Introduction
Human primary squamous cell carcinoma of the thyroid
is a rare and aggressive type of neoplasm and although optimal
treatment strategies are adopted, survival time is not expected to
surpass six months. An effective therapeutic agent is therefore
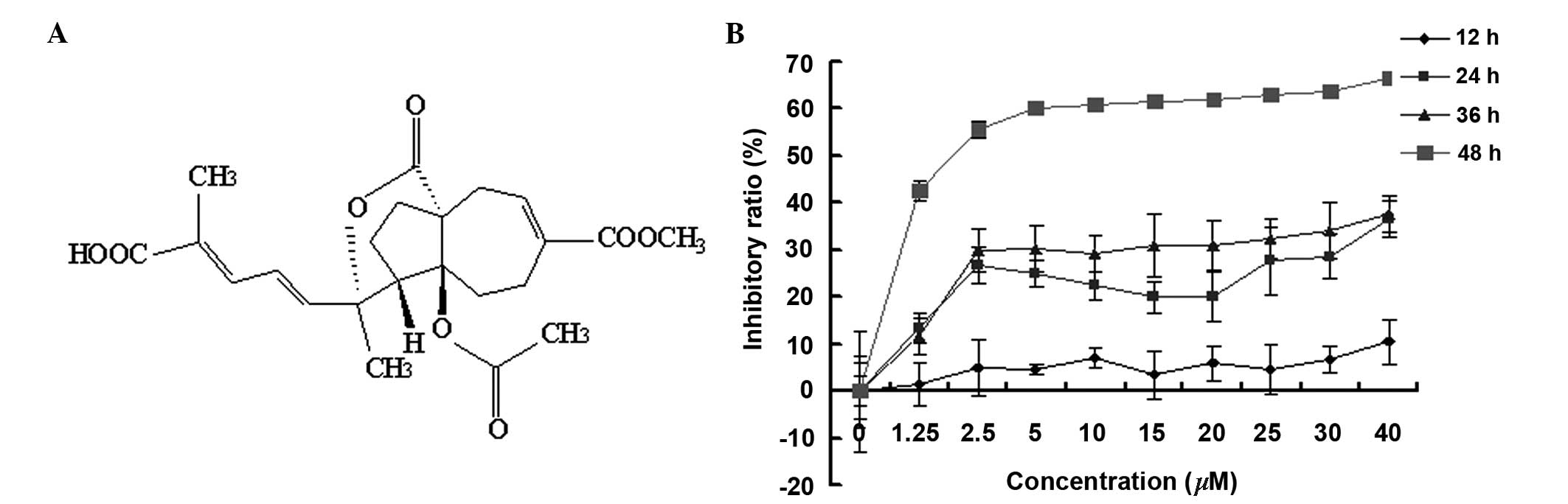
required. Pseudolaric acid B (PAB) (Fig. 1A) is a diterpene acid isolated from
the root and trunk bark of Pseudolarix kaempferi Gordon
(Pinaceae), known in Chinese as Tu-Jin-Pi, which may be
administered to treat dermatological fungal infections. PAB has
demonstrated potent inhibition of cell growth in vitro in a
number of tumor cell lines (1–6).
Thus, the aim of the present study was to investigate the antitumor
effect of PAB on squamous cell carcinoma of the thyroid.
PAB is an antitubulin therapeutic agent (7–9)
which, similar to other tubulin-associated agents, including
taxanes (paclitaxel and docetaxel), the vinca alkaloids
(vincristine and vinblastine) and nocodazole (10–12),
suppresses microtubule dynamics to inhibit tumor growth in
different cancer cell lines (7–9).
Apoptosis, as one type of antitumor mechanism, has been the focus
of many previous studies into antitumor therapeutic agent
development (13,14). Cell cycle arrest is another type of
antitumor mechanism where cells are blocked from entering the next
phase of the cell cycle and cannot proliferate. It has been
reported that cell cycle arrest is often associated with apoptosis
(15,16) and/or autophagy (17,18).
Autophagy is the process by which cellular components are delivered
to lysosomes for bulk degradation (19), in certain cases it appears to
promote cell death and morbidity, however, in the majority of
circumstances, autophagy promotes cell survival by adapting cells
to stress (20). In addition,
autophagy has been demonstrated to inhibit apoptosis, thereby
decreasing the antitumor effect of therapeutic agents (21). The present study assessed the
effect of PAB on the proliferation and autophagy-mediated cell
survival of human primary squamous cell carcinoma.
Materials and methods
Materials
PAB was purchased from the National Institute for
the Control of Pharmaceutical and Biological Products (Beijing,
China) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich,
St. Louis, MO, USA) to make a stock solution. The concentration of
DMSO was maintained at <0.01% in all the cell cultures, and no
detectable effect on cell growth or cell death was observed.
Propidium iodode (PI), monodansylcadaverine (MDC), rhodamine 123,
3-methyladenine (3-MA), Hoechst 33258, RNase A and MTT were
purchased from Sigma-Aldrich. An Annexin V:FITC apoptosis detection
kit I was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Mouse anti-human LC3A/B monoclonal antibody (66139-1-Ig), rabbit
anti-human Beclin 1 polyclonal antibody (11306-1-AP), rabbit
anti-human B-cell lymphoma 2 (Bcl-2) polyclonal antibody
(12789-1-AP) and rabbit anti-human p53 polyclonal antibody
(10442-1-AP) were purchased from ProteinTech Group, Inc
(ProteinTech, Chicago, IL, USA). Rabbit anti-human histone H3
polyclonal antibody (A01502-40) was purchased from GenScript, Inc
(Piscataway, NJ, USA). Mouse anti-human α-tubulin monoclonal
antibody (sc-23948), mouse anti human caspase-3 monoclonal antibody
(sc-65497), fluorescein isothiocyanate (FITC)-labeled mouse
secondary antibody (sc-2339), alkaline phosphatase (AP)-labeled
rabbit anti-mouse (sc-358915) and goat anti-rabbit (sc-2057)
secondary antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, Dallas, TX, USA).
Cell culture
SW579 human thyroid squamous cell carcinoma cells
were obtained from American Type Culture Collection (Manassas, VA,
USA), and cultured in L-15 medium (GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal calf serum (Gibco,
Grand Island, NY, USA), 2 mM glutamine (Gibco), penicillin (100
U/ml; Sigma-Aldrich) and streptomycin (100 µg/ml; Amresco,
Solon, OH, USA), and maintained at 37°C without CO2 in a
humidified atmosphere.
Cell growth inhibition test
The inhibition of cell growth was determined using
an MTT assay. SW579 cells (1.0×104 cells/well) were
seeded onto 96-well culture plates (Nalge-Nunc, Int., Penfield, NY.
