Introduction
Insulin resistance is a pathological condition that
precedes several disease states, including hypertension, obesity,
dyslipidemia and type 2 diabetes (1,2).
Insulin resistance is characterized by inadequate regulation of
nutrient metabolism and glucose uptake, in numerous tissues and
organs, including the liver.
Tumor necrosis factor-α (TNF-α) is synthesized as a
membrane-anchored 26-kDa precursor and is cleaved to generate a
secretary 17-kDa form by TNF-α-converting enzyme (TACE) (3–6). The
level of TNF-α is controlled by the TACE/tissue inhibitor of
metalloproteinase 3 (TIMP3) system (TIMP3 inhibits TACE activity)
(4). TNF-α is important in
regulating insulin sensitivity at the intracellular level and in
liver and muscle tissue (7).
Several studies have demonstrated that TNF-α contributes to the
genesis and development of insulin resistance by promoting the
phosphorylation of insulin receptor substrate (IRS)-1 at Ser307,
inhibiting tyrosine phosphorylation of IRS-1 and downregulating the
expression of glucose transporter type 4 (GLUT4) at the cellular
membrane (3,8–13).
Trigonella foenum-graecum is an herb in the
leguminoseae family and is commonly termed fenugreek. Fenugreek is
one of the oldest medicinal plants that is widely cultivated across
Africa, Asia and Europe (14,15).
The amino acid 4-hydroxy-isoleucine (4-HIL) is extracted from
fenugreek seeds and is 80% free amino acids (16–18).
4-HIL exists predominantly as the isomeric forms 2S, 3R, 4S and 2R,
3R, 4R (15). According to Broca
et al, the major isomeric form of 4-HIL (2S, 3R, 4S) induces
insulin secretion by directly affecting pancreatic B cells in rats
and humans (18). Certain studies
have found that 4-HIL also improved insulin resistance in skeletal
muscle, the liver and in fat tissue (15,19–22).
However, the molecular mechanisms underlying how 4-HIL improves
insulin resistance remain to be elucidated. A previous study
indicated that 4-HIL activated phosphoinositide 3-kinase (PI3K) in
the insulin-signaling pathway without affecting the level of
insulin in blood plasma (19). The
present study was conducted to understand and define the molecular
mechanisms underlying how 4-HIL improves insulin resistance in
HepG2 cells. Specifically, the aim of the present study was to
address the effect of 4-HIL on crosstalk between the inflammatory
cytokine TNF-α and insulin signaling transduction in
hepatocytes.
Materials and methods
Cell culture and glucose uptake
The established human HepG2 hepatoma cell line was
obtained from the American Type Culture Collection (Rockville, MD,
USA). The HepG2 cells were grown in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich, Shanghai, China) supplemented with 10%
fetal bovine serum (Gibco, Beijing, China) under standard cell
culture conditions (humidified atmosphere, 5% CO2 and
37°C). The following protocol was used to determine the optimal
dose of insulin and treatment duration required to establish
insulin-resistant (IR) cells: Initially, 2 days prior to the
experiment, the cells were seeded in 24-well plates (with
1.5×104 cells/well) with certain wells remaining empty.
Secondly, once the cells reached confluence, the medium was
replaced with DMEM supplemented with a high glucose concentration
(25 mmol/l) and insulin at various concentrations (10−9,
10−8, 10−7, 10−6 and
10−5 mmol/l) for 36 h. Subsequently, the cells were
treated with DMEM supplemented with a high glucose concentration
(25 mmol/l) and insulin (10−7 mmol/l) for 24, 48 and 72
h. At the end of the incubation periods, the medium was removed and
glucose concentrations were determined using the glucose oxidase
method. HepG2 cell glucose uptake was calculated by subtracting the
glucose concentration of the wells with cells from the glucose
levels of the blank wells. The established IR HepG2 cells were then
treated with different concentrations of 4-HIL (0, 5, 10, 20 and 40
µmol/l) for 24 h and glucose uptake was determined as
described above.
Enzyme-linked immunosorbent assay
(ELISA)
The culture medium was collected and centrifuged at
200 × g for 10 min to remove undue particles and the supernatant
was analyzed for TNF-α levels using a human TNF-α ELISA kit
(Neobioscience, Shenzhen, China).
Western blotting
Whole-cell lysates were extracted using
radioimmunoprecipitation assay buffer (Abcam, Shanghai, China)
supplemented with phosphatase inhibitors diluted 1:100 and a
protease inhibitor cocktail diluted 1:50 (Wuhan Huge Biotechnology
Co., Ltd., Wuhan, China). The cell lysates (30 µg of
protein) were dissolved in 5X SDS-PAGE protein sample buffer
(Beyotime Institute of Biotechnology, Haimen, China) and were
boiled for 5–10 min at 100°C. The samples were then separated by
SDS-polyacrylamide gel electrophoresis and electrotransferred onto
polyvinylidene difluoride membranes (Millipore, Beijing, China).
