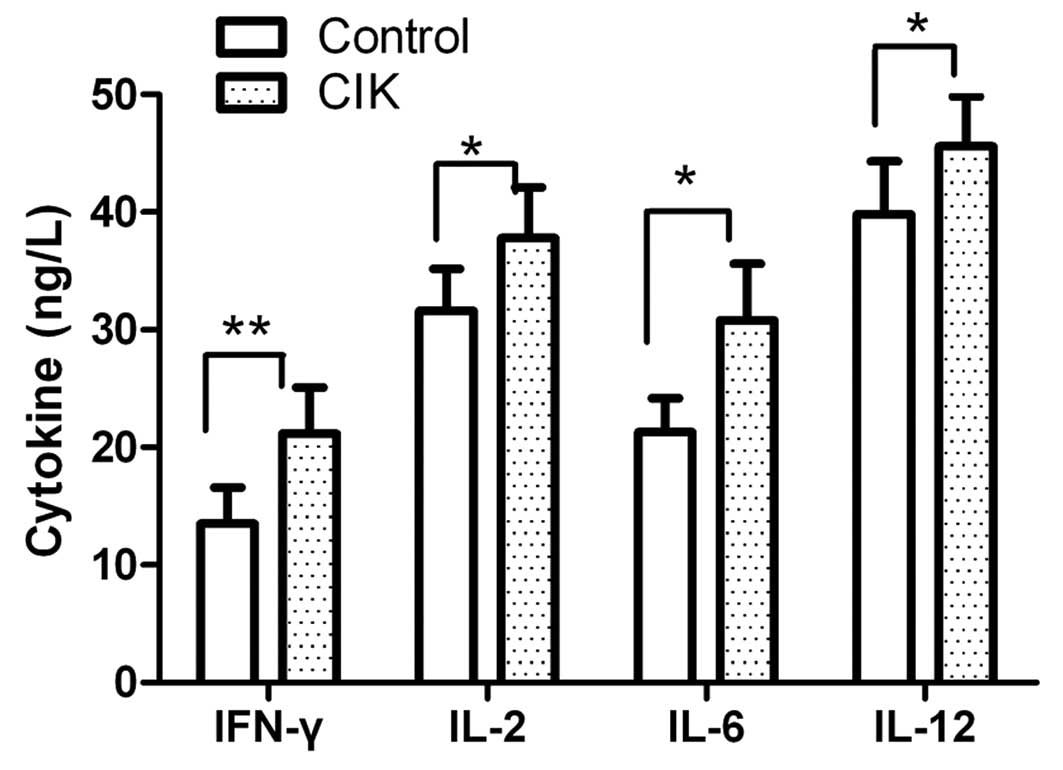
Cell cytokine secretion by CIK
The cell cytokine secretion of CIK was detected
using an ELISA assay. The CIK mainly produced IFN-γ, IL-2, IL-6 and
IL-12 at the end of the culturing period. Cytokine secretion levels
in each group were assessed prior to and after inductive cytokine
treatments. As shown in Fig. 1,
the levels of IFN-γ, IL-2, IL-6 and IL-12 were slightly enhanced
following culture for 72 h.