USA). Following incubation for 24 h, different concentrations of
PAB (0, 1.25, 2.5, 5, 10, 15, 20, 25, 30 or 40 µM) were
added to the plates. Cell growth was measured at different
time-points (12, 24, 36 and 48 h) by addition of 20 µl MTT
(5 mg/ml) at 37°C for 3 h, and DMSO (150 µl) was added to
dissolve the formazan crystals. Absorbance was measured at 492 nm
using an enzyme-linked immunosorbent assay plate reader (iMark™;
Bio-Rad, Hercules, CA, USA). The percentage of inhibition was
calculated as follows: Cell death (%) =
[A492(control)−A492(sample)]/[A492(control)−A492(blank)]
×100.
Cell counting using a hemocytometer
Trypan blue (Sigma-Aldrich) was used to stain the
cells. Live cells appeared colorless and bright (refractile) under
phase contrast microscopy and the dead cells stained blue
(non-refractile). Subsequent to staining with a final concentration
0.2% trypan blue, live cells were visualized and counted using a
hemocytometer (Shanghai Qiujing Xue Qiu Ji Shu Ban, Shanghai,
China). Decreasing ratio was calculated as follows: Decreasing
ratio (%) = [cell number (control) − cell number (sample)]/cell
number (control) ×100.
Immunofluorescence
SW579 cells (5×105) were placed on cover
slips in a 6-well plate. Following 24 h of cell culture, the cells
were treated with 4 µM PAB for 24 h, washed with
phosphate-buffered saline (PBS), fixed in 3.7% formaldehyde
(Sigma-Aldrich) and rinsed three times in 1X PBS. The specimen was
placed in blocking buffer (1X PBS, 5% normal serum and 0.3% Triton
X-100) for 60 min, and subsequently incubated with α-tubulin
antibody (1:100 dilution) overnight at 4°C. Following rinsing three
times with 1X PBS, cells were incubated with FITC-conjugated mouse
secondary antibody (1:1,000 dilution) for 2 h at room temperature
in the dark. The secondary antibodies were aspirated and the cover
slips were rinsed once in 1X PBS prior to staining with 5 mg/l
Hoechst 33258 for 30 min. The intensity of FITC staining was
measured by fluorescence microscopy (Olympus CKX31, Olympus Corp.,
Tokyo, Japan) at an excitation wavelength of 505 nm with a 534-nm
emission filter (Leica, Mannheim, Germany). Nuclear changes were
observed by fluorescence microscopy at an excitation wavelength of
350 nm with a 460-nm emission filter (Leica).
Flow cytometric analysis of the cell
cycle
SW579 cells (1.0×106) were harvested and
rinsed with PBS. The cell pellets were fixed in 70% ethanol at 4°C
overnight. Following two washes with PBS, the cells were stained
with 1.0 ml PI solution containing 50 mg/l PI, 1 g/l RNase A and
3.8 mM 0.1% Triton X-100 in sodium citrate, followed by incubation
on ice in the dark for 30 min. The samples were analyzed using a
FACScan flow cytometer (BD Biosciences).
Observation of morphologic changes by
light microscopy
SW579 cells (5×105 cells/well) were
cultured in 6-well plates for 24 h. The cells were treated with 4
µM PAB for 24 and 48 h, and morphologic changes were
observed by phase contrast microscopy (Olympus IX51, Olympus
Corp.).
Observation of nuclear morphologic
changes by fluorescence microscopy
SW579 cells (5×105) were placed on cover
slips in a 6-well plate. Following a 24-h cell culture, the cells
were treated with 4 µM PAB for 24 and 48 h, washed with PBS,
fixed in 3.7% formaldehyde for 1 h and stained with 5 mg/l Hoechst
33258 for 30 min. Nuclear changes were observed by fluorescence
microscopy (Olympus CKX31, Olympus Corp.) at an excitation wave
length of 350 nm using a 460-nm emission filter (Leica).
Annexin V-PI staining
Phosphatidylserine was detected using the Annexin
V:FITC apoptosis detection kit I according to the manufacturer's
instructions. The cells were trypsinized, washed twice with cold
PBS and resuspended in 200 µl 1X binding buffer. Cell
suspension (100 µl) was transferred to a 5-ml culture tube
and incubated with 5 µl FITC-Annexin V and 10 µl PI
(10 µg/ml) for 15 min at room temperature in the dark.
Binding buffer (1X; 500 µl) was added to each tube and the
cells were analyzed by flow cytometry (FACSCalibur; BD
Biosciences). Analysis was conducted according to the protocol of
the Annexin V-PI apoptosis detection kit.
Determination of DNA fragmentation by
agarose gel electrophoresis
Cells were trypsinized and the adherent and floating
cells were collected by centrifugation at 1,000 × g for 5 min. The
procedure was conducted as described by Yu et al (4).
Observation of MDC staining by
fluorescence microscopy
A fluorescent compound, MDC has been proposed as a
tracer for autophagic vacuoles. SW579 cells were treated with 4
µM PAB for 36 h and incubated with 0.05 mM MDC at 37°C for 1
h. Following incubation, the cells were washed once with PBS.
Intracellular MDC was measured by fluorescence microscopy (Olympus
CKX31, Olympus Corp) at an excitation wavelength of 380 nm with a
525-nm emission filter (Olympus Corp).
Cell migration
Cells derived from human primary squamous cell
carcinoma of the thyroid were cultured for 24 h. Lines were
arbitrarily scratched using a pipette tip, those of a similar width
were selected, and the width was recorded at 12, 24, 36 and 48 h
after PAB treatment by phase contrast microscopy (Olympus IX51,
Olympus Corp.).
Western blot analysis of total protein
expression and nuclear protein
SW579 cells (1×106) were cultured in a
25-ml culture bottle for 24 h and were subsequently treated with 4
µM PAB for 24 h. Adherent and floating cells were collected
by trypsinization and centrifugation, and frozen at −80°C. Western
blot analysis was performed for total, cytoplasmic and nuclear
proteins as described by Yu et al (4). Protein expression was detected using
the corresponding primary polyclonal antibody at 1:1,000 dilution,
except for LC3A/B monoclonal antibody, which was used at 1:200
dilution. Subsequently, blots were incubated with the corresponding
AP-conjugated secondary antibody at 1:1,000 dilution. Proteins were
visualized using nitro blue tetrazolium chloride and
5-bromo-4-chloro-3-indoylphosphate and then scanned (Hp G4050;
Hewlett-Packard, Palo Alto, CA, USA).
Statistical analysis
Statistical analyses were performed using Student's
t-test through Microsoft Excel 2007 (Microsoft, Franklin, TN, USA).
