Introduction
Slow transit constipation (STC) is usually
considered an intractable colonic motor type of functional
constipation. Patients who suffer from the disease tend to be less
affected by drug therapy (1) and
the most severe cases can require surgery (2,3).
Although the pathogenesis of STC remains to be elucidated, numerous
theories have attempted to explain its pathogenesis, including a
lack of fiber, autonomic neuropathy as well as disorders of the
enteric nervous system (ENS) and neuroendocrine system. The ENS
derived from neural crest cells is an independent comprehensive
nervous network system that consists of ganglionated plexuses,
where almost all intrinsic nerve cells reside (4,5). Two
ganglionated plexuses are located in the intestine, submucosa and
myenteric plexuses, and are known to be involved in all
gastrointestinal functions, including motility, secretion, blood
flow, mucosal growth and aspects of the local immune system. In
pathological situations, the number and type of the enteric neurons
may alter. For example, a previous study have demonstrated that the
tyrosine hydroxylase phenotype of enteric neurons can be regulated
by neuronal activity (6). A marked
difference in the expression of several messengers, including
vasoactive intestinal peptide, has also been observed in the
hypertrophic ileum of patients with Crohn's disease (7). It has been reported that apoptotic
phenomena significantly reduced the number of neurons and glial
cells in the myenteric plexus in STC patients (8,9).
Similar to these findings, the decreased density of neuronal cells
was also observed in patients with STC and acquired megacolon
(10). A previous study
demonstrated that BMP-2 promotes the differentiation of the
nitrergic and catecholaminergic enteric neurons in immorto fetal
enteric neuronal (IM-FEN) cell lines (11). However, the function of BMP-2 in
the differentiation of neuronal nitric oxide synthase (nNOS) in
patients with STC has not yet been reported.
Several studies have indicated that bone
morphogenetic protein-2 (BMP-2), a member of the transforming
growth factor (TGF)-β superfamily, is important in the process of
regulating the specification, migration, differentiation and
aggregation of enteric neurons (12–14).
BMPs, for example, promote the development of neurotrophin-3
(NT-3)-dependent, TrkC enteric dopaminergic neurons (15). The differentiation of neural
ectoderm and the establishment of the neural crest are also
regulated by BMPs (16,17). Inhibition of BMP activity using its
antagonist Noggin prevents normal migration of enteric neural crest
cells in vivo and in vitro (15). Furthermore, neural crest cell
migration and ganglion formation in the enteric nervous system were
regulated by BMP-2 and the absence of BMP signaling also leads to
failure of ganglion formation, with crest cells unable to aggregate
into clusters (18,19). Cellular responses to BMP-2 are
mediated by the formation of hetero-oligomeric complexes of type I
and type II serine/threonine kinase receptors, which are important
in the binding and signaling of BMPs (20). Smad1 is an immediate downstream
molecule of the BMP-2 receptors (21). Previous studies have revealed that
BMP-2 receptors lead to phosphorylation of Smad1/5/8 in a
ligand-dependent manner. When phosphorylated, a heteromeric complex
is formed by Smad1/5/8 with Smad4, which translocates to the
nucleus to control the expression of the target genes.
In the present study, using the primary enteric
neurons (from E15 rat embryos), the morphological and neurochemical
differentiation effect of BMP-2 was assessed. In addition, the
effect of BMP-2 on the expression of nNOS in primary enteric
neurons was assessed. Finally, the signal transduction pathways
involved were examined, focusing on the Smad1 signaling
pathways.
Materials and methods
Ethical statement
The protocol for this study was approved by the
Ethics Committee of Qingdao Municipal Hospital, (Qingdao, China),
Shandong Jiaotong Hospital (Jinan, China) and Shandong University,
(Jinan, China), and written informed consent was obtained from all
study patients.
All animal studies were approved by the
Institutional Animal Care and Use Committee of Shandong University.
Pregnant Sprague-Dawley rats were obtained from the Animal
Experiment Center of Shandong University according to the
Institutional Animal Care and Use Committee guidelines and all
efforts were made to minimize suffering.
Primary enteric neuron culture
Three Pregnant Sprague-Dawley rats (average weight,
201 g) were purchased from the Animal Experiment Center of Shandong
University. The rats were kept in a temperature-controlled
environment on a 12-h light/dark cycle with free access to food and
water. Rats were treated with an overdose of CO2
followed by severing the carotid arteries. All efforts were made to
minimize suffering. The embryos (E15; 35–45 per isolation from
three pregnant rats) were removed and sacrificed by decapitation.
Subsequently, the colon of embryos was removed and finely diced in
Hank's balanced salt solution (Sigma-Aldrich, St. Louis, MO, USA).
Tissue fragments were collected in 5 ml of medium (DMEM-F12 1:1
medium) and digested at 37°C for 15 min in 0.1% trypsin
(Sigma-Aldrich). The trypsin reaction was terminated by adding 10
ml of medium containing 10% fetal calf serum and then treated with
DNAse I (0.01%; Sigma-Aldrich) for 10 min at 37°C. Following
triturating with a 10 ml pipette, cells were centrifuged at 10,620
× g for 10 min. Cells were counted and then seeded at a density of
2.4×105 cells cm−2 on 24-well plates
previously coated for 6 h with a solution of gelatin (0.5%;
Sigma-Aldrich) in sterile phosphate-buffered saline (PBS). After 24
h, the medium was replaced with serum-free medium (DMEM-F12 1:1
containing 1% of N-2 supplement; Sigma-Aldrich). Cells were
maintained in culture for 7 days. Half of the medium was replaced
every other day.