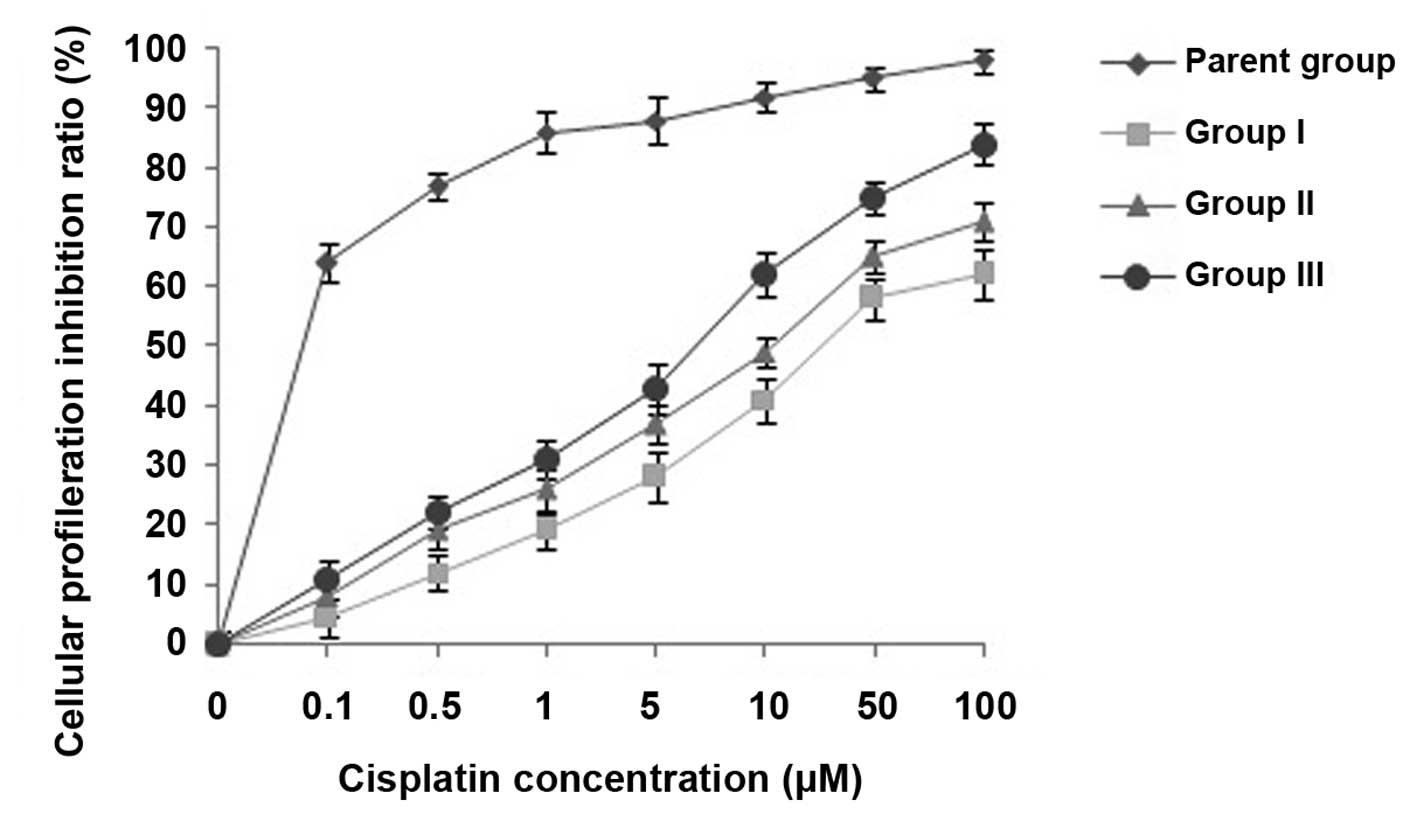
Effects of CIK cells on MDR reversal in
U87MG/DDP cells
The viability of U87MG/DDP cells treated with
cisplatin at various concentrations (0, 0.1, 0.5, 1, 5, 10, 50 and
100 µM) for 72 h was assessed and the effects of
co-incubation with CIK on MDR reversal in U87MG/DDP were evaluated
using an MTS assay. As shown in Fig.
2, the cytotoxicity of cisplatin to group-II cells was higher
than that to group-I cells, while that to group-III cells was even
higher, indicating an MDR-reversing effect of CIK on U87MG/DDP.
