Introduction
Mast cells (MCs), containing large quantities of
mediators, are widely distributed throughout gastrointestinal
tracts (1) and previous studies
have demonstrated that MC degranulation may exacerbate injury in a
rodent model of small intestinal ischemia-reperfusion (IIR)
(2,3). Furthermore, tryptase, uniquely
released in MC activation, is important in the process of small IIR
injury (2). In addition, tryptase
is involved in various types of intestinal disease, such as acute
colitis and inflammatory bowel disease (4). However, the role of tryptase in the
pathogenesis of small intestinal mucosal injury induced by ischemia
has been difficult to define, as oxidative stress and inflammation
are involved in vivo (5),
and whether tryptase directly induces small intestinal mucosal
injury remains to be elucidated.
Protease-activated receptor (PAR) is a family of G
protein-coupled receptors that are activated by proteolysis, and
thereby act as sensors for extracellular proteases (6). To date, four PARs have been
identified, among which PAR-2 is specifically activated by trypsin
and MC tryptase, while others are activated by thrombin (7). Various lines of evidence have so far
demonstrated that PAR-2,highly expressed in the intestinal
epithelial cells, functions in small IIR injury (8) and inhibition of tryptase following
ischemia limits this by downregulation of PAR-2 (2). However, the precise mechanism of the
direct role of tryptase in small intestinal mucosal injury remains
to be elucidated.
β-arrestins (β-arrestin-1 and -2), originally
identified as terminators of the G protein-coupled receptor
signaling pathway, interact with PAR-2 as signal scaffolds
(9) and a previous study
determined that β-arrestins participate in PAR-2-mediated signal
transduction (10). As a subtype
of the β-arrestin family, Jacob et al (11) demonstrated that β-arrestin-2 is
essential for PAR2-induced activation of extracellular
signal-related kinase (ERK) 1/2 in colonocytes triggered by
tryptase. Furthermore, β-arrestin-2 is significant in
PAR-2-stimulated immune cell migration (12) and β-arrestin-2 may negatively
regulate inflammation in polymicrobial sepsis (13). However, to the best of our
knowledge, no studies have yet elucidated the role of β-arrestin-2
in tryptase-induced intestinal mucosal injury under
pathophysiological conditions.
Based on previous findings, it was hypothesized that
tryptase alone may directly result in small intestinal mucosal
injury via PAR-2 activation, and that β-arrestin-2 may participate
in the process of injury. This hypothesis was evaluated in the
IEC-6 rat intestinal epithelial cell line challenged by tryptase
stimulation in the presence or absence of specific PAR-2 and ERK
inhibitors, as well by knockdown of β-arrestin-2 in
vitro.
Materials and methods
Reagents
IEC-6 cells were purchased from Xiehe Cell Resource
Center (Beijing, China). Dulbecco's modified Eagle's medium (DMEM),
DMEM with high glucose and fetal bovine serum (FBS) were purchased
from Gibco Life Technologies (Carlsbad, CA, USA). Tryptase was
obtained from Promega Corporation (Madison, WI, USA). PAR-2
inhibitor, FSLLRY-NH2 (FS) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). The 3-(4,5-dimethylthiazol-2-yl)
-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay,
Cytotoxicity Detection kit and LDH Detection kit were purchased
from KeyGen Biotech Co., Ltd. (Nanjing, China). Specific monoclonal
antibodies against p44/42 mitogen-activated protein kinases (MAPK;
ERK1/2), phospho (p)-p44/42 MAPK (p-ERK1/2), total caspase-3,
cleaved caspase-3, and MEK1/2 inhibitor, U0126 were purchased from
Cell Signaling Technology, Inc., (Beverly, MA, USA). Mouse specific
polyclonal antibodies against β-arrestin 2 (mouse/human; dilution,
1:1,000, cat no. ab54790; Abcam, Cambridge, MA, USA) and mouse
specific monoclonal antibodies against PAR2 (mouse/rat/human;
dilution, 1:500; cat no. sc-13504; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) were used. Furthermore, rabbit monoclonal
antibodies against p44/42 (human/mouse/rat; dilution, 1:2,000; cat
no. 4695), rabbit monoclonal antibodies against caspase-3
(human/mouse/rat/monkey; dilution, 1:2,000; cat no. 9665), mouse
monoclonal antibodies against β-actin (human, mouse, rat; dilution,
1:1,000; cat no. 3700), rabbit monoclonal antibodies against
phospho (p)-p44/42 (human/mouse/rat; dilution, 1:2,000; cat no.
8544), rabbit monoclonal antibodies against cleaved caspase-3
(human/mouse/rat/monkey; dilution, 1:2,000; cat no. 9654),
horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin (Ig)G (dilution, 1:2,000; cat no. 4410) and
HRP-conjugated goat anti-rabbit IgG (dilution, 1:2,000; cat no.
7074) were all purchased from Cell Signaling Technology, Inc.,
(Beverly, MA, USA). Control small interfering (si)RNA, β-arrestin-2
siRNA and siRNA Transfection Reagent were obtained from Santa Cruz
Biotechnology, Inc.
Cell cultures
IEC-6 cells (rat small intestinal epithelial cell
line) were cultured in DMEM with 4.5 g/l glucose supplemented with
5% FBS, 0.01 mg/ml insulin, supplemented with 100 U/ml penicillin
and 100 µg/ml streptomycin (all from Sigma-Aldrich) in a
humidified atmosphere containing 5% CO2 at 37°C. The
culture medium was replaced twice each week and all cells used in
the experiments were in passage (P)24-P50.