Tissue preparation
All samples were acquired from Qingdao Municipal
Hospital and Shandong Jiaotong Hospital. Normal tissues of colon
samples were acquired from 20 patients with colon cancer as the
control group and 20 patients with STC who received surgery (STC
group). Normal samples were collected from areas adjacent to the
colon tumor tissue but outside the tumor margins. The control group
included 12 males and 8 females, and the STC group comprised 9
males and 11 females. The age of the control group was 67 years and
the STC group was 60 years. For histological examination, tissue
samples were fixed in 4% paraformaldehyde in PBS for 24 h and
dehydrated with gradually increasing concentrations of ethanol and
embedded in paraffin. A microtome was then used to obtain 5
µm-thick sections. These prepared slices were used for
hematoxylin and eosin (H&E; Beijing Solarbio Science &
Technology Co Ltd., Shanghai, China) and immunohistochemistry (IHC)
staining.
H&E staining for morphological
observation
The paraffin sections were deparaffinized and
hydrated in water. The paraffin sections were stained with
hematoxylin for 5 min, rinsed several times with distilled water
and then stained with eosin for 3 min. Finally, the slides were
dehydrated through graded alcohol, made transparent in xylene and
mounted with neutral gum. Alterations in basic morphology of the
myenteric neuron plexus and smooth muscles were observed under a
light microscope (Leica DM3000; Leica Microsystems, Wetzlar,
Germany).
IHC
The paraffin-embedded tissue sections were hydrated
in xylene (Guangcheng Chemical Reagent Co., Ltd., Tianjin, China)
and a graded alcohol series. Antigen retrieval was performed in a
water bath at 95°C for 20 min with citric acid buffer (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China),
and endogenous peroxidase activity was blocked with 3%
H2O2. Next, the tissue sections were
incubated with goat serum (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 45 min and stained with rabbit
anti-BMP-2 polyclonal antibody (cat. no. SAB1411278; 1:200
dilution; Sigma-Aldrich, Shanghai, China) and mouse anti-nNOS
monoclonal antibody (cat. no. sc-5302; 1:200 dilution Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) at 4°C overnight.
Following washing with PBS, the tissues were respectively incubated
with the biotin-labeled goat anti-rabbit IgG secondary antibody
(cat. no. ZDR-5306; 1:800 dilution; Zhongshan Golden Bridge
Biological Technology Inc.) and the biotin-labeled goat anti-mouse
IgG secondary antibody for 30 min at 37°C (cat. no. ZDR-5307; 1:800
dilution; Zhong Shan Golden Bridge Biological Technology Inc,
Beijing, China). Then tissues were stained with
3,3′-diaminobenzidine and hematoxylin (Zhongshan Golden Bridge
Biological Technology Inc, Beijing, China). The experiment was
repeated three times.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Primary enteric neurons were cultured in the
presence of BMP-2 (0–20 ng/ml) for 48 h. Total RNA was isolated
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). Total RNA (l µg per sample) was reverse-transcribed
using the ImProm-II Reverse Transcription System (Promega
Corporation, Madison, WI, USA). β-actin was used as a control. The
sequences of the primers used for PCR are shown as follows: nNOS,
forward 5′-GGTCGCTTTGAGTACCAGCCT-3′ and reverse
5′-GGTCGCTTTGACTCTCTTGG-3′; β-actin, forward
5′-AACAGTCCGCCTAGAACACA-3′ and reverse 5′-CGTTGACATCCGTAAAGACC-3′.
The product was analyzed on a 1.5% agarose gel stained with
ethidium bromide and the amplified products were visualized by
ultraviolet transillumination.
Western blotting
Proteins were obtained from primary enteric neurons
treated with BMP-2 (0–20 ng/ml) or its antagonist Noggin (100
ng/ml) for 48 h. Proteins were resolved on a 10% SDS-polyacrylamide
gel and electroblotted onto a polyvinylidene difluoride membrane
(Pall Corporation, Port Washington, NY, USA). The blots were then
incubated with rabbit polyclonal anti-pSmad1 (cat. no. sc-101800,
1:500 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), mouse monoclonal anti-β-actin (cat. no. sc-47778 1:2,000
dilution; Santa Cruz Biotechnology, Inc.) or mouse monoclonal
anti-nNOS antibody (cat. no. sc-5302 1:500 dilution; Santa Cruz
Biotechnology, Inc.). β-actin was used as a loading control. The
membranes were washed and incubated goat anti-rabbit IgG secondary
antibody (1:400 dilution; cat. no. sc-45101, Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG secondary antibody
(1:400 dilution cat. no. sc-395763; Santa Cruz Biotechnology Inc.)
for 1 h at 37°C. The experiment was performed using the SNAP i.d.
protein detection system (Millipore, Billerica, MA, USA) according
to the manufacturer's instructions and was repeated at least six
times. Finally, the signal was visualized following exposure to
X-ray film and the relative optical density (OD) ratio was
calculated with Image J software 1.4.3.67 (National Institutes of
Health, Bethesda, MD, USA) by comparison to β-actin.
Immunocytochemistry
Primary enteric neurons were treated with the
control, BMP-2 (10 ng/ml) or Noggin (100 ng/ml) for 24 h. Cells
were fixed using 4% paraformaldehyde (30 min) and permeabilized (10
min) with 0.3% Triton X-100. This was followed by blocking the
cells with 3% normal goat serum (20 min) and overnight incubation
with different primary antibodies (anti-nNOS dilution 1:500;
anti-pSmad1 dilution 1:500). Secondary detection was performed in
conjunction with goat anti-rabbit or anti-mouse IgG conjugated to
fluoresce. Fluorescent photomicrographs were obtained on a Leica
microscope (Leica DM1000B).
Morphological assessment of neuronal
differentiation
Primary enteric neurons were cultured for 1–4 days
in the presence of BMP-2 (0–20 ng/ml). Neuron differentiation was
assessed by measurement of neurite length using a phase contrast
microscope (Leica DMI3000B).