Biotinylated markers (Fermentas, Vilnius, Lithuania) were used as a
molecular weight indicator. The polyvinylidene difluoride membranes
were then blocked for 1 h using 5% bovine serum albumin in
Tris-buffered saline and Tween 20 (TBST). Following being washed
three times with TBST, the membranes were incubated overnight with
the following primary antibodies: Polyclonal anti-TIMP3 rabbit
anti-human antibody (ab39184; 1:1,000; Abcam, Cambridge, UK),
polyclonal anti-TACE rabbit anti-human antibody (ab2051; 1:1,000;
Abcam), monoclonal anti-IRS-1 rabbit anti-human antibody (#3407;
1:1,000; Cell Signaling Technology, Inc., Boston, MA, USA),
monoclonal anti-IRS-2 rabbit anti-human antibody (#4502; 1:1,000;
Cell Signaling Technology, Inc.), monoclonal anti-Phospho-IRS-1
(Ser307) rabbit anti-human antibody (#2381; 1:1,000; Cell Signaling
Technology, Inc.), polyclonal anti-Glut4 goat anti-human antibody
(sc-1608; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), and monoclonal anti-β-actin mouse anti-human antibody
(sc-47778; 1:1,000; Santa Cruz Biotechnology, Inc.). Subsequently,
the membranes were washed three times with TBST and were then
incubated with secondary antibodies (1:1,000; goat anti-mouse
IgG-HRP, sc-2005; goat anti-rabbit IgG-HRP, sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h. The membranes were then washed three
times with TBST and the immunoreactive proteins were detected using
ECL plus western blotting detection reagents (Beyotime Institute of
Biotechnology, Nantong, China).
Statistical analysis
All experiments were performed in duplicate and were
repeated at least two or three times. Each experiment yielded
almost identical results and the data are expressed as the mean ±
standard deviation. The differences between groups were determined
by a one-way analysis of variance. Statistical analysis was
performed using SPSS software v. 19 (SPSS Inc., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
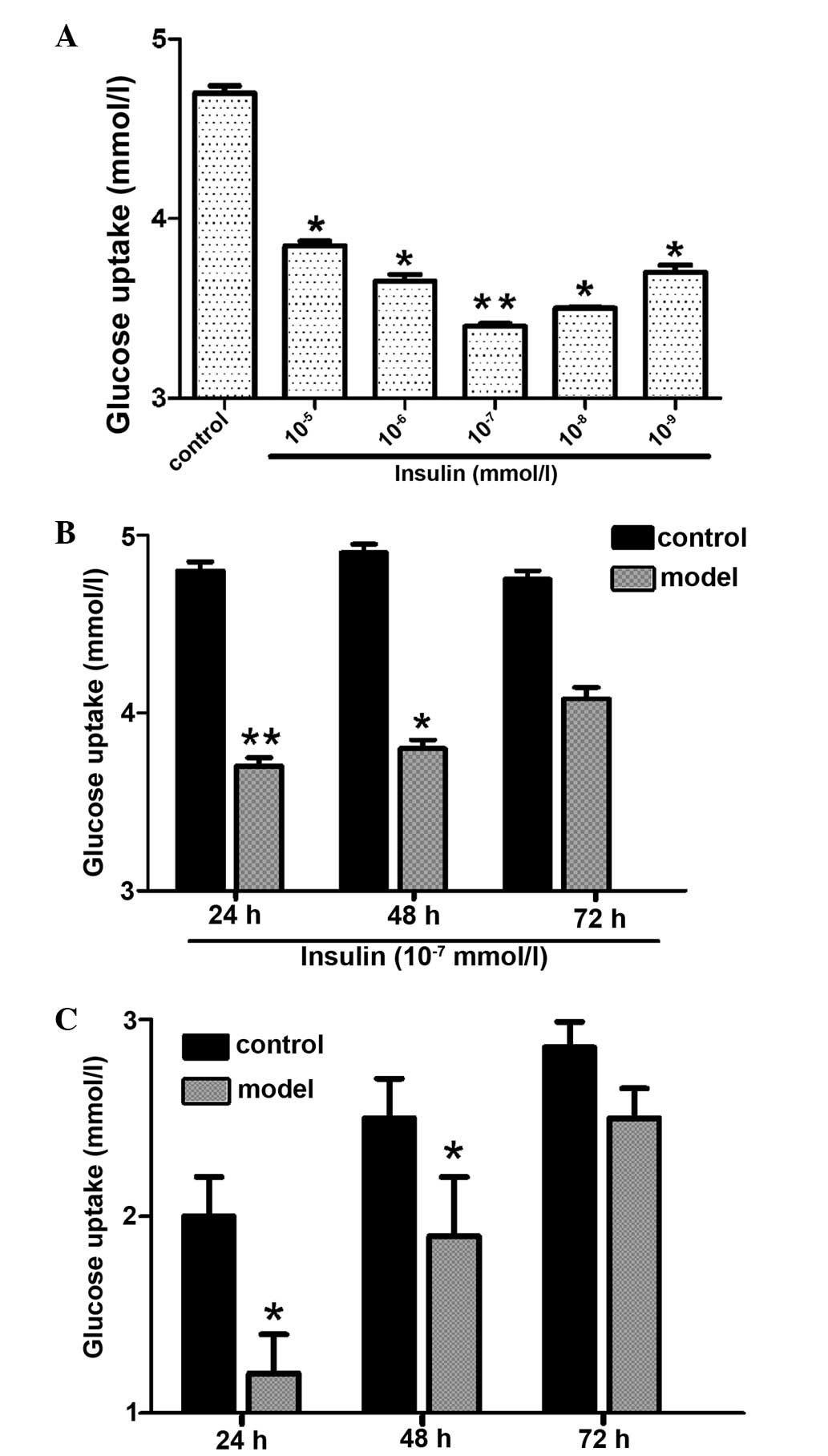
Establishing an IR cell line
In order to develop an IR cell line, the HepG2 cells
were initially treated with different concentrations of insulin for
36 h (Fig. 1A). The results
indicate that the lowest glucose uptake occurred at an insulin dose
of 10−7 mmol/l (Fig.