Cell proliferation assay
Cell proliferation was measured by MTT assay, as
described previously (14). Cells
were collected and seeded at a density of 5,000 cells/well, in
96-well plates, at 37°C in a humid chamber with 5% CO2
for 24 h. The cells were treated with tryptase (0, 10, 100 or 1,000
ng/ml) in medium containing 1% FBS for 0, 6, 12 and 24 h. MTT (1X;
50 µl) was added to each well and incubated with cells at
37°C for 4 h. The medium was replaced with 150 µl dimethyl
sulfoxide (DMSO) per well to dissolve the formazan crystals. The
optical density (OD) was detected with a ThermoMax microplate
reader (Thermo Fisher Scientific, Waltham, MA, USA) at a wavelength
of 490 nm. All experiments were performed in triplicate.
LDH activity assay
To prepare samples for the LDH activity assay,
1×104 cells were seeded in 96-well plates for 24 h prior
to stimulation. The cells were washed twice with PBS and exposed to
different concentrations of tryptase (0, 10, 100 or 1,000 ng/ml) in
DMEM containing 1% FBS. After 12 h exposure, the supernatant was
collected and centrifuged at 12,000 × g at 4°C for 15 min to remove
cell debris and the tryptase. Each sample (50 µl) was used
to determine the activity of LDH according to the instructions of
the LDH detection kit. The absorbance was measured at 440 nm using
the ThermoMax microplate reader.
β-Arrestin-2 knockdown
The protocol followed that of previous studies with
small modifications (15,16). The following solutions were
prepared: Solution A, for each transfection, 6 µl siRNA
duplex was diluted in 100 µl siRNA transfection medium
(reduced-serum medium); Solution B, for each transfection, 6
µl siRNA transfection reagent was diluted in 100 µl
siRNA transfection medium. The siRNA duplex solution (solution A)
was added directly to the diluted transfection reagent (solution B)
using a pipette, mixed gently by repetitive pipetting, and
incubated for 30 min at room temperature prior to use. Cells
(2×105 per well) were seeded in a 6-well tissue culture
plate in 2 ml antibiotic-free normal growth medium supplemented
with FBS and incubated at 37°C in a CO2 incubator until
the cells were 60–80% confluent. This took 18–24 h. The cells were
washed with 2 ml siRNA transfection medium. For each transfection,
0.8 ml siRNA transfection medium was added to each tube containing
the siRNA transfection reagent mixture (solution A + solution B).
After incubating for 6 h at 37°C in a CO2 incubator, the
transfection mixture was removed and replaced with 1X normal growth
medium for an additional 24 h, and the cells were subjected to
western blot analysis of β-arrestin-2.
Western blot analysis
Cells were scraped and collected in 80 µl
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
per well and maintained at 4°C. Cell lysate was centrifuged at
12,000 × g at 4°C for 15 min and protein concentration in the
extracts was determined by bicinchoninic acid assay. Proteins were
denatured with loading buffer [30mM EDTA, 36% (v/v) glycerol, 0.05%
(w/v) xylene cyanol FF and 0.05% (w/v) bromophenol blue] and
separated by 10 or 12% SDS-polyacrylamide gel (Beyotime Institute
of Biotechnology) at 200 V for 40 min. Proteins were
electro-transferred to 0.22- or 0.44-µm nitrocellulose
membranes (Beyotime Institute of Biotechnology) at 100 V for 60
min. The membranes were blocked with 5% non-fat milk dissolved in
Tris-buffered saline with Tween-20 (TBST; pH 7.5, 150 mM NaCl, 10
mM Tris-HCl and 0.1% Tween-20; Beyotime Institute of Biotechnology)
at room temperature for 1 h. The membranes were incubated with
different antibodies (anti-ERK, -p-ERK, -PAR-2, -β-arrestin-2,
-caspase-3, -cleaved caspase-3 and -β-actin) overnight at 4°C. The
membranes were washed at least three times with TBST and incubated
with a horseradish peroxidase-conjugated anti-rabbit IgG or
anti-mouse IgG for 1 h at room temperature. Densitometric analyses
were performed using Quantity One software v 4.62 (Bio-Rad
Laboratories, Hercules, CA, USA).
Statistical analysis
The data are presented as means ± standard error of
the mean. Analyses of variance (ANOVA) were performed using
Graphpad Prism software v. 5.01 (GraphPad Software, Inc., La Jolla,
CA, USA). One-way ANOVA was used for multiple comparisons, followed
by the Bonferroni method and Student's t-test for unpaired values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tryptase directly induced intestinal
epithelial cell injury
A previous study demonstrated that tryptase
exacerbated small intestinal injury in a rodent model of IIR
(17), however, in vivo
results cannot determine the roles of oxidative stress and
inflammation in the pathogenesis of small IIR injury (5). Thus, the aim of the present study was
to investigate the direct role of tryptase alone in intestinal
epithelial cells. As shown in Fig. 1A
and B, 100 or 1,000 ng/ml tryptase stimulation for 12 h reduced
the cell viabilities of IEC-6 cells, furthermore, incubating IEC-6
cells with 100 or 1,000 ng/ml tryptase exhibited marked injury, as
demonstrated by significant increases in LDH activity; however,
stimulation with 10 ng/ml tryptase indicated a smaller increase in
LDH activity as compared with non-stimulated cells. Furthermore, no
statistical differences were observed between the 100 and 1,000
ng/ml groups. These results suggest that tryptase at higher
concentrations may directly damage intestinal epithelial cells.