Immunohistochemical analysis
The expression of BMP-2 and nNOS in the myenteric
nerve plexus of STC and control tissues was subjected to
microscopic analysis. Following IHC staining, if a cell or tissue
was stained from light yellow to brown, it was recorded as positive
immunostaining. The areas from STC and its control normal tissue
were selected for analysis. The intensity of the staining signal
was measured and documented using Image-Pro Plus 6.0 image analysis
software (Media Cybernetics, Inc. Silver Spring, MD, USA). The mean
densitometry of the digital image (magnification, ×400) is
designated as representative BMP-2 and nNOS staining intensity
(indicating relative BMP-2 and nNOS expression level). The signal
density of tissue areas from five randomly selected fields were
counted blindly and subjected to statistical analysis.
Statistical analysis
Statistical analysis was performed using Student's
t-test or by one-way analysis of variance with Dunnett's t-test.
SPSS 17.0 (SPSS Inc., IBM, Armonk, NY, USA) was used to conduct
statistical analysis. Statistical tests were two sided. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of BMP-2 and nNOS in myenteric
neurons in STC
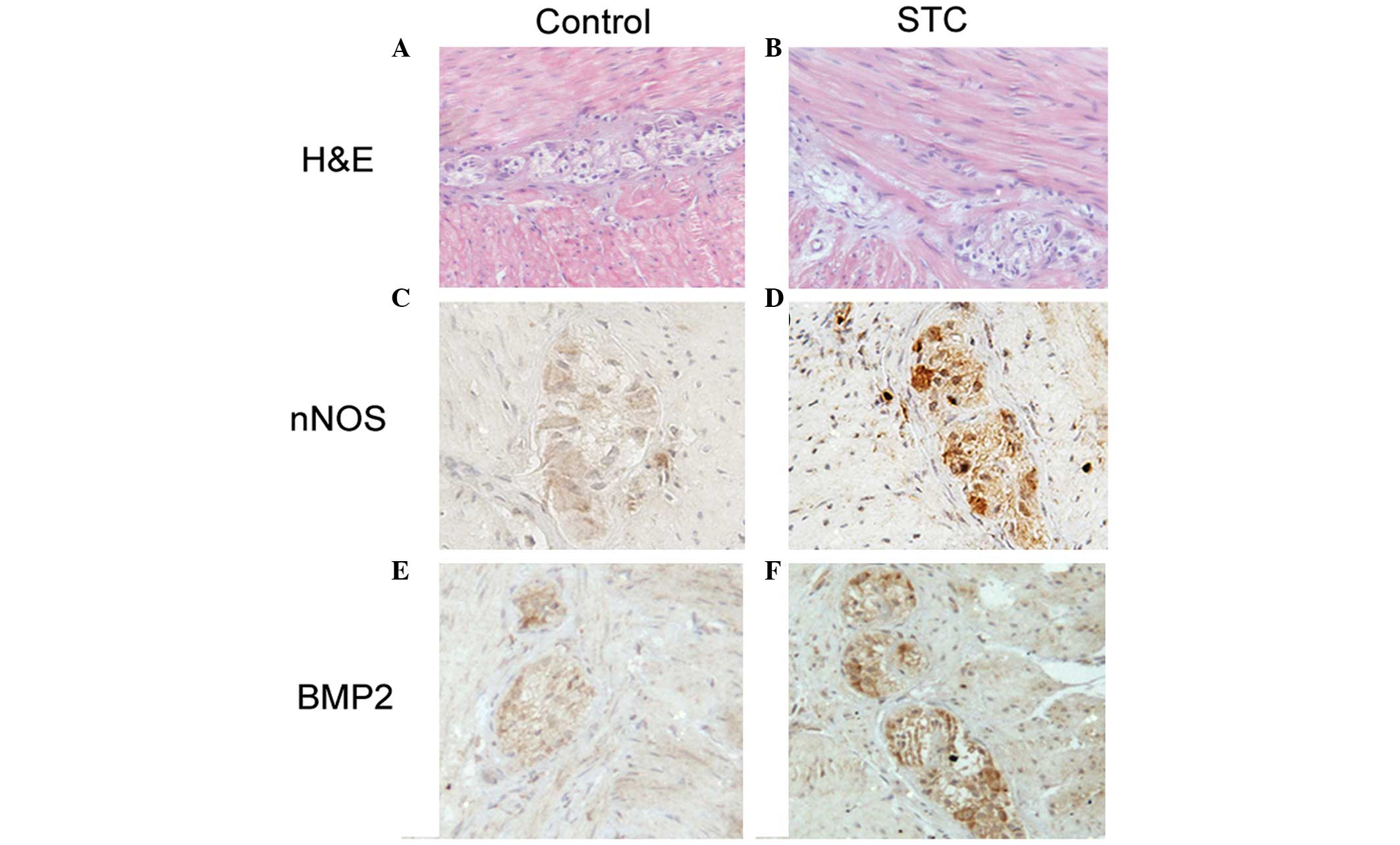
In order to determine the difference between the
control group and the STC group, the morphological changes of
myenteric neurons were examined. No apparent morphological
difference was identified in the myentric nerve plexus from H&E
staining (Fig. 1A and B). The
expression of nNOS increased in the STC group and was predominantly
located in the myenteric neurons, however, a small number of
positive nNOS-expressing neuronal cells were observed in the
control group (Fig. 1C and D). In
order to determine the association between BMP-2 and the
abovementioned variation of nNOS, BMP-2 was detected by IHC
staining and it was found that the expression of BMP-2 clearly
increased in the STC group compared with the control group
(Fig. 1E and F). The mean density
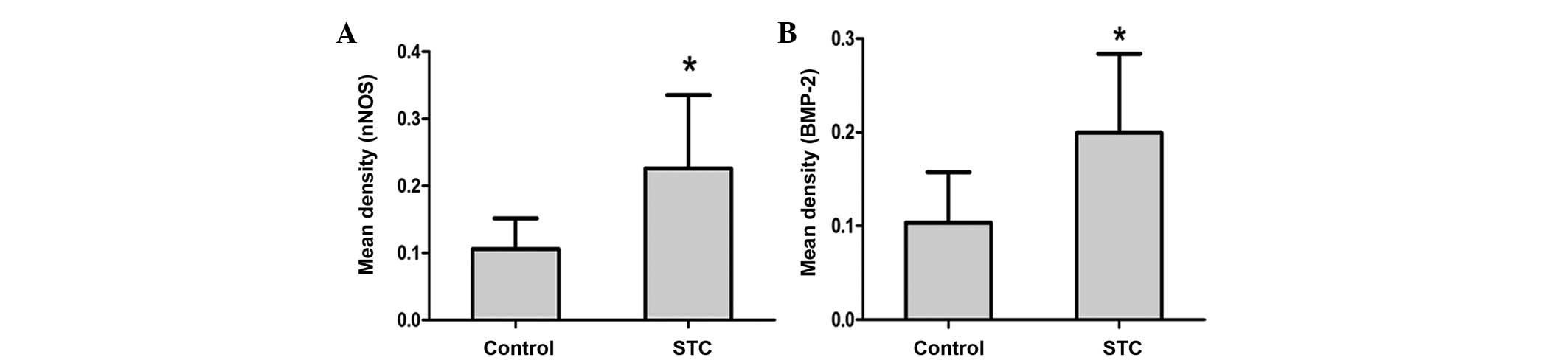
of nNOS and BMP-2 was also calculated in STC and control tissues.
