Introduction
Spinal cord injury (SCI) is a serious complication
of trauma often caused by car accidents, falls and other causes of
spinal trauma, of which the symptoms include sensory and motor
dysfunction below the damage plane, autonomic nerve dysfunction,
difficulty in restoring nerve function following injury and high
rates of morbidity (1,2). The causes of these symptoms are
predominantly secondary to violent injury, however, secondary SCI
includes the integrity of blood-spinal cord barrier, neutrophil
inflammatory cell infiltration following primary trauma, neuronal
necrosis, apoptosis and glial scar formation (3). In previous years, the incidence of
the disease has exhibited a gradually increasing trend, due to a
lack of effective treatment options, and surgery cannot restore
lost nerve functions (4).
The pathophysiological processes of SCI include the
initial primary injury and the consequent secondary injury.
Secondary injury is involved in a variety of molecular mechanisms,
including Ca2+ influx overload, excitatory amino acid
toxicity and oxidative stress (5).
Oxidative stress directly damages the structure and the function of
nerve cells by attacking biological macromolecules in the cells,
and causes death of the nerve cells, which is closely associated
with neurodegenerative disease following SCI (6). Therefore, antioxidative stress is an
effective strategy for SCI therapeutic intervention. Cavus et
al reported that montelukast and methylprednisolone have a
neuroprotective effect on SCI by downregulating the levels of
malondialdehyde (MDA), superoxide dismutase (SOD), glutathione
peroxidase (GSH-PX) and catalase (7). Tavukçu et al (8) revealed that melatonin and tadalafil
treatment improve erectile dysfunction following SCI through
suppression of the levels of MDA, SOD and glutathione (GSH) in
rats.
The induction of secondary damage following SCI can
lead to tissue hemorrhage, edema and apoptosis, and immune and
inflammatory grade-linking reactions are further expanded, with
inflammation being important in SCI. Early relief of inflammation
following SCI is involved in neural protection and the promotion of
functional recovery. Nuclear factor (NF)-κB, tumor necrosis factor
(TNF)-α and interleukin (IL)-1β have been reported to be
significantly downregulated following plumbagin treatment in SCI
rats (9). Nacar et al
(10) revealed the beneficial
effect of atorvastatin in rat SCI through anti-inflammatory
effects.
The mitogen-activated protein kinase (MAPK) family
is a conservative group of serine/threonine protein kinase, and
comprise a major group of signaling molecules in the transduction
process, which is important in the development and occurrence of
disease. MAPK activation is the final step in the intracellular
phosphorylation cascade (11). P38
is a phosphoric acid protein tyrosine kinase, which is purified and
isolated from mammalian cells stimulated by endotoxin. P38 is the
most important member of the MAPK family in the control of the
inflammatory response, which may be activated due to physiological
stress, lipopolysaccharides, osmotic stress or ultraviolet
irradiation (11). Galan-Arriero
et al (12) reported that
oral administration of an p38α MAPK inhibitor inhibited affective
pain behavior following SCI. Qu et al (13) also reported that inhibition of
p38-MAPK signaling reduces the microglial inflammatory response in
rats following SCI.
Asiaticoside is a white needle-like crystal, which
is extracted from Centellaasiatica and has a swelling and
detoxification effect, which is reported to have certain antitumor
activities (14). It has been
demonstrated that asiaticoside has potent pharmacological activity
and broader pharmacological effects, having antioxidant,
antidepressant and hepatic protective effects, and functioning in
the inhibition of tumor cell proliferation. As these previous data
presented only indirect evidence on the effects of asiaticoside on
SCI, the present study aimed to investigate the mechanisms
underlying the action of asiaticoside in neurological function
using SCI model rats.
Materials and methods
Chemicals
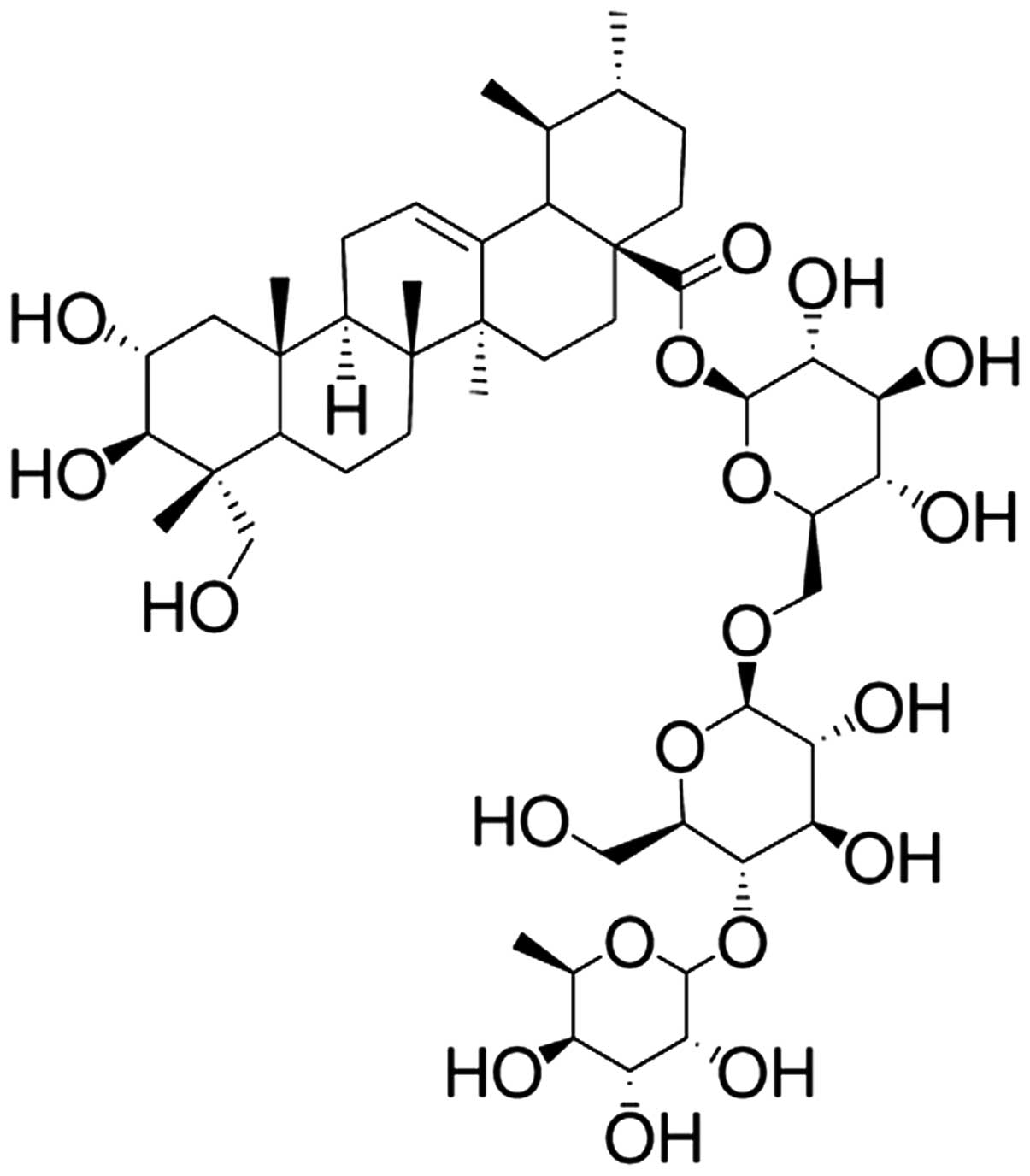
Asiaticoside (purity >95%) was purchased from
Nanjing Traditional Chinese Medicine Institute of Chinese Material
Medica (Nanjing, China) and the chemical structure is indicated in
Fig. 1. MPSS was purchased from
the First Hospital of Jilin University (Changchun, China). MDA,
