Introduction
Liver fibrosis is the pathophysiological consequence
of the excessive accumulation of extracellular matrix (ECM)
proteins in response to chronic liver injury or disease (1,2).
Advanced liver fibrosis results in liver cirrhosis and can
eventually progress to liver failure and hepatocellular carcinoma,
diseases which have a poor outcome and high mortality (3,4). As
early liver fibrosis is asymptomatic, a large percentage of
patients present with advanced and irreversible liver fibrosis or
even cirrhosis at the time-point of diagnosis (5). Therefore, anti-fibrotic therapies
that are capable of halting or reversing the progression of liver
fibrosis in patients with advanced disease are urgently required
(5,6).
Liver fibrosis is a continuous remodeling process
involving numerous cells types, inflammatory cytokines and
signaling pathways (5,6). The key step in the genesis of liver
fibrosis is the activation of hepatic stellate cells (HSCs), which
are the primary source of ECM and are characterized by the
expression of α-smooth muscle actin (α-SMA) (1,4).
Following the activation of HSCs, a number of cytokines are
secreted to activate associated intracellular signaling pathways
and regulate liver fibrosis (6);
these secreted cytokines include transforming growth factor
(TGF)-β, tumor necrosis factor (TNF)-α, interferon (IFN)-γ,
adiponectin and leptin. Target signaling pathways include the
TGF-β/SMAD, TNF-α/NF-κB, leptin, IFN-γ/signal transducer and
activator of transcription 3, adipoR/mitogen-activated protein
kinase and peroxisome proliferator-activated receptor-α signaling
pathways. These signaling pathways are all potential targets for
anti-fibrotic treatments.
Resveratrol is a plant-derived polyphenol that has
anti-oxidant and anti-inflammatory properties (7–9).
Evidence has suggested that resveratrol protects against heart
diseases (10), autoimmune
diseases (11), skin disorders
(12), diabetes (13) and numerous cancer types (14). Furthermore, it has been evidenced
that resveratrol protects against numerous liver diseases,
including alcoholic fatty liver disease (15,16),
non-alcoholic fatty liver disease (17), high-fat diet-induced fatty liver
(18), liver fibrosis (19,20)
and hepatocellular carcinoma (9).
It is thought that resveratrol primarily prevents liver damage by
increasing the hepatic glutathione content, scavenging free
radicals and inhibiting the expression or activity of inflammatory
factors, including TNF-α and NF-κB (19–22).
It has been reported that resveratrol can prevent
liver fibrosis by inhibiting the activity of NF-κB (19); however, the mechanisms by which
resveratrol modulates NF-κB have remained elusive. It has also
remained elusive whether other signaling pathways are involved in
the preventive effects of resveratrol against liver fibrosis and
the implication of these signaling pathways in the pathology of
liver fibrosis. The present study used a mouse model of carbon
tetrachloride (CCL4)-induced liver fibrosis to study the
inhibitory effects of resveratrol on liver fibrosis and to reveal
the underlying mechanisms.
Materials and methods
Cell lines and treatments
The human stellate cell line LX-2 was obtained from
the Institute of Biochemistry and Cell Biology (Chinese Academy of
Sciences, Shanghai, China) and maintained in RPMI 1640 culture
medium (Invitrogen Life Technologies, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS; Invitrogen Life Technologies) and 1%
penicillin/streptomycin (Invitrogen Life Technologies) in 5%
CO2 at 37°C. Resveratrol was purchased from
Sigma-Aldrich (St. Louis, MO, USA). A stock solution of resveratrol
in dimethylsulfoxide (DMSO; Aladdin Reagents Co., Ltd., Shanghai,
China) at a concentration of 100 mg/ml was prepared.
MTT assay
LX-2 cells were seeded at a density of
5×103 cells per well in 96-well plates. After 24 h,
various concentrations of resveratrol were added to the wells (0,
3.125, 6.25, 12.5, 25.0, 50.0, 75.0, 100 and 125 µg/ml) and
the plates were incubated for 72 h. After treatment, MTT
(Sigma-Aldrich) was added to each well at a final concentration of
0.5 mg/ml. Plates were incubated at 37°C for an additional 4 h.
After incubation, the supernatant was removed and the cells were
lysed in 150 µl DMSO. Absorbance of the blue formazan
derivative was measured at 570 nm using a microplate reader (VICTOR
X Multilabel; PerkinElmer, Waltham, MA, USA). All measurements were
performed in triplicate and all experiments were repeated three
times.
Immunofluorescence assay
Cells were seeded in 96-well plates at a density of
5×103 cells per well. After 24 h, the cells were
incubated for 48 h with resveratrol (0, 10, 20 and 50
µg/ml). Cells were then fixed at room temperature (RT) in 4%
paraformaldehyde (Aladdin Reagents Co., Ltd.) for 20 min,
permeabilized at RT in 0.1% Triton-X 100 (Sigma-Aldrich) in 0.01 M
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) for 10 min and then blocked at RT in 5% horse
serum in PBS for 20 min. After blocking, the cells were incubated
at 4°C overnight with a primary antibody against α-SMA (1:400,
rabbit polyclonal; cat no. ab5694; Abcam, Cambridge, MA, USA).