The results demonstrated that the mean density of nNOS in STC
(0.2257±0.1095) was significantly higher than that of the control
tissues (0.1060±0.0454, n=20; P<0.05; Fig. 2A). The results also demonstrated
that the mean density of BMP-2 in STC (0.1993±0.0847) was
significantly higher than that of the control tissues
(0.1036±0.5391, n=20; P<0.05, Fig.
2B). The results demonstrated that nNOS and BMP-2 are
significantly upregulated in STC tissues compared with control
tissues.
BMP-2 induces the differentiation of
primary enteric neurons
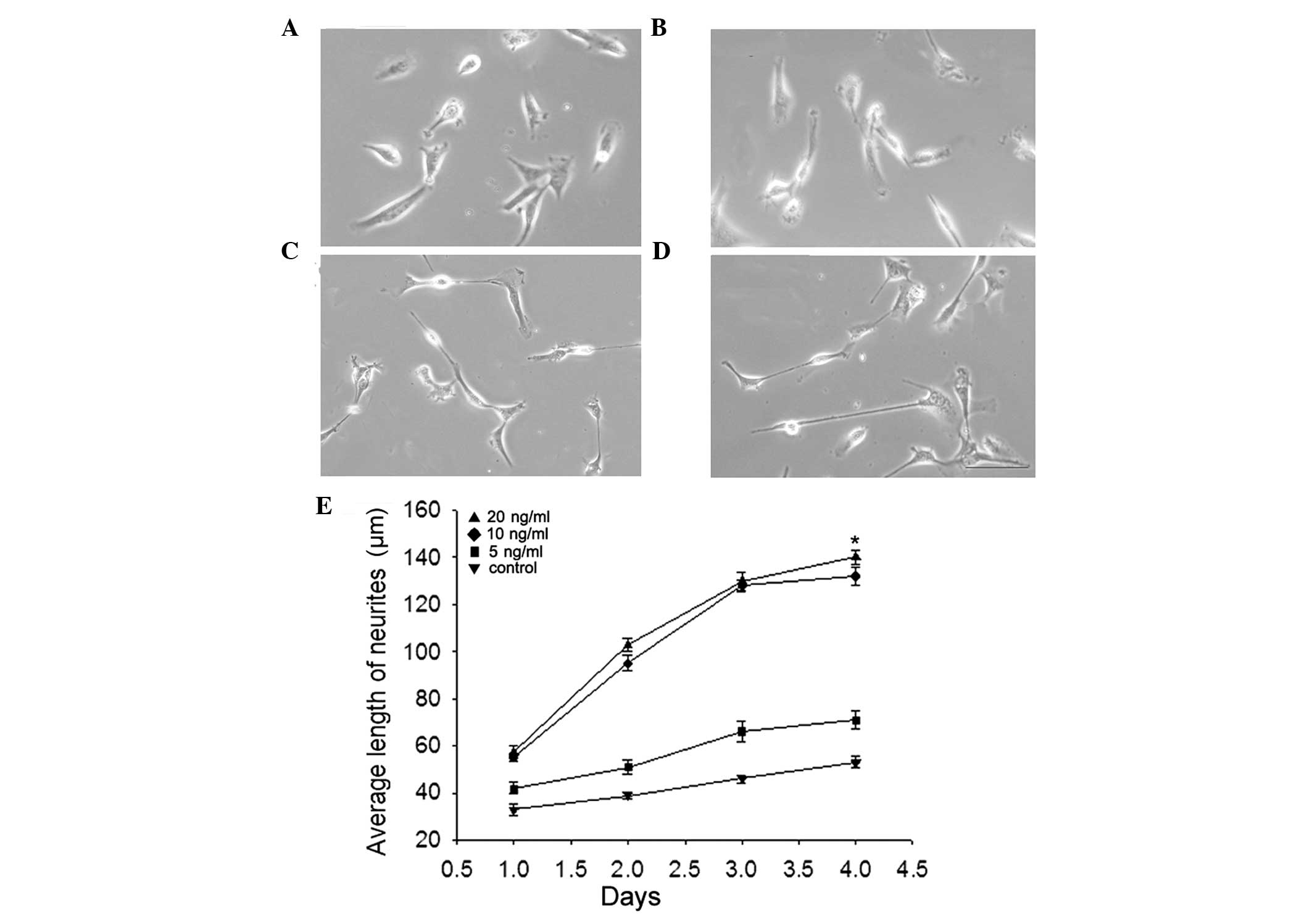
The effect of BMP-2 on the differentiation of
primary enteric neurons (from E15 rat embryos) was examined by
measuring neurite length. Neurons were treated with different
concentrations of BMP-2 for 1–4 days. The average neurite length
was not significantly altered in the 5 ng/ml BMP-2 group compared
with the control group (Fig. 3A and
B). However, in the 10 ng/ml (Fig.