SOD, GSH, GSH-PX, NF-κB p65 unit, TNF-α, IL-1β and IL-6 commercial
immunoassay kits were purchased from Elabscience Biotechnology Co.,
Ltd. (Wuhan, China). The Inducible Nitric Oxide Synthase (iNOS)
commercial kit was purchased from Sangon Biotech Co., Ltd.
(Shanghai, China).
Animals and ethical statement
The animals used in the present study were male
Sprague-Dawley rats (8–10 weeks old; 250–280 g), which were
obtained from the Animal Resource Center of the First Hospital of
Jilin University and approved by the ethics committee of the First
Hospital of Jilin University. The rats were individually housed in
Plexiglas bins with food and water continuously available, and were
maintained under a controlled environment at 23 ± 1°C with a 12-h
light/dark cycle and 60–80% humidity. All experimental procedures
were approved by the guidelines of the Care and Use of Laboratory
Animals of the First Hospital of Jilin University.
Experimental groups and induction of the
SCI rat model
The 50 male Sprague-Dawley rats were randomly
assigned into five groups. The sham group (n=10), received only
physiological saline (0.1 ml/100 g, intraperitoneally). The
remaining four groups underwent SCI at the T10 spinal segment
impactor. The SCI group (n=10) received no treatment following SCI,
the ASI (30) group (n=10)
received asiaticoside at doses of 30 mg/kg once a day for 7 days
following SCI (15), the ASI (60)
group (n=10) received asiaticoside at a dose of 60 mg/kg once a day
for 7 days following SCI, the MPSS group (n=10) received 100 mg/kg
MPSS following SCI.
The rat model of SCI was induced, as previously
described (16). The rats were
anesthetized with an intraperitoneal injection of sodium
pentobarbital (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA), and a
laminectomy was performed at the T10 level to expose the cord
beneath, without disrupting the dura. Subsequently, a rat model of
spared cord injury was created by performing a laminectomy, during
which the T8 and T9 vertebral peduncles were removed. The control
model rats underwent the same laminectomy, but without
compression.
Evaluation of neuronal function
recovery
Following SCI, the evaluation of locomotor recovery
was evaluated using the Basso, Beattie and Bresnahan (BBB),
locomotor rating scale of 0–21, in which 0, indicated no observable
hind-limb movements and 21 indicated normal locomotion (17).
Assessment of the water content of the
spinal cord following SCI
At 72 h post-SCI, the spinal cord was collected, the
wet weight was obtained and the spinal cord was placed in an oven
at 80°C for 48 h. The dry weight of the spinal cord was then
measured. The plasma supernatant was collected following
centrifugation at 5,000 × g for 10 min at 4°C. The nitrite
concentration was spectrophotometrically determined using a
CM-2600d spectrophotometer (Konica Minolta Sensing Singapore Pte
Ltd., Jurong East, Singapore) and the following formula: Spinal
cord water content (%) = (wet weight − dry weight) / wet weight ×
100%.
Measurement of levels of MDA, SOD, GSH
and GSH-PX
Following treatment with asiaticoside for seven
consecutive days, the peripheral blood was collected from each
group. Subsequently, the supernatant was centrifuged at 12,000 × g
for 20 min at 4°C. The serum levels of MDA, SOD, GSH and GSH-PX
were analyzed using commercial immunoassay kits (cat. nos.
E-EL-0060c, E-EL-R1424c, E-EL-R0440c and E-EL-R2491c,
respectively), according to the manufacturer's instructions
(Elabscience Biotechnology Co., Ltd.
Measurement of iNOS activity
Following treatment with asiaticoside for seven
consecutive days, the rats were sacrificed via cervical dislocation
and spinal cord tissue was collected from each group. The activity
of iNOS was determined using a commercial kit, according to the
manufacturer's instructions (Sangon Biotech Co., Ltd.).
Measurement of the activities of NF-κB
p65 unit, TNF-α, IL-1β and IL-6
Following treatment with asiaticoside for seven
consecutive days, the peripheral blood was collected from each
group. Subsequently, the supernatants were centrifuged at 12,000 ×
g for 20 min at 4°C. The serum activities of NF-κB p65 unit, TNF-α,
IL-1β and IL-6 (cat. nos. E-EL-R0674c, E-CL-R0019c, E-EL-R0012c and
E-EL-R0015c, respectively) were analyzed using the respective
commercial immunoassay kits, according to the manufacturer's
instructions (Elabscience Biotechnology Co., Ltd.).