All data are representative of at least three independent
experiments, and are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhibitory effect of PAB on SW579
cells
PAB is a diterpene acid (Fig. 1A), which has demonstrated an
antitumor effect in diverse cancer cell lines (1–6). MTT
analysis indicated that PAB inhibited SW579 cell growth in human
thyroid squamous carcinoma cell line, in a time- and dose-dependent
manner. The inhibitory effect of PAB was greater at 48 h compared
with 12, 24 and 36 h, and the half maximal inhibitory concentration
of PAB at 48 h was 4.26 µM (Fig. 1B). During the current study, 4
µM PAB was used in all experiments.
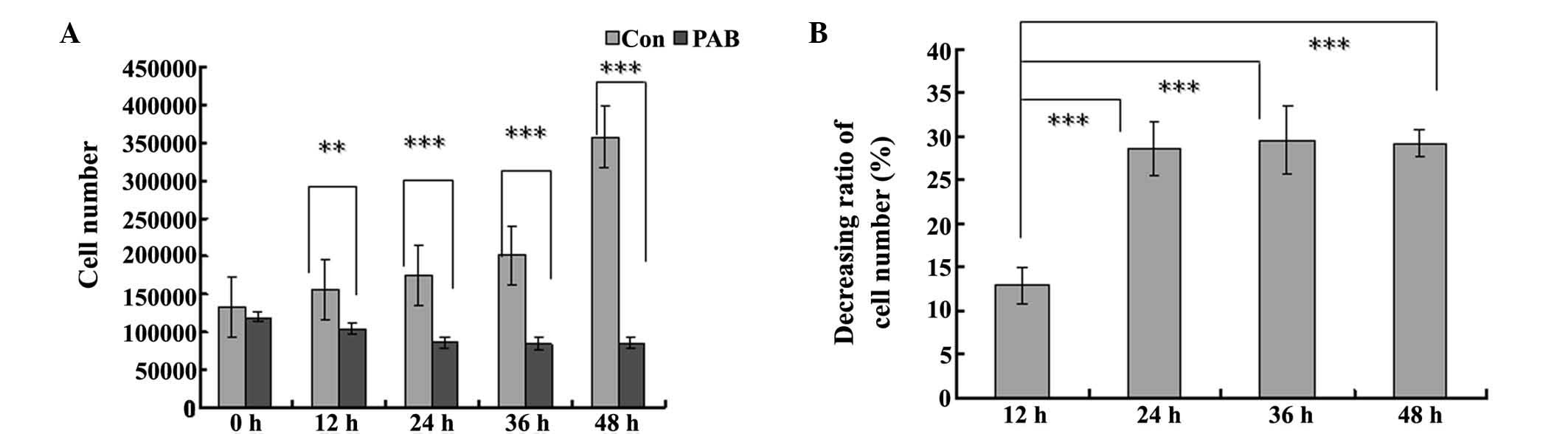
In the cell counting test, the cell number in the
PAB-treated group was reduced at 12, 24, 36 and 48 h, compared with
the control group and the difference became more pronounced over
time (Fig. 2A). In the PAB-treated
group, the cell number at 12 and 24 h was decreased compared with
that at 0 h, with a decreasing ratio of 12.47 and 28.64%,
respectively (Fig. 2B), while from
24 h the cell number was stable, which indicated that from 24 h,
the cells had entered cytostatic status (Fig. 2B).
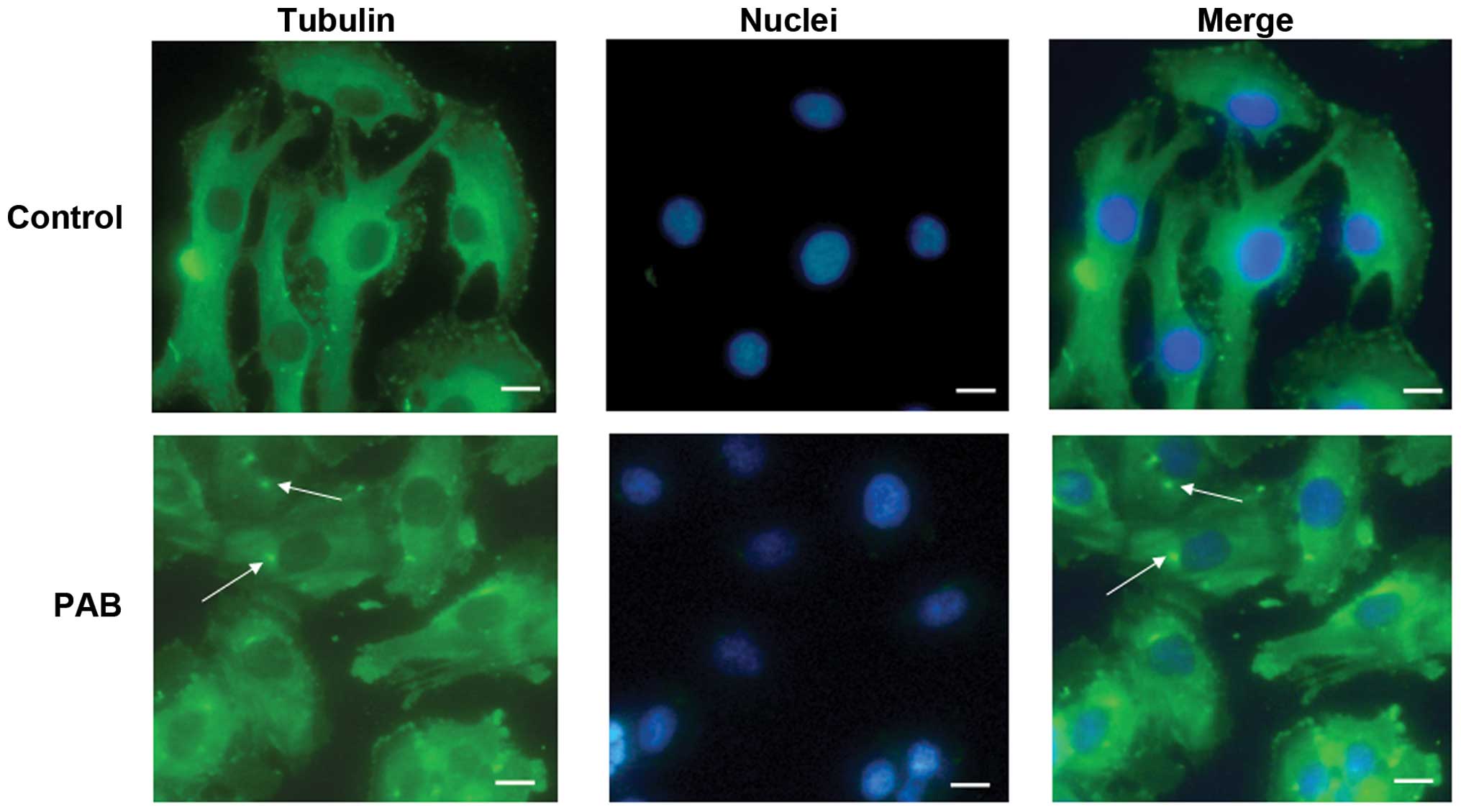
PAB exposure altered tubulin
distribution
Previous studies have reported that PAB is a
tubulin-targeting agent (7),
therefore, the effect of PAB on microtubule networks in SW579 cells
was investigated by immunofluorescence staining of α-tubulin. As
presented in Fig. 3, treatment of
SW579 cells with 4 µM PAB for 24 h resulted in aggregation
of the microtubule fibers. This disruption was accompanied by
cellular deformation, consistent with the role of microtubules in
the maintenance of cell shape (22). The cell nuclei were stained and the
dots of aggregation of the microtubule fibers were observed to be
located near to the cell nuclei (Fig.