3C) or 20 ng/ml BMP-2 group (Fig.
3D), the average neurite length was significantly increased
(P<0.05). This indicated that in high concentrations, BMP-2
significantly regulated the differentiation of primary enteric
neurons and increased the length of neurites compared with the
control group (Fig. 3E).
High-concentration BMP-2 markedly
increase the expression of nNOS
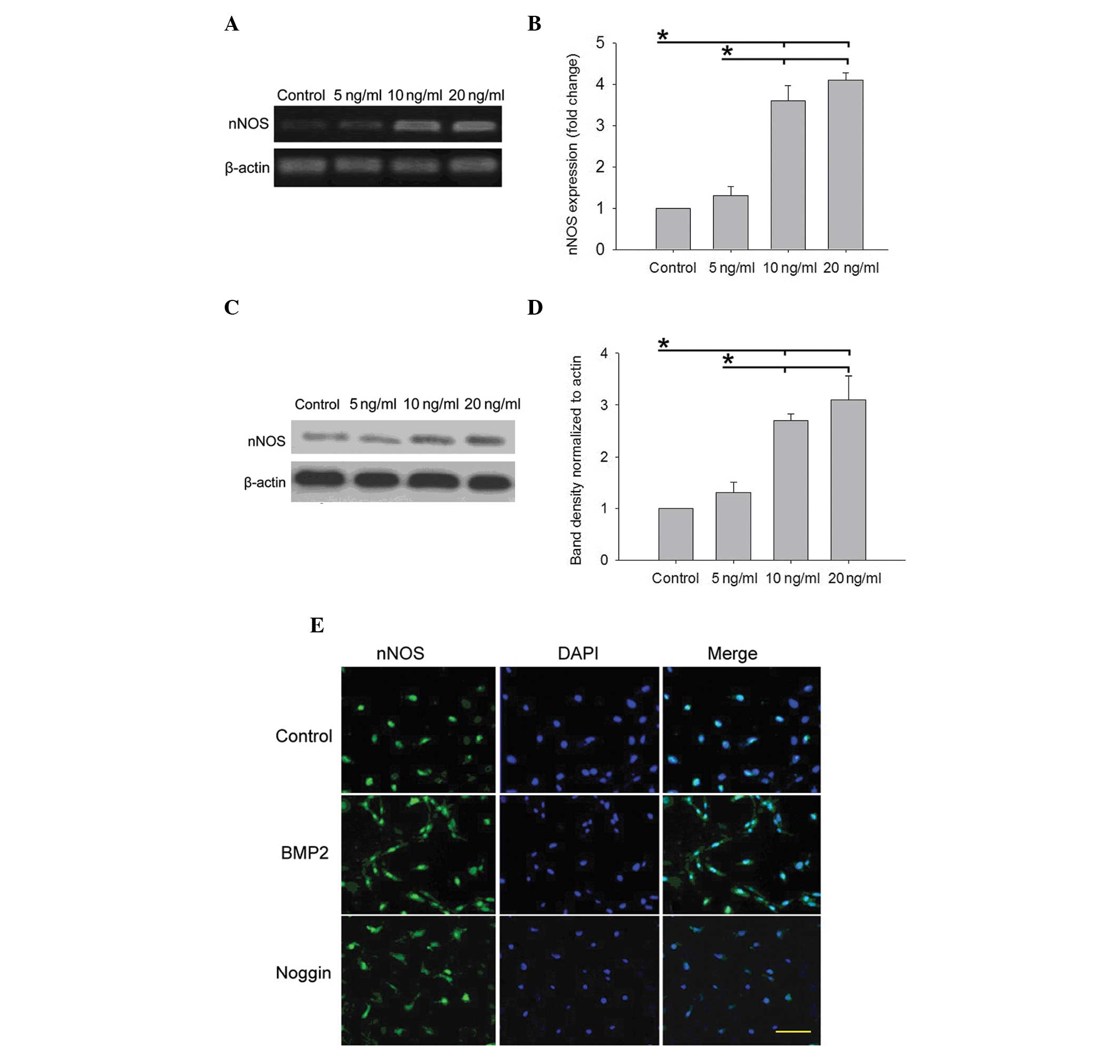
To examine the association between BMP-2 and nNOS in
STC, the effect of different concentration of BMP-2 on nNOS was
examined in primary enteric neurons (from E15 rat embryos) in
vitro. The primary enteric neurons were treated with BMP-2
(0–20 ng/ml) for 3 days. The results demonstrated that the
induction of nNOS expression by BMP-2 was concentration dependent
(Fig. 4). The present study
subsequently assessed the effect of BMP-2 on the expression of nNOS
by RT-qPCR. The expression of nNOS at the RNA level was almost
undetectable in the control or 5 ng/ml BMP-2 group, however, it
significantly increased in the 10 and 20 ng/ml group (P<0.05;
Fig. 4A and B). Subsequently, the
level of nNOS protein was examined by western blot analysis.
Similar to the trend of transcripts for nNOS, the protein
expression of nNOS was significantly increased in the presence of
10 and 20 ng/ml BMP-2 compared with the control group and 5 ng/ml
group (P<0.05; Fig. 4C and D).
Furthermore, the effect of BMP-2 or its antagonist Noggin on
regulating nNOS expression was determined in primary enteric
neurons and it was found that BMP-2 significantly increased the
expression of nNOS compared with the control group. However, the
effect of BMP-2 on nNOS expression was decreased in the presence of
Noggin (Fig. 4E).