1A). HepG2 cells were then exposed to 10−7 mmol/l
insulin for 24, 48 and 72 h (Fig.
1B) and the results demonstrated that insulin-induced glucose
uptake was lowest at 24 h (Fig.
1B). Therefore, the IR HepG2 cells used in the present study
were established using 10−7 mmol/l insulin administered
to the cells for 24 h. In order to determine the stability of the
model, the IR cells were grown in medium without insulin for 24, 48
and 72 h (Fig. 1C) and the results
show that the IR cells remained stable for 48 h.
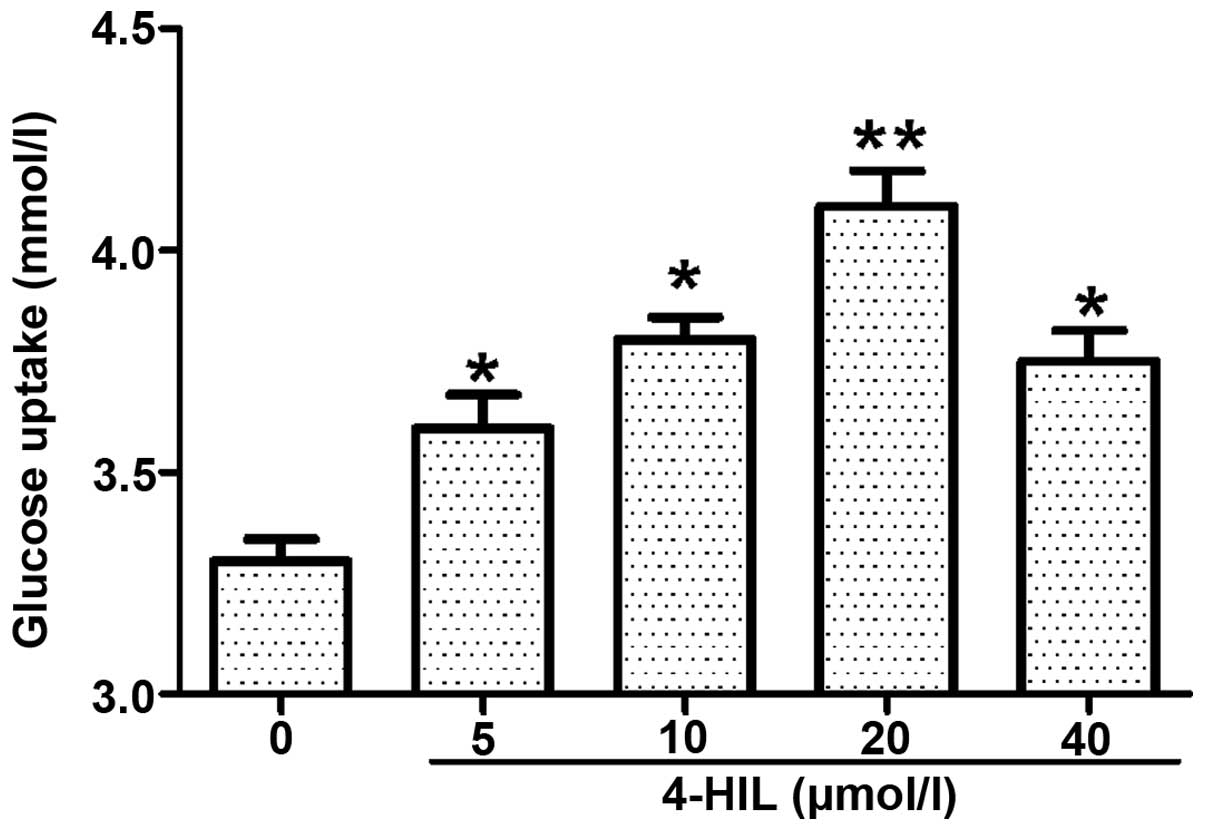
4-HIL improves insulin resistance in IR
HepG2 cells
The IR HepG2 cells were treated with different doses
of 4-HIL for 24 h (Fig. 2) and it
was found that insulin-induced glucose uptake was increased in a
dose-dependent manner with the maximal effect observed at 20
µmol/l 4-HIL. These data suggest that 4-HIL improves insulin
sensitivity in IR HepG2 cells in a dose-dependent manner.