Western blot analysis
Samples (~10 mg) of the exposed spinal cord tissue
were removed from the rats and incubated with 100 µl
ice-cold lysis buffer, containing 2 mM EDTA, 10 mM EGTA, 0.4% NaF,
20 mM Tris-HCl and protease inhibitors (pH 7.5) for 10–15 min on
ice. Subsequently, the homogenates were centrifuged at 12,000 × g
for 20 min at 4°C. The protein concentrations of the soluble
materials were determined using a Bicinchoninic Acid protein assay
(Beyotime Institute of Biotechnology, Nanjing, China). Equal
quantities of protein (30 µg) were separated on 12% sodium
dodecyl sulfate-polyacrylamide gels (Beyotime Institute of
Biotechnology), followed by transfer onto polyvinylidene fluoride
membranes (0.22 mm; EMD Millipore, Billerica, MA, USA). The
membranes were blocked with phosphate-buffered saline with 5%
non-fat milk to block nonspecific binding sites. Subsequently, the
membranes were incubated with monoclonal mouse anti-human
anti-p38-MAPK (cat. no. sc-4708 1:2,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and monoclonal anti-β-actin (cat. no.
D110001; 1:500; Sangon Biotech Co., Ltd.) overnight at 4°C. The
membrane was then washed three times with Tris-buffered saline with
Tween 20 (Senbeijia Biotech Co., Nanjing, China) for 2 h, and then
detected by incubation with anti-mouse IgG (cat. no. sc-358922;
1:1,000; Santa Cruz Biotechnology, Inc.) conjugated with
horseradish peroxidase for 2 h at room temperature. The relative
band intensity was determined using a gel image analysis system
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Statistical analysis
All the data were analyzed using SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance, followed by Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of asiaticoside on neurological
function
In the present study, BBB scores were used to assess
neurological function at 24 h, 48 h and 72 h following SCI, the
results of which are presented in Fig.
2. The mean BBB scores of the SCI group were lower than the
sham-operated group. However, it was noted that the injured rats of
the asiaticoside-treated group had particularly high BBB scores. As
shown in Fig. 2, the BBB scores
following treatment with asiaticoside at a dose of 60 mg/kg were
similar to those of the MPSS group (P>0.05).
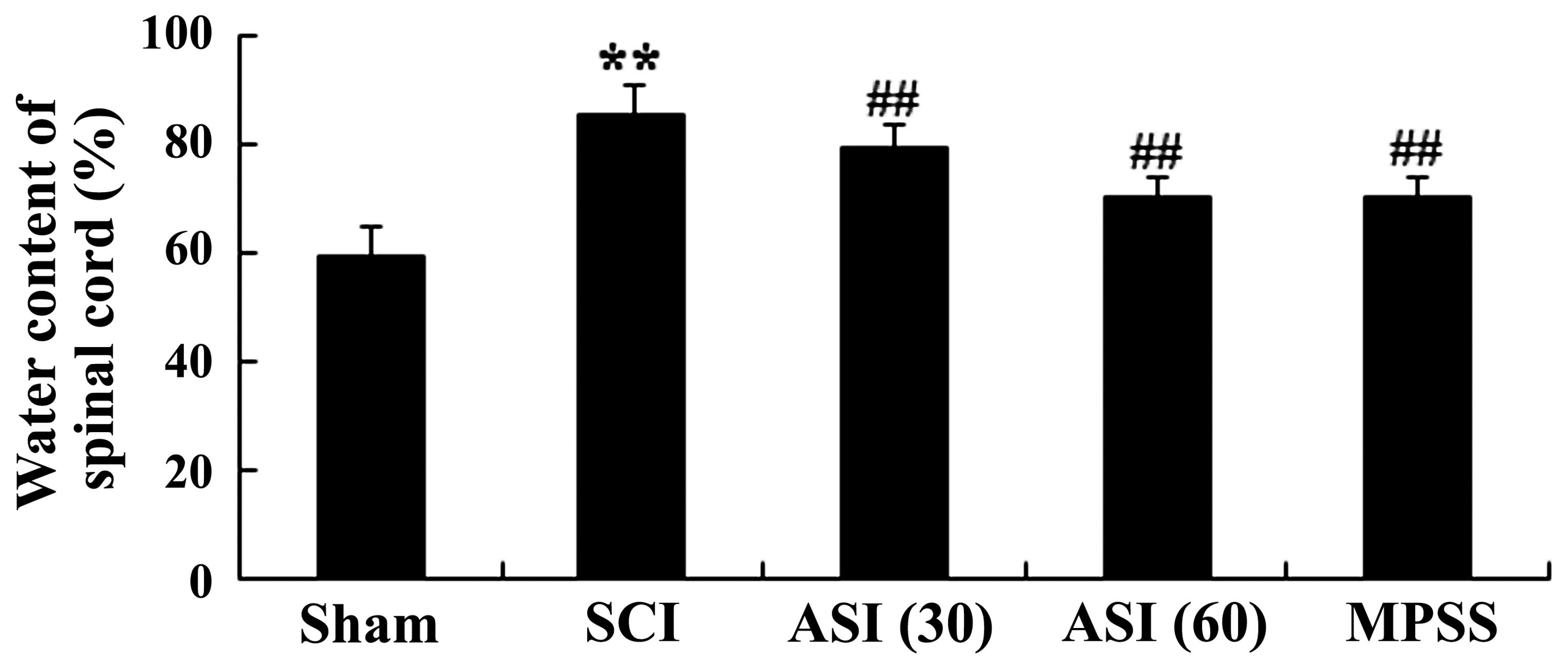
Effects of asiaticoside on the water
content of the spinal cord following SCI
The rats induced by SCI exhibited severe impairment
with marked increase in water content of the spinal cord following
SCI (Fig. 3). However, treatment
with asiaticoside at different doses (30 and 60 mg/kg) of the
injured rats significantly reduced the water content of the spinal
cord, compared with the SCI model group (Fig. 3). No significant difference was
observed between the MPSS group and the 60 mg/kg asiaticoside group
(P>0.05).