3).
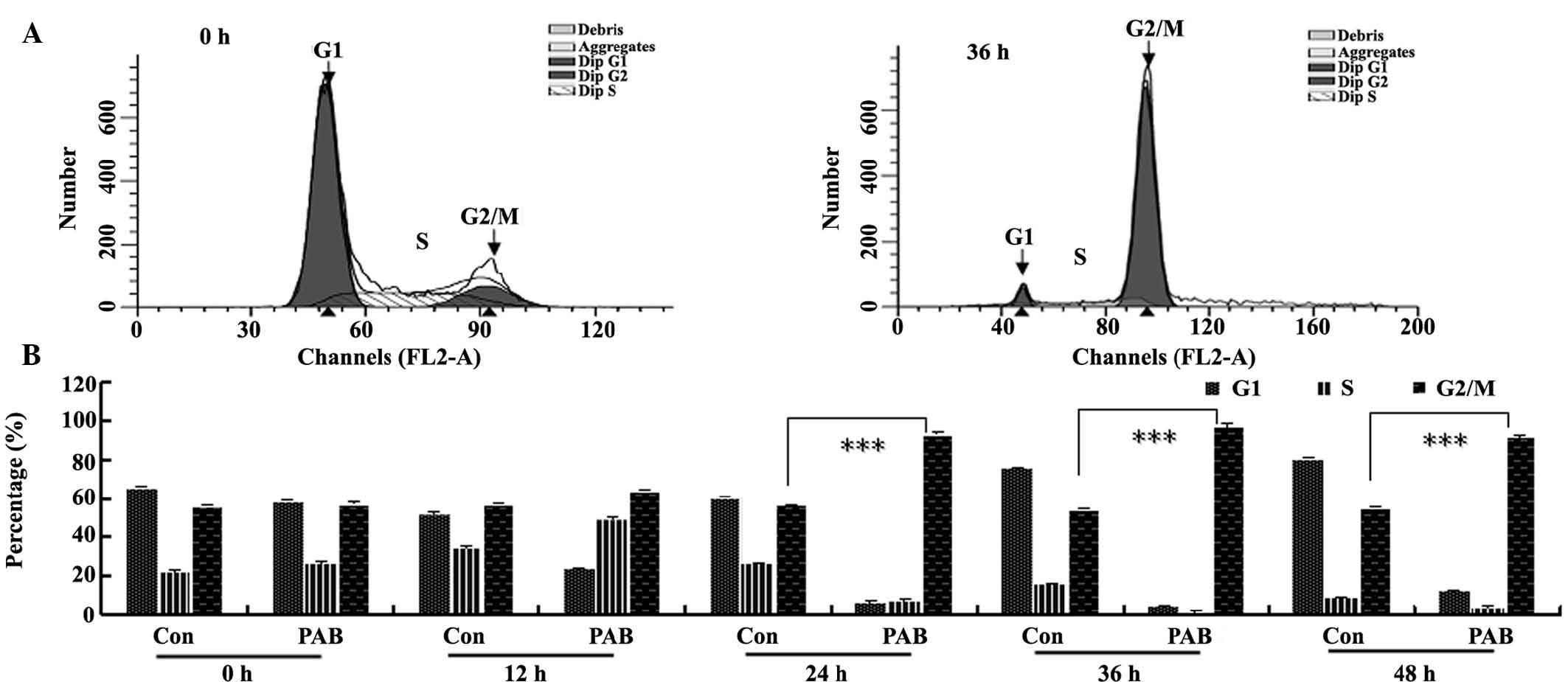
PAB induced G2/M cell cycle
arrest
To further investigate the mechanism of cell growth
inhibition by PAB, the cell cycle was analyzed flow cytometry.
Following 4 µM PAB treatment for 36 h, the quantity of DNA
doubled compared with the control group (Fig. 4A), indicating that PAB-treated
cells may be arrested at the G2/M phase. In the
histogram analysis, it was observed that from 12 h, PAB began to
increase the percentage of cells in the G2/M phase of
the cell cycle (Fig. 4B).
