Introduction
In recent years, stress-associated neuropsychiatric
disorders have been increasing, and stress has become an important
feature of modern society. Stress-associated neuropsychiatric
disorders, including anxiety and post-traumatic stress disorder
(PTSD), appear to be increasing. In the regulation of physiological
function and response to stressful stimuli, the nervous, endocrine
and immune systems are closely integrated. The
hypothalamic-pituitary-adrenal axis (HPA axis) is a major feature
of the neuroendocrine system, which controls reactions to stress.
Steroid hormone production by the HPA axis increases in response to
stressful stimuli, with the hypersecretion of corticoids
contributing to stress-associated neuropsychiatric disease
(1). Steroid receptors in the
brain are classified into glucocorticoid receptors (GR) and/or
mineralocorticoid receptors (MR). The hippocampus is one of the
most stress-susceptible regions in the brain. GR and MR are
abundant in the hippocampus (2).
These receptors exhibit a difference in affinity for
corticosteroids. GR are activated by stressful stimuli; however, MR
are activated under physiological conditions. Blood glucocorticoid
levels also increase in response to stressful stimuli; however, the
behavior of MR under stress-induced conditions remains to be
elucidated.
Therefore, an investigation was conducted with a
focus on the following three points: MR are abundant in the
hippocampus compared with other parts of the brain (3); the hippocampal CA regions exhibit
significant cytotoxic atrophy, particularly in pyramidal cells in
the CA3 region, due to social stress (4) and only MR are expressed in the CA3
region, whereas GR are not expressed (5). The aim of the present study was to
develop novel treatment strategies for neuropsychiatric disease
with impaired learning and memory functions and psychiatric
disorders utilizing the hippocampal MR function.
Materials and methods
Drug treatment
Fludrocortisone (FD), aldosterone (Aldo),
spironolactone (Spi), methyl green and diaminobenzidine (all from
Sigma-Aldrich, St. Louis, MO, USA), cresyl violet acetate (MP
Biomedicals, Illkirch, France) and Luxol Fast Blue
(Chroma-Gesellschaft Schmidt and Co., Stuttgart, Germany) were used
in the present study. Cholesterol, lithium carbonate, pentobarbital
sodium and sodium hydroxide were purchased from (Nacalai Tesque
(Kyoto, Japan).
Animals
Male mice of the ddY strain (six weeks old) were
obtained from Japan SLC Inc. (Hamamatsu, Japan) and maintained
under a constant temperature and humidity (25±1°C, 55±5% humidity)
with a 12-h light/dark cycle (7:00 to 19:00, 19:00 to 7:00). Solid
feed (CE-2, CLEA Japan Inc, Osaka, Japan) and hypochlorous acid
water (10 ppm) were available ad libitum. The mice were
acclimated to the environment for at least 1 week prior to the
initiation of the experiment. Experiments were approved by the
Institutional Animal Care and Use Committee of Tohoku
Pharmaceutical University (Sendai, Japan) according to the
guidelines for animal experiments prescribed by the Science Council
of Japan.
After FD (20 mg) and cholesterol (80 mg) were
pressed into tablets (0.5 mm3/5 mm) using a tableting
machine (Spectrum One, Perkin Elmer Co. Ltd., Waltham, MA, USA),
these were embedded in mouse dorsal subcutaneous tissues for 1
week. Mice were sacrificed by decapitation. The right hippocampus
was removed to collect the floating cells, then the left
hippo-campus was used to prepare the brain slices. The fresh and
frozen tissues were placed in liquid nitrogen and then stored at
−80°C. An adrenalectomy was performed on a proportion of the mice
under pentobarbital [60 mg/kg, intraperitoneally (i.p,)] anesthesia
to remove the left and right adrenal glands followed by seven days
of rearing.
Comet assay
The comet assay was performed using the
CometAssay® kit (Trevigen, Gaithersburg, MD, USA).
Briefly, following the collection of mouse hippocampal floating
cells (1×105 cells/ml) via centrifugation at 300 x g for
10 min, agarose was added and the samples were sequentially
subjected to alkaline lysis. Electrophoresis was performed at 50 V
for 10 min, followed by the addition of SYBR green 1 nucleic acid
stain. The gel was observed using a fluorescence microscope
(Eclipse E-800, C1-LU3, C1-SHV; Nikon, Tokyo, Japan). Following
capturing the PC image with Image Gauge version 4.0 (Fuji Photo
Film Co. Ltd., Tokyo, Japan) on the fluorescence camera, the tail
length was calculated to compare the cytotoxic intensity.
Preparation of cryostat sections
Sagittal sections (12 µm) were cut using a
cryostat from the frozen samples of brain tissue embedded in
cryomold optimum cutting temperature compound (Sakura Finetech
Inc., Tokyo, Japan). The frozen blocks were sliced sequentially
using a cryostat microtome (Microm HM505N; Microm International
GmbH, Walldorf, Germany) to prepare serial sections. These sections
were then mounted on (3-aminopropyl)triethoxysilane-coated micro
slide glasses (Matsunami Glass Ind. Ltd., Tokyo, Japan) for
assessment of the anatomical regions. Anatomical brain regions and
brain areas were identified using a mouse brain atlas (6). The respective sections were
maintained at −80°C until use.
Kluver-Barrera (KB) staining
After 20 min of air-drying at room temperature, the
slices were incubated in 0.1% Luxol Fast Blue solution for 30 min
at 60°C, then washed successively in ethanol, lithium carbonate and
deionized water. After the 90 min incubation at 37°C in 10% cresyl
violet acetate solution, the slices were washed in ethanol at 4°C,
followed by air-drying, then they were observed under an optical
microscope (CX-41; Olympus Co. Ltd., Tokyo, Japan).
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick end labeling (TUNEL) staining
TUNEL staining was performed using the in
situ apoptosis detection kit of Takara-Bio Inc. (Otsu, Japan).
Briefly, the slices were fixed for 20 min in 4% formaldehyde
solution and then the endogenous peroxidase was blocked in a 0.3%
H2O2 methanol solution. Following incubation
with the TdT enzyme (containing labeling safe buffer) in 50
µl/slice, the reaction adjustment solution (anti-fluorescein
isothiocyanate-horseradish peroxidase conjugate) was added to the
sample (70 µl/slice), followed by staining with
3,3′-diaminobenzidine-H2O2 solution. The
counterstaining was performed with 3% methyl green solution.
Serum creatinine measurement
The Wako-Creatinine kit from Wako Pure Chemical
Industry (Osaka, Japan) was used. The absorbance was measured at
492 nm (Immuno-mini, NJ-300; Biotec Co. Ltd., Tokyo, Japan) by
addition of picric acid reagent and 0.75 M sodium hydroxide.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. The Kruskal-Wallis analysis of variance rank test
followed by Dunnett's test was performed to assess statistical
significance using the Sigma Stat Statistical Software version 2.03
(Systat Software, Inc., Chicago, IL USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
MR exhibit an affinity for cortisol
MR are present in the cytoplasm of epithelial cells,
producing an aldosterone-MR complex, which affects Na or water
storage via movement into the nucleus. By contrast, cortisol is
present at a concentration of ~1,000 times that of aldosterone in
the blood, exhibiting a binding capacity equal to that of
aldosterone for MR. The selective binding of MR and aldosterone in
epithelial tissues, such as the kidney, is protected by type 2
11β-hydroxylase (11β-HSD2) (7),
which rapidly converts cortisone to a low MR-affinity steroid,
cortisol. MR have been revealed to be present in non-epithelial
cells, including the brain and heart, and the levels of 11β-HSD2 in
these organs are lower as compared with those in other epithelial
tissues, particularly the hippocampus (8). Since cortisol cannot be converted to
cortisone in the hippocampus, learning and memory disorders are
caused by the excessive secretion of glucocorticoids in an acute
stress disorder, such as PTSD. Therefore, it is possible that
hippocampal neuron damage is not only due to the glucocorticoid-GR
system, but is also regulated by the MR system. Based on this
evidence, the effect of the administration of FD, a synthetic
mineralocorticoid, on hippocampal neurons was investigated in mice.
The effect of spironolactone on FD-induced hippocampal neuron
damage in vitro was also examined.