Anti-oxidative effects of
asiaticoside
It was previously reported that serum cytokine
levels are relevant to SCI (18).
Therefore, the serum levels of oxidant stress were determined in
the present study. As shown in Fig.
4A, the MDA concentrations of the SCI group were higher than
those in the sham-operated group. In the asiaticoside-treated
group, the serum levels of MDA were also lower than those in the
SCI group (Fig. 4A). The levels of
SOD, GSH and GSH-PX in the SCI group were lower than those of the
sham-operated group (Fig. 4B–D).
The expression levels of SOD, GSH and GSH-PX in the
asiaticoside-treated group were gradually increased, compared with
those of the SCI group. However, no significant changes in cytokine
levels between the MPSS group and 60 mg/kg asiaticoside treatment
group were observed (Fig.
4A–D).
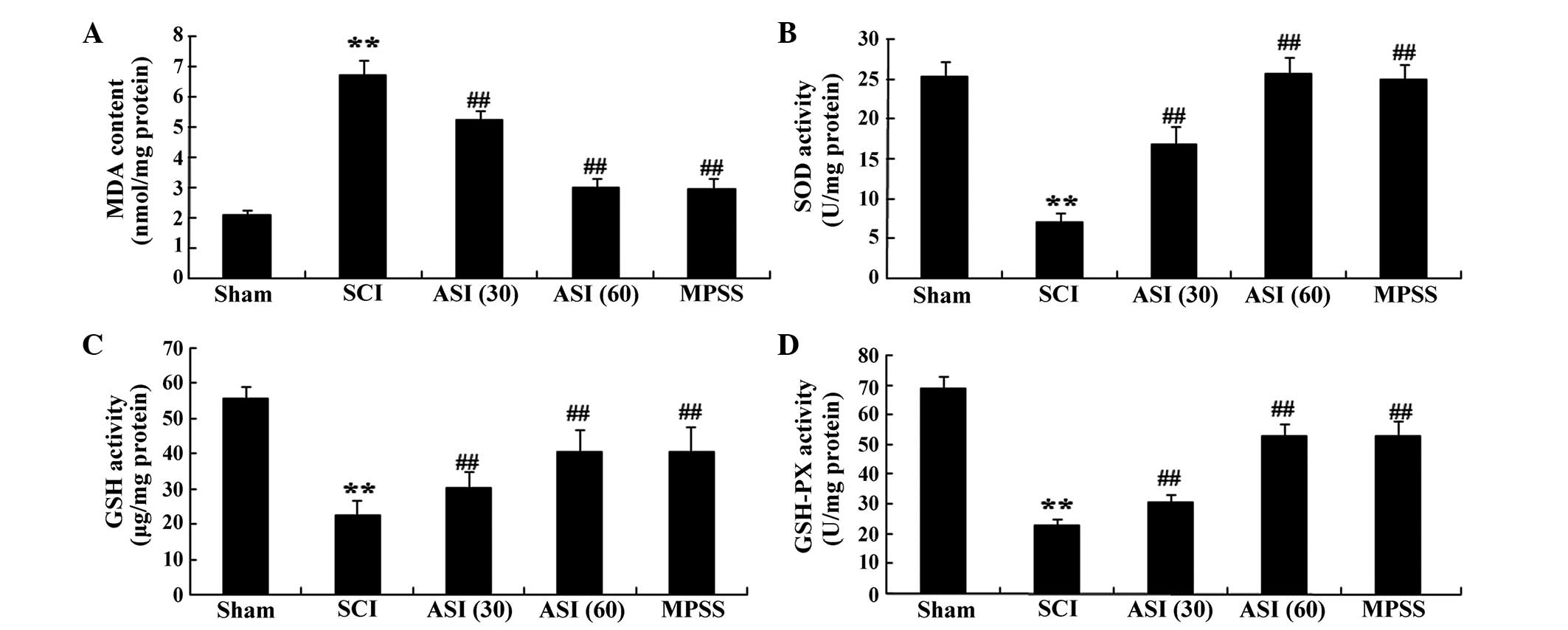 | Figure 4Anti-oxidative effects of asiaticoside
following SCI. Anti-oxidative effects of asiaticoside on the
concentrations of (A) MDA, (B) SOD, (C) GSH and (D) GSH-PX in the
SCI model rats. Data are expressed as the mean ± standard
deviation. **P<0.01, compared with the control group;
##P<0.01, compared with the SCI group. Con, control;
SCI, spinal cord injury; ASI (30), asiaticoside (30 mg/kg); ASI (60),
asiaticoside (60 mg/kg); MPSS, methylprednisolone; MDA,
malondialdehyde; SOD, superoxide dismutase; GHS, glutathione;
GSH-PX, glutathione peroxidase. |
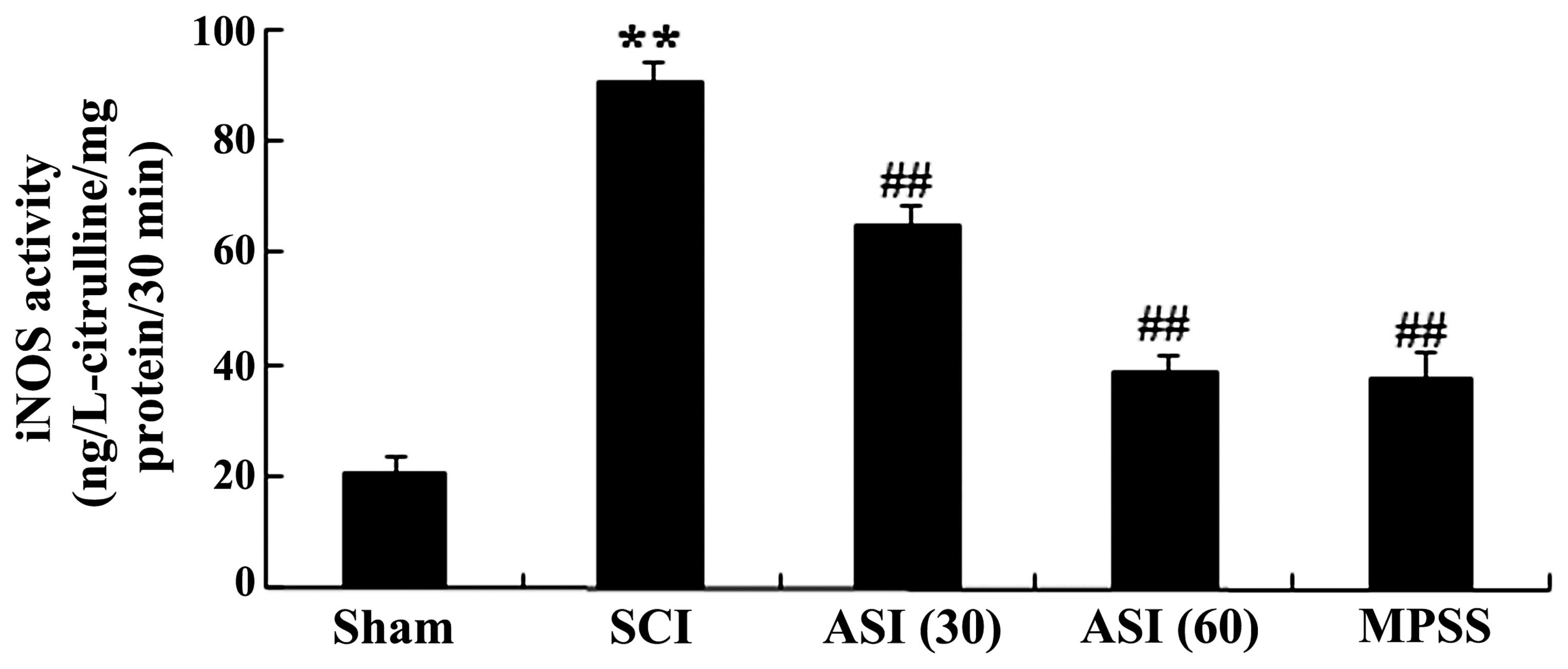
Anti-oxidative effects of asiaticoside on
iNOS
The present study further investigated whether
asiaticoside exerted protection against SCI through the mediation
of nitric oxide. As shown in Fig.
5, iNOS activity was markedly increased in the spinal cord
tissues of the SCI group. However, administration with asiaticoside
(30 and 60 mg/kg) generated a more pronounced reduction in iNOS
activity in the SCI-induced rats (Fig.
5). In addition, the anti-oxidative effect of asiaticoside at a
dose of 60 mg/kg was equipotent to that of the MPSS group (Fig. 5).
Anti-inflammatory effects of
asiaticoside
The present study used ELISA commercial immunoassay
kits to determine the expression levels of inflammatory factors,
for assessment of the progression of the SCI. There were increases
