Introduction
Human induced pluripotent stem (hiPS) cells are a
promising cell source for regenerative medicine. To avoid exposure
of hiPS cells to proteins from species other than human,
feeder-free culture conditions have been developed (1). Several factors have been identified,
which are part of the signaling network of hiPS cells (2). Leukemia inhibitory factor (LIF) is
involved in the pluripotency of mouse embryonic stem cells
(3). In human embryonic stem (hES)
cells, Nodal/activin A is involved in pluripotency (3). Activin A maintains the pluripotency
of hiPS cells under feeder-free conditions by regulating the
expression of Nanog (4–6). Basic fibroblast growth factor (bFGF)
sustains the self-renewal capacity of hES cells (7). CHIR99021 (CHIR), a glycogen synthase
kinase (GSK)-3β inhibitor, improves the ability of hiPS cells to
maintain pluripotency by acting synergistically with activin A
(8). The development of hiPS cells
from human embryonic keratinocytes via the activation of Oct3/4 and
Klf4 was reported to be driven by culture with CHIR (9). Mouse iPS cells can also be generated
in culture medium containing Oct3/4 and CHIR (10). Finally, mouse embryonic stem cells
maintain pluripotency when cultured with CHIR (11). Taken together, these reports
suggest that CHIR is involved in the maintenance of pluripotency.
Since GSK-3β is involved in the activation of the Wnt signaling
pathway, it is likely that the Wnt pathway is important in
pluripotency (12).
The Wnt signaling pathway is mediated by Disheveled
(Dsh) proteins in Xenopus. Dsh proteins inhibit GSK-3β and
promote the accumulation of β-catenin. Dsh homolog (Dvl)2, a
mammalian homolog of Dsh, is phosphorylated upon stimulation by the
Wnt signaling pathway and mediates endocytosis of the frizzled
receptor (13,14). Once GSK-3β has been inactivated via
phosphorylation of Ser9 by Dvl2, β-catenin accumulates in the
cytoplasm and subsequently translocates into the nucleus (15).
The role of the Wnt signaling pathway in hiPS cells
is controversial. The Wnt signaling pathway has been demonstrated
to be involved in pluripotency (16) and accumulation of β-catenin by
inhibition of GSK-3β sustains pluripotency in conjunction with
other factors (17). Indeed, the
addition of the GSK-3β inhibitor to the culture facilitates the
efficient generation of hiPS cells (18). On the other hand, the Wnt signaling
pathway has been demonstrated to promote the differentiation of
hiPS cells in conjunction with other factors. For instance,
stage-specific activation of bone morphogenetic protein and the Wnt
signaling pathway promotes the differentiation of iPS cells into
epicardial cells (19). The
combination of the Wnt signaling pathway and bFGF promotes the
differentiation of iPS cells into nephron progenitor cells
(20). Therefore, the present
study investigated the direct role of the Wnt signaling pathway in
maintaining hiPS pluripotency under feeder-free culture conditions
(8).
Materials and methods
Cell culture
The hiPS cells (201B7; Riken Cell Bank, Tsukuba,
Japan) were cultured in 6-well plates (Asahi Techno Glass, Tokyo,
Japan) coated with Matrigel™ (Becton Dickinson, Franklin Lakes, NJ,
USA) in ReproFF medium (Reprocell, Yokohama, Japan) for feeder-free
culture in 5% CO2 at 37°C in a humidified chamber. The
cells were harvested using Accutase® (Innovative Cell
Technologies, Inc., San Diego, CA, USA) for experiments. A total of
10 ng/ml activin A (R&D Systems, Inc., Minneapolis, MN, USA), 2
µM GSK-3β inhibitor (CHIR; Wako Pure Chemicals Industries,
Ltd., Osaka, Japan), 1,000 U/ml human LIF (Sigma-Aldrich, St.
Louis, MO, USA) or the SB431542 activin A inhibitor (Wako Pure
Chemicals Industries, Ltd.) was added to Dulbecco's Minimum
Essential Medium-F12 (Sigma-Aldrich), supplemented with 20%
Knockout Serum Replacement (Life Technologies, Grand Island, NY,
USA), 10% Minimum Essential Amino Acids (Life Technologies), 2 mM
l-Glutamine (Life Technologies), and 1 mM 2-Mercaptoethanol
[iPSm(-); Sigma-Aldrich].
