Introduction
The cornea is a layer of transparent film consisting
of a fibrous membrane covering the front of the eye, and while the
cornea appears round from behind, it appears elliptical from the
front (1–5). The cornea is divided into the
following five layers, from front to back: Epithelium, lamina
elastica anterior (Bowman membrane), stroma, lamina elastica
posterior (Descemet membrane) and endothelium (1–5). The
endothelium is a monolayer of hexagonal flat corneal endothelial
cells (CECs). Its matrix layer of water molecules are discharged
into the anterior chamber, and the matrix in the dehydrated state
is transparent, which is key for its refractive properties
(1–5). The CECs maintain the corneal
structure, enable corneal refraction, provide a barrier function,
maintain the osmotic pressure and guarantee normal corneal
metabolism (1–5). CECs are end-stage differentiated
cells that can not regenerate (1–5).
Damage to a large area of CECs causes edema, corneal degeneration,
corneal decompensation and sometimes blindness (1–5).
There are numerous factors that can cause corneal damage, such as
mechanical, irradiation, chemical and freezing injuries (6,7).
Injury caused by freezing of CECs is common in cold regions of the
world and can affect vision (7).
However, to date, the mechanism of damage caused by low temperature
freezing of CECs is not clear. Skt11, also termed LKB1, is a
serine/threonine protein kinase, which has been implicated in the
regulation of multiple biological processes and signaling pathways
(8–11). Mutation of Stk11 causes
Peutz-Jeghers syndrome (8–11). A previous study reported that Stk11
was recruited directly to the p21/WAF1 promoter, as well as other
p53 activated promoters, in a p53-dependent manner (9). Furthermore, Stk11 could activate the
p53 and p16 pathways to arrest cell cycle progression from the
G0/G1 phase to S phase (8).
However, it is unknown whether low-temperature freezing activates
the Stk11-p53 pathway to induce the apoptosis of CECs. Therefore, a
liquid nitrogen mouse model was used to investigate whether
low-temperature damage of mouse CECs induces expression and
activation of the Stk11-p53 signaling pathway.
Materials and methods
Animals and cryoinjury treatment
Female C57BL/6 mice (n=30; age, 4–5 weeks of age)
were obtained from the Animal Research Center, Shanghai First
People's Hospital of Shanghai JiaoTong University (Shanghai,
China). This study was approved by the Animal Ethics Committee of
Shanghai JiaoTong University in compliance with the Experimental
Animal Regulations of the National Science and Technology
Commission, China (Permit no. SJTAEC201401). All mice were housed
for 14 days, 3–4 per cage, in a temperature-controlled colony room
under standard light-dark cycle conditions with access to food and
water ad libitum. Cryoinjury was induced as previously
described (7). In brief, the
animals were divided into 2 groups: The untreated control group (6
animals not exposed to liquid nitrogen) and the cryoinjury
experimental group (24 animals exposed to liquid nitrogen). A
cryoprobe (Shanghai Qiujing Biochemical Reagent and Instrument Co.
Ltd., Shanghai, China) with a diameter of 2.5 mm [similar in
diameter to C57BL/6 mouse corneas (2.6 mm)] was frozen in liquid
nitrogen. First, the mice were intraperitoneally injected with 1.5%
of 0.1 ml/20 g nembutal (Sigma-Aldrich, St. Louis, MO, USA). After
anesthetizing, the cryoprobe was placed on the mouse cornea three
times at 1 min intervals.
RNA extraction and analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
All steps were conducted as previously described
(12). In brief, total cellular
RNA was isolated using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Then, the RNA samples were reverse-transcribed into
cDNA using the ReverTra Ace-α First Strand cDNA Synthesis kit
(TOYOBO, Osaka, Japan). RT-qPCR was conducted using a RealPlex4
real-time PCR detection system from Eppendorf Co. Ltd. (Hamburg,
Germany), with SYBR Green RealTime PCR Master mix and detection dye
(TOYOBO). RT-qPCR amplification was performed using the following
steps: Denaturation at 95°C for 120 sec; followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 58°C for 45 sec, and
extension at 72°C for 42 sec. Target cDNA was quantified using the
relative quantification method. A comparative threshold cycle (Ct)
was used to determine gene expression relative to a control
(calibrator) and steady-state mRNA levels are reported as an n-fold
difference relative to the calibrator. For each sample, the marker
gene Ct values were normalized using the formula
ΔCt=Ct_genes-Ct_18SrRNA. To determine relative expression levels,
the following formula was used: ΔΔCt=
ΔCt_treated_group-ΔCt_control_group. The values used to the plot
relative expression of markers were calculated using the expression
2−ΔΔCt. The mRNA levels were calibrated based on levels
of 18S rRNA. The cDNA of each gene was amplified using primers as
follows (Table I).