in the serum levels of the NF-κB p65 unit, TNF-α, IL-1β and IL-6 in
the SCI group, compared with the sham group (Fig. 6A–D). However, asiaticoside
treatment of the SCI-induced rats reversed these indices (Fig. 6A–D). The anti-inflammatory effect
of asiaticoside (60 mg/kg) was equipotent to that in the MPSS group
(Fig. 6A–D).
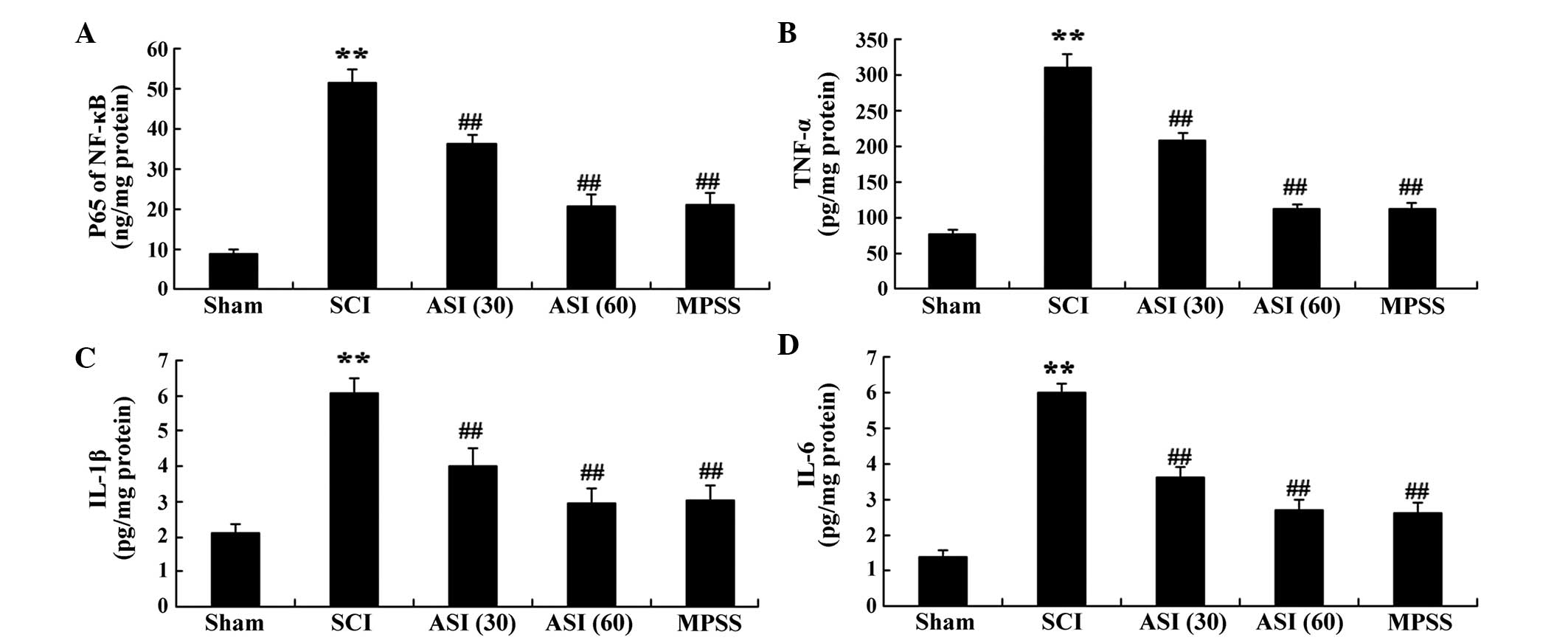 | Figure 6Anti-inflammatory effects of
asiaticoside following SCI. Anti-inflammatory effects of
asiaticoside on the serum activities of (A) NF-κB p65, (B) TNF-α
(B), (C) IL-1β (C) and (D) IL-6 in the SCI model rats. Data are
expressed as the mean ± standard deviation. **P<0.01,
compared with the control group; ##P<0.01, compared
with the SCI group. Con, control; SCI, spinal cord injury; ASI
(30), asiaticoside (30 mg/kg);
ASI (60), asiaticoside (60 mg/kg); MPSS, methylprednisolone; IL,
interleukin. |
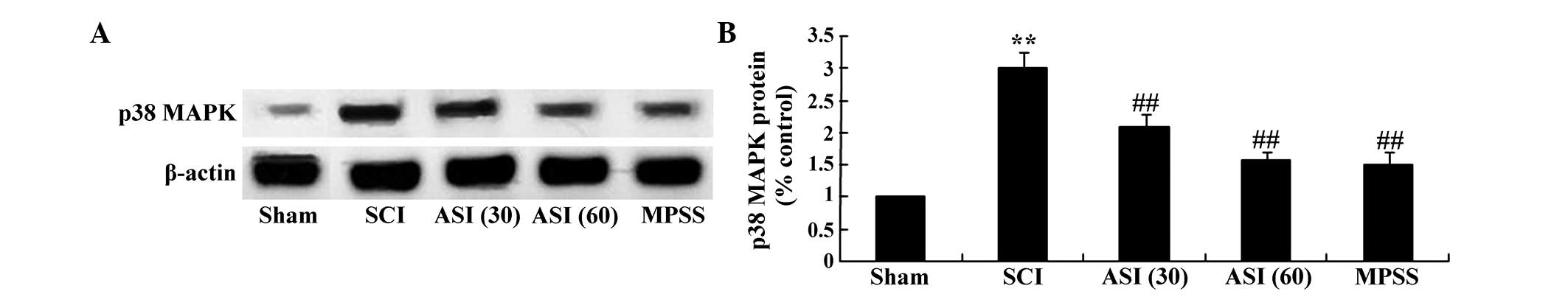
Astaxanthin adjusts the expression of
p38-MAPK
The present study further investigated whether
asiaticoside exerted protection against SCI through mediation of
the expression of p38-MAPK. As shown in Fig. 7A, western blot analysis using the
p38-MAPK antibody demonstrated the anticipated bands of 43 kDa.
Quantitative analysis disclosed an evident elevation of p38-MAPK
protein in the SCI group, compared with the sham group (Fig. 7B). However, asiaticoside treatment
(30 and 60 mg/kg) markedly decreased the protein expression of
p38-MAPK, compared with the SCI group (Fig. 7A–B). No significant inter-group
differences were observed between the MPSS group and asiaticoside
treatment (60 mg/kg) group in the protein expression of p38-MAPK in
the SCI model rat (Fig. 7A–B).
Discussion
SCI is a trauma-induced disease, the causes of which
predominantly include injury from car accidents and falls from
heights (18). The treatment of
SCI is limited, and the majority of patients have various degrees
of sensory and motor nerve dysfunction, autonomic dysfunction, a
reduction or loss of self-care ability and difficulty recovering,
significantly affecting quality of life and introducing a serious
burden for individuals, families and society (19). In the present study, it was first