Luciferase assay
The 201B7 cells were seeded into 24-well plates,
coated with Matrigel™, and cultured for 24 h. Once the cells
reached 70% confluence, they were transfected with Lipofectamine
LTX (Life Science Technologies), and 0.5 µg TOPflash
reporter plasmid or FOPflash reporter plasmid (EMD Millipore,
Temecula, CA, USA) and 0.05 µg pRL-TK (Promega, Madison, WI,
USA) was added to the medium. The transcriptional activity was
measured using a dual luciferase reporter assay system (Promega)
using Gene Light (GL-200A; Microtech Co. Ltd., Funabashi, Japan).
Non-transfected cells were used as a negative control. The ratio of
the luciferase activity of TOPflash against that of FOPflash was
calculated. The relative Wnt activity was determined by normalizing
this ratio against that of 201B7 cells cultured in ReproFF
medium.
Western blot analysis
The total protein was isolated from the cells
following culture for 48 h. A 10 µg protein sample was
separated on 12 %sodium dodecyl sulfate (Wako Pure Chemicals
Industries, Ltd.)-polyacrylamide (Bio-Rad, Hercules, CA, USA) gel
electrophoresis gels and was subsequently transferred to a nylon
filter (EMD Millipore). Following blocking for 30 min with 5%
non-fat milk, the filters were incubated with rabbit monoclonal
anti-human β-catenin antibodies (1:2,500; cat. no. #9582; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h at room
temperature. The filters were washed in phosphate-buffered saline
(PBS) three times, and subsequently incubated with horseradish
peroxidase (HRP)-conjugated anti-rabbit antibody (1:2,500; cat. no.
NA934-100UL; GE Healthcare Life Sciences, Little Chalfont, UK) at
room temperature for 1 h. After three washes with PBS, the specific
antigen-antibody complexes were visualized using enhanced
chemiluminescence (cat. no. RPN2232; GE Healthcare Life Sciences).
The filter was reprobed with 10 Minute Western Re-probe kit (cat.
no. JZ-008; Jacksun Easy Biotech Inc., New York, NY, USA), and
incubated with mouse monoclonal anti-human α-tubulin antibody (cat.
no. MS-581-P0; 1:2,500; Lab Vision Corporation, Fremont, CA, USA)
for 1 h to use as a loading control. The filters were washed with
PBS, and incubated with HRP-linked anti-mouse antibody (cat. no.
NA931- 100UL; GE Healthcare Life Sciences) for 1 h. The specific
antigen-antibody complexes were visualized using enhanced
chemiluminescence (cat. no. RPN2232; GE Healthcare Life Sciences)
after three washes with PBS. The expression levels of β-catenin
were normalized against that of α-tubulin and were analyzed using
ImageJ 64 imaging software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed by one-factor
analysis of variance using JMP 10.0.2 (SAS Institute, Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
To investigate activity of the Wnt signaling
pathway, a luciferase assay was performed using TOPflash and
FOPflash as reporter plasmids (Fig.
1). Following transfection, activin A, CHIR and/or LIF was
added to the cell culture medium. The relative Wnt activity was
lower in the cells cultured in iPSm(-) (0.18±0.05; P<0.05)
compared with the ReproFF cultured cells. The addition of activin A
suppressed the activity of Wnt when compared with the cells
cultured in iPSm(-) alone (0.11±0.05). By contrast, the Wnt
signaling pathway was markedly activated in cells cultured with
activin A and CHIR (5.28±0.23; P<0.05). These results suggested
that the combination of activin A and CHIR in the culture medium
clearly stimulated the Wnt pathway.