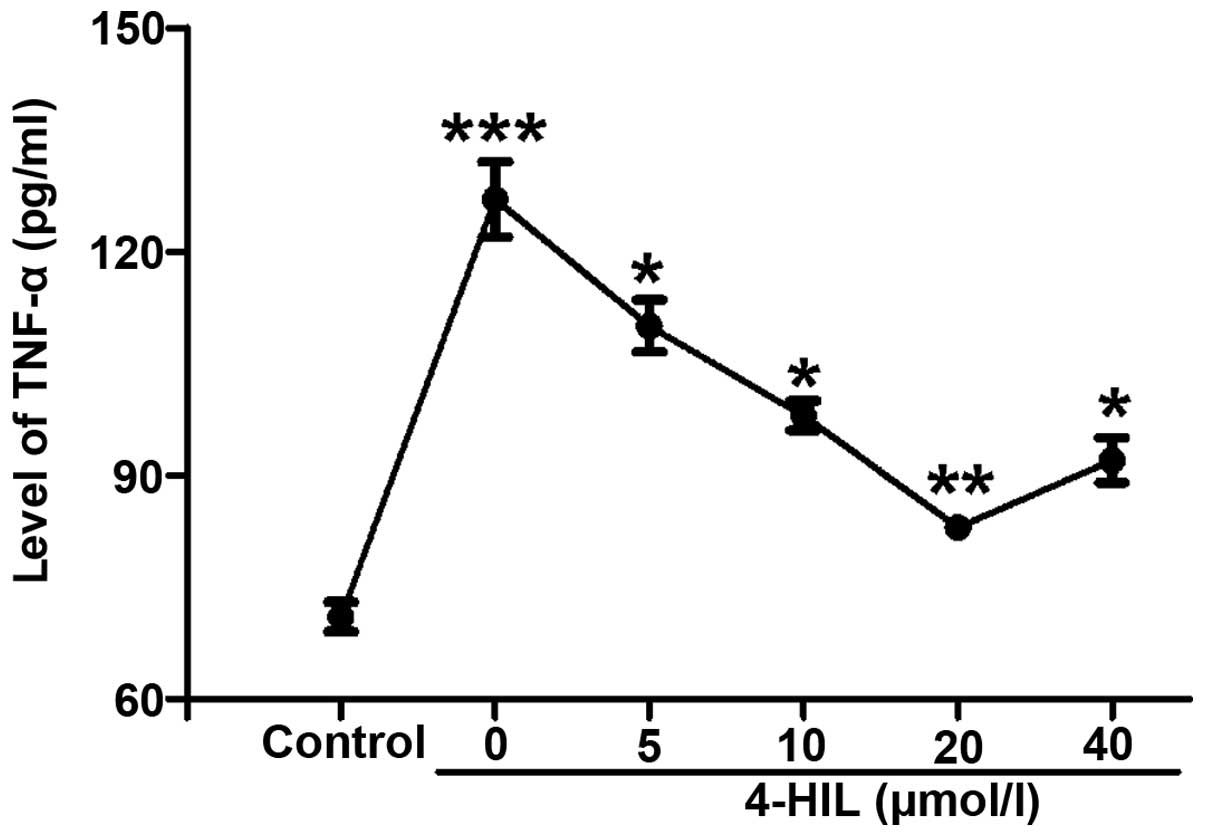
4-HIL inhibits TNF-α in IR HepG2
cells
In order to understand the potential mechanisms
underlying how 4-HIL improves insulin resistance, the effect of
4-HIL on TNF-α levels in IR HepG2 cells was investigated. The
results in Fig. 3 show that TNF-α
levels were markedly increased in IR HepG2 cells (0 µmol/l)
compared with the control cells (P<0.001), but decreased
significantly in a dose-dependent manner with increasing
concentrations of 4-HIL. These results indicated that 4-HIL
dose-dependently inhibited the overproduction of TNF-α in IR HepG2
cells.
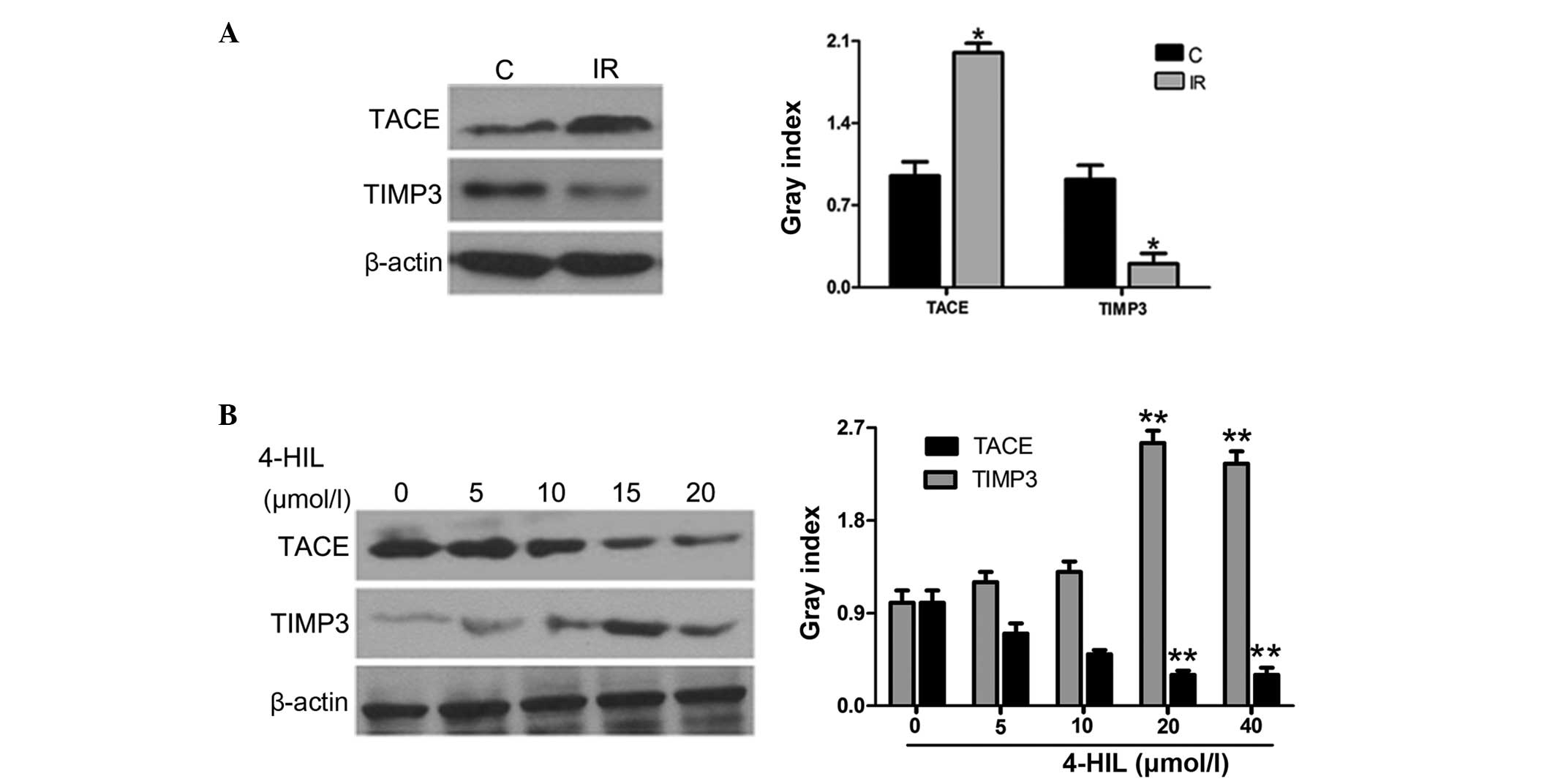
4-HIL upregulates the expression of TIMP3
and downregulates the expression of TACE in IR HepG2 cells
Since TNF-α release is controlled by the TACE/TIMP3
system, the role of 4-HIL in regulating the protein expression of