 | Table IReverse transcription-quantitative
polymerase chain reaction primers. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primers.
| Gene product | Primers (5′→3′) | Size (bp) |
|---|
| Stk11 | F:
GGGCAACCTGCTACTCACC | 103 |
| R:
CCAGATGTCCACCTTGAAAC |
| p53 | F:
ATGAACCGCCGACCTATC | 98 |
| R:
AGGGCAGGCACAAACACG |
| p21 | F:
GCCTTGTCGCTGTCTTGC | 95 |
| R:
GCTGGTCTGCCTCCGTTTT |
| 18S Rrna | F:
AGGGGAGAGCGGGTAAGAGA | 241 |
| R:
GGACAGGACTAGGCGGAACA |
Histopathology
The cornea tissues were stained with hematoxylin and
eosin (H&E) for analysis by histopathology. Briefly, fresh
tissues were washed three times with phosphate-buffered saline
(PBS), fixed in 4% paraformaldehyde (Sigma-Aldrich) for 30 min,
dehydrated through a graded series of ethanol, vitrified in xylene
and embedded in paraffin. Next, 6-µm sections were cut in
serial succession and stained with H&E. The sections were
analyzed using a microscope (DMI3000; Leica, Allendale, NJ,
USA).
Immunohistochemistry
All steps were conducted as previously described
(13). Briefly, fresh tissues were
washed 3 times with PBS, fixed with 4% paraformaldehyde
(Sigma-Aldrich) for 30 min, dehydrated through a graded series of
ethanol, vitrified in xylene and embedded in paraffin. Next,
6-µm sections were cut in serial succession, rinsed with 3%
phosphate buffer, and underwent microwave heat repairing. Rabbit
anti-mouse Stk11 polyclonal antibody (cat. no. sc-28788; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA; dilution, 1:100); rabbit
anti-mouse p53 Ser15-pho polyclonal antibody (cat. no. sc-101762;
Santa Cruz Biotechnology Inc.; dilution, 1:100); rabbit anti-mouse
p21 polyclonal antibody (cat. no. sc-397; Santa Cruz Biotechnology
Inc.; dilution, 1:100); and rabbit anti-mouse active caspase-3
polyclonal antibody (cat. no. 9661S; Cell Signaling Technology
Inc., Danvers, MA, USA; 1:200) were added and incubated for 45 min,
followed by incubation with horseradish peroxidase-conjugated
secondary antibody (cat. no. sc-2004; Santa Cruz Biotechnology
Inc.; dilution, 1:100). Antibody detection was achieved with a
color reaction using an ABC chromogenic reagent (Sigma-Aldrich).
PBS (pH 7.4) was used in the place of primary antibody as a
negative control. Five randomly selected fields (×200
magnification) from each tissue section were observed and analyzed
by Image-Pro Plus 6.0 software (Media Cybernetics Co. Ltd.,
Rochville, MD, USA).
Chromatin immunoprecipitation (ChIP)
assays
To perform ChIP experiments primary antibodies as
used for IH and normal rabbit IgG (Upstate Biotechnology, Lake
Placid, NY, USA) as a negative control were used. In brief, all
steps were conducted as previously described (9). Cells were fixed in 1% formaldehyde
for 30 min at 37°C and then quenched with 125-mM glycine
(Sigma-Aldrich) for 10 min at room temperature to create
DNA-protein cross-links. Samples were sonicated on ice until
chromatin fragments became 200–1,000 bp in size and were then
incubated with antibodies at 4°C overnight. PCR amplification was
performed under the following conditions: 33 cycles run by
denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 30 sec.
Statistical analysis
Each experiment was performed at least three times.
Data are shown as the mean ± standard error and analyzed by
Student's t-test when appropriate. P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism
5.00 (GraphPad Software Inc., La Jolla, CA, USA) was used for
statistical analysis.