After the overnight incubation, cells were washed three times with
PBS for 10 min each prior to incubation for 30 min at RT with Alexa
488-conjugated secondary antibody (1:800; goat anti-rabbit; Sangon
Biotech Co. Ltd, Shanghai, China). Cells were then washed with PBS
and the nuclei were counterstained with DAPI (Invitrogen Life
Technologies) in PBS for 10 min at RT. Immunofluorescently labelled
cells were observed and images were captured under a fluorescence
microscope (BX71; Olympus, Tokyo, Japan) equipped with a DP70
digital camera (Olympus). All measurements were performed in
triplicate and all experiments were repeated three times.
Flow cytometric analysis
Cells were seeded in 6-cm dishes at a density of
5×105 cells per well. After 24 h, the cells were
incubated with resveratrol (0, 10, 20 and 50 µg/ml) for 48
h. For fluorescence detection, a single-cell suspension was
prepared by treatment with 0.25% trypsin (Invitrogen Life
Technologies). Single cells were fixed in ice-cold methanol
(Aladdin Reagents Co., Ltd.) for 30 min and then washed three times
in PBS. For antibody staining, ~0.2×106 cells were
incubated with α-SMA primary antibody at 4°C for 1 h in independent
reactions. Afterwards, cells were washed three times with PBS
buffer, followed by incubation at 4°C for 30 min in the dark with
AlexaFluor 488-labeled rabbit-specific secondary antibody
(Invitrogen Life Technologies, Inc.). Subsequently, cells were
washed and re-suspended in 0.2 ml sheath fluid. Flow-cytometric
analysis was performed using a BD FACSCalibur fluorescence-assisted
cell sorting machine (BD Biosciences, Franklin Lakes, NJ, USA)
using FlowJo software 7.6 (FlowJo LLC, Ashland, OR, Canada). All
measurements were performed in triplicate and all experiments were
repeated three times.
Animals and liver fibrosis model
Male C57BL/6 mice (weight, 20–25 g; age, 8–12 weeks;
n=25) were obtained from the Animal Division of Fudan University,
Shanghai Medical College (Shanghai, China). The mice were
maintained at 24°C on a 12-h light/dark cycle and had access to
rodent chow and water ad libitum. All experimental
procedures were approved by the Ethics Committee for Animal Care of
Fudan University (Shanghai, China). Mice were randomly divided into
five groups, including a normal group, a resveratrol (50 mg/kg)
treatment group, a CCl4 (Aladdin Reagents Co., Ltd.)
treatment group, a combined resveratrol (20 mg/kg) plus
CCl4 treatment group and a combined resveratrol (50
mg/kg) plus CCl4 treatment group. Resveratrol was
dissolved in 2% DMSO and saline prior to administration. To induce
liver fibrosis, mice were intraperitoneally injected with 0.3 ml/kg
CCl4 (mixed 1:1 with vegetable oil) twice a week for
four weeks and then once a week for the following four weeks. In
the combined resveratrol plus CCl4 treatment groups,
mice were given an intragastric administration of resveratrol (20
or 50 mg/kg) everyday, and were also intraperitoneally injected
with CCl4 three times per week, for a total
co-administration time of eight weeks. Resveratrol dosages were
selected according to guidelines established by previous studies
(18,23). Mice were sacrificed at eight weeks
and blood samples were collected for serum biochemistry. The liver
was dissected, weighed, frozen in liquid nitrogen and stored at
−80°C until analysis.
ELISA
Serum levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and TNF-α were determined using
ELISAs according the manufacturer's instructions. The ELISA kit for
ALT, AST and TNF-α was obtained from Shanghai Kemin Bioscience Ltd.
(Shanghai, China). All measurements were performed in triplicate
and all experiments were repeated three times.
Western blot analysis
Cells and tissues were homogenized in a commercial
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China).
For isolation of cytoplasmic and nuclear fractions, all tissue
samples were processed using a Cytoplasmic/Nuclear Extraction kit
(Fermentas, Thermo Scientific, Pittsburg, PA, USA) according to the
manufacturer's instructions. Protein concentrations were quantified
using a bicinchoninic acid protein assay kit (Sangon Biotech Co.,
Ltd.) with bovine serum albumin (Wuhan Boster Biological
Technology, Ltd.) as the standard. Protein was denatured by heating
at 100°C for 5 min and the cellular debris was removed by
centrifuging at 12,000 × g for 10 min. Equal amounts of protein (30
µg) were loaded and subjected to 10% SDS-PAGE followed by
electotransfer onto a nitrocellulose membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with Tris-buffered
saline containing 0.1% Tween-20 (TBST; Sigma-Aldrich) and 5% (w/v)
non-fat dry milk (Wuhan Boster Biological Technology, Ltd.) for 1
h. After blocking, the membranes were incubated overnight at 4°C
with primary antibodies against α-SMA (1:400; rabbit polyclonal;
cat. no. ab5694; Abcam), Collagen I (1:800; mouse monoclonal; cat.