TIMP3 and TACE was further investigated in IR HepG2 cells. The
results in Fig. 4A show that IR
HepG2 cells overexpressed TACE and underexpressed TIMP3 when
compared with the control cells. Notably, 4-HIL dose-dependently
downregulated and upregulated TACE and TIMP3 expression in IR HepG2
cells, respectively (Fig. 4B). The
observed 4-HIL-mediated down-regulation of TACE and upregulation of
TIMP3 expression in IR HepG2 cells provides evidence that the
TACE/TIMP3 system may be the determining factor for overproduction
of TNF-α in IR HepG2 cells.
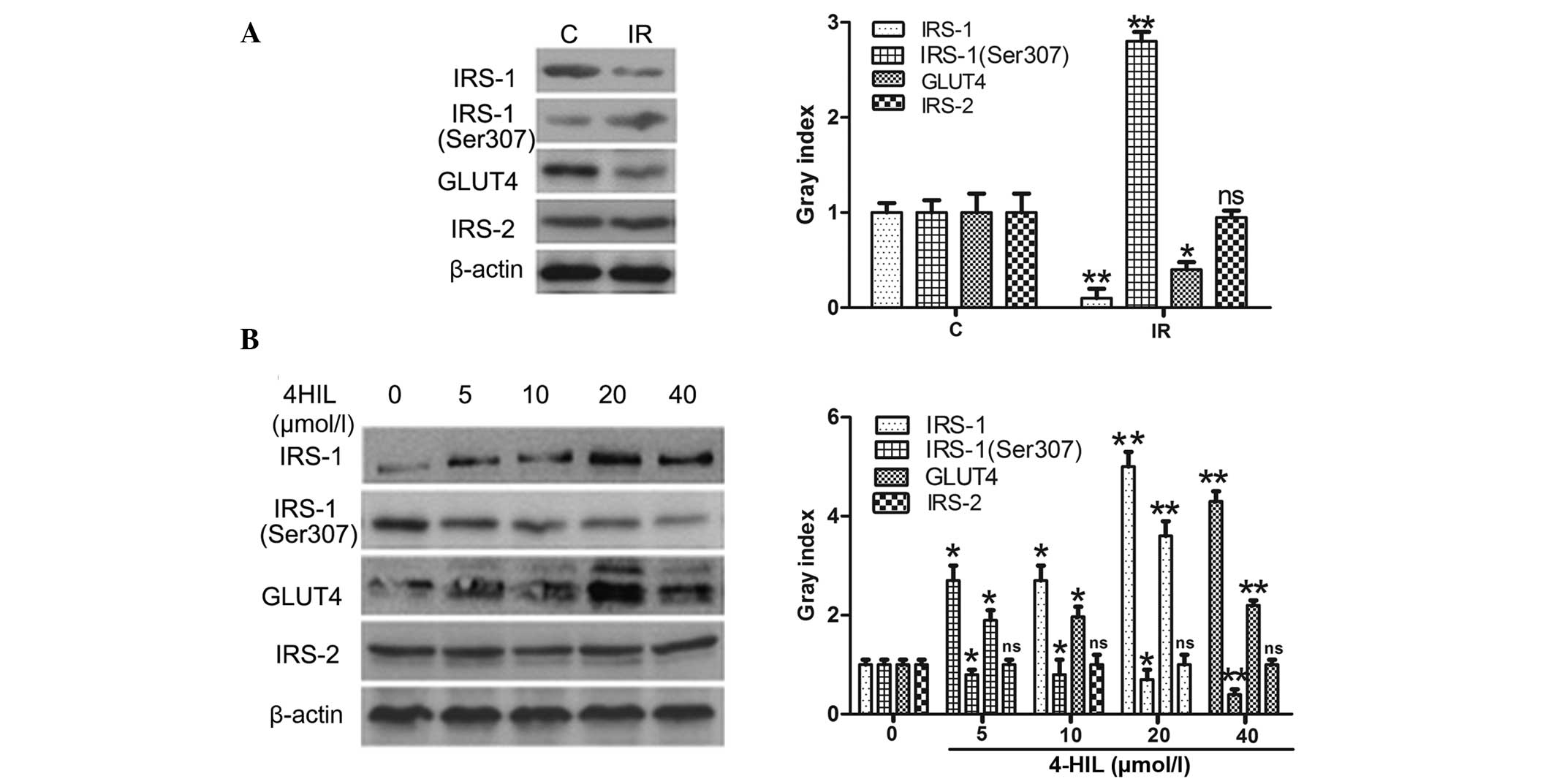
4-HIL regulates the expression of key
proteins involved in the insulin-signaling pathway in IR HepG2
cells
Whether 4-HIL directly affected the expression of
the insulin signal transduction proteins IRS-1/2 was next examined,
including phosphorylation of IRS-1 at Ser307 (p-IRS-1) and GLUT4.