Results
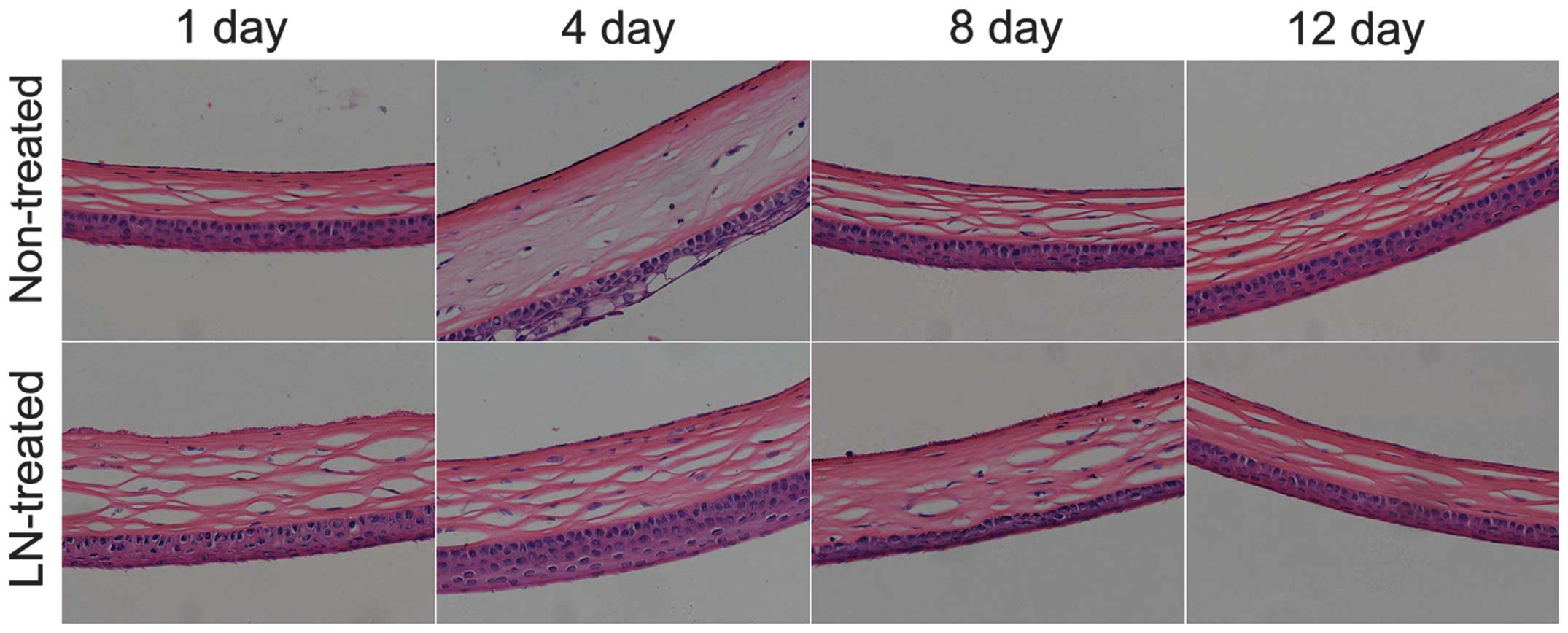
Liquid nitrogen freezing causes
significant damage to the mouse CECs
The healthy mouse CECs are round or polygonal, of
similar size and tightly aligned. After low-temperature freezing
with liquid nitrogen, injury to the corneal tissues and CECs was
significant. First, the corneal tissues were observed to be
swollen; and CECs within the tissue were observed to swell,
fragment and shed (Fig. 1). By the
12th day after liquid nitrogen treatment, CEC damage was partly
repaired, and a number of new CECs were identified.