no. ab6308; Abcam), NF-κB (1:200; rabbit polyclonal; cat. no.
ab7972; Abcam); IκB-α (1:500; mouse monoclonal; cat. no. sc-1643;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), pIκB-α (1:500;
mouse monoclonal; cat. no. sc-101713; Santa Cruz Biotechnology,
Inc.), GAPDH (1:10,000; rabbit monoclonal; cat. no. ab181603;
Abcam), p65 (1:500; rabbit polyclonal; cat. no. ab7970; Abcam),
α-tubulin (1:500; rabbit polyclonal; cat. no. ab126165; Abcam),
β-tubulin (1:1,000; rabbit monoclonal; cat. no. ab179513; Abcam).
After washing with TBST, the membranes were incubated with constant
agitation with horseradish peroxidase-conjugated secondary
antibodies (Sangon Biotech Co., Ltd.) at a dilution of 1:2,000 at
RT for 1 h. The membranes were visualized using an enhanced
chemiluminescence kit (Pierce Biotechnology, Rockford, IL, USA)
following the manufacturer's instructions. Chemiluminescent signals
were captured digitally using a chemiluminescence imaging system
(Shanghai Clinx Science Instruments, Shanghai, China). The
intensity of each band was quantified using ImagePro Plus 6.0
software (Media Cybernetics, Rockville, MD, USA). All measurements
were performed in triplicate and all experiments were repeated
three times.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Data were analyzed using SPSS 20 (IBM, Armonk, NY, USA).
Comparisons between groups were performed using analysis of
variance with Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol decreases the expression of
α-SMA in LX-2 cells
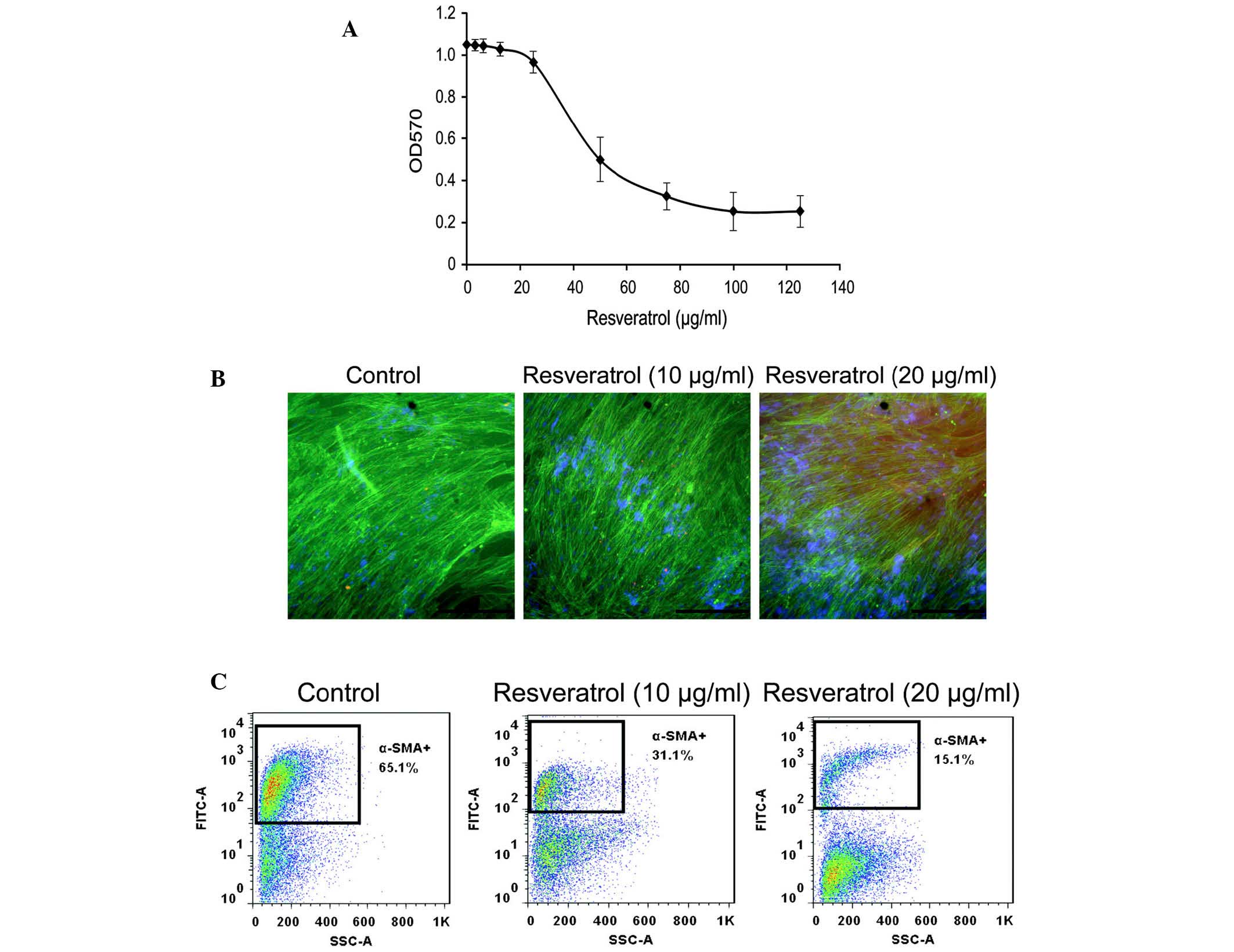
The cytotoxicity of resveratrol in LX-2 cells was
determined using an MTT assay. Resveratrol was not cytotoxic below
a concentration of 20 µg/ml. The IC50-value of
resveratrol on LX-2 cells was 51.8 µg/ml (Fig. 1A). Therefore, the non-cytotoxic
concentrations of 10 and 20 µg/ml were selected as the
experimental conditions of all subsequent experiments.