The results shown in Fig. 5A
demonstrated that IR HepG2 cells expressed lower levels of IRS-1
and GLUT4, higher levels of p-IRS-1 and equivalent levels of IRS-2
when compared with the control cells. The results shown in Fig. 5B demonstrate that 4-HIL
dose-dependently upregulated IRS-1 and GLUT4 expression,
downregulated the expression of p-IRS-1 and had no effect on IRS-2
expression in IR HepG2 cells. These results indicate that 4-HIL
directly affected expression of key insulin signal transduction
proteins in addition to regulating TNF-α expression.
Discussion
4-HIL is an atypical branched-chain amino acid
derived from fenugreek that stimulates glucose-dependent insulin
secretion by directly affecting pancreatic islets and reduces
insulin resistance in muscle and/or the liver (15,16,19,23).
However, the molecular mechanisms underlying how 4-HIL improves
insulin resistance remain to be elucidated. Broca et al
indicated that a potential mechanism of action mediated by 4-HIL
may be through activation of PI3K in the insulin signaling pathway
(19). In order to further
delineate the molecular mechanisms regulating 4-HIL-mediated
improvements in insulin resistance, the effect of 4-HIL on
crosstalk between the inflammatory cytokine TNF-α and proteins in
the insulin signal transduction pathway was investigated in IR
HepG2 cells.
An IR HepG2 cell line was established by initially
treating HepG2 cells with 10−7 mmol/l insulin for 24 h.
The IR HepG2 cell line demonstrated markedly decreased glucose
uptake, thus indicating successful establishment of an IR cell
line. The molecular mechanism underlying how 4-HIL improves insulin
resistance was then examined following identifying that 4-HIL
significantly increased glucose uptake in a dose-dependent manner
in IR HepG2 cells.
Insulin binding to its receptor induces activation
of downstream molecules, including insulin receptor tyrosine
kinase, phosphorylation of IRS-1 on multiple tyrosine residues,
PI3K and the serine/threonine kinase PI3K-linked protein kinase B
(Akt/PKB) (24,25). The activation of Akt/PKB stimulates
GLUT4, which results in enhanced glucose uptake (26). Previous studies have demonstrated
that insulin resistance is most likely attributed to a defect in
the insulin receptor/IRS-1/PI3K cascade (19). This defect is initiated by Ser/Thr
phosphorylation of IRS-1, which inhibits insulin-stimulated
tyrosine phosphorylation of IRS-1 and consequently inhibits the
insulin signal transduction pathway, ultimately leading to insulin
resistance (19,27).
The present study demonstrated that IR HepG2 cells
exhibited a high expression of p-IRS-1 (Ser307), a low expression
of IRS-1 and GLUT4, and decreased glucose uptake. The present study
also demonstrated that 4-HIL dose-dependently down-regulated the
expression of p-IRS-1 (Ser307), upregulated the expression of IRS-1
and GLUT4, and increased glucose uptake in IR HepG2 cells. These
findings suggest for the first time, to the best of our knowledge,
that 4-HIL improves insulin sensitivity by directly affecting the
insulin signaling pathway.
Insulin resistance is strongly associated with
obesity and other pathological stress conditions, including
inflammatory diseases, hemorrhage, thermal injury, sepsis and
cancer cachexia. These pathological states are characterized by an
increased inflammatory response as indicated by high levels of
pro-inflammatory cytokines, including TNF-α (28). Several studies have demonstrated
that TNF-α has a central role in obesity-induced insulin resistance
by promoting serine phosphorylation of IRS-1, which impairs insulin
receptor and IRS-1 interactions and compromises insulin signal
propagation (29,30).
The present study determined that TNF-α was
significantly increased in IR HepG2 cells and assessed whether
4-HIL affected levels of the cytokine. It was found that 4-HIL
significantly decreased TNF-α expression in IR HepG2 cells and a
secondary mechanism involved in how 4-HIL improved insulin
resistance in IR cells was highlighted. The present study next
aimed to determine the mechanism involved in decreased TNF-α in IR
HepG2 cells.
Several studies have indicated that TNF-α secretion
is dependent on the interaction between TACE and its endogenous
inhibitor TIMP3 (13,31–34).
In the present study, expression of the TACE/TIMP3 system was
detected using immunoblotting and it was demonstrated that
expression of TACE was higher, whereas TIMP3 expression was lower
in IR HepG2 cells compared with the control cells (Fig. 4A). In addition, 4-HIL
dose-dependently upregulated TIMP3 and downregulated TACE
expression (Fig. 4B), suggesting
that 4-HIL may decrease TNF-α expression in IR HEpG2 cells via its
effects on the TACE/TIMP3 system.