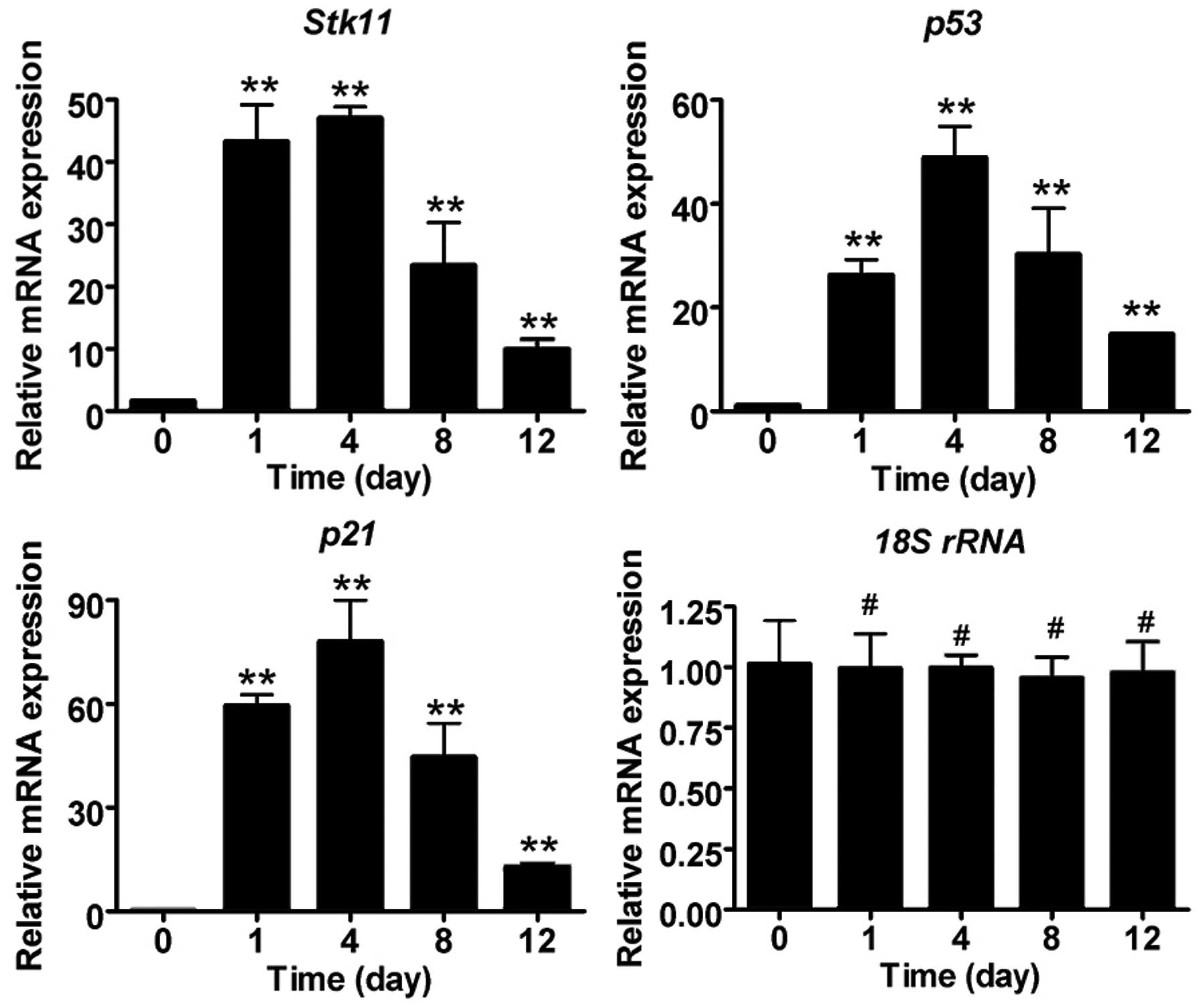
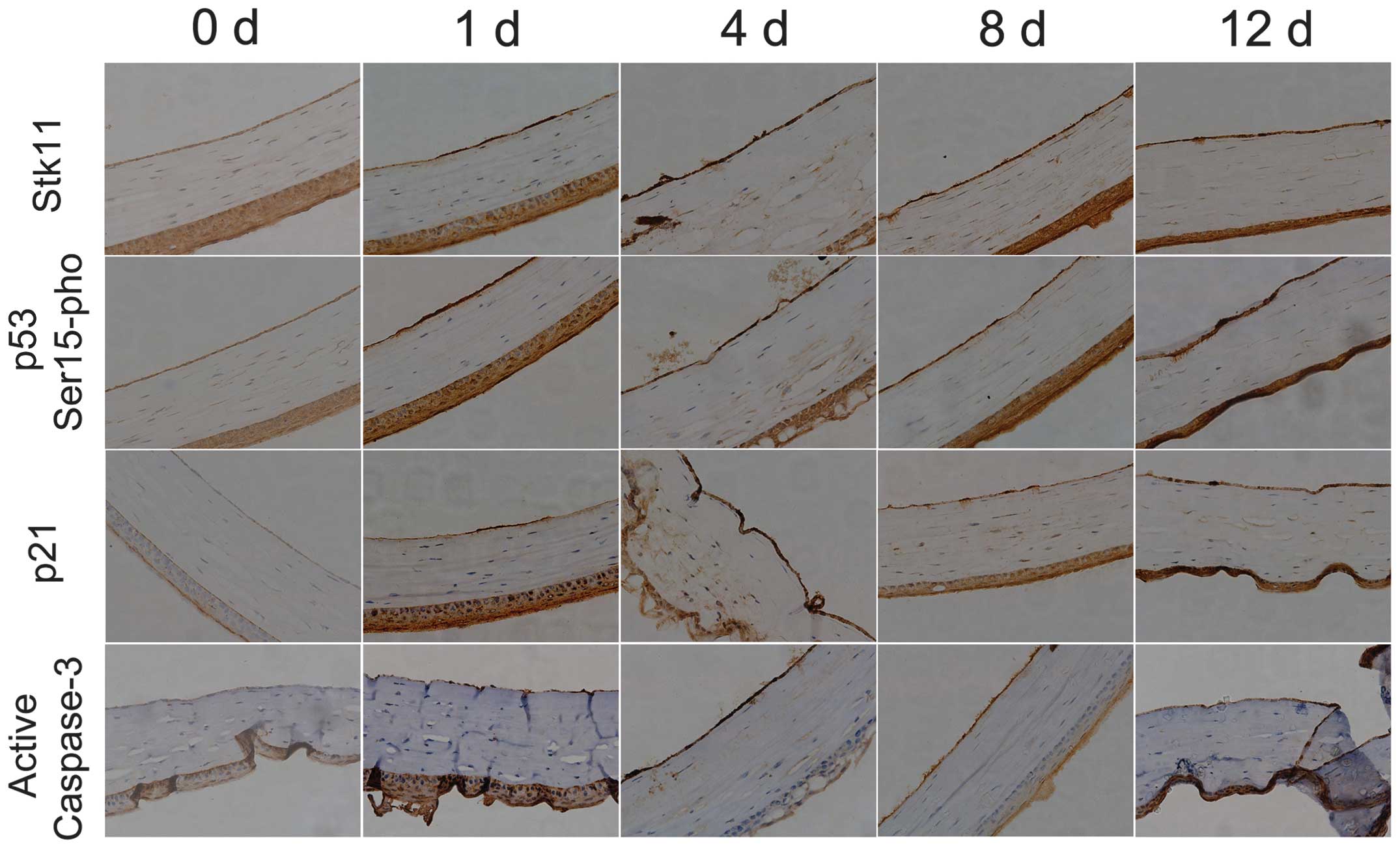
Liquid nitrogen freezing stimulates
significant expression of Stk11-p53 signaling pathway
components
To determine whether the expression of Stk11-p53
signal pathway components is induced in mouse CECs by liquid
nitrogen freezing, RT-qPCR and IHC analysis were conducted. The
mRNA expression levels of core Stk11-p53 signaling factors
(Stk11, p53 and p21) in mouse CECs were
significantly elevated after cryoinjury compared with the untreated
group (day 0) (Fig. 2; Table II). In addition, IHC confirmed
that protein expression of Stk11, p53 and p21 was elevated, and
caspase-3 was activated following cryoinjury (Fig. 3).
 | Table IIReverse transcription-quantitative
polymerase chain reaction results. |
Table II
Reverse transcription-quantitative
polymerase chain reaction results.