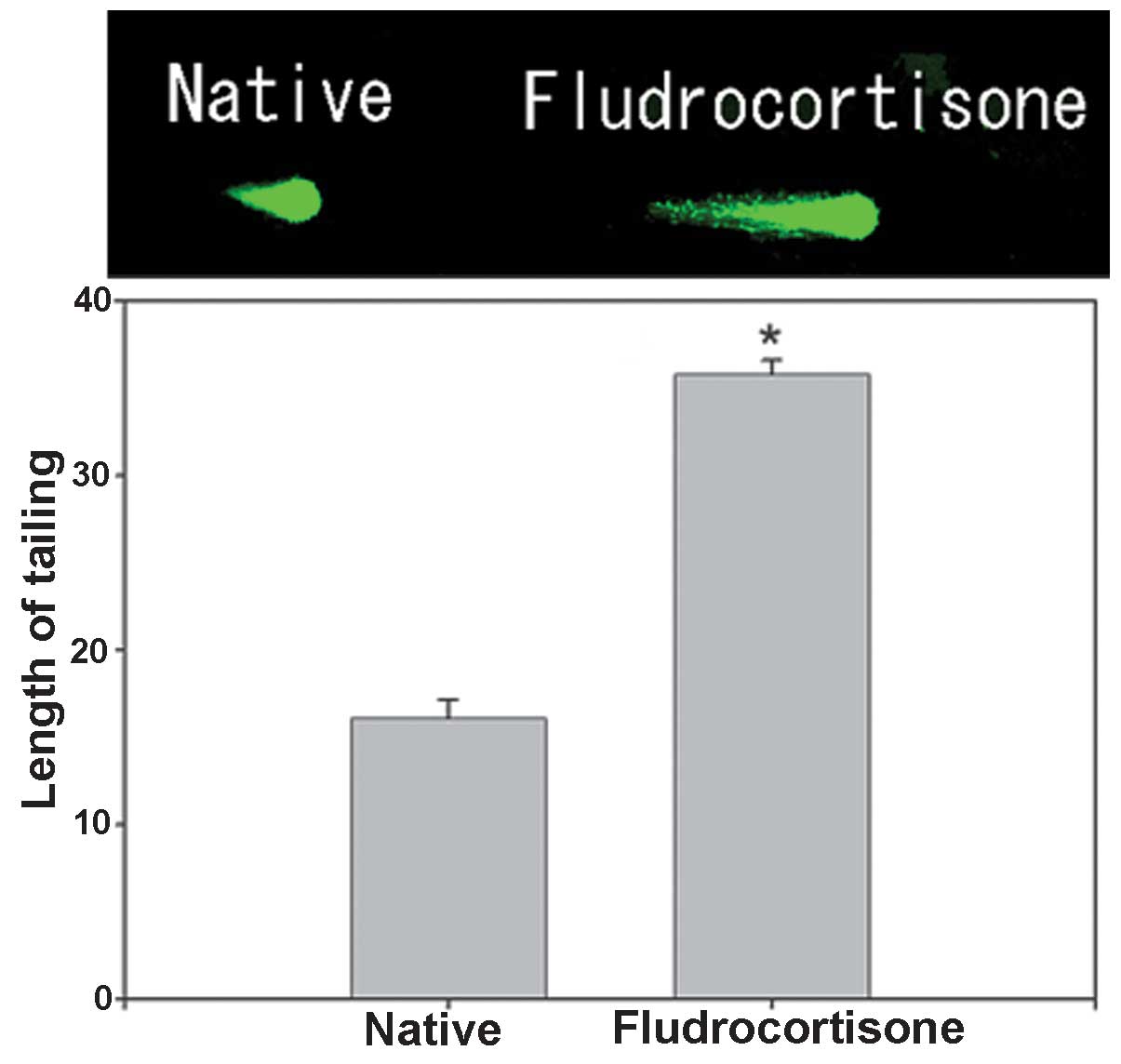
Effect of FD on hippocampal neurons
The effect of FD on hippocampal neurons caused by
embedding FD-containing cholesterol pellets subcutaneously in the
backs of mice (FD pellet group, 80 mg cholesterol and 20 mg FD) was
investigated using the comet assay (n=15 for each mouse; total, 75
per group). A significant extension of the tail length by ~2.22
fold was noted in the FD pellet group compared with that in the
control group (Fig. 1).
Subsequently, morphological changes in hippocampal neurons based on
KB staining and functional changes based on TUNEL staining were
investigated.
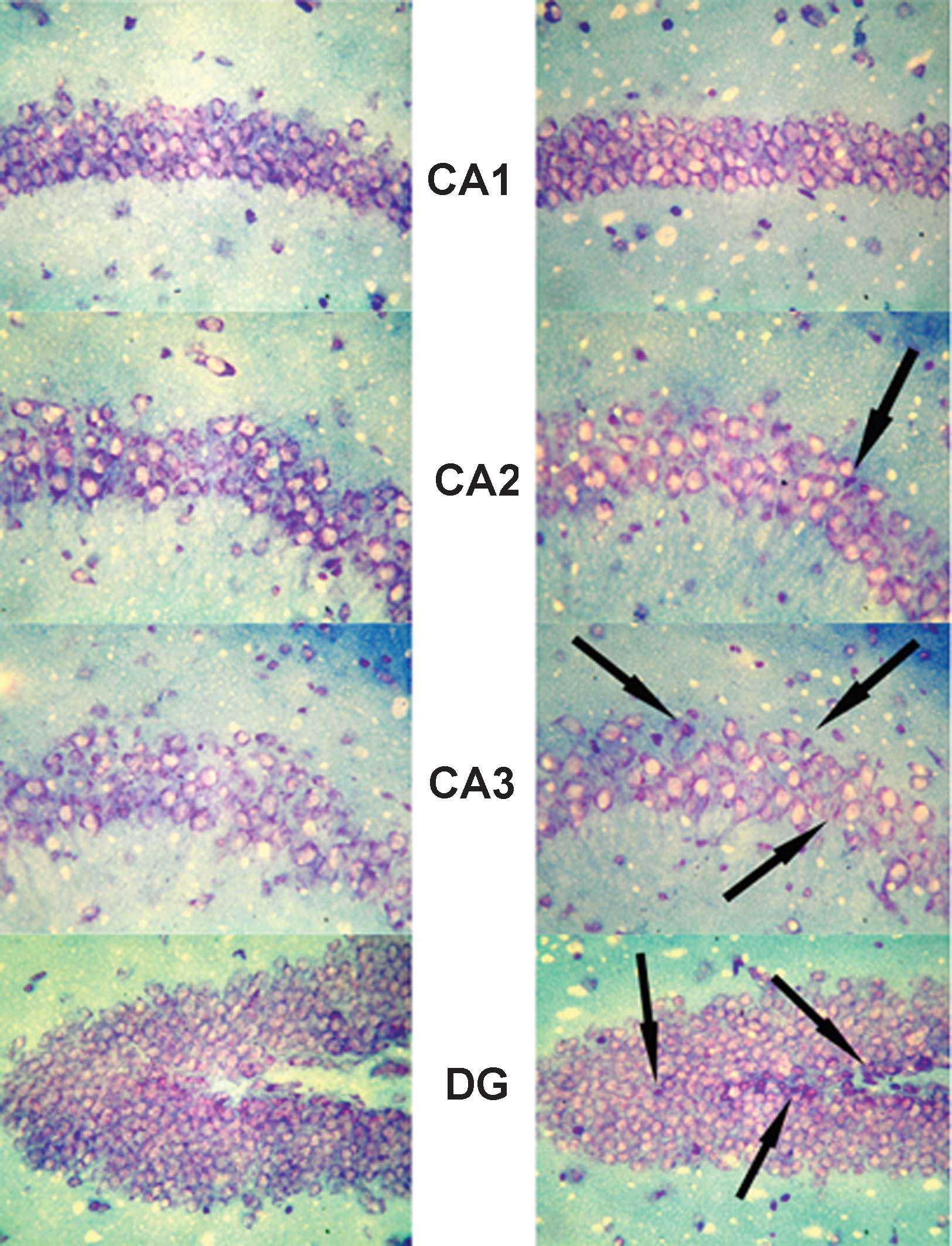
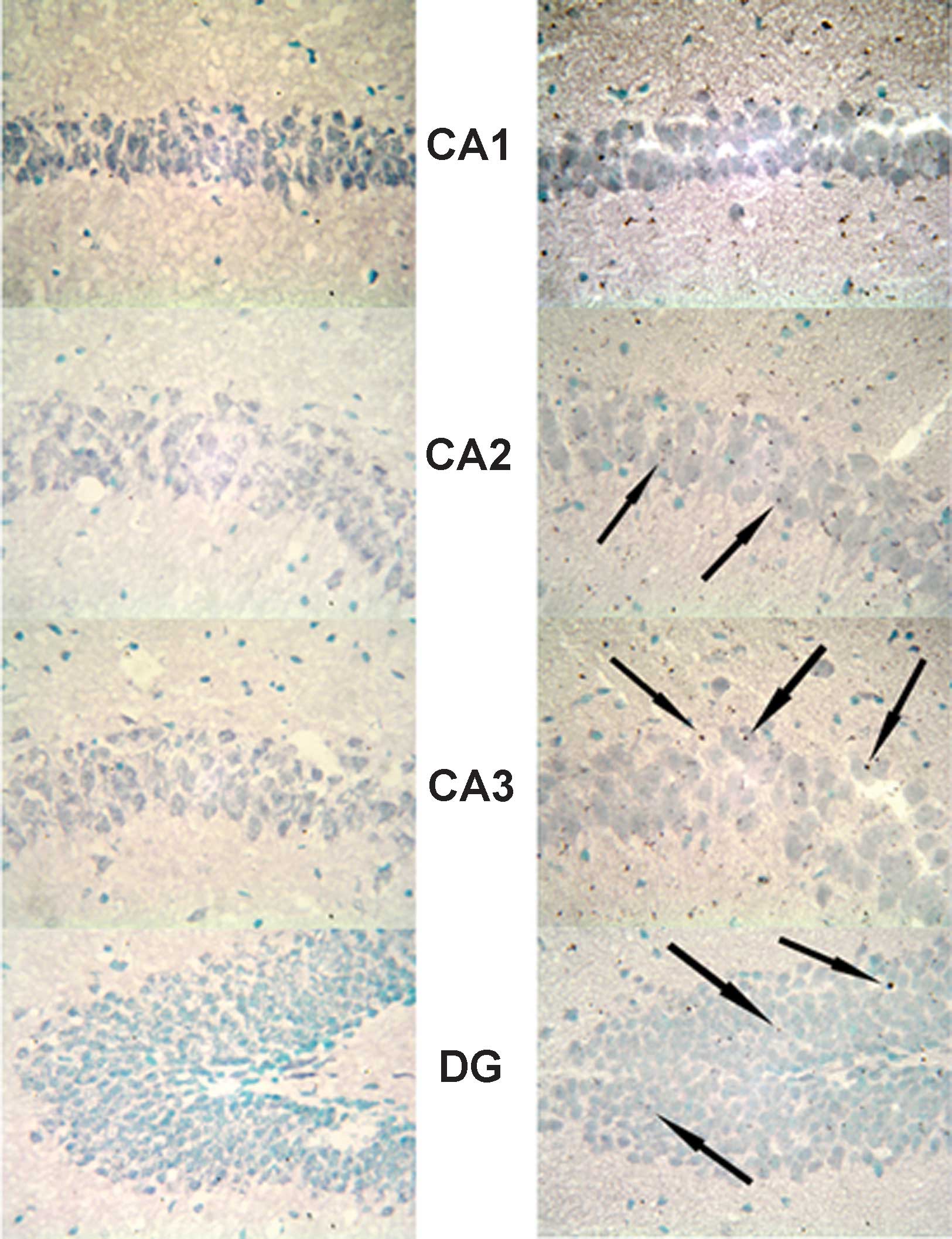
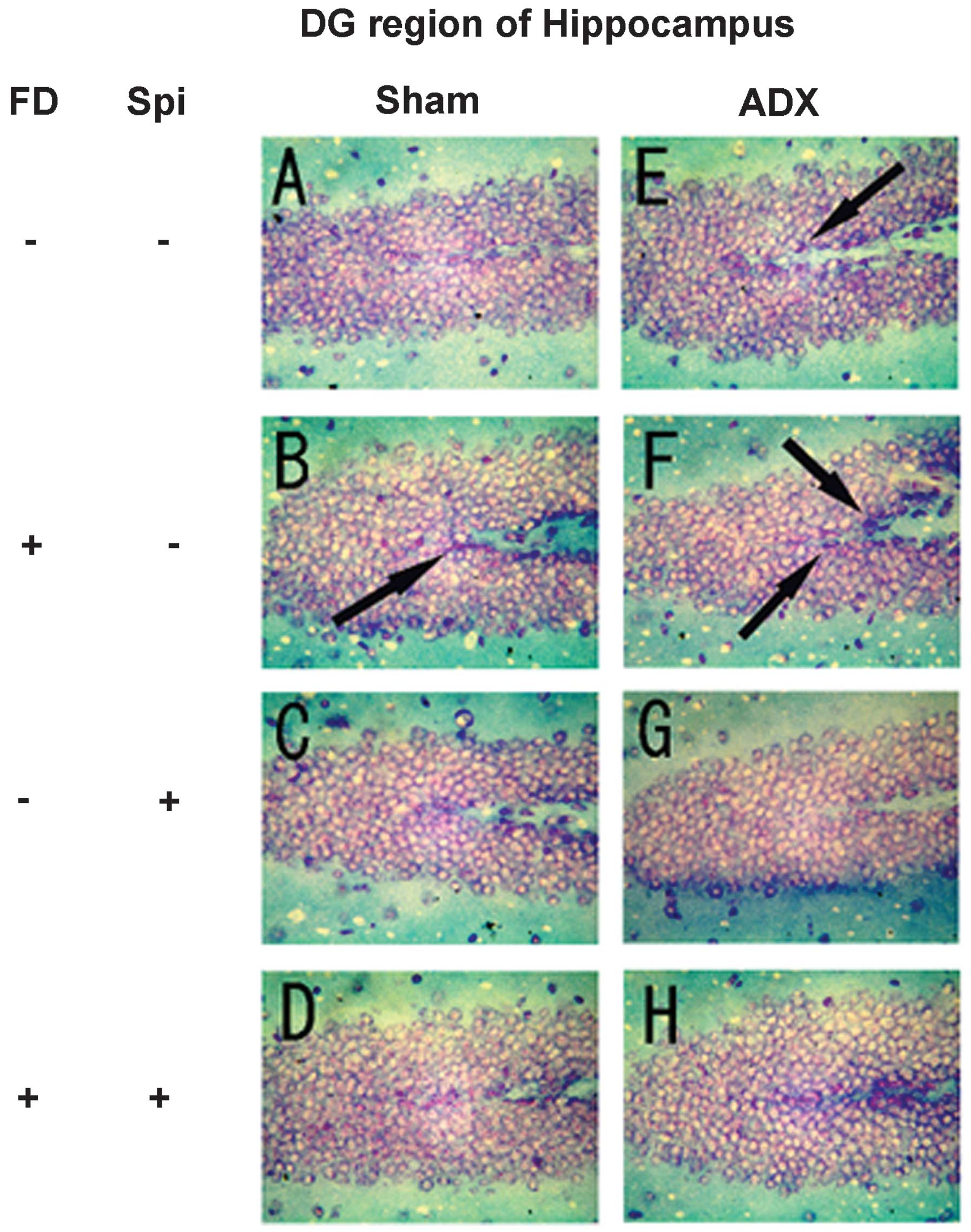
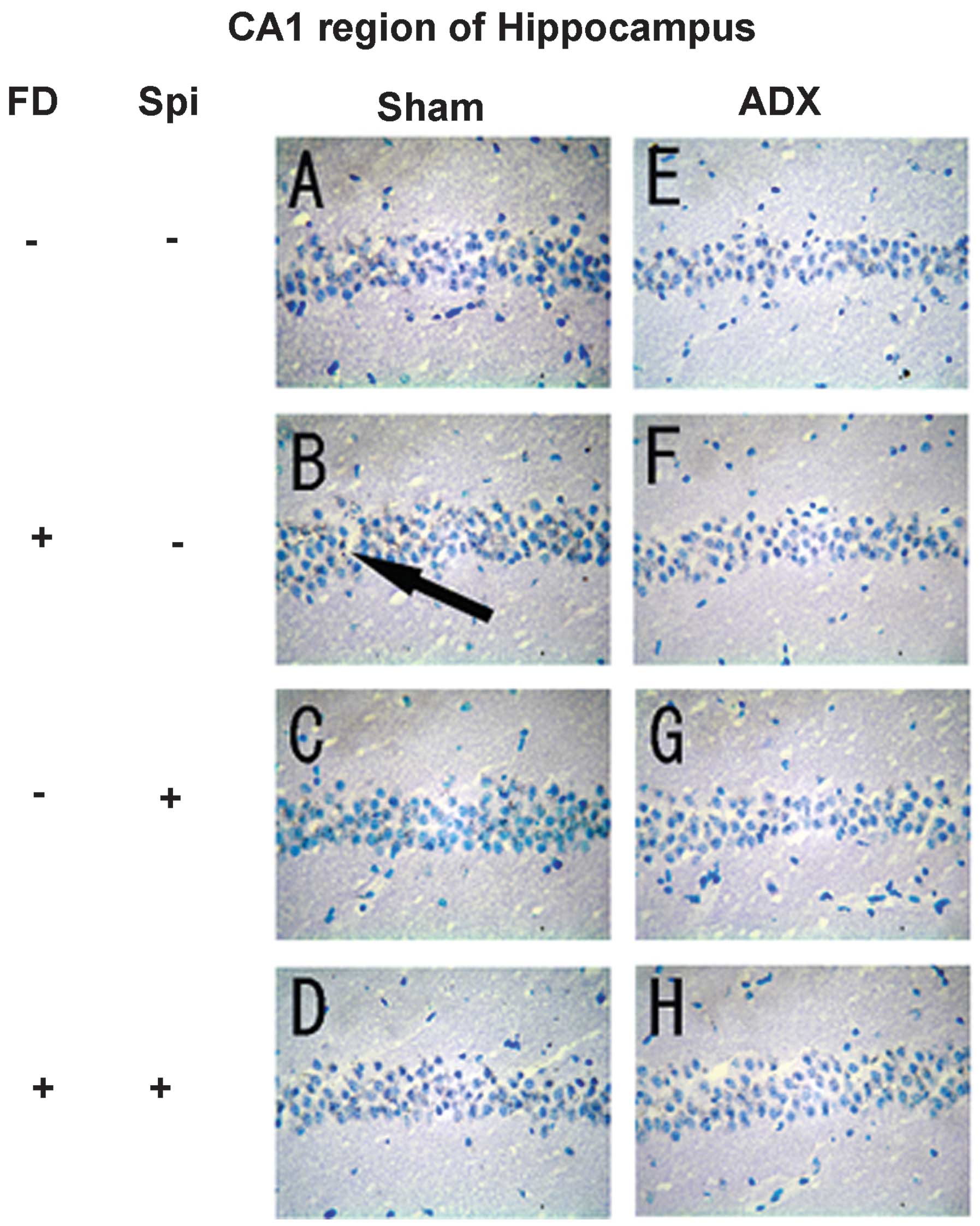
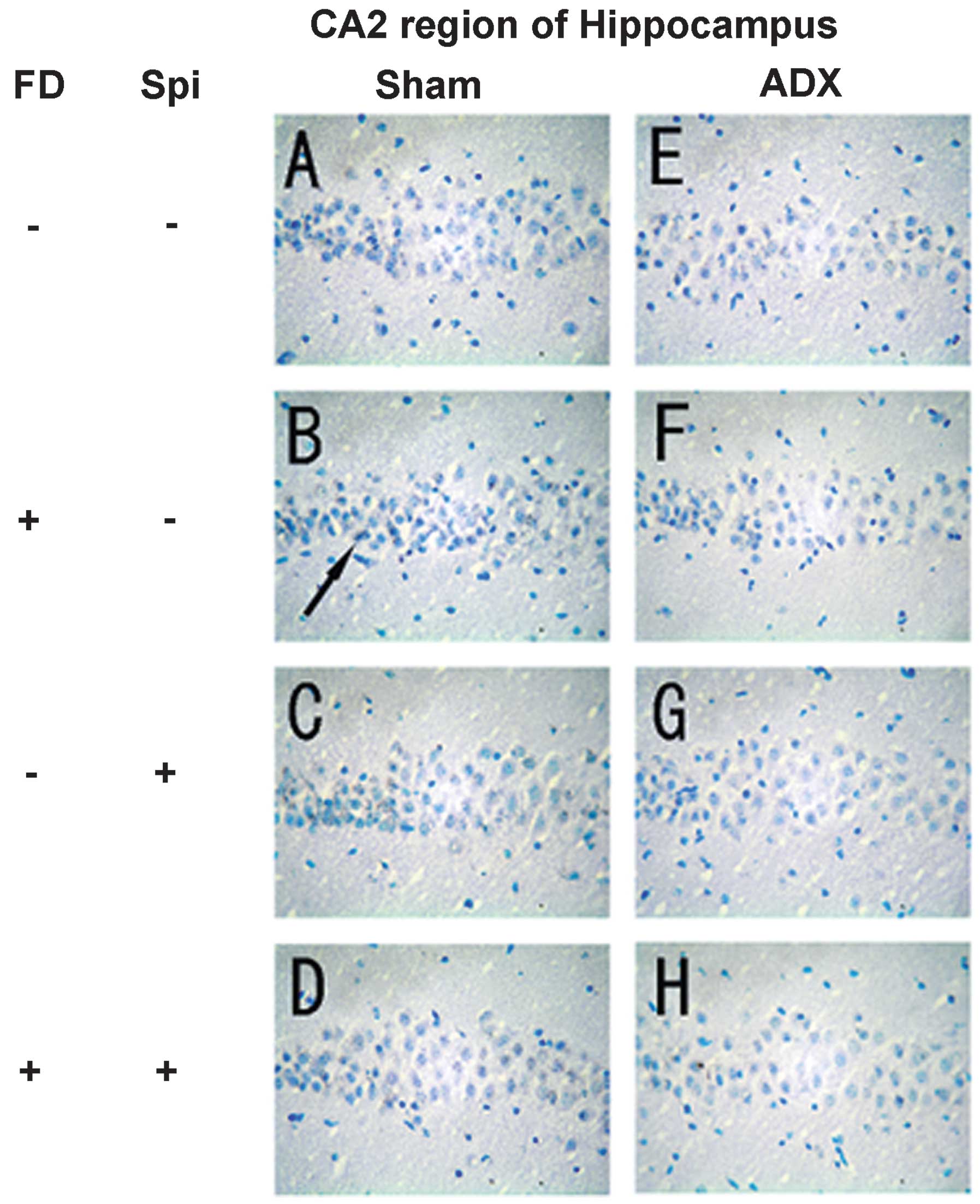
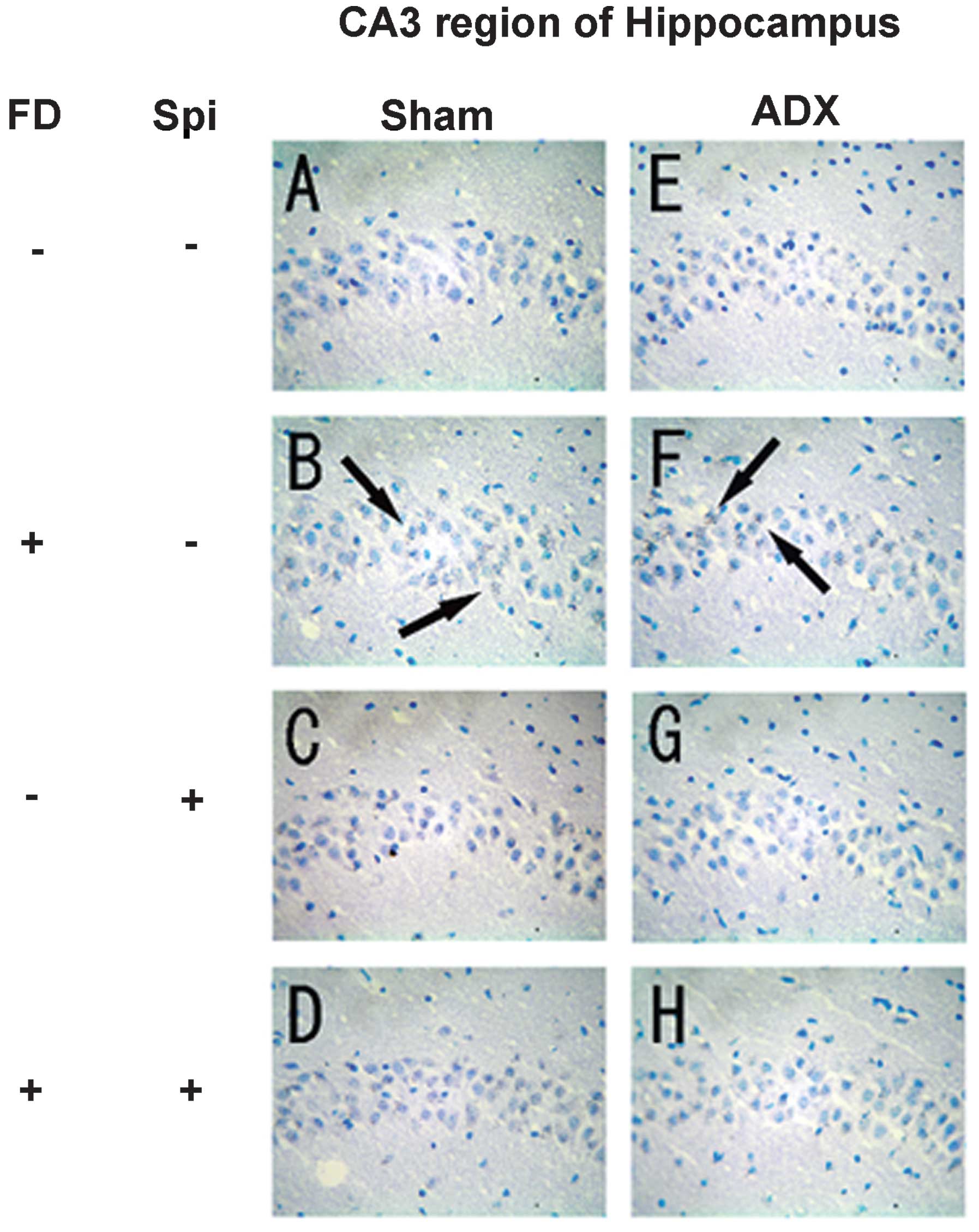
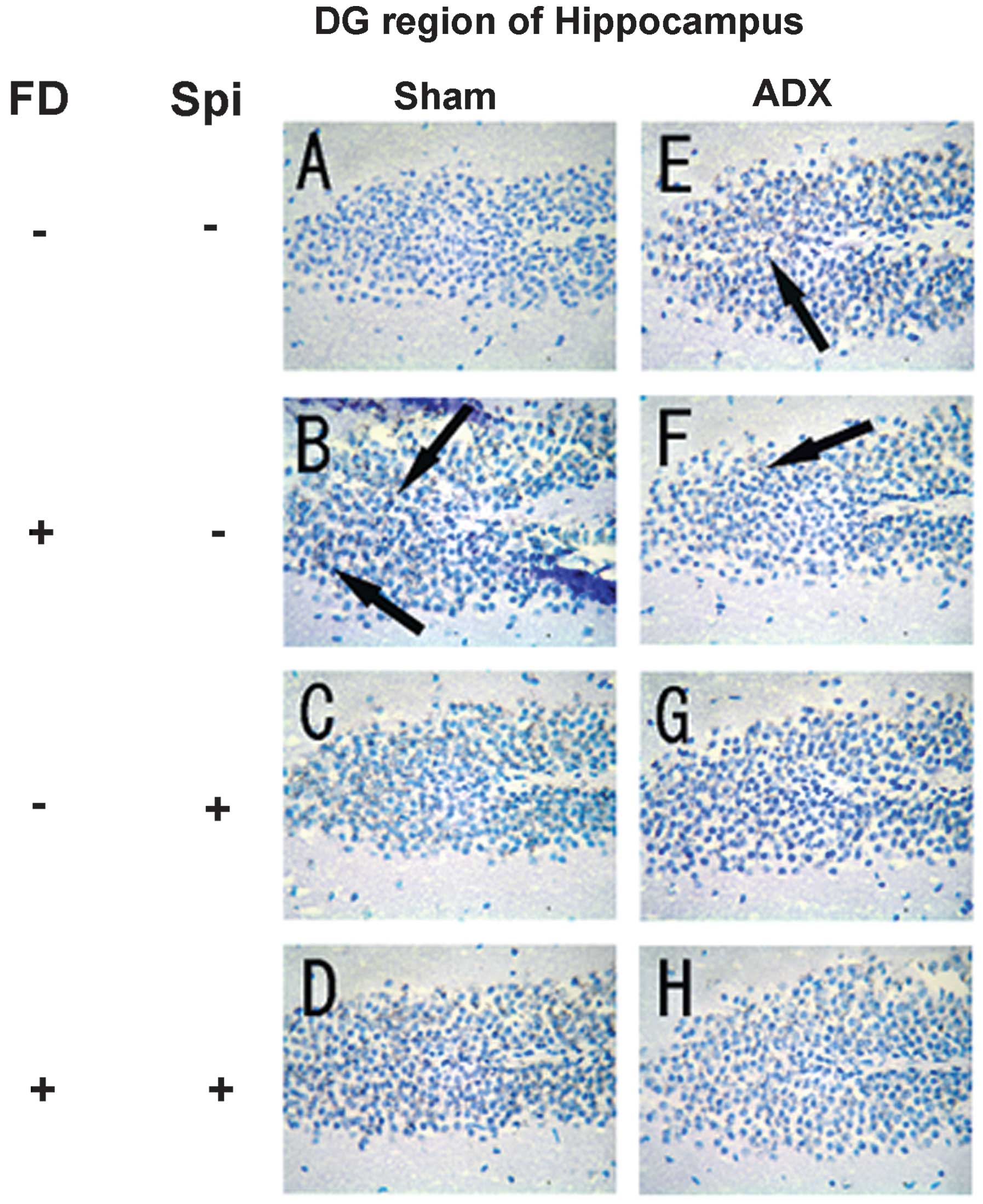
Expression of MR in the hippocampus
In the hippocampus, MR numbers are equal to those of
GR in the dentate gyrus (DG), CA1 and CA2 regions, but GR are