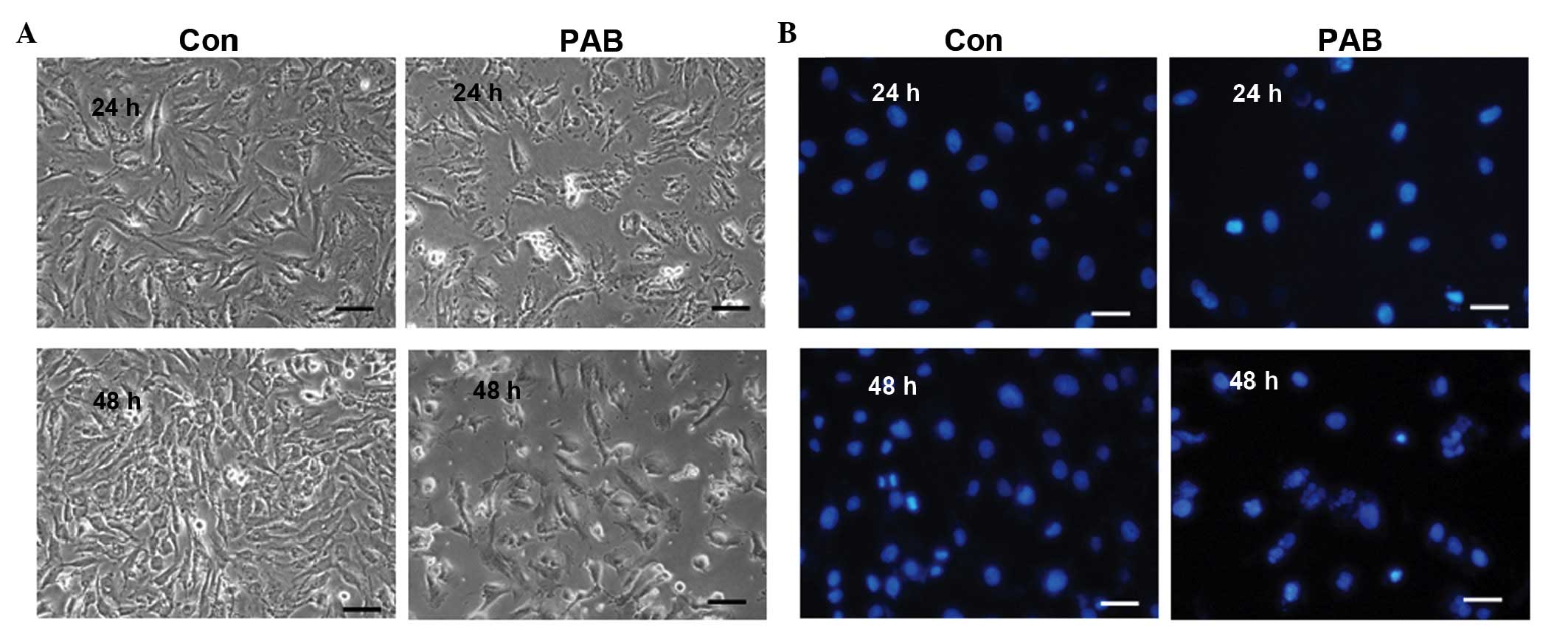
PAB did not induce apoptosis
To determine that there was no apoptosis occurring,
cell and nuclear morphology were observed. At 24 and 48 h no
characteristics of cell apoptosis were evident, such as the
appearance of apoptotic bodies or condensed cell nuclei in the
PAB-treated group (Fig. 5A). While
at 24 and 48 h after PAB treatment, cell nuclei did not become
brighter indicating that no apoptosis was occurring; however,
multinuclear cells appeared at 48 h (Fig. 5B). Further analysis was conducted
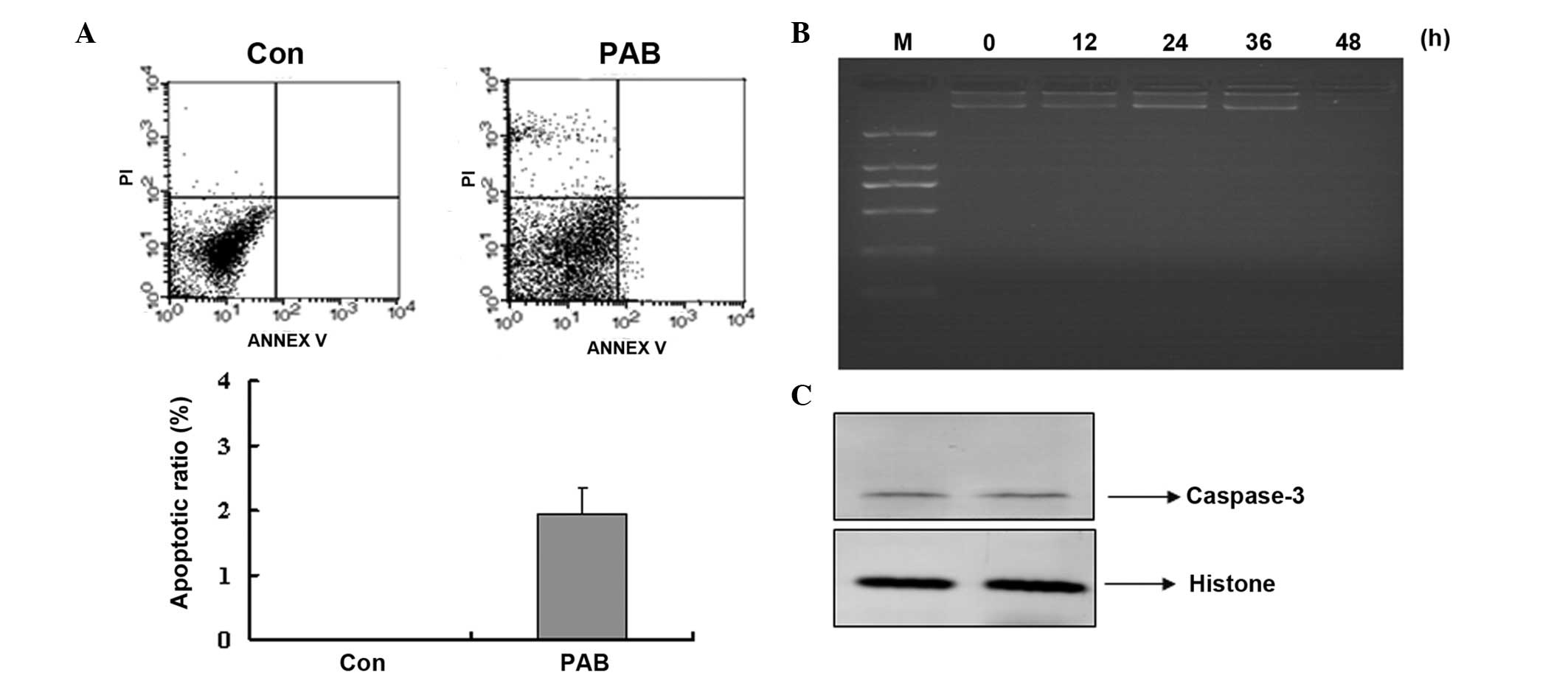
to confirm whether apoptosis was involved in the decreasing cell
number following PAB treatment. Annexin V-PI staining indicated
that no apoptosis was occurring following PAB treatment for 12 h,
and the early apoptotic ratio increased from 0 to 1.95% in the
PAB-treated cells compared with the control group (Fig. 6A). Agarose gel electrophoresis was
also conducted and no DNA ladder was observed at 0, 12, 24, 36 or
48 h subsequent to PAB treatment (Fig.
6B). In addition, at 24 h following PAB treatment, the
expression of active caspase-3 was not increased compared with the
control group (Fig. 6C).