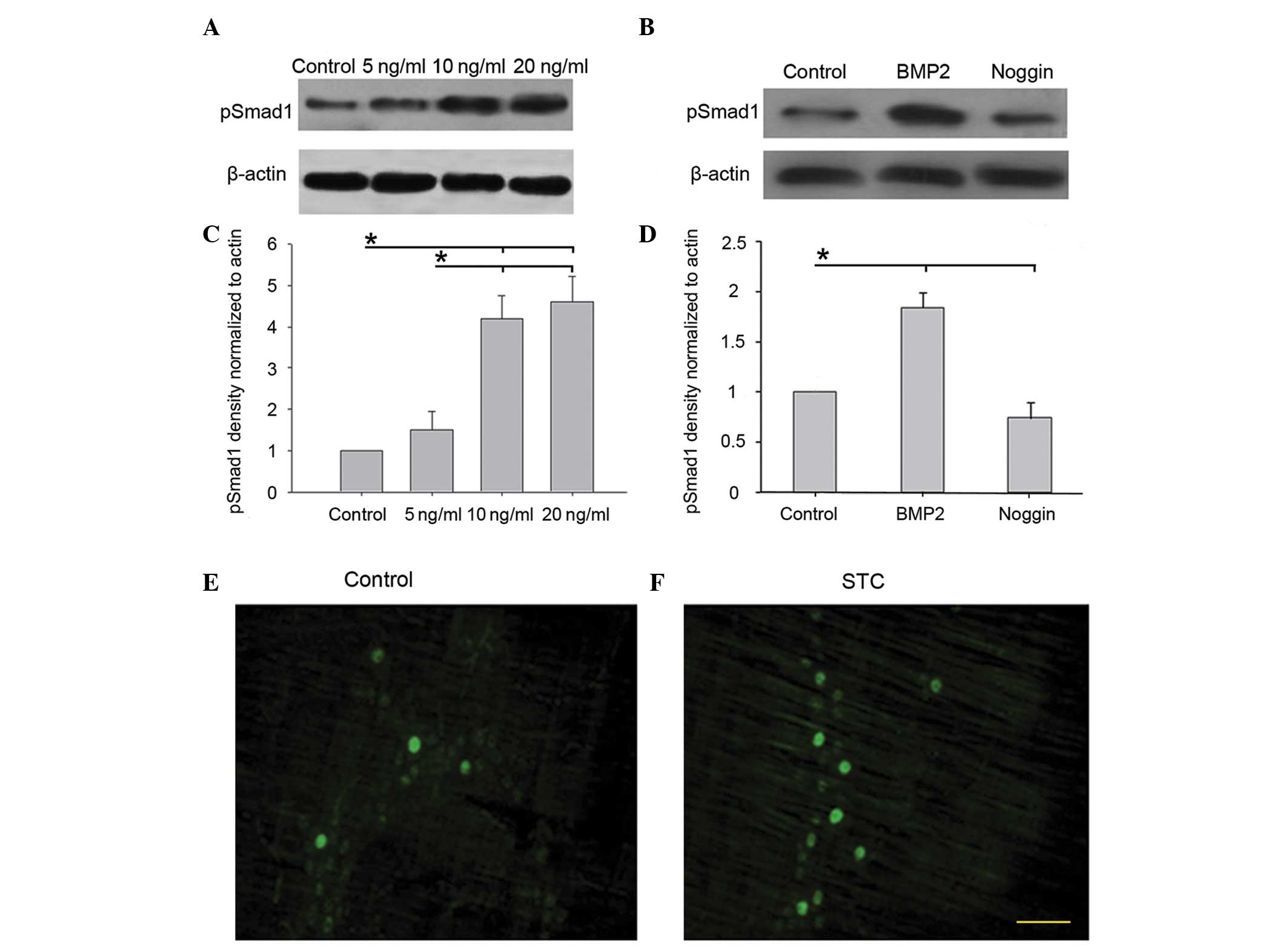
BMP-2 induces the phosphorylation and
expression of Smad1
Smad1 is an immediate downstream molecule of the
BMP-2 receptors (21). To
elucidate the signaling pathways involved in BMP-2-induced
upregulation of nNOS expression, the effects of BMP-2 stimulation
on the phosphorylation of Smad1 protein was examined. The results
demonstrated that the phosphorylation of Smad1 protein induced by
BMP-2 was concentration dependent. To validate these results, the
primary enteric neurons (from E15 rat embryos) were treated with
BMP-2 (0–20 ng/ml) for 4 days. The presence of phosphorylated Smad1
was analyzed by western blotting. No significant alterations were
observed in p-Smad1 protein content between the control group and 5
ng/ml BMP-2 group (P>0.05). However, p-Smad1 protein increased
markedly in the 10 and 20 ng/ml BMP-2 group compared with the
control and 5 ng/ml BMP-2 group (P<0.05; Fig. 5A and C). In order to confirm the
results, the primary enteric neurons were treated with BMP-2 (10
ng/ml) and its antagonist Noggin (100 ng/ml). Western blot analysis
demonstrated that phosphorylation of Smad1 protein was
significantly increased by BMP-2 and decreased by Noggin compared
with the control group (P<0.05; Fig. 5, B and D). In addition, the
percentage of pSmad-positive neurons in the STC group was observed
to be more than that in the control group by immunofluorescence
(Fig. 5E). These results indicated
that the Smad1 pathway is important in BMP-2-induced upregulation
of nNOS expression.
Discussion
The present study demonstrated that BMP-2 regulates
the differentiation of nitrergic enteric neurons through the Smad1
pathway in STC. It has been demonstrated that BMPs are essential
not only for the development of the neuronal components of the ENS,
as previously established, but also for the glial component as well
(15,22,23).
Using primary enteric neurons, it was demonstrated that BMP-2
regulated the differentiation of nNOS-expressing enteric neurons
and may have induced the dysfunction of bowel in STC.