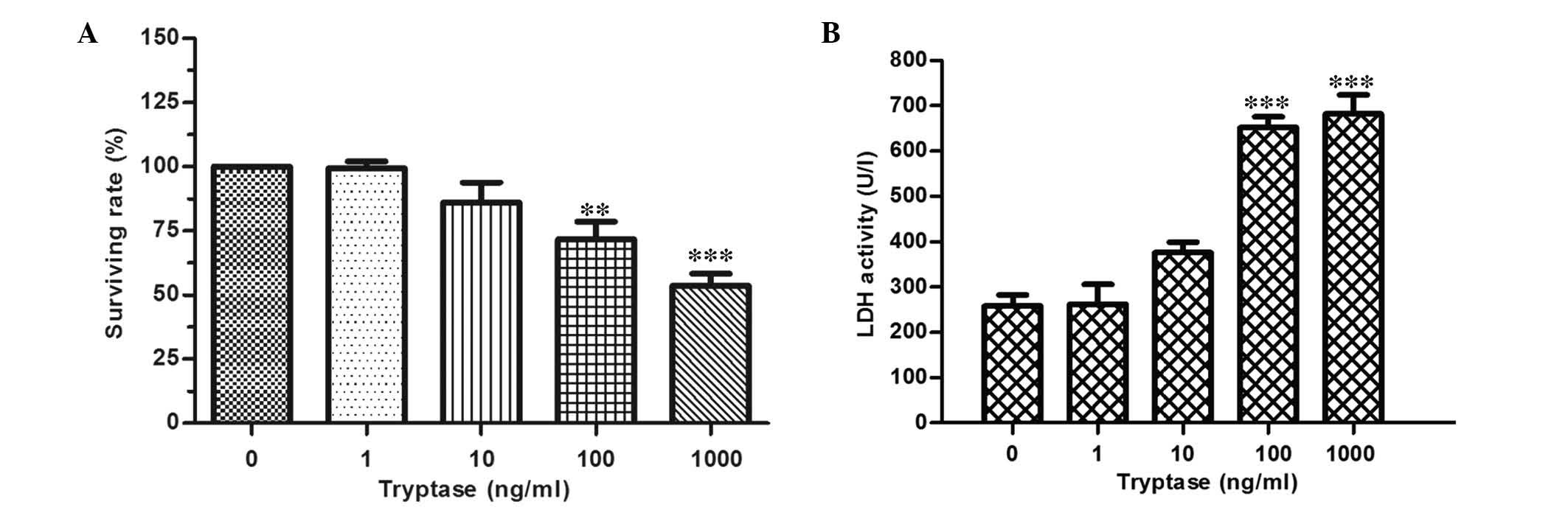 | Figure 1IEC-6 cell injury induced by different
concentrations of tryptase stimulation. (A) Cell viabilities
measured by MTT assay of IEC-6 cells following tryptase stimulation
at different concentrations (0, 1, 10, 100 and 1,000 ng/ml) for 12
h. (B) LDH activity resulting from IEC-6 cells stimulated by
different concentrations of tryptase (0, 1, 10, 100, and 1,000
ng/ml) for 8 h. Data are expressed as percentages of the control
(non-stimulated) group (n=4). **P<0.01,
***P<0.005 vs. control group. LDH, lactate
dehydrogenase. |
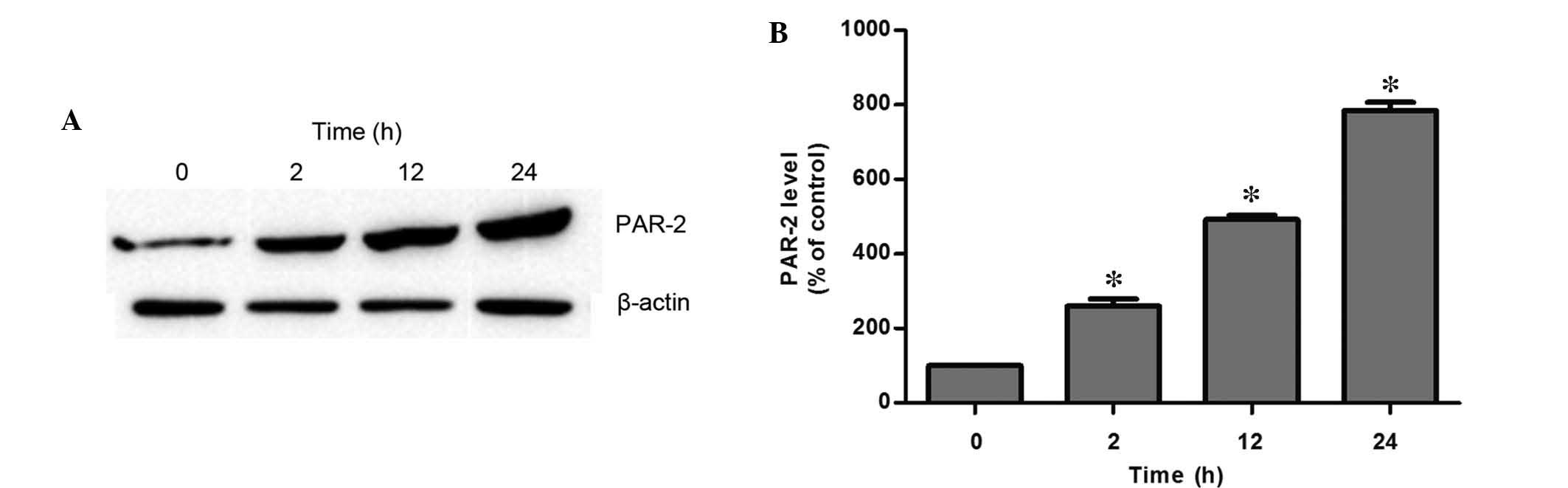
Tryptase enhanced PAR-2 protein
expression
It has been demonstrated that PAR-2 is critical in
the process of tryptase-induced inflammation in the bladder
(18), thus, the involvement of
PAR-2 in tryptase-mediated IEC-6 cell injury was evaluated in the
present study. PAR-2 protein expression was markedly upregulated in
a time-dependent manner following exposure to 1,000 ng/ml tryptase
(Fig. 2).
IEC-6 cell injury was induced by tryptase
via PAR-2
As tryptase may upregulate PAR-2 protein expression,
the current study evaluated whether tryptase mediates IEC-6 cell
injury via the PAR-2 signaling pathway. Thus, a specific PAR-2
inhibitor, FS, was employed. Treatment with 200 or 400 µM FS
blocked significant increases in LDH activity and cleaved caspase-3
protein expression when challenged by 1,000 ng/ml tryptase
stimulation. Furthermore, FS alone at concentrations of 200 and 400
µl had no effect on LDH activity, as compared with the
control group. In addition, no significant differences between FS
at concentrations of 200 and 400 µM were identified
(P>0.05; Fig. 3A).