demonstrated that asiaticoside increased the BBB scores and reduced
the water content of the spinal cord following SCI. No
statistically significant differences were identified between the
asiaticoside (60 mg/kg) and MPSS groups.
Currently, it is considered that a lot of oxygen
free radicals (OFRs) are generated in SCI pathogenesis. OFR damage
to the body may act with a trigger-like effect, with calcium
overload being a final common pathway for cell damage (20). SOD is an enzyme, which catalyzes
disproportionation of superoxide anions, as a major intracellular
antioxidant enzyme and free radical scavenger, which can protect
cells against oxygen free radicals, and the level of which
indicates the strength of the effectiveness in protecting cells
from toxic oxygen free radical damage (21). MDA is the end product of lipid
peroxidation, and can be measured to directly reflect the level of
free radicals and is an important indicator of the level of tissue
injury (22). Measuring the
activities of MDA and SOD can indirectly reflect the antioxidant
abilities of the body (23). In
the present study, it was demonstrated that asiaticoside reduced
the activities of MDA and iNOS and induced the levels of SOD, GSH
and GSH-PX in the SCI rats. The anti-oxidative effects of
asiaticoside induce the levels of SOD, GSH and GSH-PX in healing
wounds (24), and the
anti-inflammatory effects of asiaticoside dependently inhibit liver
myeloperoxidase (MPO) activity and the protein expression of brain
cyclooxygenase-2 (COX-2) (25). Xu
et al (26) indicated that
asiaticoside is effective in reversing
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine-induced Parkinsonism
through the levels of MDA and GSH, whereas Guo et al
(27) indicated that the
inhibitory effects of asiaticoside in gastric ulcer healing in rats
via the inhibition of iNOS.
The SCI process is associated with the production
and release of inflammatory mediators, microvascular endothelial
function disorder, inflammatory cell infiltration and accumulation
in the spinal cord tissue, and increased expression of inflammatory
cytokines and adhesion molecules (16). The combined effect of these factors
trigger a cascade of inflammation, thereby increasing secondary
injury in the ischemic area (28).