In conclusion, an IR HepG2 cell line that exhibited
down-regulated TIMP3, IRS-1 and GLUT4, upregulated TACE, TNF-α and
p-IRS-1 (Ser307), and low glucose uptake was successfully
established. The present study also identified that 4-HIL
dose-dependently improved insulin resistance in IR HepG2 cells by
two potential mechanisms. Firstly, 4-HIL affected expression of the
TACE/TIMP3 system that has been previously demonstrated to
negatively regulate TNF-α production, thus indirectly increasing
insulin sensitivity of the IR cell line (32). Secondly, 4-HIL directly affected
protein expression of p-IRS-1 (Ser307), IRS-1 and GLUT4 in the
insulin signaling pathway in IR HepG2 cells, thus highlighting an
additional mechanism. Notably, IRS-2 expression was not altered in
IR HepG2 cells or affected by exposure to 4-HIL. The results from
the present study provide evidence that 4-HIL may be a novel and
useful clinical tool to combat insulin resistance.
Abbreviations:
|
TNF-α
|
tumor necrosis factor-α
|
|
TACE
|
TNF-α converting enzyme
|
|
TIMP
|
tissue inhibitor of
metalloproteinase
|
|
IRS
|
insulin receptor substrate
|
|
p-IRS
|
phosphorylated insulin receptor
substrate
|
|
Ser
|
serine
|
|
GLUT
|
glucose transporter
|
|
PI3K
|
phosphoinositide 3-kinase
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81001670).
References
|
1
|
Cordain L, Eades MR and Eades MD:
Hyperinsulinemic diseases of civilization: More than just Syndrome
X. Comp Biochem Physiol A Mol Integr Physiol. 136:95–112. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie W, Wang W, Su H, Xing D, Pan Y and Du
L: Effect of ethanolic extracts of Ananas comosus L. leaves on
insulin sensitivity in rats and HepG2. Comp Biochem Physiol C
Toxicol Pharmacol. 143:429–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peraldi P, Hotamisligil GS, Buurman WA,
White MF and Spiegelman BM: Tumor necrosis factor (TNF)-alpha
inhibits insulin signaling through stimulation of the p55 TNF
receptor and activation of sphingomyelinase. J Biol Chem.
271:13018–13022. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss ML, Jin SL, Milla ME, Bickett DM,
Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et
al: Cloning of a disintegrin metalloproteinase that processes
precursor tumour-necrosis factor-alpha. Nature. 385:733–736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bu R, Borysenko CW, Li Y, Cao L, Sabokbar
A and Blair HC: Expression and function of TNF-family proteins and
receptors in human osteoblasts. Bone. 33:760–770. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreira AP, Dias-Melicio LA, Peraçoli MT,
Calvi SA and Victoriano de Campos Soares AM: Killing of
Paracoccidioides brasiliensis yeast cells by IFN-gamma and
TNF-alpha activated murine peritoneal macrophages: Evidence of H
(2)O (2) and NO effector mechanisms. Mycopathologia. 166:17–23.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi E, Okumura A, Unoki-Kubota H,
Hirano H, Kasuga M and Kaburagi Y: Differential proteome analysis
of serum proteins associated with the development of type 2
diabetes mellitus in the KK-A(y) mouse model using the iTRAQ
technique. J Proteomics. 84:40–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saghizadeh M, Ong JM, Garvey WT, Henry RR
and Kern PA: The expression of TNF alpha by human muscle.
Relationship to insulin resistance. J Clin Invest. 97:1111–1116.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuki A, Sumida Y, Murashima S, Murata
K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K
and Yano Y: Serum levels of tumor necrosis factor-alpha are
increased in obese patients with noninsulin-dependent diabetes
mellitus. J Clin Endocrinol Metab. 83:859–862. 1998.PubMed/NCBI
|
|
10
|
Rui L, Aguirre V, Kim JK, Shulman GI, Lee
A, Corbould A, Dunaif A and White MF: Insulin/IGF-1 and TNF-alpha
stimulate phosphorylation of IRS-1 at inhibitory Ser307 via
distinct pathways. J Clin Invest. 107:181–189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
del Aguila LF, Claffey KP and Kirwan JP:
TNF-alpha impairs insulin signaling and insulin stimulation of
glucose uptake in C2C12 muscle cells. Am J Physiol. 276:E849–E855.
1999.PubMed/NCBI
|
|
12
|
Krogh-Madsen R, Plomgaard P, Møller K,
Mittendorfer B and Pedersen BK: Influence of TNF-alpha and IL-6
infusions on insulin sensitivity and expression of IL-18 in humans.
Am J Physiol Endocrinol Metab. 291:E108–E114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang ZY, Loss G, Carmody I and Cohen AJ:
TIMP-3 ameliorates hepatic ischemia/reperfusion injury through
inhibition of tumor necrosis factor-alpha-converting enzyme
activity in rats. Transplantation. 82:1518–1523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narender T, Puri A, Shweta Khaliq T,
Saxena R, Bhatia G and Chandra R: 4-hydroxyisoleucine an unusual
amino acid as anti-dyslipidemic and antihyperglycemic agent. Bioorg
Med Chem Lett. 16:293–296. 2006. View Article : Google Scholar
|
|
15
|
Jetté L, Harvey L, Eugeni K and Levens N:
4-Hydroxyisoleucine: A plant-derived treatment for metabolic
syndrome. Curr Opin Investig Drugs. 10:353–358. 2009.PubMed/NCBI
|
|
16
|
Sauvaire Y, Petit P, Broca C, Manteghetti
M, Baissac Y, Fernandez-Alvarez J, Gross R, Roye M, Leconte A,
Gomis R and Ribes G: 4-Hydroxyisoleucine: A novel amino acid
potentiator of insulin secretion. Diabetes. 47:206–210. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haefelé C, Bonfils C and Sauvaire Y:
Characterization of a dioxygenase from Trigonella foenum-graecum
involved in 4-hydroxyisoleucine biosynthesis. Phytochemistry.