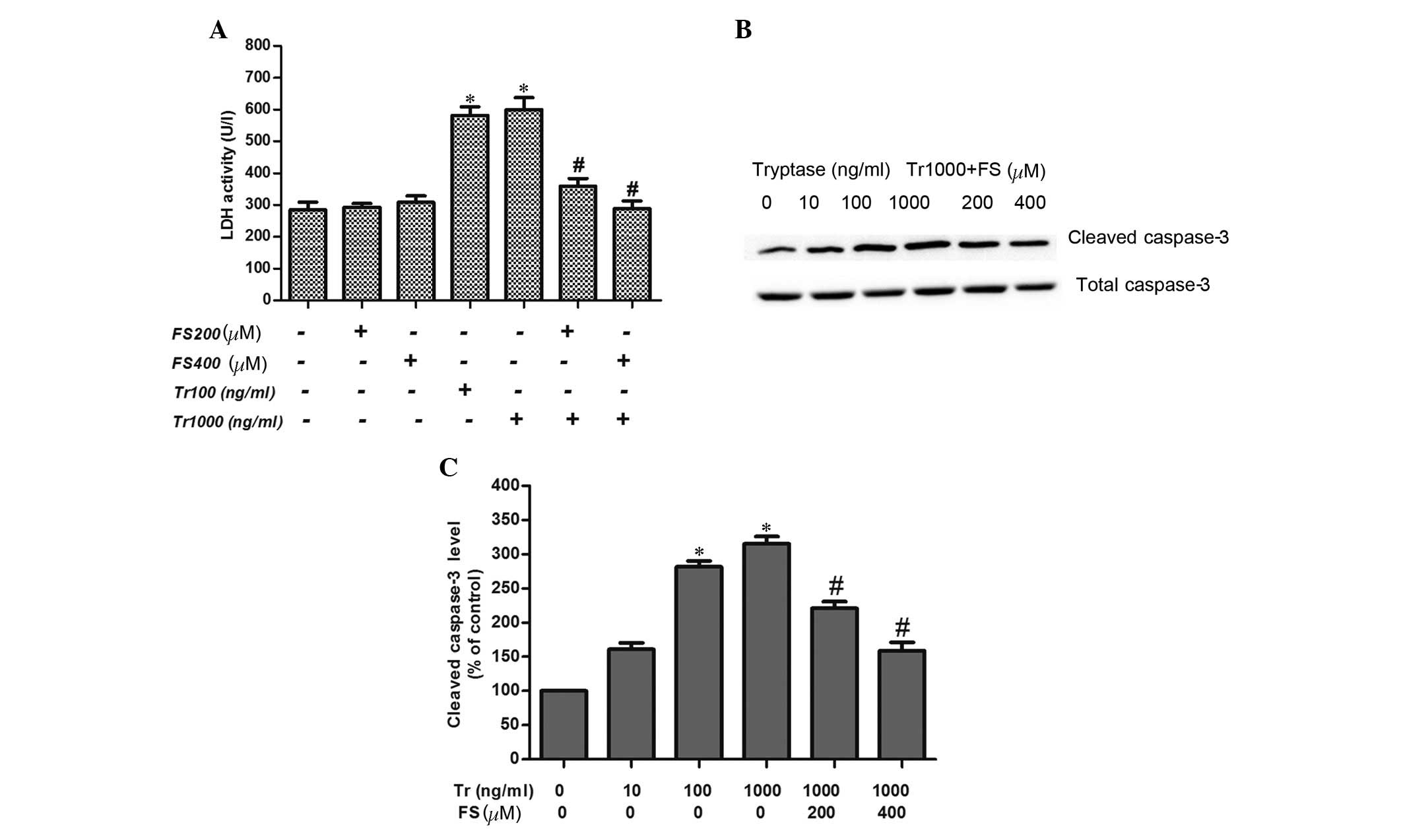 | Figure 3Effect of protease-activated receptor
2 inhibitor, FS on LDH release and the protein expression of
cleaved caspase-3. (A) LDH activities of IEC-6 cells following
exposure to different tryptase concentrations (100 and 1,000 ng/ml)
in the presence or absence of FS (200 and 400 µM) for 8 h
(n=4). (B) Band of cleaved caspase-3 protein expression in IEC-6
cells stimulated by different concentrations of tryptase (10, 100,
and 1,000 ng/ml) in the presence or absence of FS (200 and 400
µM) for 12 h. (C) Variations in cleaved caspase-3 protein
expression (n=3). *P<0.05 vs. control
(non-stimulated) group, #P<0.05 vs. tryptase
stimulated (concentration, 1,000 ng/ml) group. FS. FSLLRY-NH2; LDH,
lactate dehydrogenase; Tr, tryptase. |
ERK-2 was involved in the PAR-2 signaling
pathway
As MAPK is one of the downstream signal pathways of
PAR-2, the current study examined the role of ERK, an MAPK. The
results demonstrated that p-ERK protein expression was
significantly increased and peaked 30 min subsequent to tryptase
(1,000 ng/ml) exposure; however, p-ERK protein expression was
reduced to baseline at 60–90 min. Furthermore, as shown in Fig. 4, the specific ERK inhibitor (U0126)
blocked tryptase-mediated IEC-6 cell injury, as demonstrated by
markedly downregulating LDH activity.