The effects of resveratrol on α-SMA expression in
LX-2 cells was determined using immunofluorescence and flow
cytometric analyses. Immunofluorescent microscopic observation
revealed that resveratrol (20 µg/ml) markedly decreased
α-SMA expression (Fig. 1B).
Similarly, flow cytometric analysis demonstrated that resveratrol
treatment decreased the expression of α-SMA in LX-2 cells (Fig. 1C). The percentage of α-SMA-positive
cells was 65.1, 31.0 and 15.1% when cells were treated with 0, 10
and 20 µg/ml resveratrol, respectively. These results
demonstrated that resveratrol decreased α-SMA expression in LX-2
cells.
Resveratrol reduces the expression of
liver fibrosis markers in a CCl4-induced mouse model of
liver fibrosis
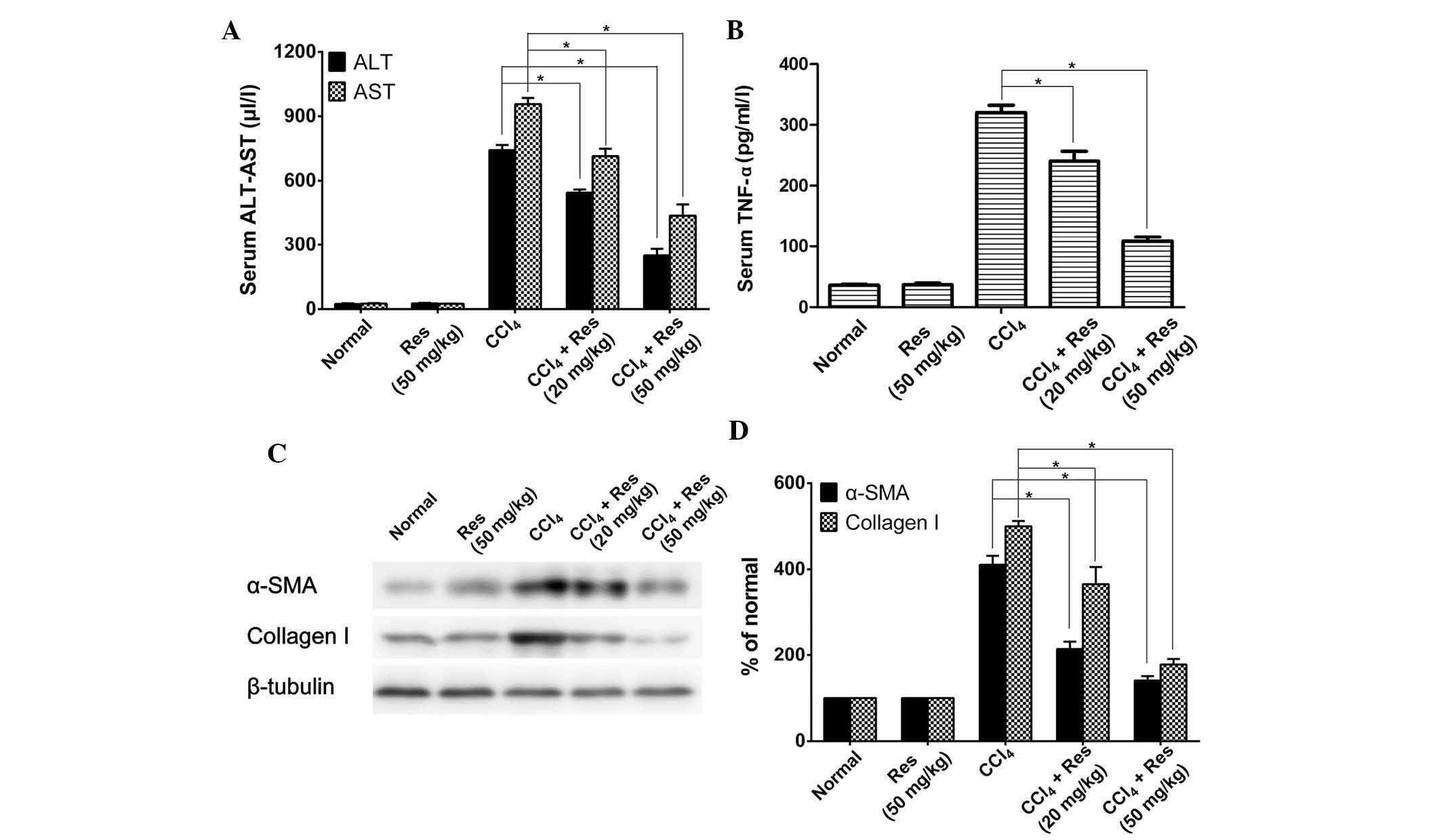
To investigate the potential anti-liver fibrosis
activity of resveratrol in vivo, the present study used a
CCl4-induced mouse model of liver fibrosis. Serum levels
of ALT and AST were determined in animals treated with or without
CCl4 and resveratrol. As shown in Fig. 2A, CCl4-induced mice had
significantly higher levels of ALT and AST when compared with those
in the untreated control mice. Resveratrol treatment decreased the
levels of ALT and AST in a dose-dependent manner in the
CCl4-induced mice; however, in mice that were not
treated with CCl4, resveratrol treatment had no effect
on ALT or AST expression.
Serum levels of the inflammatory factor TNF-α as
well as other markers of liver fibrosis were also detected. IThe
serum levels of TNF-α were significantly decreased by resveratrol
treatment in CCl4-induced mice when compared with those
in the untreated mice (Fig. 2B).
The effects of resveratrol treatment on the expression of the liver
fibrosis markers α-SMA and collagen-I were also examined. As shown
in Fig. 2C and D, the expression
of α-SMA and collagen-I was markedly increased in the