absent in the CA3 region, where only MR are present (5). In the FD pellet group, cytotoxicity
(pyknosis and degranulation) and DNA fragmentation due to the death
of nerve cells were observed using the TUNEL method and KB staining
in the CA3 region and DG, whereas the CA1 and CA2 regions barely
exhibited cell damage (Figs. 2 and
3). It was clearly indicated that
dysfunction of the hippocampal neurons is caused by MR, also
showing differences in vulnerability and sensitivity to mineral
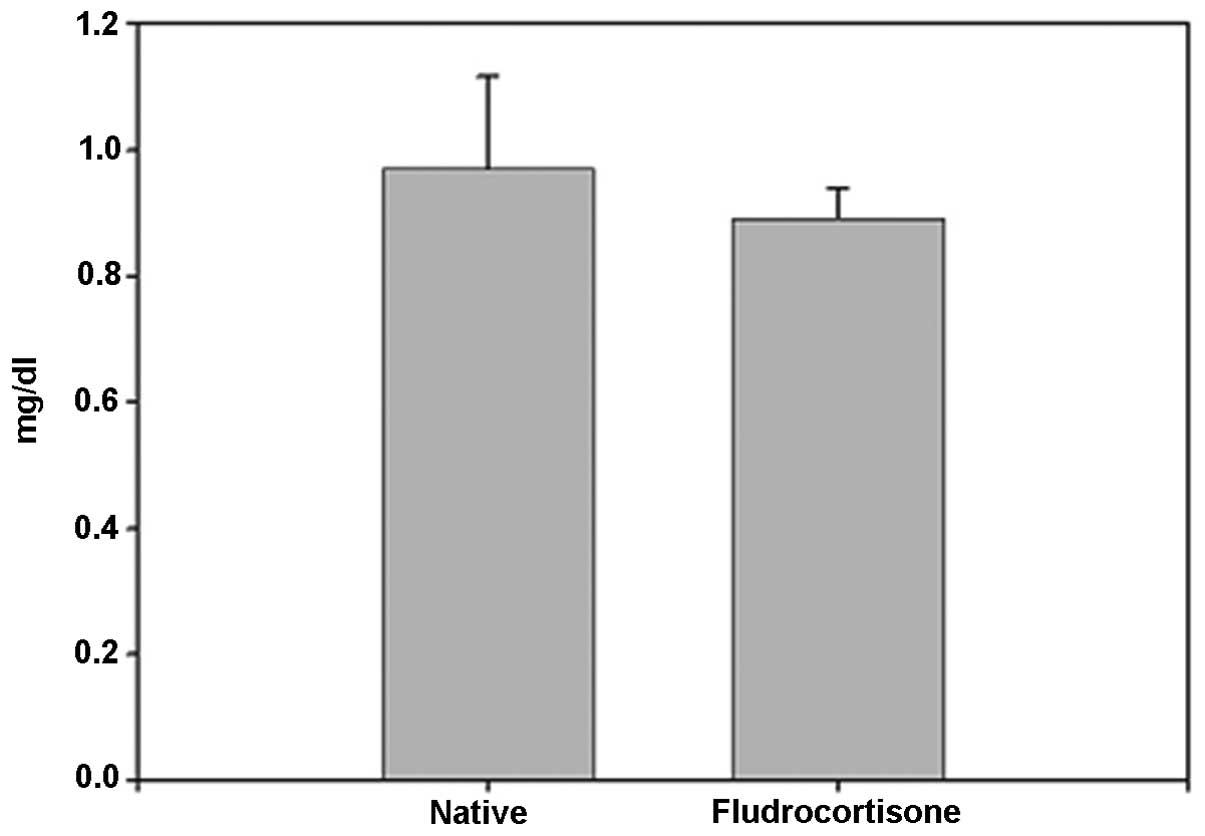
corticoids in MR-containing regions in the brain. No significant
difference was identified between the FD pellet and control groups
regarding serum creatinine; thus, induction by hippocampal MR was
suggested (Fig. 4).
Comet assay
Subsequently, the effect of Spi, an MR antagonist,
and Aldo, an MR agonist, on normal mouse hippocampal floating cells
was examined using the comet assay. No significant difference was
identified in the tail length compared with that of the control
group following Spi addition alone. No significant difference was
observed at 1, 10 or 50 nM with Aldo addition alone, but the tail