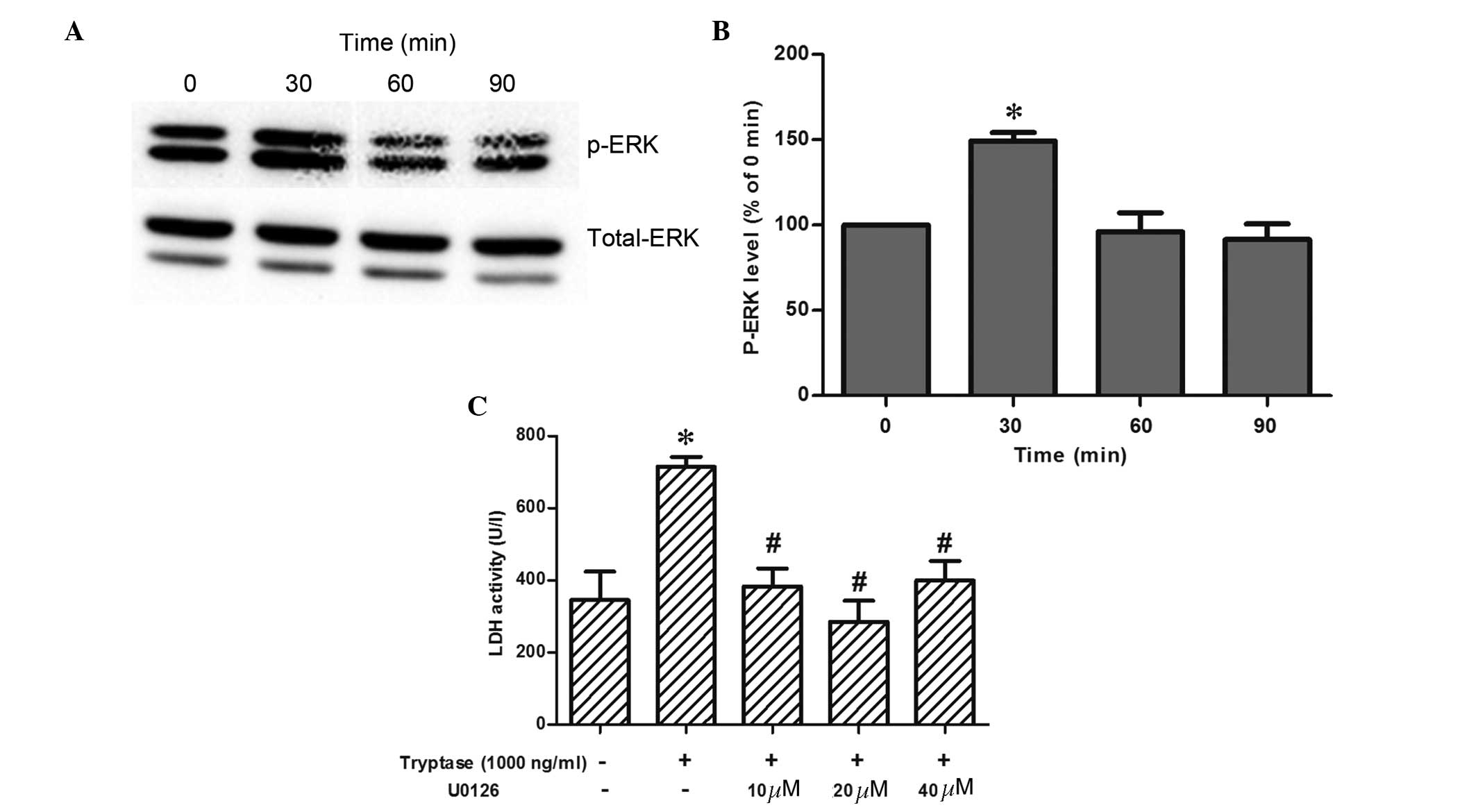 | Figure 4Changes in, and the role, of p-ERK in
LDH release in IEC-6 cells following tryptase stimulation. (A) Band
of time course changes of p-ERK protein expression in IEC-6 cells
at different time-points (0, 30, 60 and 90 min) with 1,000 ng/ml
tryptase stimulation. (B) Variations in p-ERK protein expression
(n=3). *P<0.05 vs. baseline. (C) LDH activity in
IEC-6 cells exposed to 1,000 ng/ml tryptase for 8 h in the absence
or presence of variations in specific p-ERK inhibitor, U0126 (10,
20 and 40 µM; n=4). *P<0.05 vs. control
(non-stimulated) group, #P<0.05 vs. 1,000 ng/ml
tryptase stimulated group (1,000 ng/ml). p-ERK,
phosphorylated-extracellular signal-related kinases; LDH, lactate
dehydrogenase; Tr, tryptase. |
Role of β-arrestin-2 in tryptase-mediated
IEC-6 cell injury
Previous studies have demonstrated that β-arrestin-2
is involved in PAR-2-associated signaling pathways, and affects the
structure and function of cells (10). In the present study, the
β-arrestin-2 protein expression exhibited no changes within 24 h of
1,000 ng/ml tryptase stimulation. Following knock-down of
β-arrestin-2 (Fig. 5C and D), the
IEC-6 cell injury was significantly attenuated, as demonstrated by
a marked reduction in LDH activity, and the cleaved caspase-3
protein expression when compared with the control groups in the
presence of 100–1,000 ng/ml tryptase (Fig. 5E–G). Additionally, after 12 h
exposure to 1,000 ng/ml tryptase, β-arrestin-2 knockdown
significantly reduced the increases in PAR-2 protein expression
that were induced by tryptase (Fig. 5H
and I). These results collectively indicate that
tryptase-mediated IEC-6 cell injury via PAR-2 activity requires
β-arrestin-2.