CCl4-induced mice, while resve-ratrol treatment
significantly inhibited the CCl4-induced increase of
α-SMA and collagen-I in a dose-dependent manner. Treatment with 50
µg/ml resveratrol decreased the expression levels of α-SMA
and collagen-I to almost basal levels of the normal control.
Resveratrol inhibits the activation of
NF-κB in a mouse model of CCl4-induced liver fibrosis
and LX-2 cells
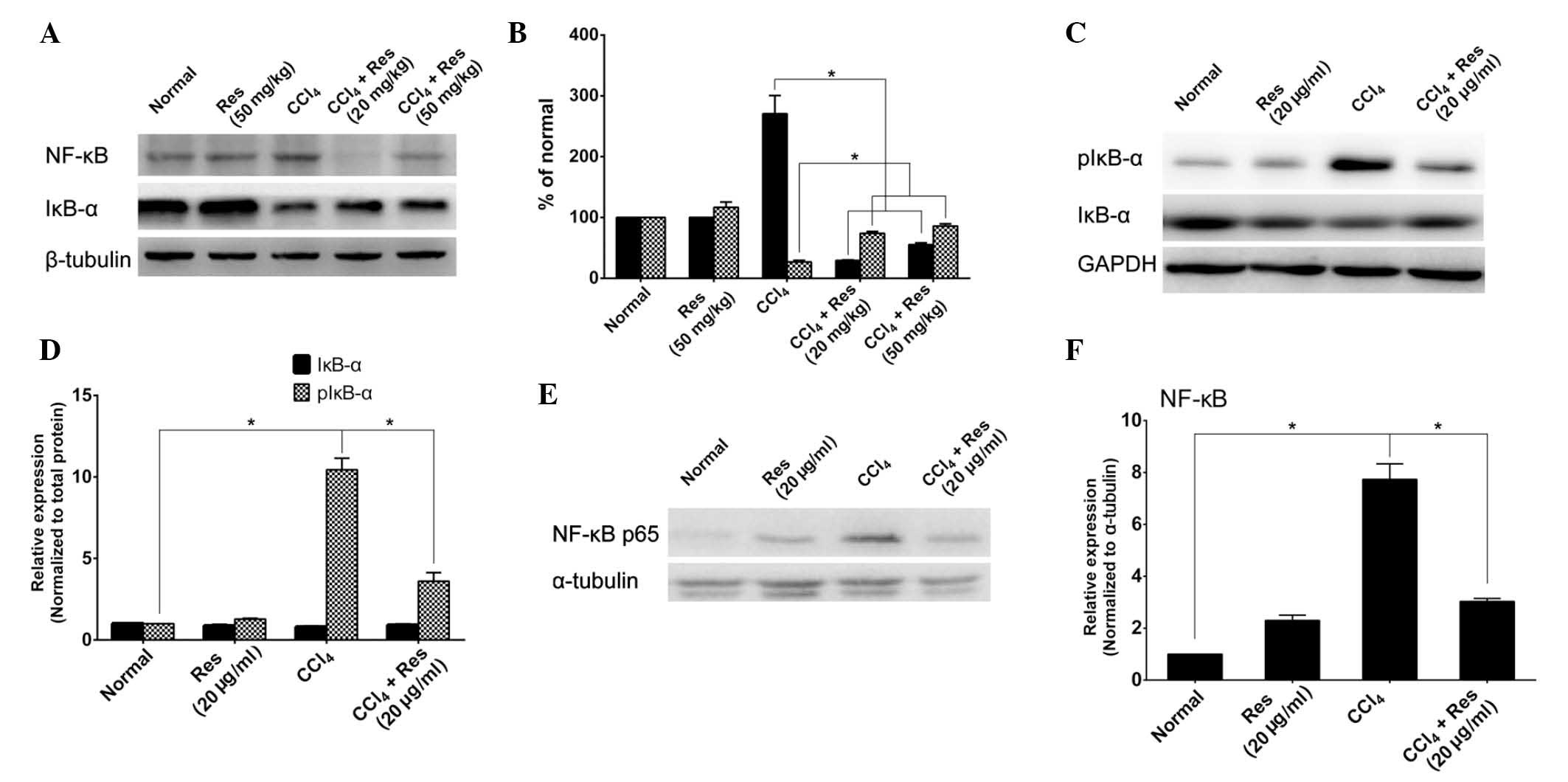
As it has been reported that resveratrol prevents
liver fibrosis by inhibiting the activity of NF-κB (19), the effects of resveratrol on NF-κB
activity were investigated in the mouse model of liver fibrosis as
well as in LX-2 cells. As NF-κB is activated through
phosphorylation of IκB and the translocation of p65 from the
cytoplasm to the nucleus (24),
the levels of IκB, pIκB and p65 were assessed. As shown in Fig. 3A and B, the expression of IκB-α was
markedly decreased in CCl4-induced mice; however,
resveratrol treatment partially rescued the expression of IκB-α.
Furthermore, NF-κB was markedly increased by CCl4
stimulation, while resveratrol treatment reduced NF-κB to levels
below those of the control group. In a further experiment, LX-2
cells were induced with CCl4. While resveratrol or
CCl4 treatment had no significant effects on the
expression of IκB-α, the levels of pIκB-α were markedly increased
in CCl4-induced cells, which was attenuated by
resveratrol treatment (Fig. 3C and
D). Furthermore, the expression of NF-κB in
CCl4-induced LX-2 cells was assessed (Fig. 3E and F). The levels of the NF-κB
p65 sub-unit were markedly increased in the nuclei of
CCl4-induced cells, which was significantly inhibited by
resveratrol treatment. All of these results suggested that
resveratrol inhibits the activation of NF-κB during liver
fibrosis.
Resveratrol inhibits the activation of
Akt in LX-2 cells
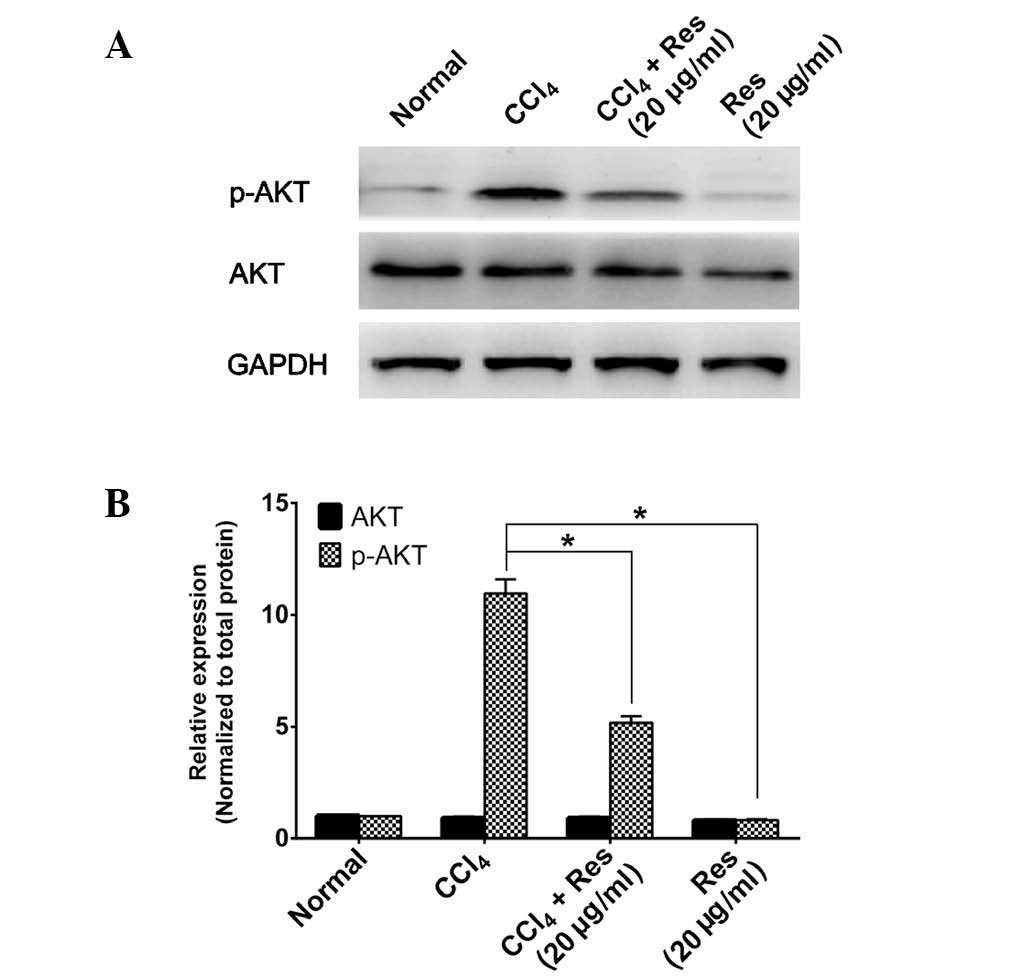