Avascular necrosis of neurons, glial cells and endothelial cells
following SCI can induce the production of large quantities of
inflammatory cytokines, including TNF-α, IL-1β and IL-6, which can
stimulate the production of cytokines and other inflammatory
mediators, affecting gene expression in glial cells (29). These cytokines can be used as a
signaling molecule of endothelial cell activation, thereby
stimulating the secretion of cell adhesion molecules and the
adhesion between leukocytes and endothelial cells, which induce the
infiltration of leukocytes to the damage zone (30). Leukocyte infiltration damages the
blood brain barrier, further aggravating SCI. Studies have
demonstrated that there are several factors involved in the process
of apoptosis following SCI, in which inflammatory cytokines are
important. The present study demonstrated decreased activities of
the NF-κB p65 Unit, TNF-α, IL-1β and IL-6 in SCI rats. Zhang et
al (31) suggested that the
protective effects of asiaticoside effectively protected against
septic lung injury through the regulation of the iNOS, TNF-α, IL-6
and NF-κB pathway. Bhaumik et al (32) reported that asiaticoside induces
TNF-α to treat experimental visceral leishmaniasis via nitric oxide
production.
The P38-MAPK signaling pathway is one of the three
classical branches of the MAPK signaling pathway, widely involved
in stress responses, including inflammation and radioactive injury.
Studies have reported that activation of the p38-MAPK pathway
enables the expression of downstream MAPK (MK)2 to be increased,
promoting the expression of MMP-9 following SCI, leading to
destruction of the blood-spinal cord barrier (33–35).
The findings of the present study provide the first direct
evidence, to the best of our knowledge, that asiaticoside
restrained the protein expression of p38-MAPK in SCI rats. Chen
et al (15) suggested that
asiaticoside attenuates memory impairment through anti-inflammatory
effects and inhibiting the overactivation of the p38-MAPK pathway.
Zhang et al (36) reported
that the protective effects of asiaticoside on acute liver injury
are induced by restricting TNF-α and the p38-MAPK pathway in
mice.
In conclusion, the major finding of the present
study was that asiaticoside successfully decreased water content in
SCI rats. Asiaticoside appeared to inhibit oxidative damage, nitric
oxide activity, pro-inflammatory cytokine production and the
p38-MAPK pathway. Further investigations on the signaling pathways
and cross-talk consequent to asiaticoside administration may
provide further insights into its therapeutic action in terms of
SCI, and provide a starting point for developing novel strategies
for pain control.
References
|
1
|
Liu C, Wu W, Zhang B, Xiang J and Zou J:
Temporospatial expression and cellular localization of glutamine
synthetase following traumatic spinal cord injury in adult rats.
Mol Med Rep. 7:1431–1436. 2013.PubMed/NCBI
|
|
2
|
Ravikumar R, Fugaccia I, Scheff SW, Geddes
JW, Srinivasan C and Toborek M: Nicotine attenuates morphological
deficits in a contusion model of spinal cord injury. J Neurotrauma.
22:240–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furlan JC, Sakakibara BM, Miller WC and
Krassioukov AV: Global incidence and prevalence of traumatic spinal
cord injury. Can J Neurol Sci. 40:456–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong Z, Hong H, Chen H, Wang Z and Hong D:
Investigation of the protective effect of erythropoietin on spinal
cord injury in rats. Exp Ther Med. 2:837–841. 2011.
|
|
5
|
Oyinbo CA: Secondary injury mechanisms in
traumatic spinal cord injury: A nugget of this multiply cascade.
Acta Neurobiol Exp (Wars). 71:281–299. 2011.
|
|
6
|
Smith JA, Park S, Krause JS and Banik NL:
Oxidative stress, DNA damage and the telomeric complex as
therapeutic targets in acute neurodegeneration. Neurochem Int.
62:764–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cavus G, Altas M, Aras M, Ozgür T,
Serarslan Y, Yilmaz N, Sefil F and Ulutas KT: Effects of
montelukast and methylprednisolone on experimental spinal cord
injury in rats. Eur Rev Med Pharmacol Sci. 18:1770–1777.
2014.PubMed/NCBI
|
|
8
|
Tavukçu HH, Sener TE, Tinay I, Akbal C,
Erşahin M, Cevik O, Cadirci S, Reiter RJ and Sener G: Melatonin and
tadalafil treatment improves erectile dysfunction after spinal cord
injury in rats. Clin Exp Pharmacol Physiol. 41:309–316. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Cheng L, Hou Y, Si M, Zhao YP and
Nie L: Plumbagin protects against spinal cord injury-induced
oxidative stress and inflammation in wistar rats through Nrf-2
upregulation. Drug Res (Stuttg). 2014.
|
|
10
|
Nacar OA, Eroglu H, Cetinalp NE, Menekse
G, Yildirim AE, Uckun OM, Daglioglu E, Turkoglu OF and Belen AD:
Systemic administration of atorvastatin improves locomotor
functions and hyperacute-acute response after experimental spinal
cord injury: An ultrastructural and biochemical analysis. Turk
Neurosurg. 24:337–343. 2014.PubMed/NCBI
|
|
11
|
Kanno A, Ozawa T and Umezawa Y:
Bioluminescent imaging of MAPK function with intein-mediated
reporter gene assay. Methods Mol Biol. 574:185–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galan-Arriero I, Avila-Martin G,
Ferrer-Donato A, Gomez-Soriano J, Bravo-Esteban E and Taylor J:
Oral administration of the p38α MAPK inhibitor, UR13870, inhibits
affective pain behavior after spinal cord injury. Pain.
155:2188–2198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu WS, Tian DS, Guo ZB, Fang J, Zhang Q,
Yu ZY, Xie MJ, Zhang HQ, Lü JG and Wang W: Inhibition of EGFR/MAPK
signaling reduces microglial inflammatory response and the
associated secondary damage in rats after spinal cord injury. J
Neuroinflammation. 9:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin X, Huang R, Zhang S, Wei L, Zhuo L, Wu
X, Tang A and Huang Q: Beneficial effects of asiaticoside on
cognitive deficits in senescence-accelerated mice. Fitoterapia.