44:563–566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broca C, Manteghetti M, Gross R, Baissac
Y, Jacob M, Petit P, Sauvaire Y and Ribes G: 4-Hydroxyisoleucine:
Effects of synthetic and natural analogues on insulin secretion.
Eur J Pharmacol. 390:339–345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Broca C, Breil V, Cruciani-Guglielmacci C,
Manteghetti M, Rouault C, Derouet M, Rizkalla S, Pau B, Petit P,
Ribes G, et al: Insulinotropic agent ID-1101 (4-hydroxyisoleucine)
activates insulin signaling in rat. Am J Physiol Endocrinol Metab.
287:E463–E471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Savage DB, Petersen KF and Shulman GI:
Mechanisms of insulin resistance in humans and possible links with
inflammation. Hypertension. 45:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haeri MR, Izaddoost M, Ardekani MR, Nobar
MR and White KN: The effect of fenugreek 4-hydroxyisoleucine on
liver function biomarkers and glucose in diabetic and fructose-fed
rats. Phytother Res. 23:61–64. 2009. View
Article : Google Scholar
|
|
22
|
Henkel J, Neuschäfer-Rube F,
Pathe-Neuschäfer-Rube A and Püschel GP: Aggravation by
prostaglandin E2 of interleukin-6-dependent insulin resistance in
hepatocytes. Hepatology. 50:781–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ogawa J, Kodera T, Smirnov SV, Hibi M,
Samsonova NN, Koyama R, Yamanaka H, Mano J, Kawashima T, Yokozeki K
and Shimizu S: A novel L-isoleucine metabolism in Bacillus
thuringiensis generating (2S,3R,4S)-4-hydroxyisoleucine, a
potential insulinotropic and anti-obesity amino acid. Appl
Microbiol Biotechnol. 89:1929–1938. 2011. View Article : Google Scholar
|
|
24
|
Zhang WY, Lee JJ, Kim IS, Kim Y, Park JS
and Myung CS: 7-O-methylaromadendrin stimulates glucose uptake and
improves insulin resistance in vitro. Biol Pharm Bull.
33:1494–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan AH and Pessi JE: Insulin regulation
of glucose uptake: A complex interplay of intracellular signalling
pathways. Diabetologia. 45:1475–1483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J,
Woodgett JR and Klip A: Protein kinase B/Akt participates in GLUT4
translocation by insulin in L6 myoblasts. Mol Cell Biol.
19:4008–4018. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miura A, Ishizuka T, Kanoh Y, Ishizawa M,
Itaya S, Kimura M, Kajita K and Yasuda K: Effect of tumor necrosis
factor-alpha on insulin signal transduction in rat adipocytes:
Relation to PKCbeta and zeta translocation. Biochim Biophys Acta.
1449:227–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tal R, Pavlovsky L and David M: Psoriasis
and cardiovascular risk factors. Harefuah. 151:573–575. 2012.In
Hebrew.
|
|
29
|
Xin-Long C, Zhao-Fan X, Dao-Feng B and Wei
D: mTOR partly mediates insulin resistance by phosphorylation of
insulin receptor substrate-1 on serine (307) residues after burn.
Burns. 37:86–93. 2011. View Article : Google Scholar
|
|
30
|
Hotamisligil GS, Peraldi P, Budavari A,
Ellis R, White MF and Spiegelman BM: IRS-1-mediated inhibition of
insulin receptor tyrosine kinase activity in TNF-alpha- and
obesity-induced insulin resistance. Science. 271:665–668. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monroy A, Kamath S, Chavez AO, Centonze
VE, Veerasamy M, Barrentine A, Wewer JJ, Coletta DK, Jenkinson C,
Jhingan RM, et al: Impaired regulation of the TNF-alpha converting
enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system
in skeletal muscle of obese type 2 diabetic patients: A new
mechanism of insulin resistance in humans. Diabetologia.
52:2169–2181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Federici M, Hribal ML, Menghini R, Kanno
H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo
V, et al: Timp3 deficiency in insulin receptor-haploinsufficient
mice promotes diabetes and vascular inflammation via increased
TNF-alpha. J Clin Invest. 115:3494–3505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serino M, Menghini R, Fiorentino L,
Amoruso R, Mauriello A, Lauro D, Sbraccia P, Hribal ML, Lauro R and
Federici M: Mice heterozygous for tumor necrosis factor-alpha
converting enzyme are protected from obesity-induced insulin
resistance and diabetes. Diabetes. 56:2541–2546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smookler DS, Mohammed FF, Kassiri Z,
Duncan GS, Mak TW and Khokha R: Tissue inhibitor of
metalloproteinase 3 regulates TNF-dependent systemic inflammation.
J Immunol. 176:721–725. 2006. View Article : Google Scholar : PubMed/NCBI
|