The effects of resveratrol on Akt were also examined
in vitro. As shown in Fig. 4A
and B, neither CCl4-induced nor resveratrol-treated
cells showed a change in total Akt expression compared with that in
the control group. However, Akt phosphorylation was markedly
increased in CCl4-induced cells, which was attenuated by
resveratrol treatment. These results indicated that resveratrol
inhibited the activation of Akt during liver fibrosis.
Discussion
The present study reported that resveratrol
downregulated the expression of α-SMA in HSCs. Furthermore,
resveratrol decreased the serum levels of ALT, AST and TNF-α, and
the protein expression of α-SMA and collagen-I in a mouse model of
liver fibrosis. Furthermore, resveratrol inhibited the activation
of NF-κB and Akt during liver fibrosis.
Liver fibrosis is a dynamic wound-healing response
to chronic liver injury that can result in serious and
life-threatening consequences for affected patients; however, it
has been indicated that even advanced fibrosis is a potentially
reversible process (2). Activation
of HSCs is the initial step in the process of liver fibrosis and is
characterized by the expression of α-SMA (1). Downregulation of α-SMA expression is
widely thought to be a promising potential method of liver fibrosis
inhibition (25). Using
immunofluorescence and flow cytometry, the present study revealed
that resveratrol decreased the expression of α-SMA in HSCs,
indicating that resveratrol inhibited liver fibrosis. Furthermore,
resveratrol decreased serum levels of ALT, AST and TNF-α as well as
the expression of α-SMA and collagen-I in a CCl4-induced
mouse model of liver fibrosis. The results of the present study
were in accordance with those of previous studies and indicate that
resveratrol inhibits liver fibrosis (19,20).
Inflammation is an integral part of the
wound-healing response in the liver and chronic inflammation is