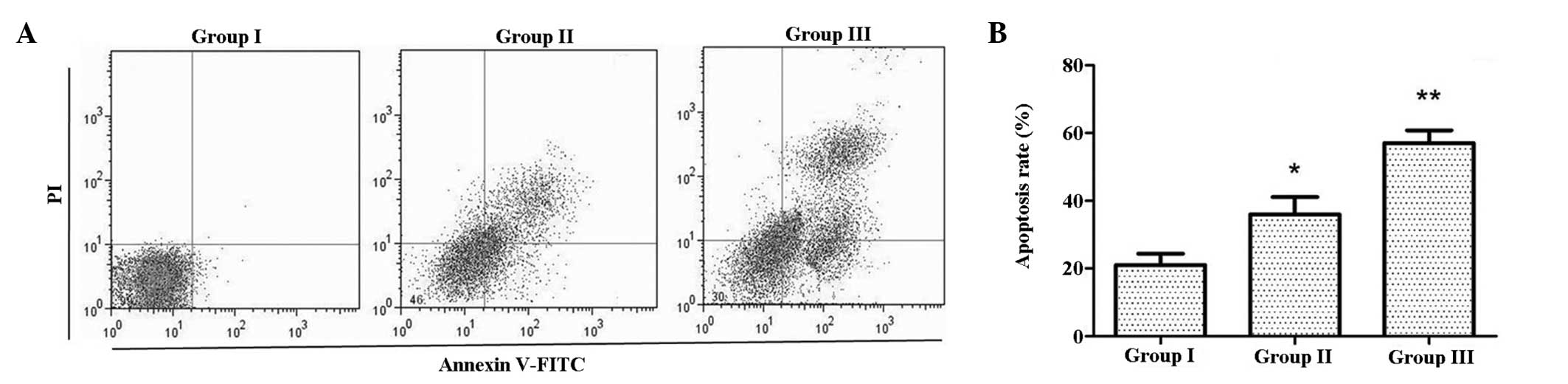
CIK induce apoptosis in U87MG/DDP
To investigate the effects of CIK on apoptosis,
Annexin V-FITC/PI double staining was employed. As shown in
Fig. 3, the apoptotic rate in
group II was higher than that in group I, while that in group III
was even higher. This indicated that co-culture with CIK increased
the apoptotic rate of U87MG/DDP.
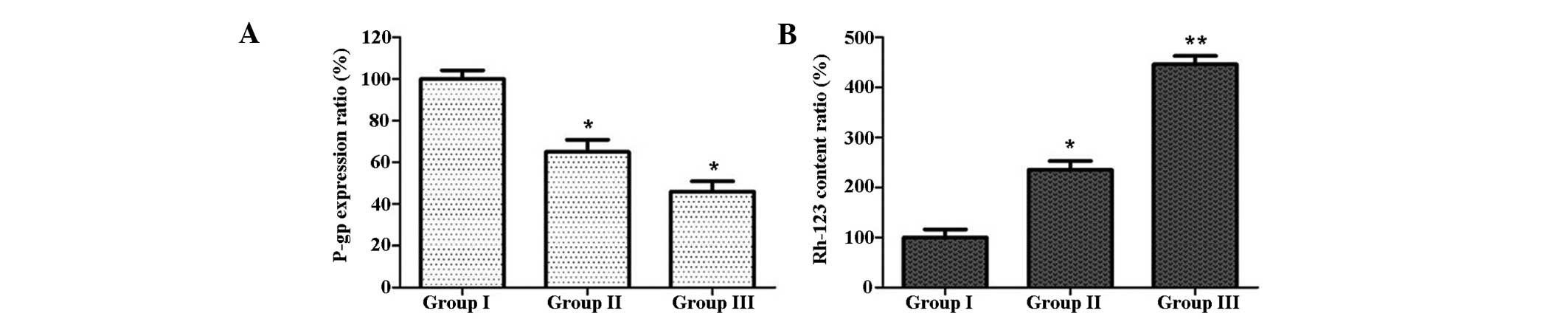
CIK induce Rh-123 production and reduce
P-gp expression in U87MG/DDP
The intracellular Rh-123 content and expression of
P-gp were analyzed by flow cytometry. As shown in Fig. 4, the intracellular Rh-123 content
in group II was increased compared with that in group I, and the
expression of P-gp in group II was lower than that in group I;
these effects were even more marked in group III. These results
indicated that co-incubation with CIK increased the intracellular
Rh-123 content, while having an inhibitory effect on P-gp
expression in U87MG/DDP cells.