length was extended by ~2.7 times compared with that in the control
group at 100 nM (Fig. 5). The
cytotoxicity of Aldo was suppressed by the addition of Spi, as in
the combination group receiving Spi (100 nM) and Aldo (100 nM), the
tail length was extended by ~1.3 times compared with that in the
control group. Spi was added at increasing rates in addition to
Aldo (100 nM), and the tail length in the combination group at was
reduced to ~0.6 times and to ~0.3 times of that following Aldo
treatment alone upon addition of 50 or 100 nM Spi, respectively
(Fig. 6). Therefore, it was
suggested that the cell damage caused by Aldo was induced through
the action of the MR agonist and it may be suppressed by inhibiting
MR.
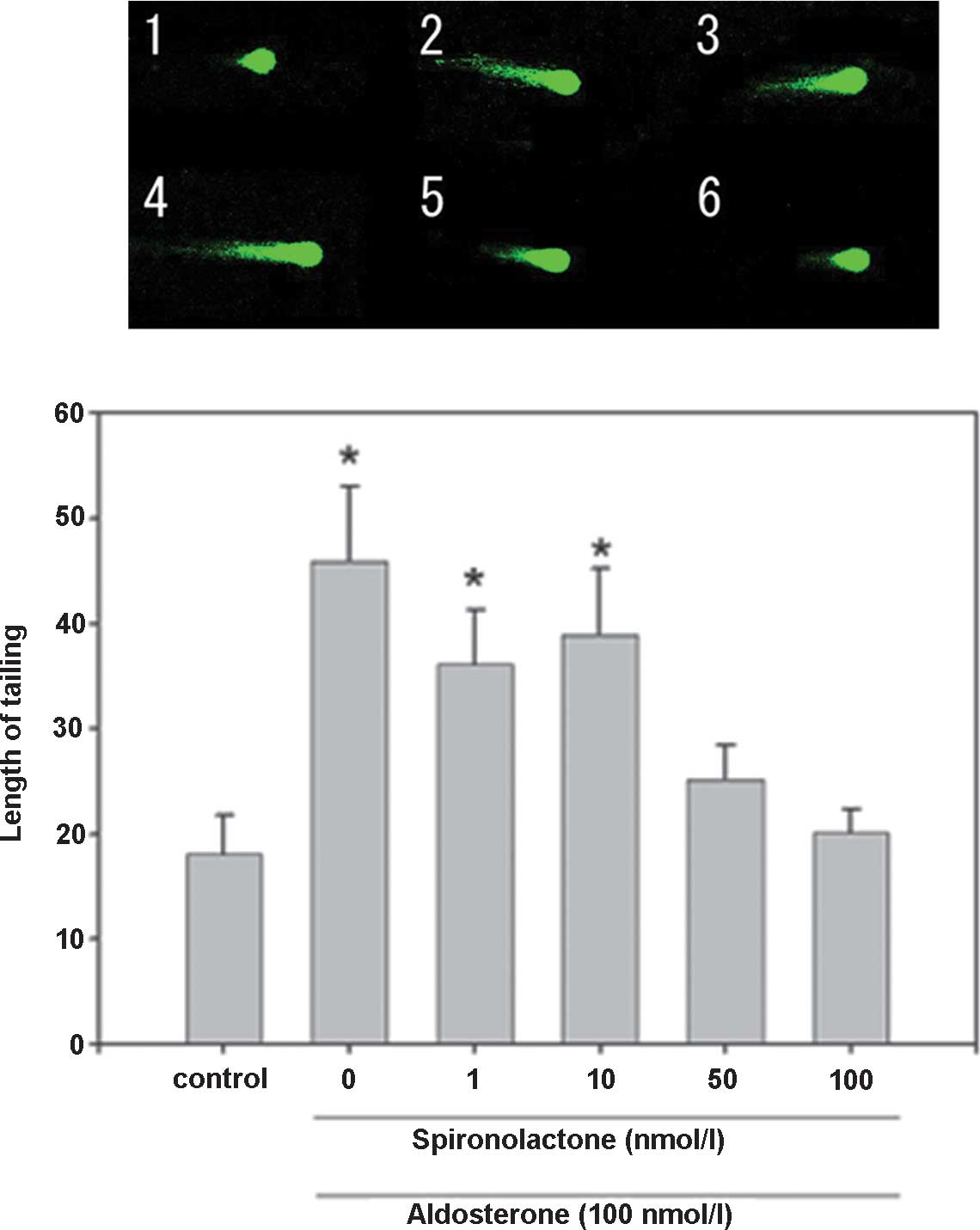
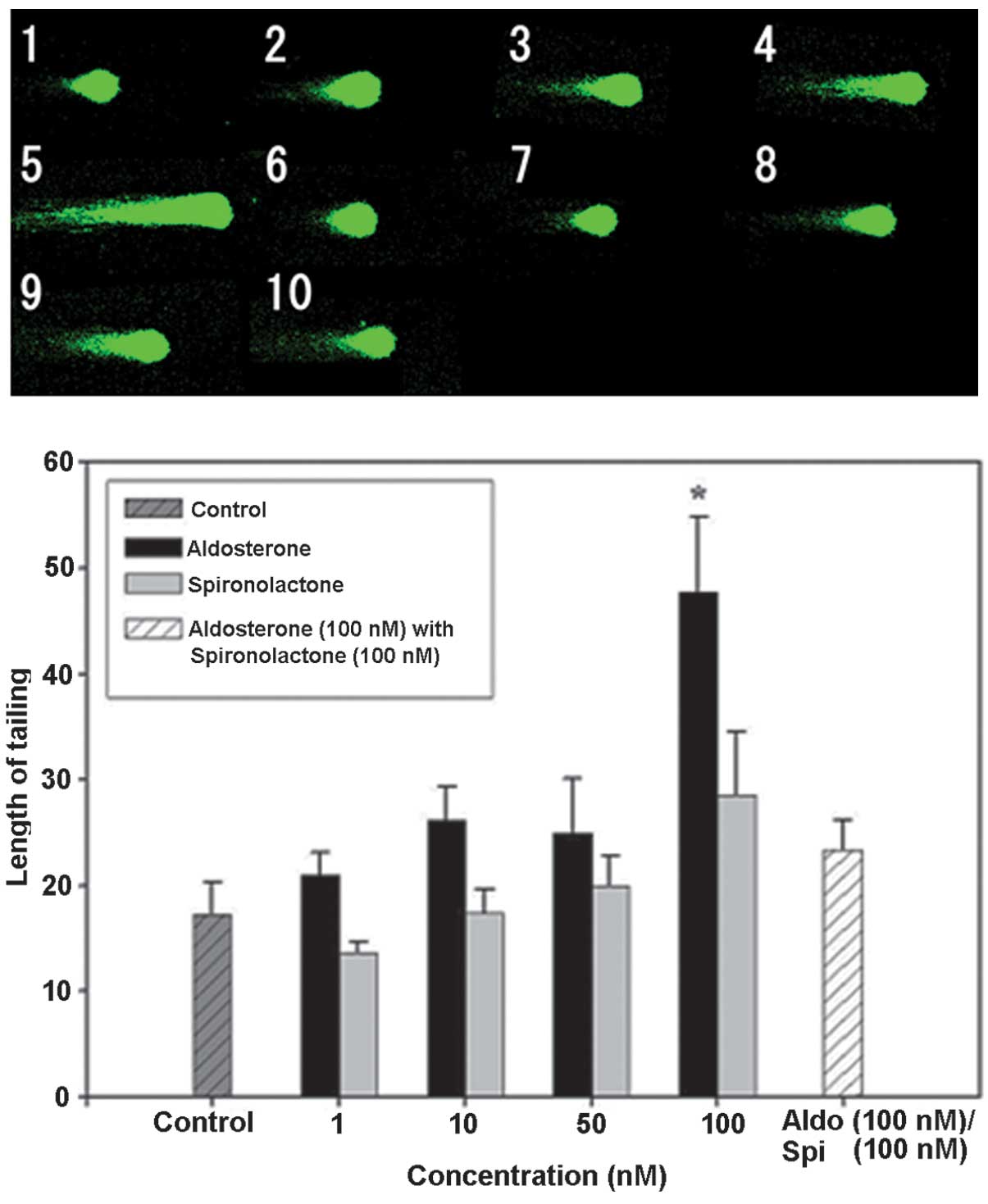 | Figure 5DNA damage in hippocampal floating
cells caused by aldosterone and its inhibition by spironolactone
(magnification, ×400). Example of comet tail in floating
hippocampal cells of (1) a native
mouse, (2) with aldosterone 1 nM,