tightly associated with liver fibrosis (26). The NF-κB signaling pathway is a
highly evolutionarily conserved pathway that has a pivotal role in
the regulation of immune and inflammatory responses (24). In accordance with this known
function, the NF-κB signaling pathway appears to have a central
role in liver homeostasis (24).
The NF-κB family of proteins are Rel family proteins. They are
transcription factors that can exist as either heterodimers or
homodimers, and they regulate the transcription of genes with the
common κB binding motif. There are five DNA-binding Rel family
sub-units: p50, p52, cRel, p65 and RelB. The most common form of
NF-κB is the p50:p65 heterodimer. NF-κB is activated through two
different pathways - the classical pathway, which depends on the
phosphorylation of IκB and the translocation of p65 from cytoplasm
to nucleus, and the non-canonical pathway, which is based on the
inducible processing of NF-κB2/p100 to p52:RelB (24,26).
It was recently reported that inhibition of NF-κB alleviated
CCl4-induced liver fibrosis via suppression of activated
HSCs (27).
A previous study postulated that the prevention of
liver fibrosis by resveratrol is likely to be associated with its
ability to reduce NF-κB activation (19). While this previous study observed
DNA-binding activity of NF-κB in liver tissue, it did not elucidate
the regulatory mechanism of NF-κB activation. The present study
demonstrated that in the CCl4-induced mouse model of
liver fibrosis, resveratrol attenuated the fibrosis-induced
decrease in IκB-α expression and the increase in NF-κB expression.
Furthermore, in activated LX-2 cells, resveratrol attenuated the
CCl4-induced increase in pIκB-α levels and inhibited the
nuclear translocation of NF-κB p65. These results indicated that
resveratrol reduces liver fibrosis via the inhibition of NF-κB
through the classical pathway.