Although the pathogenesis of STC remains to be
elucidated, numerous theories have attempted to explain STC,
including a lack of fiber, autonomic neuropathy and disorders of
the ENS and neuroendocrine system. A previous study indicated that
a genetic basis may be present in a subgroup of patients with STC
(24). Severely constipated
patients have important neuroenteric abnormalities, not confined to
ganglion cells and the interstitial cells of Cajal (8). However, it remains unclear whether
alterations of enteric neurotransmitters have a role in the
pathophysiology of STC. A previous study demonstrated that an
excessive production of the neurotransmitter NO in the colonic
myenteric plexus of patients with STC contributed to the persistent
inhibition of propulsive contractile activity (25). NO is an inorganic molecule with
numerous physiological functions and is synthesized from L-arginine
by nitric oxide synthase (NOS) (26). Similar to these findings, the
increase of NO may be associated with the impaired motility
observed in the STC colon (27,28).
The expression of nNOS and BMP-2 in the myenteric nerve plexus in
patients with STC and control patients was determined using IHC. As
a result, it was found that BMP-2 and nNOS were significantly
increased in patients with STC compared with patients with colon
cancer.
Goldstein et al demonstrated that BMP
signaling is necessary for neural crest cell migration and ganglion
formation in the ENS and the inhibition of BMP activity leads to
hypoganglionosis and failure of enteric ganglion formation, with
crest cells unable to cluster into aggregates (19). Similar to these findings, in the
present study, BMP-2 was found to promote the differentiation of
enteric neurons and increase the expression of nNOS in enteric
neurons. In addition, the effect of BMP-2 on promoting the
expression of nNOS was concentration dependent. Thus, it appears
that BMP-2 has a marked effect on the inhibitory subset of enteric
neurons. This has therapeutic implications for disease, including
STC where the expression of inhibitory neurotransmitters results in
dysfunction of intestinal diastole and contraction.
As a result of neurons releasing nNOS, which
represent the nitrergic population of enteric neurons, the effect
of different concentrations of BMP-2 on the expression of nNOS was
next examined in primary enteric neurons. No significant
alterations were identified in the protein expression of nNOS and
p-Smad1 in the control group and 5 ng/ml BMP-2 group. However, in
the 10 and 20 ng/ml BMP-2 group nNOS expression was significantly
increased. BMP-2 increased the population of nNOS enteric neurons
and the expression of nNOS in primary enteric neurons. It has been
reported that BMP-6 stimulates macrophages to produce iNOS through
Smad signaling pathways and BMP-2 increases nNOS-expressing neurons
in IM-FEN cell lines (11,29). These results support the present
findings that BMP-2 promotes expression of nNOS in enteric neurons
in STC. On the basis of these studies, BMP-2 antagonists may be of
potential therapeutic value to reduce the population of
nNOS-expressing enteric neurons and ameliorate the dysfunction of
bowel in STC disease. Previous studies have demonstrated that BMPs
regulate migration and neurite fasciculation within the developing
ENS and BMP-2, BMP-4, BMPR-IA (BMP receptor subunit), BMPR-IB,
BMPR-II, and the BMP antagonist, Noggin, were all expressed in ENS
precursors and intestinal non-neural crest-derived cells at
embryonic day 12 (E12) in rats (15,30).
Future studies are required to determine whether components of the
TGF or BMP signaling pathways can be used as therapeutic targets to
prevent or treat dysmotility syndromes, including STC that
compromise the function of the ENS. In addition, the effect of
BMP-2 on the differentiation of nNOS-expressing enteric neurons and
induction of STC has also not been examined in rat models.
BMPs function mainly through two signal transduction
pathways, the Smad-dependent pathway and Smad-independent pathway.
Smad signaling is activated and phosphorylated by type I and type
II BMP receptor serine/threonine kinases, which form a complex with
the common mediator of Smad signaling, Smad4 (16). The Smad complexes subsequently
translocate into the nucleus to trigger target gene transcription
(31,32). It has been verified that Smad1
signaling is essential for the differentiation of nitrergic enteric
neurons induced by BMP-2 in IM-FEN cell lines (12). The present study revealed that
BMP-2 can promote the phosphorylation of Smad-1 in primary enteric
neurons and the expression of phosphorylated Smad-1 proteins was
antagonized by adding Noggin to the cultures of primary enteric
neurons. In conclusion, BMP-2 may regulate the expression of nNOS
through the Smad1 signaling pathway in enteric neurons of STC.
In conclusion, the results demonstrated that BMP-2
promoted the differentiation of ENS through the Smad1 pathway in
STC. In the presence of BMP-2, the expression of nNOS increased
significantly in primary enteric neurons. The involvement of BMP-2
in the differentiation of ENS in STC patients is important for a
more correct clinical and therapeutic approach.
Acknowledgments
This study was supported by projects from the
Science and Technology Development Program of Shandong Province
(grant no. 2012G0021828), the Qingdao Outstanding Health
Professional Development Fund, Shandong Provincial Natural Science
Foundation (grant no. ZR2014HL016) and the 2015 Qingdao Huimin
Project of Science and Technology.
References
|
1
|
Knowles CH and Martin JE: Slow transit
constipation: A model of human gut dysmotility. Review of possible
aetiologies. Neurogastroenterol Motil. 12:181–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bassotti G, Chistolini F, Nzepa FS and
Morelli A: Colonic propulsive impairment in intractable
slow-transit constipation. Arch Surg. 138:1302–1304. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bassotti G, Roberto GD, Sediari L and
Morelli A: Toward a definition of colonic inertia. World J
Gastroenterol. 10:2465–2467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furness JB, Nguyen TV, Nurgali K and
Shimizu Y: The enteric nervous system and its extrinsic
connections. Textbook of Gastroenterology. Yamada T, Alpers DH,
Kalloo AN, Kaplowitz N, Owyang C and Powell DW: 1. 5th edition.