| Gene | Time (days) after
liquid nitrogen freezing treatment
|
|---|
| 0 (non-treated) | 1 | 4 | 8 | 12 |
|---|
| Stk11 | 1.627±0.094 | 43.327±5.853 | 47.048±1.793 | 23.419±6.855 | 9.888±1.663 |
| p21 | 0.560±0.034 | 59.519±3.164 | 78.071±11.814 | 44.564±9.789 | 12.848±0.889 |
| p53 | 1.102±0.003 | 26.253±2.990 | 48.881±6.067 | 30.266±8.859 | 14.827±0.206 |
| 18S rRNA | 1.016±0.178 | 0.996±0.143 | 0.998±0.052 | 0.953±0.089 | 0.976±0.129 |
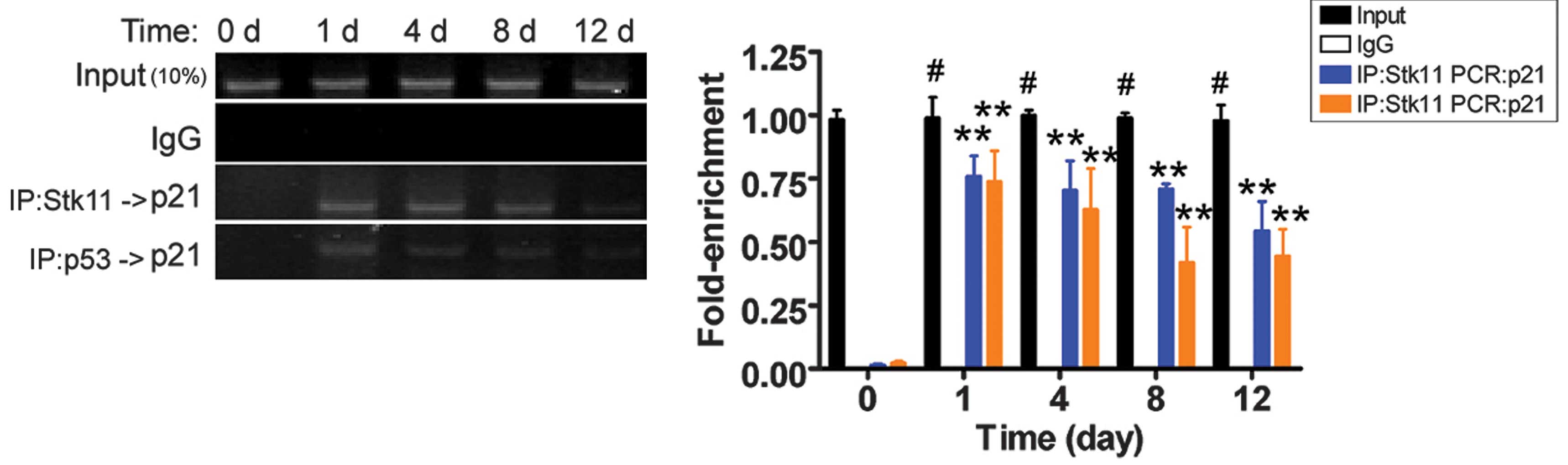
Cryoinjury stimulates p21 gene
transcription
Previously, the Stk11-p53 complex was shown to
specifically bind to the p21 gene promoter (9). The results of the ChIP-PCR assay
revealed that PCR amplification bands of the p21 gene
promoter were weaker on the 12th day after liquid nitrogen
treatment. However, the PCR signals were not present in the
untreated group (Fig. 4). These
results suggest that cryoinjury induced Stk11-p53-mediated
transcription of p21, which may eventually induce apoptosis.
Discussion
Extremely low temperatures can lead to corneal
injury, and damage to CECs can affect vision (7,14).
Very brief contact with cold material can be sufficient to cause
frostbite. Furthermore, in addition to possibly disrupting vision,
cryoinjuries have relatively long recovery periods. Although the
outcome of cryoinjury to CECs may be obvious, the mechanism of
injury is not clear. In this study, the Stk11-p53 signaling pathway
was observed to be involved in the mechanism of CEC cryoinjury
based on the results of previous studies (7–9).
These studies indicated that the Stk11-p53 signal pathway regulated
cell proliferation (8–11). Usually, the Stk11-p53 signaling
pathway is silenced during cell division and proliferation or in a
stem cell state. However, when normal cells are exposed to external
stimuli (such as oxidative damage), the Stk11-p53 signaling pathway
is activated (8–11). When the Stk11-p53 signaling pathway
is activated, it inhibits cell cycle progression, arresting cells
at the G0/G1 phase to inhibit cell mitosis and ultimately resulting
in apoptosis (8–11). Stk11 is a serine/threonine kinase,
which regulates numerous physiological and pathological processes.