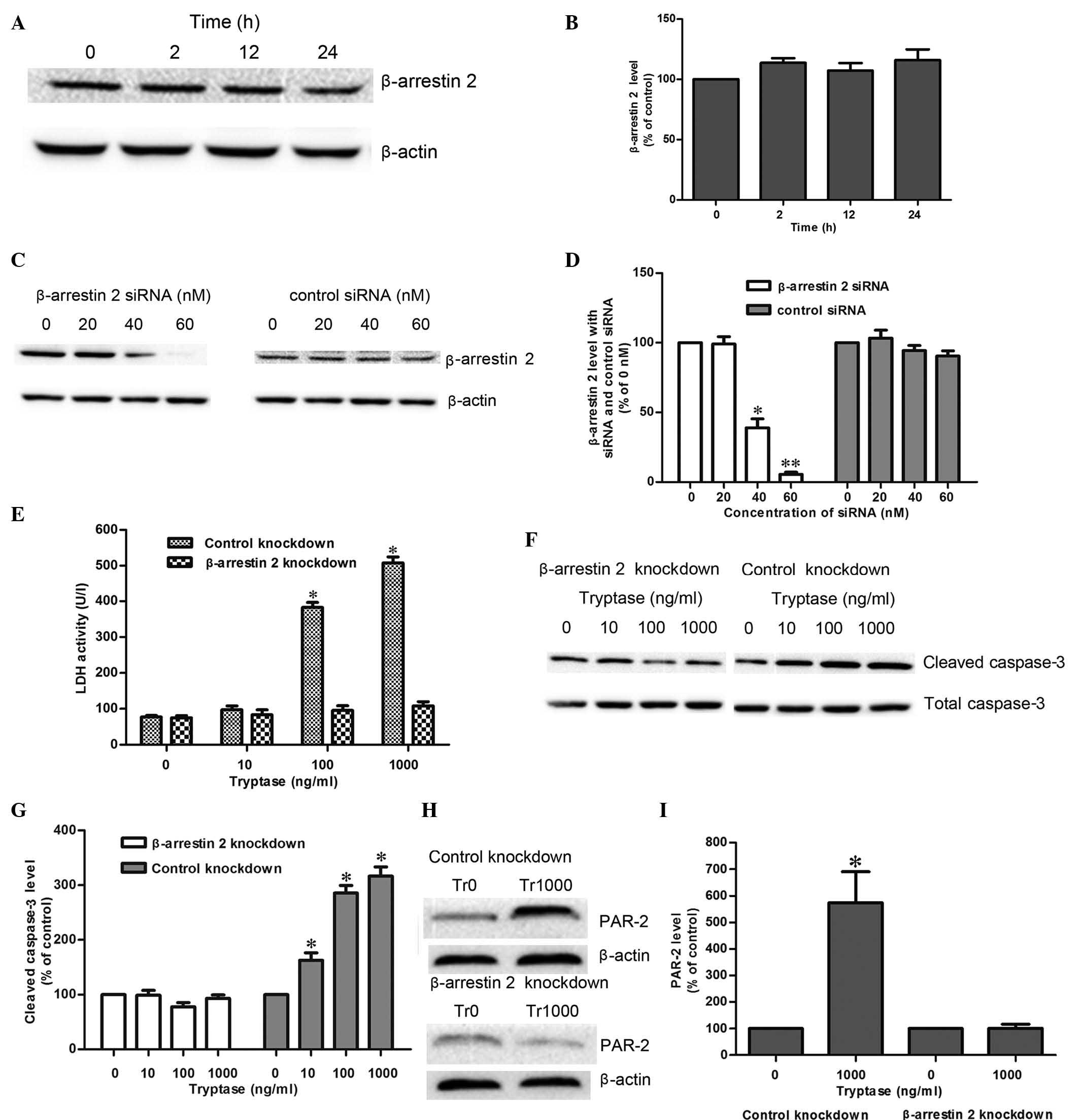 | Figure 5Roles of β-arrestin-2 in
tryptase-mediated IEC-6 injury. (A) Band of time-course changes of
β-arrestin-2 protein expression in IEC-6 cells at different
time-points (0, 2, 12, and 24 h) with 1,000 ng/ml tryptase
stimulation. (B) Changes of β-arrestin-2 protein expression (n=3).
(C) Band of β-arrestin-2 protein expression following treatment
with different dosages of siRNA and control siRNA reagents (0, 20,
40 and 60 nM). (D) Protein expression of β-arrestin-2 (n=3).
*P<0.05; **P<0.01 vs. control group (0
nM). (E) LDH activities in IEC-6 cells following tryptase
stimulation for 8 h in the absence or presence of β-arrestin-2
knockdown (n=3). *P<0.05 vs. control siRNA group. (F)
Cleaved caspase-3 protein expression in IEC-6 cells stimulated by
different concentrations of tryptase (10, 100 and 1,000 ng/ml) in
the presence or absence of β-arrestin-2 knockdown for 12 h. (G)
Changes of cleaved caspase-3 protein expression (n=3).
*P<0.05 vs. control siRNA group. (H) PAR-2 protein
expression in IEC-6 cells stimulated by tryptase (1,000 ng/ml) in
the presence or absence of β-arrestin-2 knockdown for 12 h. (I)
Changes of PAR-2 protein expression (n=3). *P<0.05
vs. the control knockdown group. PAR-2, protease-activated receptor
2; LDH, lactate dehydrogenase; Tr, tryptase; siRNA, small
interfering RNA. |
Discussion
Our previous study indicated that tryptase may
aggravate small IIR injury (2),
however, little is known of the direct role of tryptase in small
intestinal epithelial cells. In the present study, tryptase alone
was demonstrated to directly induce IEC-6 cell injury, as
demonstrated by significant increases in LDH activity and cleaved
caspase-3 protein expression following exposure to 100 or 1,000
ng/ml tryptase for 12 h. Furthermore, tryptase markedly upregulated
PAR-2 expression and specifically inhibiting PAR-2 significantly
abrogated tryptase-mediated injury. Furthermore, ERK was
demonstrated to be involved in the process of injury, as the
addition of an ERK inhibitor blocked the increase in LDH activity,
which had been induced by tryptase. Notably, tryptase stimulation
exerted no effects on the expression of β-arrestin-2 in IEC-6 cells
whereas the injury induced by tryptase was significantly reduced
following β-arrestin-2 knockdown. These results suggest that
tryptase may directly result in IEC-6 cell injury by activating the
PAR-2/ERK signaling pathway, and that the process of
tryptase-mediated injury requires β-arrestin-2.