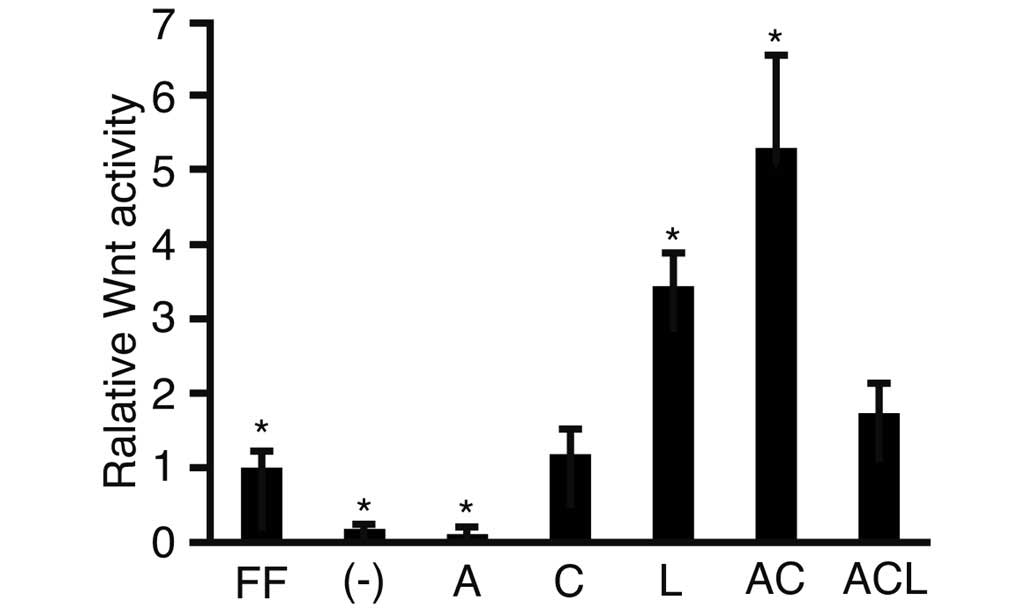 | Figure 1Luciferase reporter assay. Human
induced pluripotent stem cells were transfected with the reporter
plasmids, TOPflash or FOPflash, cultured in various media and
subjected to a luciferase reporter assay. The ratios of
TOPflash/FOPflash in the transfected cells were normalized against
the cells cultured with ReproFF alone, and the resulting value was
expressed as the relative Wnt activity. The data are presented as
the mean ± standard deviation (*P<0.05; n=3). FF,
ReproFF; (-), iPSm(-); A, activin A; C, CHIR99021; L, leukemia
inhibitory factor; AC, activin A + CHIR99021; ACL, activin A,
CHIR99021 + leukemia inhibitory factor. |
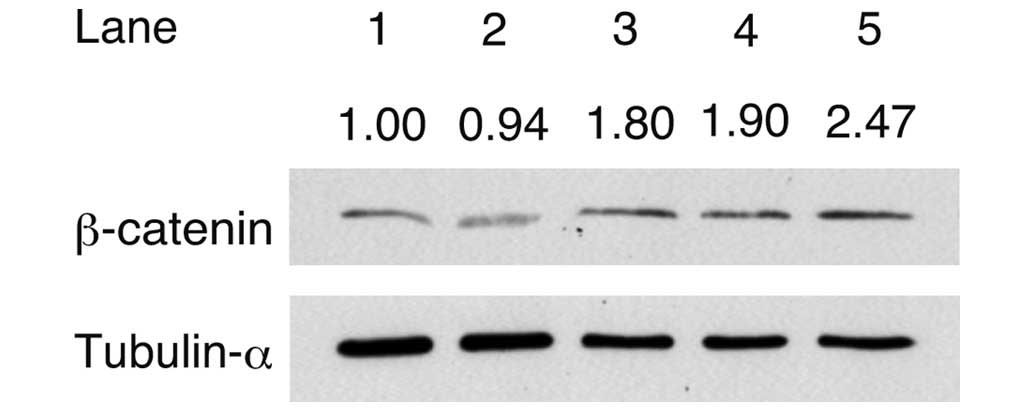
To analyze the accumulation of β-catenin, western
blot analysis was performed following the addition of activin A,
CHIR and/or LIF to the culture medium (Fig. 2). The signal intensity of β-catenin
was divided by the intensity of tubulin-α to obtain the relative
signal intensity. The relative signal intensity of β-catenin
detected in cells cultured in iPSm(-) (0.94) was lower compared
with that of the cells cultured in ReproFF. The relative signal
intensity of β-catenin detected in the cells cultured in medium
with activin A (1.80), activin A + CHIR (1.90) or the combination
of activin A, CHIR + LIF (2.47) was higher compared with that
detected in the cells cultured in iPSm(-) alone. These results
suggested that β-catenin accumulated in response to culture with
activin A and other factors.