The Stk11 gene is generally considered to be a tumor
suppressor gene (8–11). In this study, it was demonstrated
that the Stk11-p53 signaling pathway was abnormally activated
during mouse CEC cryoinjury. Four days following cryoinjury, the
damage to CECs was significant and included swelling, partial
necrosis and shedding. Simultaneously, the expression of the
Stk11-p53 signaling pathway core factors (Stk11, p53, and p21) was
significantly elevated. This suggested that the Stk11-p53 signaling
pathway was involved in the apoptosis of mouse CECs following
cryoinjury. Further investigation demonstrated found that
cryoinjury damages mouse CECs and that Stk11 catalytically modified
p53 by phosphorylation at serine residue 15, which enhanced p53
activity. p53 activation resulted in specific binding to the
p21 gene promoter, and ultimately enhanced the
transcriptional activity of the p21 gene. In conclusion,
extremely low temperatures lead to cryoinjury and apoptosis of
mouse CECs due to Stk11-p53 signaling pathway activation. In
conclusion, the Stk11-p53 signaling pathway may be a novel target
for the treatment of corneal endothelial cell damage.
Acknowledgments
This study was supported by grant from National
Natural Science Foundation of China (grant nos. 81371068 and
81202811) to Professor Yan Liu and Professor Te Liu.
References
|
1
|
Mimura T, Yamagami S and Amano S: Corneal
endothelial regeneration and tissue engineering. Prog Retin Eye
Res. 35:1–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams KA, Irani YD and Klebe S: Novel
therapeutic approaches for corneal disease. Discov Med. 15:291–299.
2013.PubMed/NCBI
|
|
3
|
Joyce NC: Proliferative capacity of
corneal endothelial cells. Exp Eye Res. 95:16–23. 2012. View Article : Google Scholar :
|
|
4
|
Lam FC, Bruinsma M and Melles GR: Descemet
membrane endothelial transfer. Curr Opin Ophthalmol. 25:353–357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peh GS, Beuerman RW, Colman A, Tan DT and
Mehta JS: Human corneal endothelial cell expansion for corneal
endothelium transplantation: An overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi T, Yamagami S, Tanaka K, Yokoo S,
Usui T, Amano S and Mizuki N: A mouse model of allogeneic corneal
endothelial cell transplantation. Cornea. 27:699–705.
2008.PubMed/NCBI
|
|
7
|
Han SB, Ang H, Balehosur D, Peh G,
Chaurasia SS, Tan DT and Mehta JS: A mouse model of corneal
endothelial decompensation using cryoinjury. Mol Vis. 19:1222–1230.
2013.PubMed/NCBI
|
|
8
|
Liang X, Wang P, Gao Q, Xiang T and Tao X:
Endogenous LKB1 knockdown accelerates G (1)/S transition through
p53 and p16 pathways. Cancer Biol Ther. 9:156–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng PY and Berger SL: LKB1 is recruited
to the p21/WAF1 promoter by p53 to mediate transcriptional
activation. Cancer Res. 66:10701–10708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaahtomeri K and Mäkelä TP: Molecular
mechanisms of tumor suppression by LKB1. FEBS Lett. 585:944–951.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krock B, Skuli N and Simon MC: The tumor
suppressor LKB1 emerges as a critical factor in hematopoietic stem
cell biology. Cell Metab. 13:8–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Chen Q, Huang Y, Huang Q, Jiang L
and Guo L: Low microRNA-199a expression in human amniotic
epithelial cell feeder layers maintains human-induced pluripotent
stem cell pluripotency via increased leukemia inhibitory factor
expression. Acta Biochim Biophys Sin (Shanghai). 44:197–206. 2012.
View Article : Google Scholar
|
|
13
|
Shen DZ, Xin SL, Chen C and Liu T: Effect
of atorvastatin on expression of TLR4 and NF-κB p65 in
atherosclerotic rabbits. Asian Pac J Trop Med. 6:493–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Zhang J, Liu CY, Hayashi Y and Kao
WW: Bone marrow mesenchymal stem cells can differentiate and assume
corneal keratocyte phenotype. J Cell Mol Med. 16:1114–1124. 2012.
View Article : Google Scholar
|