CIK reduces MDR-associated gene
expression in U87MG/DDP
To further study the mechanism of the MDR reversal
by CIK, the expression of MDR-associated proteins and genes (MDR1,
MRP1, Bcl-2, Survivin and GST-π) was analyzed by western blot and
RT-qPCR analyses. As shown in Fig.
5, the protein and mRNA expression of these genes in group II
was lower than that in group I, while it was even lower in group
III, indicating an inhibitory effect of CIK on the expression of
MDR-associated genes in U87MG/DDP.
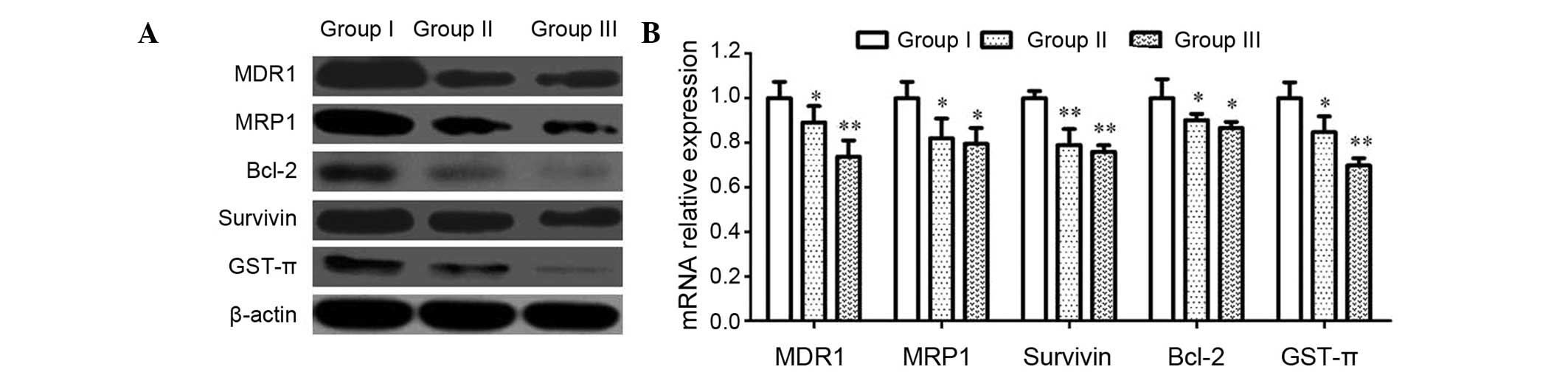 | Figure 5Effects of CIK on MDR-associated
proteins and genes in U87MG/DDP cells. (A) U87MG/DDP cells were
treated for 72 h, and the protein expression of MDR1, MRP1, Bcl-2,
Survivin and GST-π was assessed using western blotting. (B) mRNA
levels of MDR1, MRP1, Bcl-2, Survivin and GST-π were assessed by
reverse transcription-quantitative polymerase chain reaction assays
in cells. GAPDH served as an internal reference. Values are
expressed as the mean ± standard deviation (n=5).