To address the possibility that activin A stimulated
the Wnt signaling pathway to a comparable level of ReproFF and
SB431542, a specific inhibitor of activin A was added to the
culture medium, and a luciferase assay was performed (Fig. 3). The relative Wnt activity was
lower in the cells cultured in media containing activin A
(0.28±0.11) compared with those cultured in iPSm(-) (0.49±0.27).
The relative Wnt activity of the cells cultured in medium with
activin A + CHIR (2.20±0.68; P<0.05) and the combination of
activin A, CHIR + LIF (1.85±0.58; P<0.05) was higher compared
with that in the cells cultured in ReproFF alone. These results
were consistent with those shown in Fig. 1. The relative Wnt activity was
lower in the cells cultured with activin A + SB431542 compared with
those cultured in the presence of activin A alone. Addition of
SB431542 to the culture marginally increased the relative Wnt
activity simulated by culture with activin A + CHIR, and the
combination of activin A, CHIR + LIF. However, these differences
were non-significant.
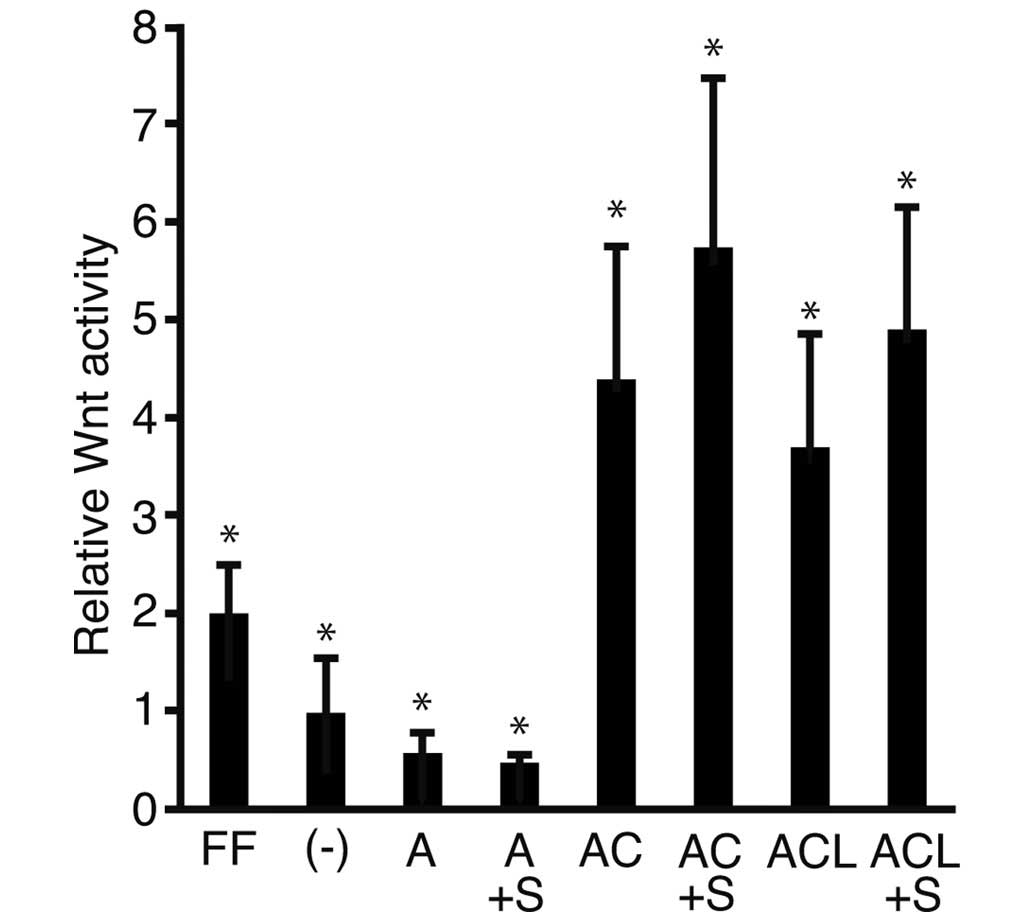 | Figure 3Luciferase reporter assay. Human
induced pluripotent stem cells transfected with the reporter
plasmids, TOPflash or FOPflash, were cultured in various media and
were subjected to a luciferase reporter assay. The ratios of
TOPflash/FOPflash in transfected cells were normalized against the
cells cultured with ReproFF alone and the resulting value was
expressed as the relative Wnt activity. The data are presented as
the mean ± standard deviation (*P<0.05; n=3). FF,
ReproFF; (-), iPSm(-); A, activin A; A + S, activin A + SB431542;
AC, activin A + CHIR99021; ACS, activin A, CHIR99021 + SB431542;
ACL, activin A, CHIR99021 + leukemia inhibitory factor; ACLS,
activin A, CHIR99021, leukemia inhibitory factor + SB431542. |
Discussion
Activin A is essential for the maintenance of the
pluripotency of hiPS cells cultured under feeder-free conditions
(4,21). In our previous study, hiPS cells
were cultured under feeder-free conditions in the presence of
activin A and CHIR (8). Since CHIR
is involved in activation of the Wnt signaling pathway, it was