87:69–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Yin ZJ, Jiang C, Ma ZQ, Fu Q, Qu R
and Ma SP: Asiaticoside attenuates memory impairment induced by
transient cerebral ischemia-reperfusion in mice through
anti-inflammatory mechanism. Pharmacol Biochem Behav. 122:7–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu JY, McKeon R, Goussev S, Werb Z, Lee
JU, Trivedi A and Noble-Haeusslein LJ: Matrix metalloproteinase-2
facilitates wound healing events that promote functional recovery
after spinal cord injury. J Neurosci. 26:9841–9850. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. ++multicenter
animal spinal cord injury study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhang C, Hong Z, Chen H, Chen W
and Chen G: C/EBP homologous protein (CHOP) mediates neuronal
apoptosis in rats with spinal cord injury. Exp Ther Med. 5:107–111.
2013.
|
|
19
|
Liu G, Wang X, Shao G and Liu Q:
Genetically modified Schwann cells producing glial cell
line-derived neurotrophic factor inhibit neuronal apoptosis in rat
spinal cord injury. Mol Med Rep. 9:1305–1312. 2014.PubMed/NCBI
|
|
20
|
Xie YG, Mu HJ, Li Z, Ma JH and Wang YL:
Supression of chronic central pain by superoxide dismutase in rats
with spinal cord injury: Inhibition of the NMDA receptor
implicated. Exp Ther Med. 8:1137–1141. 2014.PubMed/NCBI
|
|
21
|
Kurtoglu T, Basoglu H, Ozkisacik EA, Cetin
NK, Tataroglu C, Yenisey C and Discigil B: Effects of cilostazol on
oxidative stress, systemic cytokine release, and spinal cord injury
in a rat model of transient aortic occlusion. Ann Vasc Surg.
28:479–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YD, Ma YH, Zhao JX and Zhao XK:
Protection of ultra-filtration extract from Danggui Buxue Decoction
on oxidative damage in cardiomyocytes of neonatal rats and its
mechanism. Chin J Integr Med. 17:854–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Y, Liu J, Zhang F, Zhang J, Shi T and
Zeng Z: Antioxidant effect of quercetin against acute spinal cord
injury in rats and its correlation with the p38MAPK/iNOS signaling
pathway. Life Sci. 92:1215–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shukla A, Rasik AM and Dhawan BN:
Asiaticoside-induced elevation of antioxidant levels in healing
wounds. Phytother Res. 13:50–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan J, Gong X, Jiang R, Zhang Z and Zhang
L: Antipyretic and anti-inflammatory effects of asiaticoside in
lipopolysac-charide-treated rat through up-regulation of heme
oxygenase-1. Phytother Res. 27:1136–1142. 2013. View Article : Google Scholar
|
|
26
|
Xu CL, Wang QZ, Sun LM, Li XM, Deng JM, Li
LF, Zhang J, Xu R and Ma SP: Asiaticoside: Attenuation of
neurotoxicity induced by MPTP in a rat model of Parkinsonism via
maintaining redox balance and up-regulating the ratio of Bcl-2/Bax.
Pharmacol Biochem Behav. 100:413–418. 2012. View Article : Google Scholar
|
|
27
|
Guo JS, Cheng CL and Koo MW: Inhibitory
effects of Centella asiatica water extract and asiaticoside on
inducible nitric oxide synthase during gastric ulcer healing in
rats. Planta Med. 70:1150–1154. 2004. View Article : Google Scholar
|
|
28
|
Manhas A, Khanna V, Prakash P, Goyal D,
Malasoni R, Naqvi A, Dwivedi AK, Dikshit M and Jagavelu K: Curcuma
oil reduces endothelial cell-mediated inflammation in
postmyocardial ischemia/reperfusion in rats. J Cardiovasc
Pharmacol. 64:228–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen DH, Cho N, Satkunendrarajah K,
Austin JW, Wang J and Fehlings MG: Immunoglobulin G (IgG)
attenuates neuroinflam-mation and improves neurobehavioral recovery
after cervical spinal cord injury. J Neuroinflammation. 9:2242012.
View Article : Google Scholar
|
|
30
|
Casella GT, Bunge MB and Wood PM: Improved
immunocyto-chemical identification of neural, endothelial, and
inflammatory cell types in paraffin-embedded injured adult rat
spinal cord. J Neurosci Methods. 139:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang LN, Zheng JJ, Zhang L, Gong X, Huang
H, Wang CD, Wang B, Wu MJ, Li XH, Sun WJ, et al: Protective effects
of asiaticoside on septic lung injury in mice. Exp Toxicol Pathol.
63:519–525. 2011. View Article : Google Scholar
|
|
32
|
Bhaumik SK, Paul J, Naskar K, Karmakar S
and De T: Asiaticoside induces tumour-necrosis-factor-α-mediated
nitric oxide production to cure experimental visceral leishmaniasis
caused by antimony-susceptible and -resistant Leishmania donovani
strains. J Antimicrob Chemother. 67:910–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou C, Shi X, Huang H, Zhu Y and Wu Y:
Montelukast attenuates neuropathic pain through inhibiting p38
mitogen-activated protein kinase and nuclear factor-kappa B in a
rat model of chronic constriction injury. Anesth Analg.
118:1090–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghasemlou N, Lopez-Vales R, Lachance C,
Thuraisingam T, Gaestel M, Radzioch D and David S:
Mitogen-activated protein kinase-activated protein kinase 2 (MK2)
contributes to secondary damage after spinal cord injury. J
Neurosci. 30:13750–13759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li XQ, Cao XZ, Wang J, Fang B, Tan WF and
Ma H: Sevoflurane preconditioning ameliorates neuronal deficits by
inhibiting microglial MMP-9 expression after spinal cord
ischemia/reper-fusion in rats. Mol Brain. 7:692014. View Article : Google Scholar
|
|
36
|
Zhang L, Li HZ, Gong X, Luo FL, Wang B, Hu
N, Wang CD, Zhang Z and Wan JY: Protective effects of Asiaticoside
on acute liver injury induced by lipopolysaccharide/D-galactosamine
in mice. Phytomedicine. 17:811–819. 2010. View Article : Google Scholar : PubMed/NCBI
|