*P<0.05 and **P<0.01. Groups: I,
U87MG/DDP; II, U87MG/DDP with 10:1 CIK; III, U87MG/DDP with 20:1
CIK. CIK, cytokine-induced killer cells; U87MG/DDP,
cisplatin-resistant U87MG cell line; MDR, multidrug-resistance;
MRP, MDR-associated protein; Bcl-2, B-cell lymphoma 2; GST,
glutathione S-transferase. |
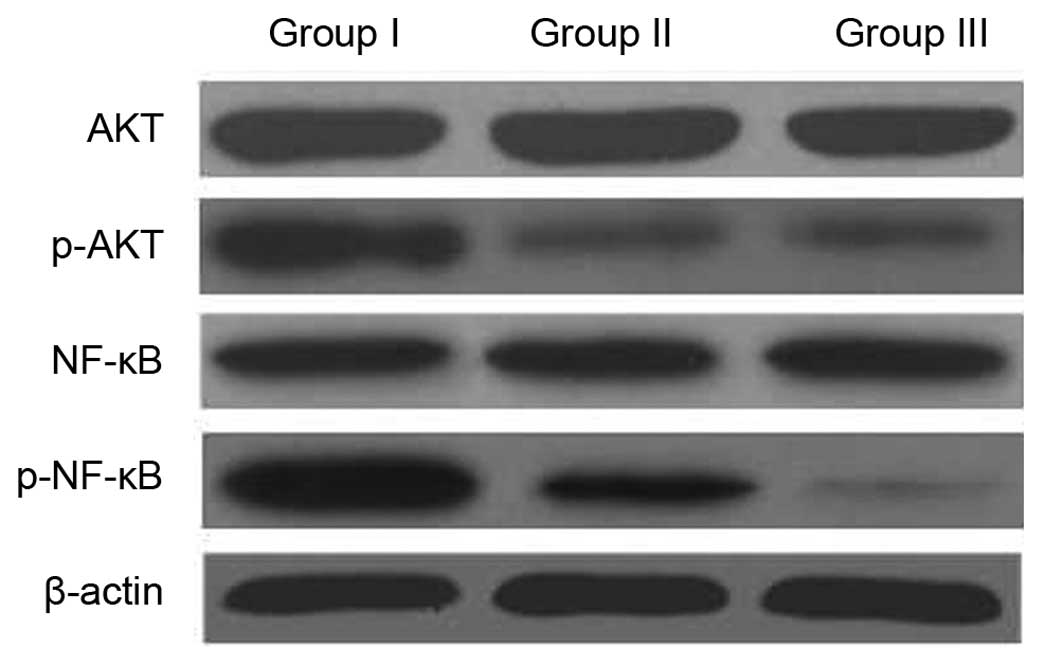
The NF-κB/AKT signaling pathway is
involved in the MDR reversal of CIK
It has been reported that activation of NF-κB
followed by Akt phosphorylation has a role in the regulation of
cell survival, apoptosis and drug resistance (8,9). The
present study therefore investigated whether the intracellular
AKT/NF-κB signaling pathway was involved in the effects of CIK on
MDR. The expression of NF-κB and AKT was assessed by western blot
analysis. As shown in Fig. 6,
co-incubation with CIK decreased the phosphorylation of AKT, but
did not affect total-Akt expression. Furthermore, the expression
levels of NF-κB were decreased in U87MG/DDP co-incubated with CIK.
These results suggested that CIK induced apoptosis and MDR reversal
by inactivation of NF-κB via the Akt/NF-κB pathway.
Discussion
Due to the insidious onset of glioma and high degree
of malignancy, most patients are already in
intermediate-to-advanced stage at the time of diagnosis, which is
characterized by a low rate of applicability of resection and
frequently occurring post-operative local recurrence and
metastasis. In addition, the poor immune function in patients with
intermediate-to-advanced stage glioma further results in poor
clinical efficacy (10,11). Accordingly, it is of high
importance to identify novel treatment protocols, improve the
quality of life in patients with intermediate-to-advanced stage
glioma and to increase their survival rate. In recent years,
biotherapy has attracted increasing attention as a treatment option
for glioma (12,13).
Based on the enormous potential of immune cells in
tumor treatment, biotherapy has become a novel and increasingly
emphasized means of comprehensive tumor treatment. CIK have the
potent anti-tumor activity of T cells as well as the non-major
histocompatibility complex-restricted cytotoxicity of natural
killer cells with a high proliferation rate and fewer toxic/side
effects (14). As experimental and
clinical studies have confirmed the efficacy of CIK on malignant
tumors, CIK have rapidly become a focus of tumor immunotherapy
research. A significant difference in cisplatin sensitivity with
10:1 and 20:1 mixtures of glioma and CIK cells was identified.
Given the fact that the mechanisms of the drug
resistance of glioma cells are closely associated with MDR genes,
effective downregulation of these genes is the primary approach to
reverse drug resistance (15–17).
MDR1 is expressed in normal tissue and cell types. P-glycoprotein
(P-gp), encoded by MDR1, is embedded in the cell membrane surface
to form an efflux pump. P-gp can also effect the secretion and
transport of lipids and exert additional functions, among which
antiport predominates (18,19).