Blackwell Publishing; Philadelphia, PA: pp. 15–39. 2003
|
|
5
|
Furness JB and Costa M: The Enteric
Nervous System. Churchill Livingstone; Edinburgh: 1987
|
|
6
|
Chevalier J, Derkinderen P, Gomes P,
Thinard R, Naveilhan P, Vanden Berghe P and Neunlist M:
Activity-dependent regulation of tyrosine hydroxylase expression in
the enteric nervous system. J Physiol. 586:1963–1975. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekblad E, Sjuve R, Arner A and Sundler F:
Enteric neuronal plasticity and a reduced number of interstitial
cells of Cajal in hypertrophic rat ileum. Gut. 42:836–844. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassotti G, Villanacci V, Maurer CA,
Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G
and Salerni B: The role of glial cells and apoptosis of enteric
neurones in the neuropathology of intractable slow transit
constipation. Gut. 55:41–46. 2006. View Article : Google Scholar
|
|
9
|
Bassotti G and Villanacci V: Slow transit
constipation: A functional disorder becomes an enteric neuropathy.
World J Gastroenterol. 12:4609–4613. 2006.PubMed/NCBI
|
|
10
|
Lee JI, Park H, Kamm MA and Talbot IC:
Decreased density of interstitial cells of Cajal and neuronal cells
in patients with slow-transit constipation and acquired megacolon.
J Gastroenterol Hepatol. 20:1292–1298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anitha M, Shahnavaz N, Qayed E, Joseph I,
Gossrau G, Mwangi S, Sitaraman SV, Greene JG and Srinivasan S: BMP2
promotes differentiation of nitrergic and catecholaminergic enteric
neurons through a Smad1-dependent pathway. Am J Physiol
Gastrointest Liver Physiol. 298:G375–G383. 2010. View Article : Google Scholar :
|
|
12
|
Katagiri T, Akiyama S, Namiki M, Komaki M,
Yamaguchi A, Rosen V, Wozney JM, Fujisawa-Sehara A and Suda T: Bone
morphogenetic protein-2 inhibits terminal differentiation of
myogenic cells by suppressing the transcriptional activity of MyoD
and myogenin. Exp Cell Res. 230:342–351. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aoyama K, Yamane A, Suga T, Suzuki E,
Fukui T and Nakamura Y: Bone morphogenetic protein-2 functions as a
negative regulator in the differentiation of myoblasts, but not as
an inducer for the formations of cartilage and bone in mouse
embryonic tongue. BMC Dev Biol. 11:442011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu A and Niswander LA: Bone morphogenetic
protein signalling and vertebrate nervous system development. Nat
Rev Neurosci. 6:945–954. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chalazonitis A, D'Autréaux F, Guha U, Pham
TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA and
Gershon MD: Bone morphogenetic protein-2 and -4 limit the number of
enteric neurons but promote development of a TrkC-expressing
neuro-trophin-3-dependent subset. J Neurosci. 24:4266–4282. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kishigami S and Mishina Y: BMP signaling
and early embryonic patterning. Cytokine Growth Factor Rev.
16:265–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roberts DJ, Johnson RL, Burke AC, Nelson
CE, Morgan BA and Tabin C: Sonic hedgehog is an endodermal signal
inducing Bmp-4 and Hox genes during induction and regionalization
of the chick hindgut. Development. 121:3163–3174. 1995.PubMed/NCBI
|
|
19
|
Goldstein AM, Brewer KC, Doyle AM, Nagy N
and Roberts DJ: BMP signaling is necessary for neural crest cell
migration and ganglion formation in the enteric nervous system.
Mech Dev. 122:821–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawabata M, Imamura T and Miyazono K:
Signal transduction by bone morphogenetic proteins. Cytokine Growth
Factor Rev. 9:49–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoodless PA, Haerry T, Abdollah S,
Stapleton M, O'Connor MB, Attisano L and Wrana JL: MADR1, a
MAD-related protein that functions in BMP2 signaling pathways.
Cell. 85:489–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chalazonitis A, Pham TD, Li Z, Roman D,
Guha U, Gomes W, Kan L, Kessler JA and Gershon MD: Bone
morphogenetic protein regulation of enteric neuronal phenotypic
diversity: Relationship to timing of cell cycle exit. J Comp
Neurol. 509:474–492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chalazonitis A, D'Autreaux F, Pham TD,
Kessler JA and Gershon MD: Bone morphogenetic proteins regulate
enteric gliogenesis by modulating ErbB3 signaling. Dev Biol.
350:64–79. 2011. View Article : Google Scholar :
|
|
24
|
Rossi E, Villanacci V, Fisogni S, Morelli
A, Salerni B, Grigolato P and Bassotti G: Chromosomal study of
enteric glial cells and neurons by fluorescence in situ
hybridization in slow transit constipation. Neurogastroenterol
Motil. 19:578–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomita R, Fujisaki S, Ikeda T and Fukuzawa
M: Role of nitric oxide in the colon of patients with slow-transit
constipation. Dis Colon Rectum. 45:593–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
27
|
Saur D, Paehge H, Schusdziarra V and
Allescher HD: Distinct expression of splice variants of neuronal
nitric oxide synthase in the human gastrointestinal tract.
Gastroenterology. 118:849–858. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porter AJ, Wattchow DA, Hunter A and Costa
M: Abnormalities of nerve fibers in the circular muscle of patients
with slow transit constipation. Int J Colorectal Dis. 13:208–216.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon SJ, Lee GT, Lee JH, Kim WJ and Kim
IY: Bone morphogenetic protein-6 induces the expression of
inducible nitric oxide synthase in macrophages. Immunology.
128(Suppl 1): e758–e765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu M, Vohra BP, Wind D and Heuckeroth RO:
BMP signaling regulates murine enteric nervous system precursor
migration, neurite fasciculation, and patterning via altered Ncam1
polysialic acid addition. Dev Biol. 299:137–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piek E, Heldin CH and Ten Dijke P:
Specificity, diversity, and regulation in TGF-beta superfamily
signaling. FASEB J. 13:2105–2124. 1999.PubMed/NCBI
|
|
32
|
Heldin CH, Miyazono K and Ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|