hypothesized that activin A and the Wnt signaling pathway cooperate
to maintain the pluripotency and proliferation of iPS cells. In the
present study, the relative Wnt activity and the expression of
β-catenin were investigated. As expected, the relative Wnt activity
was greatest in the cells cultured with a combination of activin A
and CHIR. These results indicated that the activation of the Wnt
signaling pathway was optimal for the feeder-free culture of iPS
cells.
Unexpectedly, the cells cultured in medium,
supplemented with only activin A, resulted in the accumulation of
β-catenin. It was unclear why β-catenin accumulated in the culture
supplemented with activin A, while the relative Wnt activity was
suppressed. These results may represent crosstalk between activin A
and the Wnt signaling pathway (22).
In the present study, one of the limitations was
that the expression levels of GSK3β and Dvl2 remained to be
determined. The expression levels of GSK3β and Dvl2 has been
previously demonstrated to increase when the Wnt signaling pathway
is stimulated (23). An analysis
of these molecules is required to fully elucidate the mechanism
underlying the activation of the Wnt signaling pathway, and future
studies in our laboratory will focus on this line of research.
In conclusion, culture with medium containing
activin A and CHIR stimulated the Wnt signaling pathway, as
measured using a luciferase assay with the TOPflash and FOP flash
reporter plasmids. The addition of activin A to the culture medium
was associated with β-catenin accumulation, although the underlying
mechanism remains to be elucidated. Overall, these results
suggested that the Wnt signaling pathway may be required for the
maintenance of pluripotency in hiPS cells cultured under
feeder-free conditions.
References
|
1
|
Meng G, Liu S and Rancourt DE: Synergistic
effect of medium, matrix and exogenous factors on the adhesion and
growth of human pluripotent stem cells under defined, xeno-free
conditions. Stem Cells Dev. 21:2036–2048. 2012. View Article : Google Scholar
|
|
2
|
Dalton S: Signaling networks in human
pluripotent stem cells. Curr Opin Cell Biol. 25:241–246. 2013.