The physiological significance of P-gp is to prevent cytotoxicity
resulting from intracellular accumulation of endogenous or
exogenous lipid-soluble substances, including certain cellular
metabolites, toxicants and other substances in order to maintain a
relatively stable intracellular milieu. Certain lipid-soluble and
naturally derived drugs can induce P-gp expression to easily cause
the development of multi-drug resistance. Rh-123 is a classic
substrate for the examination of P-gp activity; the in vivo
elimination of Rh-123 is only correlated with P-gp activity and can
therefore be used to determine intracellular drug concentrations
(20). CIK can effectively
downregulate the expression of intracellular MDR1 and P-gp, while
upregulating Rh-123 (21).
MRP1, a member of the adenosine triphosphate (ATP)
binding cassette transporter protein super-family, can upregulate
the expression of ATP-dependent GST-π to excrete conjugated anions
from the cells and have a role in the clearance of exogenous toxins
(22,23). In the present study, CIK
effectively downregulated the expression of intracellular MRP1 and
GST-π, which may represent one of the mechanisms for the reversal
of tumor drug resistance. Bcl-2 and Survivin are two common
anti-apoptotic proteins, which are significantly upregulated in
drug-resistant tumor cells compared with non-resistant tumor cells.
The present study confirmed that co-culture with CIK downregulated
Bcl-2 and Survivin levels and increased apoptosis in U87MG/DPP.
Furthermore, co-culture with CIK downregulated the expression of
NF-κB and p-Akt in U87MG/DPP, suggesting that the activity of this
signaling pathway was decreased and that NF-κB and p-Akt signaling
pathways were involved in CIK-mediated reversal of drug
resistance.
The drug resistance of glioma is the underlying
cause of the limited efficacy of clinical treatments. Identifying
effective reversal methods is therefore a contemporary issue to be
addressed. The results of the present study showed that co-culture
with CIK was able to reverse the resistance of glioma cells to the
chemotherapeutic drug cisplatin in vitro. This enhancement
of the sensitivity of glioma cells was mediated via the NF-κB and
p-Akt signaling pathways, the induction of apoptosis and the
reduction of the expression of MDR-associated genes. Future
clinical studies should continuously aim to discover and explore
methods with enhanced effectiveness in order to provide novel
approaches for the development of clinical treatments to overcome
drug resistance. CIK as a novel therapeutic have been employed in
the clinical treatment of tumors due to their high tumoricidal
activity, low side effects and other characteristics. Given the
promising application prospect of this method, it is necessary to
perform more in-depth studies.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81200957).
References
|
1
|
Fan QW and Weiss WA: Targeting the
RTK-PI3K-mTOR axis in malignant glioma: Overcoming resistance. Curr
Top Microbiol Immunol. 347:279–296. 2010.PubMed/NCBI
|
|
2
|
Desjardins A, Rich JN, Quinn JA,
Vredenburgh J, Gururangan S, Sathornsumetee S, Reardon DA, Friedman
AH, Bigner DD and Friedman HS: Chemotherapy and novel therapeutic
approaches in malignant glioma. Front Biosci. 10:2645–2668. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curiel TJ: Immunotherapy: A useful
strategy to help combat multidrug resistance. Drug Resist Updat.
15:106–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji J, Black KL and Yu JS: Glioma stem cell
research for the development of immunotherapy. Neurosurg Clin N Am.