Tryptase is a protease that is released by MCs and
accounts for >25% of the total MC protein (19). Tryptase has been demonstrated to
contribute to various disorders; for example, tryptase was
demonstrated to be key in the development of airway
hyper-responsiveness in a mouse model of asthma (20). Furthermore, tryptase contributes to
colitis, as stabilizing MCs, by releasing tryptase, significantly
alleviate the symptoms of colitis (21). A previous study suggests that
tryptase levels in the lower gastrointestinal tract were 33.5 ng/mg
(range, 8.0–154.6 ng/mg), and the tissue tryptase concentrations
were significantly increased to 55.7 ng/mg (range, 9.3–525.0)
subsequent to MC degranulation (22), thus, a range from 1 to 1,000 ng/ml
tryptase was used in the current study. The direct mechanism by
which tryptase alone contributes to IEC-6 cell injury was assessed
in the present study. It was identified that 100 or 1,000 ng/ml
tryptase stimulation resulted in significant damages to IEC-6
cells, which is consistent with a previous report demonstrating
that tryptase significantly contributes to inflammatory bowel
disease (4). The findings from the
present study further confirm that tryptase released by MC
activation is critical in small intestinal mucosal cell injury.
PAR-2 is highly expressed in small intestinal
mucosa, our previous studies have demonstrated that inhibiting
tryptase may reduce small IIR injury with associated downregulation
of PAR-2 (17), furthermore,
tryptase-induced itch occurs via activation of PAR-2 (23). However, since PAR-2 may be
activated by various factors, including tryptase and trypsin, it is
unknown whether the changes in PAR-2 are directly triggered by
tryptase released from MC degranulation during IIR injury in
vivo. In the present in vitro study, tryptase
stimulation was indicated to induce significant increases in PAR-2
protein expression and, furthermore, the PAR-2 inhibitor, FS
blocked the damage to IEC-6 cells, which had been triggered by 100
or 1,000 ng/ml tryptase. The results indicate that
tryptase-mediated intestinal mucosal injury is via the PAR-2
signaling pathway.
The activation of PAR-2 initiates multiple
inflammatory responses (24), and
PAR-2 activation often leads to subsequent activation of downstream
MAPK signaling pathways (7),
Groschwitz et al (25)
demonstrated that chymase stimulation of Caco-2 BBe cells resulted
in a significant increase in p38 and p44/42 (ERK1/2) activity at 15
and 60 min and, furthermore, that those changes were
PAR-2-dependent. In the present study it was found that p-ERK
expression was significantly increased and peaked at 30 min after
tryptase stimulation, and the addition of a specific ERK inhibitor
reduced the significant tryptase-induced elevations in LDH activity
and cleaved caspase-3 protein expression in IEC-6 cells. The
findings from the current study suggest tryptase may injure IEC-6
cells via PAR-2 activation and the downstream ERK signaling
pathway.
β-arrestin-2 is an adaptor protein that may promote
desensitization of G-protein signaling, it also functions as a
scaffold protein in the process of PAR-2 activation (26), and this signaling pathway is
crucial for PAR-2-stimulated cell migration (10). However, the role of β-arrestin-2 in
tryptase-mediated IEC-6 cell injury remains to be elucidated. In
the present study, the results demonstrate that there were no
changes in β-arrestin-2 protein expression resulting from tryptase
stimulation; however, the tryptase-mediated IEC-6 cell injury, as
well as upregulation in PAR-2 protein expression, was blocked by
β-arrestin-2 knockdown. These results are consistent with reports
that the PAR-2-mediated inflammatory process in the airway is
β-arrestin-2-dependent (27). The
findings from the current study further suggest that β-arrestin-2
functions as a scaffold/adaptor protein for PAR-2 activation
triggered by tryptase stimulation.
There are certain limitations of the present study;
firstly, four PARs are expressed in small intestinal mucosal cells,
and the current study did not examine the roles of PAR-1, -3, or -4
in IEC-6 cell injury, as a previous study demonstrated that PAR-2
is specifically activated by tryptase. Secondly, the downstream
MAPK signaling pathways induced by PAR-2 activation include ERK1/2,
c-Jun N-terminal kinase and p38 kinases. However, only ERK1/2 was
analyzed in the present study, as these kinases share the same
signaling pathways (28).
In conclusion, these data suggest that tryptase
alone may induce IEC-6 cell injury, via the PAR-2 and ERK signaling
pathways. Furthermore, the activation of PAR-2, which is triggered
by tryptase requires β-arrestin-2.
Acknowledgments
The present study was supported by the Fundamental
Research Funds for the Central Universities of China (grant no.
12ykpy37) and Science and Technology Program of Guangzhou, China
(grant no. 2014J4100172).
References
|
1
|
Brenner SA, Zacheja S, Schäffer M,
Feilhauer K, Bischoff SC and Lorentz A: Soluble CD14 is essential
for lipopolysaccharide-dependent activation of human intestinal
mast cells from macroscopically normal as well as Crohn's disease
tissue. Immunology. 143:174–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gan X, Liu D, Huang P, Gao W, Chen X and
Hei Z: Mast-cell-releasing tryptase triggers acute lung injury
induced by small intestinal ischemia-reperfusion by activating
PAR-2 in rats. Inflammation. 35:1144–1153. 2012. View Article : Google Scholar
|
|
3
|
Gan X, Su G, Zhao W, Huang P, Luo G and
Hei Z: The mechanism of sevoflurane preconditioning-induced
protections against small intestinal ischemia reperfusion injury is
independent of mast cell in rats. Mediators Inflamm.
2013:3787032013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamilton MJ, Sinnamon MJ, Lyng GD,
Glickman JN, Wang X, Xing W, Krilis SA, Blumberg RS, Adachi R, Lee
DM and Stevens RL: Essential role for mast cell tryptase in acute
experimental colitis. Proc of the Natl Acad Sci USA. 108:290–295.