View Article : Google Scholar :
|
|
3
|
Dowell KG, Simons AK, Bai H, Kell B, Wang
ZZ, Yun K and Hibbs MA: Novel insights into embryonic stem cell
self-renewal revealed through comparative human and mouse systems
biology networks. Stem Cells. 32:1161–1172. 2014. View Article : Google Scholar
|
|
4
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Activin A maintains
pluripotency markers and proliferative potential of human induced
pluripotent stem cells. Exp Ther Med. 2:405–408. 2011.PubMed/NCBI
|
|
5
|
Shin M, Alev C, Wu Y, Nagai H and Sheng G:
Activin/TGF-beta signaling regulates Nanog expression in the
epiblast during gastrulation. Mech Dev. 128:268–278. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyanari Y and Torres-Padilla ME: Control
of ground-state pluripotency by allelic regulation of Nanog.
Nature. 483:470–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu RH, Peck RM, Li DS, Feng X, Ludwig T
and Thomson JA: Basic FGF and suppression of BMP signaling sustain
undifferentiated proliferation of human ES cells. Nat Methods.
2:185–190. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Activin A is essential for
Feeder-free culture of human induced pluripotent stem cells. J Cell
Biochem. 114:584–588. 2013. View Article : Google Scholar
|
|
9
|
Li W, Zhou H, Abujarour R, Zhu S, Young
Joo J, Lin T, Hao E, Schöler HR, Hayek A and Ding S: Generation of
human-induced pluripotent stem cells in the absence of exogenous
Sox2. Stem Cells. 27:2992–3000. 2009.PubMed/NCBI
|
|
10
|
Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P,
Ge J, Liu C, Zhang W, Zhang X, et al: Generation of iPSCs from
mouse fibroblasts with a single gene, Oct4 and small molecules.
Cell Res. 21:196–204. 2011. View Article : Google Scholar
|
|
11
|
Ying QL, Wray J, Nichols J, Batlle-Morera
L, Doble B, Woodgett J, Cohen P and Smith A: The ground state of
embryonic stem cell self-renewal. Nature. 453:519–523. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu D and Pan W: GSK3: A multifaceted
kinase in Wnt signaling. Trends Biochem Sci. 35:161–168. 2010.
View Article : Google Scholar :
|
|
13
|
Itoh K, Brott BK, Bae GU, Ratcliffe MJ and
Sokol SY: Nuclear localization is required for Dishevelled function
in Wnt/beta-catenin signaling. J Biol. 4:32005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W, ten Berge D, Brown J, Ahn S, Hu
LA, Miller WE, Caron MG, Barak LS, Nusse R and Lefkowitz RJ:
Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated
endocytosis of Frizzled 4. Science. 301:1391–1394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Sokolosky M, Abrams SL, Montalto G, D'Assoro AB, Libra M,
Nicoletti F, et al: GSK-3 as potential target for therapeutic
intervention in cancer. Oncotarget. 5:2881–2911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sineva GS and Pospelov VA: β-Catenin in
pluripotency: Adhering to self-renewal or Wnting to differentiate?
Int Rev Cell Mol Biol. 312:53–78. 2014. View Article : Google Scholar
|
|
18
|
Bar-Nur O, Brumbaugh J, Verheul C,
Apostolou E, Pruteanu-Malinici I, Walsh RM, Ramaswamy S and
Hochedlinger K: Small molecules facilitate rapid and synchronous
iPSC generation. Nat Methods. 11:1170–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witty AD, Mihic A, Tam RY, Fisher SA,
Mikryukov A, Shoichet MS, Li RK, Kattman SJ and Keller G:
Generation of the epicardial lineage from human pluripotent stem
cells. Nat Biotechnol. 32:1026–1035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lam AQ, Freedman BS and Bonventre JV:
Directed differentiation of pluripotent stem cells to kidney cells.
Semin Nephrol. 34:445–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pauklin S and Vallier L: The cell-cycle
state of stem cells determines cell fate propensity. Cell.
155:135–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh AM, Reynolds D, Cliff T, Ohtsuka S,
Mattheyses AL, Sun Y, Menendez L, Kulik M and Dalton S: Signaling
network crosstalk in human pluripotent cells: A Smad2/3-regulated
switch that controls the balance between self-renewal and
differentiation. Cell Stem Cell. 10:312–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S, Sueishi M and Yoshida T: Niclosamide
suppresses Hepatoma cell proliferation via the Wnt pathway. Onco
Targets Ther. 6:1685–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|