21:159–166. 2010. View Article : Google Scholar
|
|
5
|
Tu W, Cheung PT and Lau YL: Insulin-like
growth factor 1 promotes cord blood T cell maturation and inhibits
its spontaneous and phytohemagglutinin-induced apoptosis through
different mechanisms. J Immunol. 165:1331–1336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JS, Chung IS, Lim SH, Park Y, Park MJ,
Kim JY, Kim YG, Hong JT, Kim Y and Han SB: Preclinical and clinical
studies on cytokine-induced killer cells for the treatment of renal
cell carcinoma. Arch Pharm Res. 37:559–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rettinger E, Kreyenberg H, Merker M, Kuci
S, Willasch A, Bug G, Ullrich E, Wels WS, Bonig H, Klingebiel T and
Bader P: Immunomagnetic selection or irradiation eliminates
alloreactive cells but also reduces anti-tumor potential of
cytokine-induced killer cells: Implications for unmanipulated
cytokine-induced killer cell infusion. Cytotherapy. 16:835–844.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X,
Lu XH, Shen L, Liu BN, Liu J, Luo HS, et al: The
AKT1/NF-kappaB/Notch1/PTEN axis has an important role in
chemoresistance of gastric cancer cells. Cell Death Dis.
4:e8472013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Li CF, Pan LM and Gao ZL:
7,8-Dihydroxycoumarin inhibits A549 human lung adenocarcinoma cell
proliferation by inducing apoptosis via suppression of Akt/NF-κB
signaling. Exp Ther Med. 5:1770–1774. 2013.PubMed/NCBI
|
|
10
|
Piil K, Juhler M, Jakobsen J and Jarden M:
Controlled rehabilitative and supportive care intervention trials
in patients with high-grade gliomas and their caregivers: A
systematic review. BMJ Support Palliat Care. pii:
bmjspcare-2013-000593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dirven L, Aaronson NK, Heimans JJ and
Taphoorn MJ: Health-related quality of life in high-grade glioma
patients. Chin J Cancer. 33:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melin B and Jenkins R: Genetics in glioma:
Lessons learned from genome-wide association studies. Curr Opin
Neurol. 26:688–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Holsinger RM, Kruse CA, Flügel A and
Graeber MB: The potential for genetically altered microglia to
influence glioma treatment. CNS Neurol Disord Drug Targets.
12:750–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zhu L, Zhang Q, He X, Yin Y, Gu
Y, Guo R, Lu K, Liu L, Liu P and Shu Y: Effects of cytokine-induced
killer cell treatment in colorectal cancer patients: A
retrospective study. Biomed Pharmacother. 68:715–720. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Queiroz RM, Takiya CM, Guimarães LP, Rocha
Gda G, Alviano DS, Blank AF, Alviano CS and Gattass CR:
Apoptosis-inducing effects of melissa officinalis L. essential oil
in glioblastoma multiforme cells. Cancer Invest. 32:226–235. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX,
Zhang GD, Wang Q and Zhang L: Alkylglyceronephosphate synthase
(AGPS) alters lipid signaling pathways and supports chemotherapy
resistance of glioma and hepatic carcinoma cell lines. Asian Pac J
Cancer Prev. 15:3219–3226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakai E, Park K, Yawata T, Chihara T,
Kumazawa A, Nakabayashi H and Shimizu K: Enhanced MDR1 expression
and chemoresistance of cancer stem cells derived from glioblastoma.
Cancer Invest. 27:901–908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goda K, Bacsó Z and Szabó G: Multidrug
resistance through the spectacle of P-glycoprotein. Curr Cancer
Drug Targets. 9:281–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lage H: MDR1/P-glycoprotein (ABCB1) as
target for RNA interference-mediated reversal of multidrug
resistance. Curr Drug Targets. 7:813–821. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu D, Tian W and Shen H: Curcumin prevents
induced drug resistance: A novel function? Chin J Cancer Res.
23:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Deng Q, Wang J, Bai X, Xiao X, Lv
HR, Zhao MF and Liu PJ: Effect of CIK on multidrug-resistance
reversal and increasing the sensitivity of ADR in K562/ADR cells.
Oncol Lett. 8:1778–1782. 2014.PubMed/NCBI
|
|
22
|
Peklak-Scott C, Smitherman PK, Townsend AJ
and Morrow CS: Role of glutathione S-transferase P1-1 in the
cellular detoxification of cisplatin. Mol Cancer Ther. 7:3247–3255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calatozzolo C, Gelati M, Ciusani E,
Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A,
Vitellaro-Zuccarello L, Croci D, et al: Expression of drug
resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human
glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|