(3) aldosterone 10 nM, (4) aldosterone 50 nM, (5) aldosterone 100 nM, (6) spironolactone 1 nM, (7) spironolactone 10 nM, (8) spironolactone 50 nM, (9) spironolactone 100 nM and (10) aldosterone 100 nM + spironolactone
100 nM. Values are expressed as the mean ± standard error of the
mean (n=15 for each mouse; total, 75 per group).
*P<0.05, compared with control value. Aldo,
aldosterone; Spi, spironolactone. |
Furthermore, the effect of FD (50 mg/kg, i.p.) and
Spi (50 mg/kg, i.p.) on normal mouse hippocampal floating cells was
examined using the comet assay with five mice in each group (n=15
for each mouse; total, 75 per group). In the present study,
adrenalectomized (ADX) mice were used to eliminate the effects of
endogenous corticosteroids (9),
with the loss of the GR function in the hippocampus. While
significant prolongation to ~2.88 times that of the control mice
subjected to sham-surgery (Sham) was observed in the
FD-administered group, the prolongation was inhibited by Spi alone
by ~1.32- and 1.31-fold that in the FD group following concomitant
administration of FD and Spi (Fig.
7). By contrast, although significant prolongation to ~1.96
times that in the control of ADX mice was observed in the
FD-administered group, the prolongation was inhibited by Spi alone
by ~1.11-fold and by FD and Spi administered concomitantly by
1.28-fold that in the FD group (Fig.
7). Thus, the FD-induced hippocampal neuronal disorder was
suppressed by Spi in the ADX group, similarly to that in the Sham
group. Subsequently, the morphological changes in hippocampal
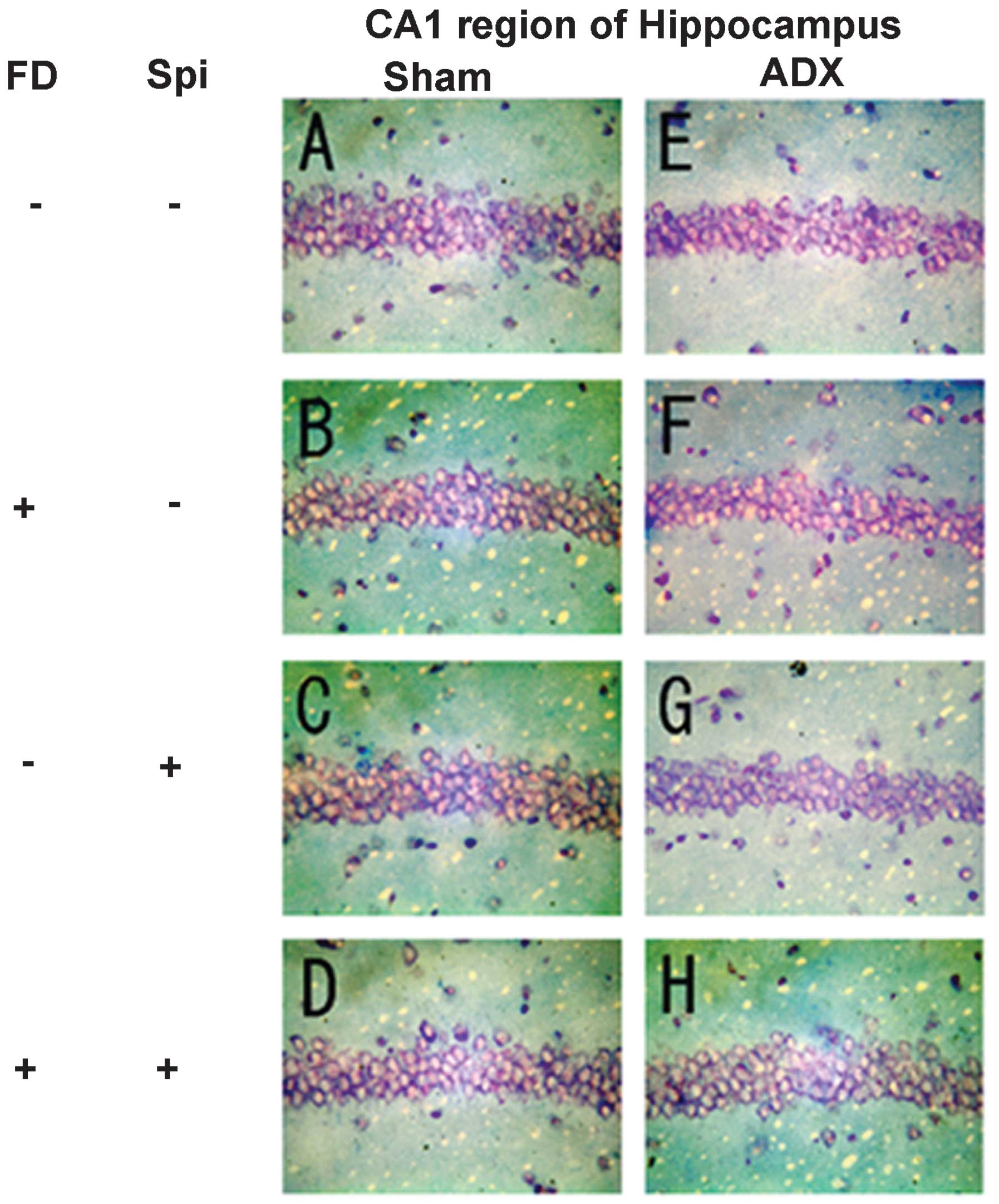
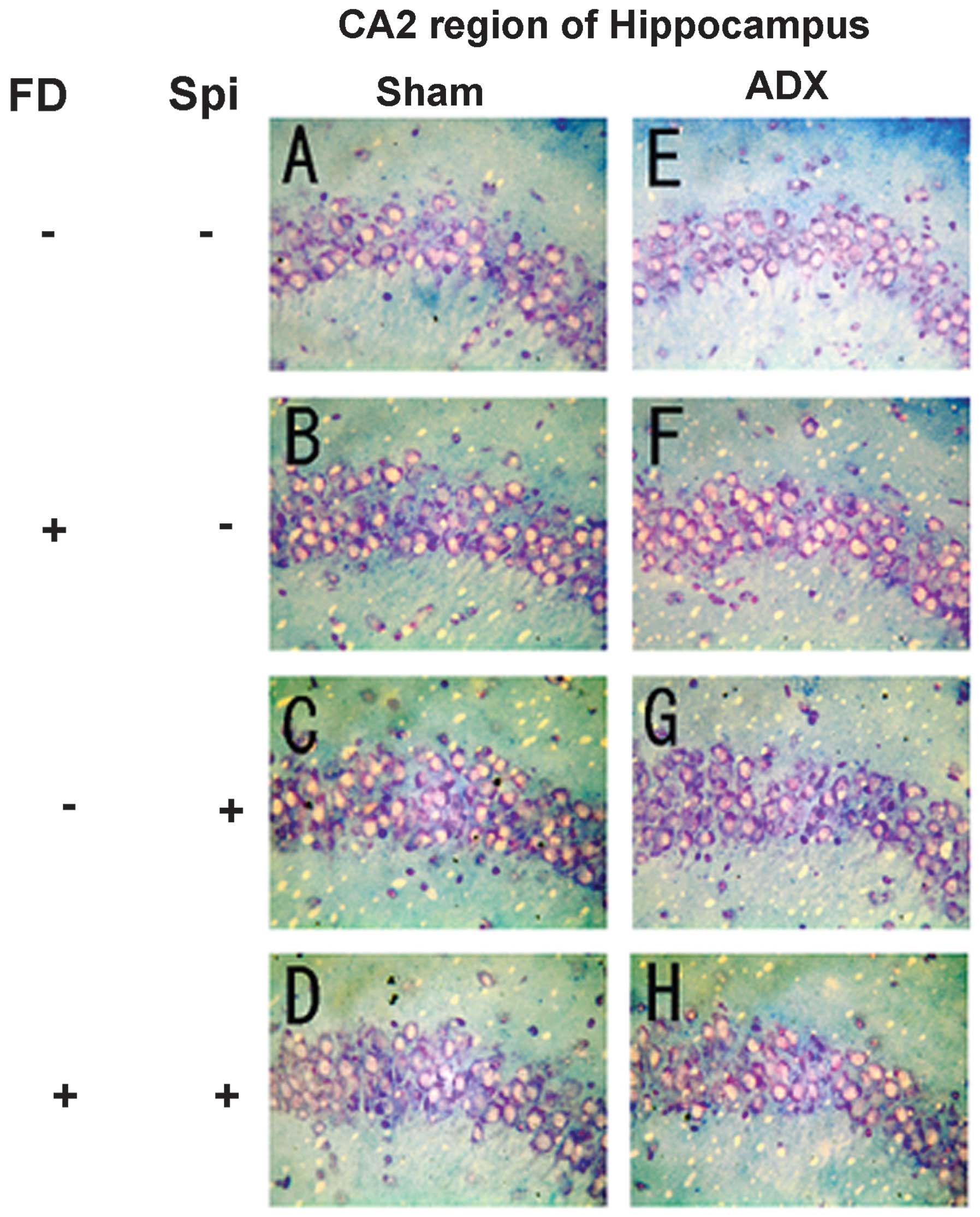
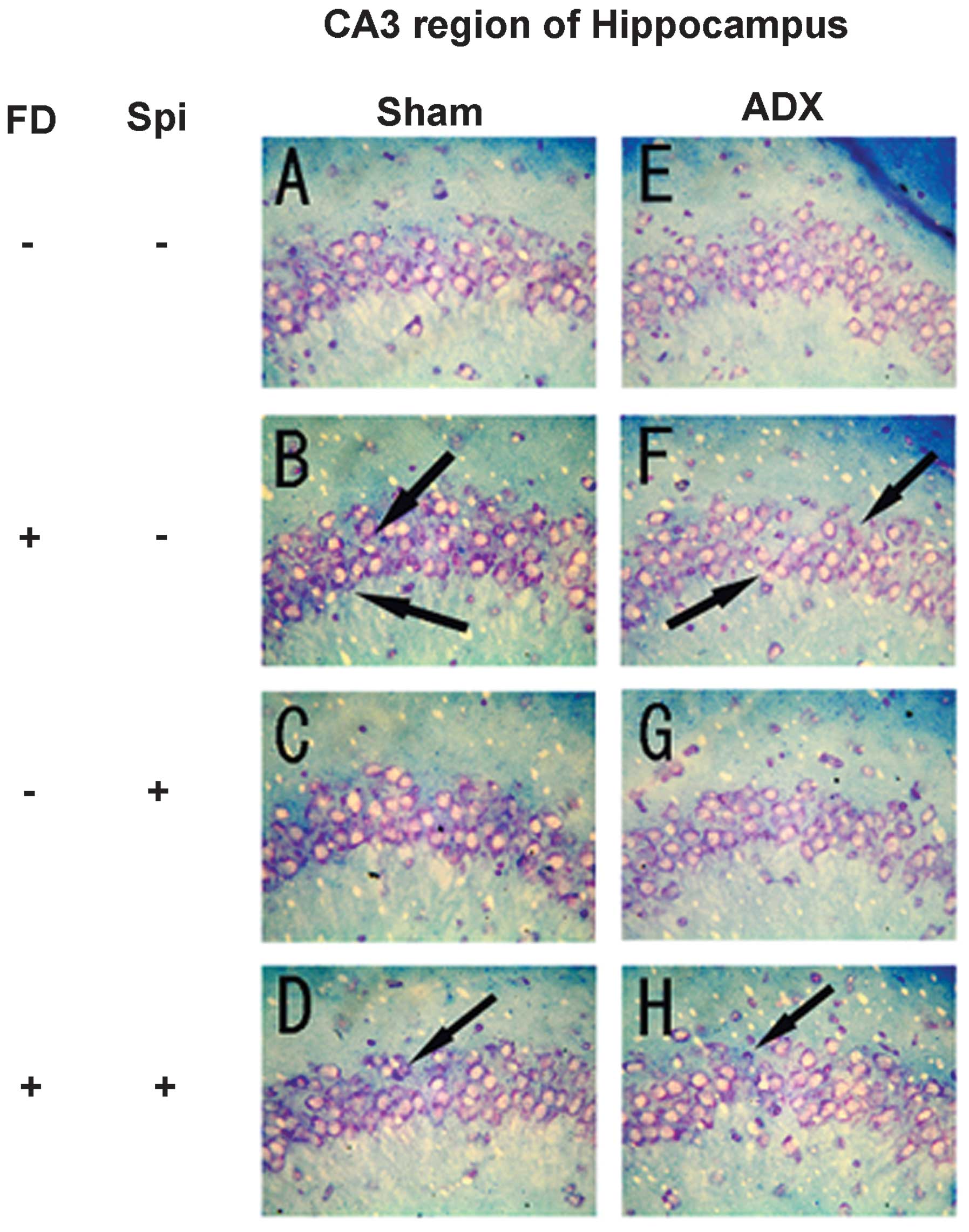
neurons caused by Spi administration and FD were examined via KB
staining. No morphological changes were observed in the hippocampal
neurons following FD administration in the ADX and Sham mice in the
CA1 and CA2 regions (Figs. 8 and
9). The granule cells in the DG
and pyramidal cells in the CA3 region did not exhibit granulation,
pyknosis or dropout (Fig. 10B and
F; Fig 11B and F). No
significant difference was observed following Spi treatment alone,
or with FD co-administered with Spi in the CA3 region (Fig. 10C, D, G and H; Fig. 11C, D, G and H). Furthermore, a
TUNEL assay was employed to assess the functional changes in
hippocampal neurons. DNA fragmentation was mildly observed in the
CA1 and CA2 regions following FD treatment alone (Figs. 12B and 13B). However, marked fragmentation of
DNA and neuronal cell death in the CA3 region and the DG were
observed in the Sham group (Figs.
14B and 15B), while
significant DNA fragmentation was noted only in the CA3 region and
the DG when FD was administered alone to ADX mice. The was no
observation of DNA fragmentation following Spi treatment alone
(Figs. 14F and 15F), or co-administration of FD and Spi
(Fig. 12C, D, G and H; Fig. 13C, D, G and H; Fig. 14C, D, G and H; Fig. 15C, D, G and H) in the Sham and ADX
mice. Therefore, it is suggested that Spi inhibits hippocampal
neuronal damage induced by administration of FD. Considering that
an adrenalectomy causes the apoptosis of granule cells in the DG,
following the promotion of neurogenesis (10), the turnover of granule cells in the
DG should be increased in ADX mice to counteract cell loss.