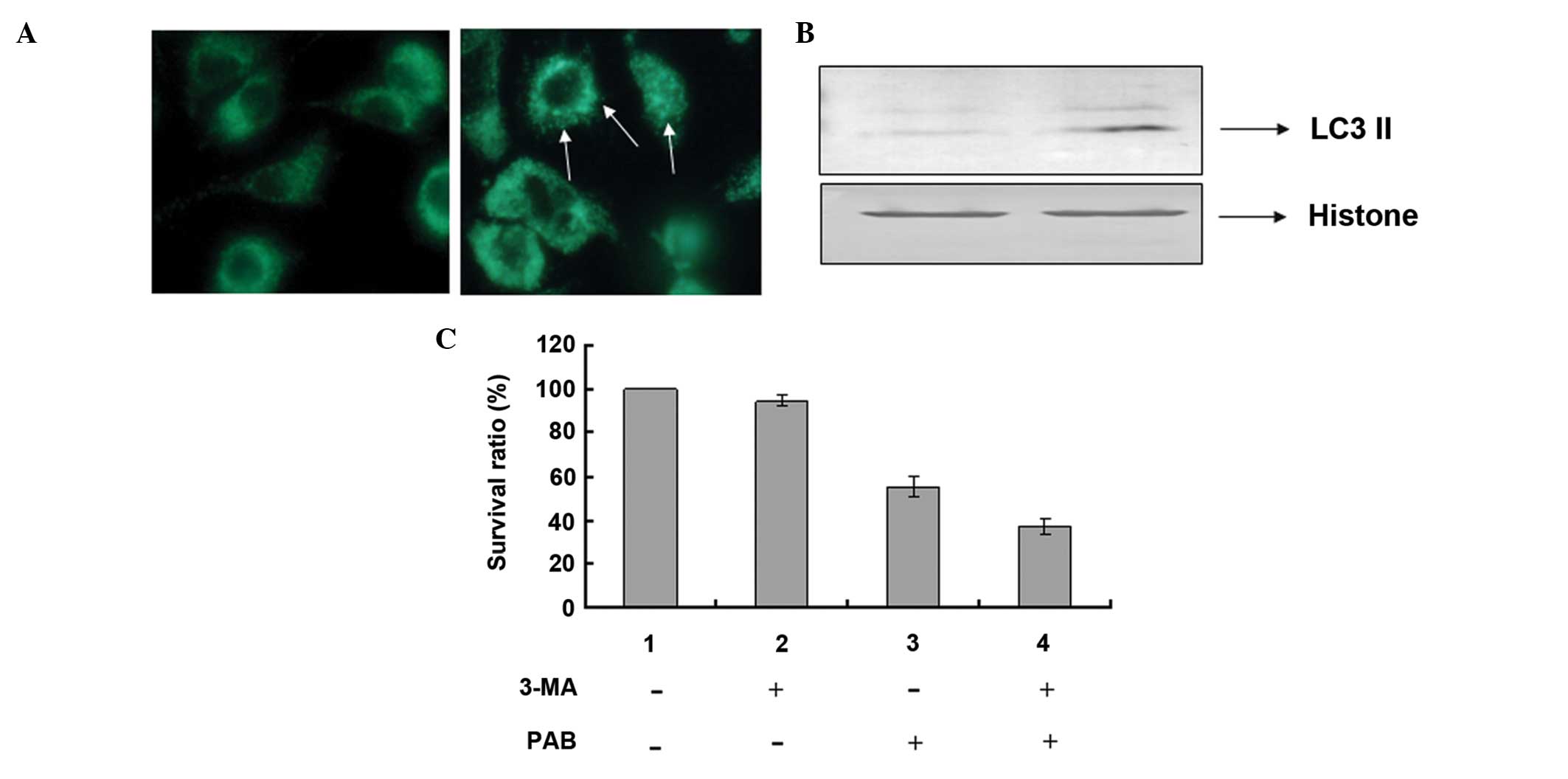
PAB induced autophagy at 24 h following
PAB treatment
It was demonstrated that 4 µM PAB increased
MDC-positive staining, which marked autophagy (Fig. 7A). Furthermore, at 24 h the level
of LC3-II expression increased, which indicated autophagy (Fig. 7B). The role of autophagy-induction
by PAB treatment was unknown, thus the autophagy inhibitor, 3-MA (2
mM) was used to inhibit autophagy and a decreased cell survival
rate was identified (Fig. 7C).
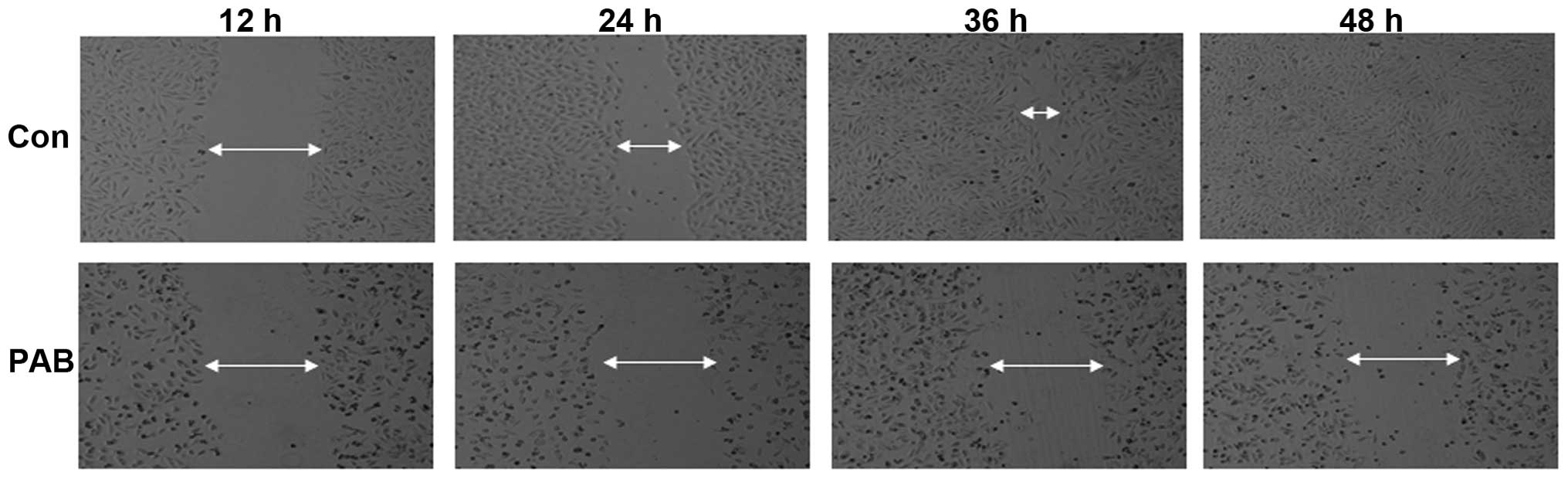
PAB inhibited cell migration
The effect of PAB exposure on inhibition of SW579
cell migration was investigated in the current study. It was
demonstrated that SW579 cells were able to migrate as, over time,
the arbitrarily scratched lines gradually disappeared in the
control cells, whereas the scratched lines remained in the
PAB-treated cells (Fig. 8).