2011. View Article : Google Scholar
|
|
5
|
Zhao W, Zhou S, Yao W, Gan X, Su G, Yuan D
and Hei Z: Propofol prevents lung injury after intestinal
ischemia-reperfusion by inhibiting the interaction between mast
cell activation and oxidative stress. Life Sci. 108:80–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao P, Metcalf M and Bunnett NW: Biased
signaling of protease-activated receptors. Front Endocrinol
(Lausanne). 5:672014.
|
|
7
|
Rothmeier AS and Ruf W: Protease-activated
receptor 2 signaling in inflammation. Semin Immunopathol.
34:133–149. 2012. View Article : Google Scholar
|
|
8
|
Yoshida N, Takagi T, Isozaki Y, Suzuki T,
Ichikawa H and Yoshikawa T: Proinflammatory role of
protease-activated receptor-2 in intestinal ischemia/reperfusion
injury in rats. Mol Med Rep. 4:81–86. 2011.PubMed/NCBI
|
|
9
|
Kang DS, Tian X and Benovic JL: Role of
β-arrestins and arrestin domain-containing proteins in G
protein-coupled receptor trafficking. Curr Opin Cell Biol.
27:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoudilova M, Min J, Richards HL, Carter D,
Huang T and DeFea KA: beta-Arrestins scaffold cofilin with
chronophin to direct localized actin filament severing and membrane
protrusions downstream of protease-activated receptor-2. J Biol
Chem. 285:14318–14329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacob C, Yang PC, Darmoul D, Amadesi S,
Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M and
Bunnett NW: Mast cell tryptase controls paracellular permeability
of the intestine. Role of protease-activated receptor 2 and
beta-arrestins. J Biol Chem. 280:31936–31948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge L, Shenoy SK, Lefkowitz RJ and DeFea K:
Constitutive protease-activated receptor-2-mediated migration of
MDA MB-231 breast cancer cells requires both beta-arrestin-1 and
-2. J Biol Chem. 279:55419–55424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan H, Bitto A, Zingarelli B, Luttrell LM,
Borg K, Halushka PV and Cook JA: Beta-arrestin 2 negatively
regulates sepsis-induced inflammation. Immunology. 130:344–351.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho J, Du Y, Wong OG, Siu MK, Chan KK and
Cheung AN: Downregulation of the gli transcription factors
regulator kif7 facilitates cell survival and migration of
choriocarcinoma cells. PLoS One. 9:e1082482014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du RW, Du RH and Bu WG: β-Arrestin 2
mediates the anti-inflammatory effects of fluoxetine in
lipopolysaccharide-stimulated microglial cells. J Neuroimmune
Pharmacol. 9:582–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lakshmikanthan V, Zou L, Kim JI, Michal A,
Nie Z, Messias NC, Benovic JL and Daaka Y: Identification of
betaArrestin2 as a corepressor of androgen receptor signaling in
prostate cancer. Proc Natl Acad Sci USA. 106:9379–9384. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu D, Gan X, Huang P, Chen X, Ge M and
Hei Z: Inhibiting tryptase after ischemia limits small intestinal
ischemia-reperfusion injury through protease-activated receptor 2
in rats. J Trauma Acute Care Surg. 73:1138–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZY, Wang P and Bjorling DE: Role of
mast cells and protease-activated receptor-2 in cyclooxygenase-2
expression in urothelial cells. Am J Physiol Regul Integr Comp
Physiol. 297:R1127–R1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pejler G, Rönnberg E, Waern I and
Wernersson S: Mast cell proteases: Multifaceted regulators of
inflammatory disease. Blood. 115:4981–4990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Y, Dahlin JS, Feinstein R, Bankova LG,
Xing W, Shin K, Gurish MF and Hallgren J: Mouse mast cell
protease-6 and MHC are involved in the development of experimental
asthma. J Immunol. 193:4783–4789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eliakim R, Karmeli F, Okon E and
Rachmilewitz D: Ketotifen effectively prevents mucosal damage in
experimental colitis. Gut. 33:1498–1503. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagel AF, deRossi T, Zopf Y, Konturek P,
Dauth W, Kressel J, Hahn EG and Raithel M: Mast cell tryptase
levels in gut mucosa in patients with gastrointestinal symptoms
caused by food allergy. Int Arch Allergy Immunol. 160:350–355.
2013. View Article : Google Scholar
|
|
23
|
Tsujii K, Andoh T, Ui H, Lee JB and
Kuraishi Y: Involvement of tryptase and proteinase-activated
receptor-2 in spontaneous itch-associated response in mice with
atopy-like dermatitis. J Pharmacol Sci. 109:388–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ebeling C, Forsythe P, Ng J, Gordon JR,
Hollenberg M and Vliagoftis H: Proteinase-activated receptor 2
activation in the airways enhances antigen-mediated airway
inflammation and airway hyperresponsiveness through different
pathways. J Allergy Clin Immunol. 115:623–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Groschwitz KR, Wu D, Osterfeld H, Ahrens R
and Hogan SP: Chymase-mediated intestinal epithelial permeability
is regulated by a protease-activating receptor/matrix
metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest
Liver Physiol. 304:G479–G489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zoudilova M, Kumar P, Ge L, Wang P, Bokoch
GM and DeFea KA: Beta-arrestin-dependent regulation of the cofilin
pathway downstream of protease-activated receptor-2. J Biol Chem.
282:20634–20646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nichols HL, Saffeddine M, Theriot BS,
Hedge A, Polley D, El-Mays T, Vliagoftis H, Hollenberg MD, Wilson
EH, Walker JK and DeFea KA: β-Arrestin-2 mediates the
proinflammatory effects of proteinase-activated receptor-2 in the
airway. Proc Natl Acad Sci USA. 109:16660–16665. 2012. View Article : Google Scholar
|
|
28
|
Darling NJ and Cook SJ: The role of MAPK
signalling pathways in the response to endoplasmic reticulum
stress. Biochim Biophys Acta. 1843:2150–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|