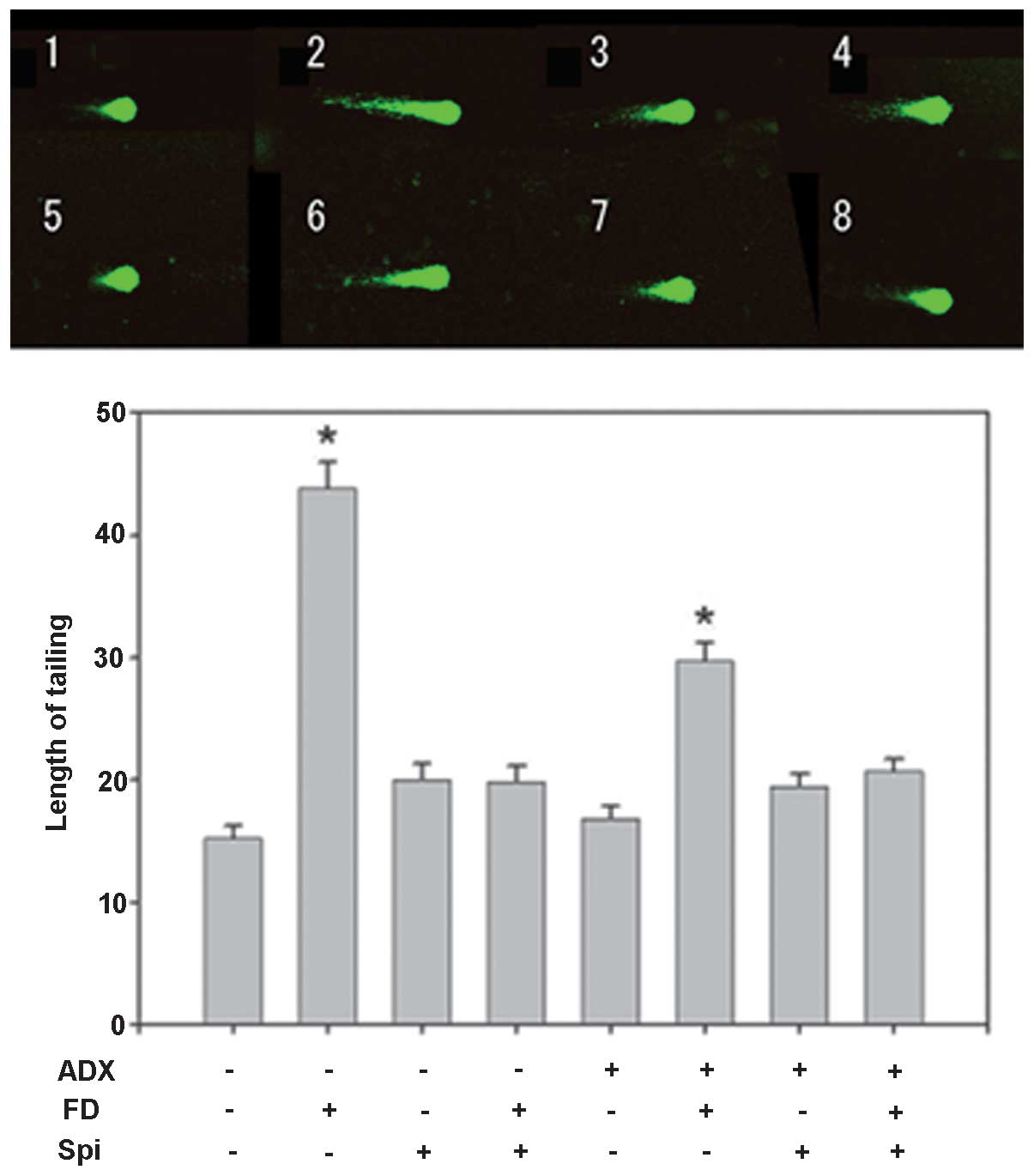 | Figure 7Effect of FD and Spi on hippocampal
floating cells in adrenalectomized mice (magnification, ×400).
Example of comet tail of hippocampal floating cell of (1) a sham mouse, (2) FD 25 mg/kg s.c., bolus administration
following sham surgery, (3) Spi 50
mg/kg i.p., bolus administration following sham, (4) FD 25 mg/kg s.c. and Spi 50 mg/kg i.p.,
bolus administration following sham, (5) ADX mouse, (6) FD 25 mg/kg s.c., bolus administration
following ADX, (7) Spi 50 mg/kg
i.p., bolus administration following ADX and (8) FD 25 mg/kg s.c. and Spi 50 mg/kg i.p.,
bolus administration following ADX. Values are expressed as the
mean ± standard error of the mean. Results are representative of
the five animals evaluated (n=15 for each mouse; total, 75 per
group). *P<0.05, compared with control. Spi,
spironolactone; FD, fludrocortisone; ADX, adrenalectomized; i.p.,
intraperitoneally; s.c., subcutaneous. |
Discussion
In addition to hippocampal neuron damage caused by
the glucocorticoid-GR system, such damage may also be directly
induced via MR by glucocorticoids or mineralocorticoids. As the
affinity of glucocorticoids for MR is higher compared with that for
GR (8) and low 11β-HSD2 levels
exist in the hippo-campus (11),
GR and MR may be highly susceptible to an increase or decrease of
glucocorticoids. However, GR are only activated to 30% with the
minimum circadian concentration of glucocorticoids, but MR are
activated to >60% in the hippo-campus (12). With the spike of blood
glucocorticoid levels due to acute stress, all MR are activated,
but only 50% of GR are activated (12). Since, as well as in Sham mice,
hippocampal neuron damage was observed following FD administration
even in the ADX mice, and the damage was suppressed by the combined
use with Spi, hippocampal neuron damage was suggested to be
mediated via MR. In addition, these results clearly indicated the
regional differences in vulnerability and/or sensitivity to
corticosteroids of MR based on the TUNEL and KB staining assays.
Since MR are only present in the CA3 region, MR sensitivity to
corticosteroids in the CA3 region is expected to be high, and
pyramidal cells are expected to be vulnerable to corticosteroids.
An increase in the density of hippocampal MR following
psychological stress has been reported (13). Further studies are required in the
future to fully elucidate these functions.
In epithelial cells, MR form a complex with heat
shock proteins (HSPs), following which they dissociate from the
HSPs to form a dimer. Following dissociation from the HSPs, MR have
been found to migrate into the nucleus from the cytoplasm, bind to
the promoter region of the target gene with the MR response element
and cause the transcriptional activation of target genes (14–16).
However, the behavior of MR in non-epithelial cells, such as in the
hippocampus, remains to be elucidated. Recently, it was reported
that reactive oxygen species (ROS) were produced following the
activation of nicotinamide adenine dinucleotide phosphate (NADPH)
oxidase with increasing intracellular Ca2+ influx and
lowering of the Mg2+ concentration by aldosterone
binding to MR in non-epithelial cells, including the heart and
blood vessels (17). Apoptosis has
also been reported to be induced in heart tissue by Aldo loading in
mice (18). In conclusion,
hippocampal neuronal dysfunction may be caused by the
overproduction of ROS induced by NADPH oxidase activation via MR
functioning. In addition to hippocampal neuronal disorders caused
by an MR agonist, the presence of disorders caused by an MR
antagonist was hypothesized in the present study. In the clinic,
organ-protective effects on the heart, blood vessels and
non-epithelial tissue mediated by MR were shown to be inhibited by
MR antagonists (19), clearly
suggesting that MR function is important in the response to stress
stimuli.
References
|
1
|
Sherin JE and Nemeroff CB: Post-traumatic
stress disorder: The neurobiological impact of psychological
trauma. Dialogues Clin Neurosci. 13:263–278. 2011.PubMed/NCBI
|
|
2
|
Hwang IK, Yoo KY, Nam YS, Choi JH, Lee IS,
Kwon YG, Kang TC, Kim YS and Won MH: Mineralocorticoid and
glucocorticoid receptor expressions in astrocytes and microglia in
the gerbil hippocampal CA1 region after ischemic insult. Neurosci
Res. 54:319–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sánchez MM, Young LJ, Plotsky PM and Insel
TR: Distribution of corticosteroid receptors in the rhesus brain:
relative absence of glucocorticoid receptors in the hippocampal
formation. J Neurosci. 20:4657–4668. 2000.PubMed/NCBI
|
|
4
|
Sousa N, Lukoyanov NV, Madeira MD, Almeida
OF and Paula-Barbosa MM: Reorganization of the morphology of
hippocampal neurites and synapses after stress-induced damage
correlates with behavioral improvement. Neuroscience. 97:253–266.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han F, Ozawa H, Matsuda K, Nishi M and
Kawata M: Colocalization of mineralocorticoid receptor and
glucocorticoid receptor in the hippocampus and hypothalamus.
Neurosci Res. 51:371–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paxinos G and Franklin KBJ: The Mouse
Brain in Stereotaxic Coordinates. Compact Third Edition. Academic
Press; Waltham: 2008
|
|
7
|
Funder JW: Mineralocorticoid receptors in
the central nervous system. J Steroid Biochem Mol Biol. 56:179–183.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Connell JM and Davies E: The new biology
of aldosterone. J Endocrinol. 186:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Z, Yuri K, Ozawa H, Lu H and Kawata M:
The in vivo time course for elimination of adrenalectomy-induced
apoptotic profiles from the granule cell layer of the rat
hippocampus. J Neurosci. 17:3981–3989. 1997.PubMed/NCBI
|
|
10
|
Krugers HJ, van der Linden S, van Olst E,
Alfarez DN, Maslam S, Lucassen PJ and Joëls M: Dissociation between
apoptosis, neurogenesis, and synaptic potentiation in the dentate
gyrus of adrenalectomized rats. Synapse. 61:221–230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Kloet ER: Hormones, brain and stress.
Endocr Regul. 37:51–68. 2003.PubMed/NCBI
|
|
12
|
Rogalska J: Mineralocorticoid and
glucocorticoid receptors in hippocampus: their impact on neurons
survival and behavioral impairment after neonatal brain injury.
Vitam Horm. 82:391–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ladd CO, Huot RL, Thrivikraman KV,
Nemeroff CB and Plotsky PM: Long-term adaptations in glucocorticoid
receptor and mineralocorticoid receptor mRNA and negative feedback
on the hypothalamo-pituitary-adrenal axis following neonatal
maternal separation. Biol Psychiatry. 55:367–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pascual-Le Tallec L and Lombès M: The
mineralocorticoid receptor: a journey exploring its diversity and
specificity of action. Mol Endocrinol. 19:2211–2221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuller PJ and Young MJ: Mechanisms of
mineralocorticoid action. Hypertension. 46:1227–1235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Connell JM and Davies E: The new biology
of aldosterone. J Endocrinol. 186:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT
and Weber KT: Aldosterone-induced inflammation in the rat heart:
role of oxidative stress. Am J Pathol. 161:1773–1781. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sam F, Xie Z, Ooi H, Kerstetter DL,
Colucci WS, Singh M and Singh K: Mice lacking osteopontin exhibit
increased left ventricular dilation and reduced fibrosis after
aldosterone infusion. Am J Hypertens. 17:188–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pitt B, Zannad F, Remme WJ, Cody R,
Castaigne A, Perez A, Palensky J and Wittes J; Randomized Aldactone
Evaluation Study Investigators: The effect of spironolactone on
morbidity and mortality in patients with severe heart failure. N
Engl J Med. 341:709–717. 1999. View Article : Google Scholar : PubMed/NCBI
|