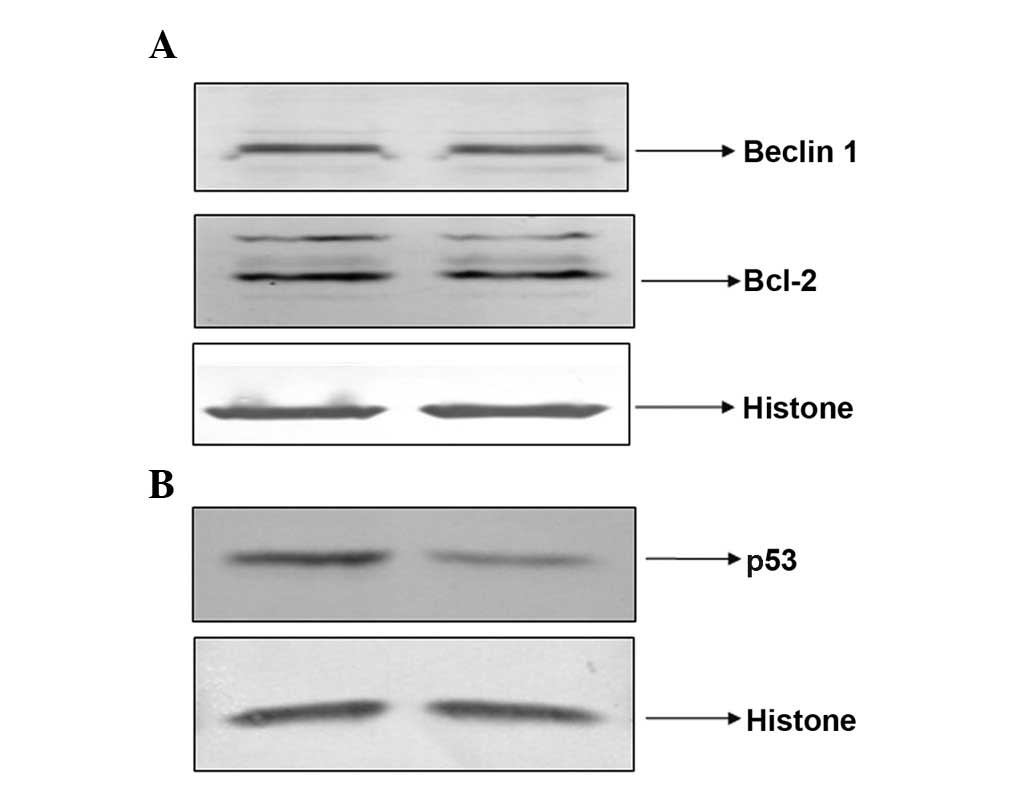
PAB did not regulate Bcl-2 and Beclin-1
expression levels, but decreased the expression of nuclear p53
It was found that PAB exposure did not regulate the
expression of Bcl-2 and Beclin-1 after 24 h treatment (Fig. 9A). However, the expression of
nuclear p53 was reduced by PAB treatment at 24 h (Fig. 9B).
Discussion
PAB has been demonstrated to exert a potent
antitumor effect on MCF-7 human breast cancer cells, HeLa human
cervical cancer cells and A375 human melanoma cells (1–6).
Compared with these cell lines, human primary squamous cell
carcinoma of the thyroid is a rare, and often ignored, example of a
cell line for a neoplasm demonstrating aggressive behavior. Despite
optimal surgical treatment being adopted, survival of greater than
six months with this aggressive malignancy is unlikely. An
effective therapeutic agent is required to improve treatment
success. In the present study, PAB was demonstrated to exert an
antitumor effect on the SW579 cells from primary squamous cell
carcinoma of the thyroid.
PAB exposure inhibited SW579 growth in a time- and
dose-dependent manner, following 48 h of PAB treatment, the
antitumor effect was marked, indicating PAB was increasingly
effective over time. Subsequently, a cell counting test was
conducted to further observe the inhibitory effect of PAB. In the
PAB-treated group, it was observed that the cell number decreased
at 12 and 24 h compared with at 0 h, however the cell number
remained stable from 24 to 48 h. This suggests that from 0 to 24 h,
a small quantity of cell death occurred, however from 24 to 48 h
cells entered a cytostatic state. Thus, indicating that the
inhibitory effect of PAB originates from control of cell growth and
cytostatic activity. It has been reported that PAB exerts its
antitumor effects via disturbing tubulin function (7), therefore the effect of PAB on tubulin
function was investigated in the SW579 cell line in the present
study. PAB exposure resulted in the aggregation of microtubule
fibers near to the nuclei, which is consistent with previous
reports (7). The cytostatic status
induced by PAB was analyzed by determining the cell cycle
distribution following PAB treatment, and it was observed that from
12 h after PAB treatment the percentage of cells in the
G2/M phase was increased, indicating that the cytostatic
status resulted from cell cycle arrest in the G2/M
phase. At 12 and 24 h, in the PAB-treated group the cell number was
reduced compared with that at 0 h, which indicated that cell death
had occurred. Apoptosis is one type of death mechanism, thus cell
and nuclear morphologic analysis, Annexin V-PI staining and gel
electrophoresis were conducted to detect apoptosis. However, in the
cell morphologic analysis, no obvious apoptotic bodies were
observed. In the nuclear morphologic analysis, the nuclei were not
condensed and bright nuclear fragmentation was not observed. The
Annexin V-PI test did not identify phosphatidylserine
translocation, and in gel electrophoresis, no DNA fragmentation was
apparent following PAB treatment for 0–48 h. Therefore, PAB did not
induce apoptosis in the SW579 cell line, thus the mechanism for
reduced cell number at 12 and 24 h after PAB treatment was not
apoptosis, but another mechanism of cell death, such as necrosis.
It has been previously reported that autophagy occurs when the cell
is arrested during the cell cycle (23). In the present study, PAB treatment
increased MDC staining and LC3-II protein expression, which marks
autophagy. Inhibition of autophagy using the autophagy inhibitor,
3-MA, increased cell death, indicating that during cytostasis
autophagy sustains cell survival. In the present study it was
observed that PAB inhibited cell migration; a notable finding due
to the aggressive nature of human primary squamous cell carcinoma
of the thyroid.
The mechanism of PAB as an antitumor therapeutic
agent was also investigated. Beclin-1 and Bcl-2 are
autophagy-associated proteins, however, the expression of these
proteins was not affected by PAB treatment, suggesting that
PAB-induced autophagy was not associated with the expression of
Beclin-1 and Bcl-2. Bcl-2 expression is decreased when apoptosis
occurs (24) and the current study
demonstrated that PAB exposure did not induce apoptosis. The p53
protein is crucial in multicellular organisms, where it regulates
the cell cycle and, thus, functions as a tumor suppressor,
preventing cancer (25). However,
it was found that following PAB treatment, the expression of
nuclear p53 was decreased. The role of decreased nuclear p53
remains unknown and requires further investigation. It was
hypothesized that decreased expression of p53 may be associated
with the lack of apoptosis in PAB-treated SW579 cells. In
conclusion, PAB exerted an antitumor effect via inducing a
cytostatic state in SW579 cells from the human thyroid squamous
cell carcinoma cell line.
In conclusion, the present study demonstrated that
the antitubulin agent PAB exerted cytostatic effects on primary
squamous cell carcinoma of the thyroid with the involvement of
autophagy and a reduced cell migratory ability. PAB is therefore a
candidate drug for treating primary squamous cell carcinoma of the
thyroid.
Acknowledgments
The present study was supported by funding from
Jilin Provincial Science and Technology Department (grant no.
20140204004YY), the National Natural Science Foundation of China
(grant no. 81301416), the Postdoctoral Science Foundation of China
(grant no. 2014M561302), the Chinese Ministry of Science and
Technology (grant nos. 2012CB911100 and 2013ZX10001005), the State
Grade III Laboratory of Traditional Chinese Medicine, the
Immunology and Molecular Biology Laboratory and the Key Laboratory
of Molecular Virology of Jilin Province (grant no. 20102209).
References
|
1
|
Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ and
Lee KH: The cytotoxic principles of Pseudolarix kaempferi:
Pseudolaric acid-A and -B and related derivatives. Planta Med.
56:383–5. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong XF, Wang MW, Tashiro S, Onodera S and
Ikejima T: Pseudolaric acid B induces apoptosis through p53 and
Bax/Bcl-2 pathways in human melanoma A375-S2 cells. Arch Pharm Res.
28:68–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong X, Wang M, Tashiro S, Onodera S and
Ikejima T: Involvement of JNK-initiated p53 accumulation and
phosphorylation of p53 in pseudolaric acid B induced cell death.
Exp Mol Med. 38:428–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces apoptosis,
senescence, and mitotic arrest in human breast cancer MCF-7. Acta
Pharmacol Sin. 28:1975–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu J, Li X, Tashiro S, Onodera S and
Ikejima T: Bcl-2 family proteins were involved in pseudolaric acid
B-induced autophagy in murine fibrosarcoma L929 cells. J Pharmacol
Sci. 107:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu JH, Wang HJ, Li XR, Tashiro S, Onodera
S and Ikejima T: Protein tyrosine kinase, JNK, and ERK involvement
in pseudolaric acid B-induced apoptosis of human breast cancer
MCF-7 cells. Acta Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong VK, Chiu P, Chung SS, Chow LM, Zhao
YZ, Yang BB and Ko BC: Pseudolaric acid B, a novel
microtubule-destabilizing agent that circumvents multidrug
resistance phenotype and exhibits antitumor activity in vivo. Clin
Cancer Res. 11:6002–6011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkar T, Nguyen TL, Su ZW, Hao J, Bai R,
Gussio R, Qiu SX and Hamel E: Interaction of pseudolaric acid B
with the colchicine site of tubulin. Biochem Pharmacol. 84:444–450.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong YG, Zhang XW, Geng MY, Yue JM, Xin
XL, Tian F, Shen X, Tong LJ, Li MH, Zhang C, et al: Pseudolarix
acid B, a new tubulinbinding agent, inhibits angiogenesis by
interacting with a novel binding site on tubulin. Mol Pharmacol.
69:1226–1233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blagosklonny MV and Fojo T: Molecular
effects of paclitaxel: Myths and reality (a critical review). Int J
Cancer. 83:151–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horwitz SB: Mechanism of action of taxol.
Trends Pharmacol Sci. 13:134–136. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li FF, Yi S, Wen L, He J, Yang LJ, Zhao J,
Zhang BP, Cui GH and Chen Y: Oridonin induces NPM mutant protein
translocation and apoptosis in NPM1c+ acute myeloid leukemia cells
in vitro. Acta Pharmacol Sin. 35:806–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Y, Yang YN, Yuan HH, Zhang TT, Sui H,
Wei XL, Liu L, Huang P, Zhang WJ and Bai YX: UCA1, a long
non-coding RNA up-regulated in colorectal cancer influences cell
proliferation, apoptosis and cell cycle distribution. Pathology.
46:396–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee H, Lee H, Chin H, Kim K and Lee D:
ERBB3 knockdown induces cell cycle arrest and activation of Bak and
Bax-dependent apoptosis in colon cancer cells. Oncotarget.
5:5138–5152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn JH, Lee YW, Ahn SK and Lee M:
Oncogenic BRAF inhibitor UAI-201 induces cell cycle arrest and
autophagy in BRAF mutant glioma cells. Life Sci. 104:38–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Xiao X, Wang PY, Wang L, Guan Q,
Du C and Wang XJ: Stimulation of autophagic activity in human
glioma cells by anti-proliferative ardipusilloside I isolated from
Ardisia pusilla. Life Sci. 110:15–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YJ, Won AJ, Lee J, Jung JH, Yoon S,
Lee BM and Kim HS: Molecular mechanism of SAHA on regulation of
autophagic cell death in tamoxifen-resistant MCF-7 breast cancer
cells. Int J Med Sci. 9:881–893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee YZ, Yang CW, Chang HY, Hsu HY, Chen
IS, Chang HS, Lee CH, Lee JC, Kumar CR, Qiu YQ, et al: Discovery of
selective inhibitors of Glutaminase-2, which inhibit mTORC1,
activate autophagy and inhibit proliferation in cancer cells.
Oncotarget. 5:6087–6101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He H, Feng YS, Zang LH, Liu WW, Ding LQ,
Chen LX, Kang N, Hayashi T, Tashiro S, Onodera S, et al: Nitric
oxide induces apoptosis and autophagy; autophagy down-regulates NO
synthesis in physalin A-treated A375-S2 human melanoma cells. Food
Chem Toxicol. 71:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Xuan A, Chen Y, Zhang J, Xu L, Yan
Q and Long D: Combined effect of nerve growth factor and
brain-derived neurotrophic factor on neuronal differentiation of
neural stem cells and the potential molecular mechanisms. Mol Med
Rep. 10:1739–1745. 2014.PubMed/NCBI
|
|
23
|
Santi SA and Lee H: Ablation of Akt2
induces autophagy through cell cycle arrest, the downregulation of
p70S6K, and the deregulation of mitochondria in MDA-MB231 cells.
PLoS One. 6:e146142011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YJ, Park IS, Lee YJ, Shim JH, Cho MK,
Nam HS, Park JW, Oh MH and Lee SH: Resveratrol contributes to
chemosensitivity of malignant mesothelioma cells with activation of
p53. Food Chem Toxicol. 63:153–160. 2014. View Article : Google Scholar
|