A recent study showed that NF-κB is inhibited by the
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway during
liver fibrosis (27). The PI3K/Akt
signaling pathway has a critical role in cell growth and survival
(28). PI3K and Akt are also
involved in the activation of innate immune cells via the
regulation of key inflammatory cytokines (29). Accumulating evidence indicated that
the de-regulation of the PI3K/AKT pathway in hepatocytes is a
common molecular event in liver diseases (28). Liver-specific activation of the
PI3K/Akt pathway promotes cytokine production and regulates the
liver's early regenerative response (30). Therefore, the present study
investigated a possible link between the PI3K/Akt signaling pathway
and the effects of resveratrol in CCl4-induced cells.
The results showed that the phosphorylation of Akt was increased in
activated LX-2 cells, and that treatment with resveratrol reversed
this activation. This result suggested that the PI3K/Akt signaling
pathway is involved the protective effects of liver fibrosis by
resveratrol.
In conclusion, the present study indicated that
resveratrol may help prevent CCl4-induced liver fibrosis
and that this effect is associated with the inhibition of Akt as
well as NF-κB activation. This mechanism may provide promising
potential targets in the treatment of human liver fibrosis. Further
study is required to verify the ability of resveratrol to prevent
or possibly reverse liver fibrosis in vivo.
Acknowledgments
The authors would like to thank Medjaden Bioscience
Ltd. (Hong Kong, China) for assisting in the preparation of this
manuscript.
References
|
1
|
Moreira RK: Hepatic stellate cells and
liver fibrosis. Arch Pathol Lab Med. 131:1728–1734. 2007.PubMed/NCBI
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Svegliati-Baroni G, De Minicis S and
Marzioni M: Hepatic fibrogenesis in response to chronic liver
injury: Novel insights on the role of cell-to-cell interaction and
transition. Liver Int. 28:1052–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar
|
|
5
|
Schuppan D and Kim YO: Evolving therapies
for liver fibrosis. J Clin Invest. 123:1887–1901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman SL: Evolving challenges in
hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 7:425–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bishayee A, Darvesh AS, Politis T and
McGory R: Resveratrol and liver disease: From bench to bedside and
community. Liver Int. 30:1103–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishayee A, Politis T and Darvesh AS:
Resveratrol in the chemoprevention and treatment of hepatocellular
carcinoma. Cancer Treat Rev. 36:43–53. 2010. View Article : Google Scholar
|
|
10
|
Raj P, Louis XL, Thandapilly SJ, Movahed
A, Zieroth S and Netticadan T: Potential of resveratrol in the
treatment of heart failure. Life Sci. 95:63–71. 2014. View Article : Google Scholar
|
|
11
|
Petro TM: Regulatory role of resveratrol
on Th17 in autoimmune disease. Int Immunopharmacol. 11:310–318.
2011. View Article : Google Scholar
|
|
12
|
Ndiaye M, Philippe C, Mukhtar H and Ahmad
N: The grape antioxidant resveratrol for skin disorders: Promise,
prospects and challenges. Arch Biochem Biophys. 508:164–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciddi V and Dodda D: Therapeutic potential
of resveratrol in diabetic complications: In vitro and in vivo
studies. Pharmacol Rep. 66:799–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: A review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bujanda L, García-Barcina M, Gutiérrez-de
Juan V, Bidaurrazaga J, de Luco MF, Gutiérrez-Stampa M, Larzabal M,
Hijona E, Sarasqueta C, Echenique-Elizondo M and Arenas JI: Effect
of resveratrol on alcohol-induced mortality and liver lesions in
mice. BMC Gastroenterol. 6:352006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasdallah-Grissa A, Mornagui B, Aouani E,
Hammami M, El May M, Gharbi N, Kamoun A and El-Fazaâ S:
Resveratrol, a red wine polyphenol, attenuates ethanol-induced
oxidative stress in rat liver. Life Sci. 80:1033–1039. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heeboll S, Thomsen KL, Pedersen SB,
Vilstrup H, George J and Grønbæk H: Effects of resveratrol in
experimental and clinical non-alcoholic fatty liver disease. World
J Hepatol. 6:188–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi YJ, Suh HR, Yoon Y, Lee KJ, Kim DG,
Kim S and Lee BH: Protective effect of resveratrol derivatives on
high-fat diet induced fatty liver by activating AMP-activated
protein kinase. Arch Pharm Res. 37:1169–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chavez E, Reyes-Gordillo K, Segovia J,
Shibayama M, Tsutsumi V, Vergara P, Moreno MG and Muriel P:
Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta
increases induced by chronic CCl4 treatment in rats. J Appl
Toxicol. 28:35–43. 2008. View
Article : Google Scholar
|
|
20
|
Hong SW, Jung KH, Zheng HM, Lee HS, Suh
JK, Park IS, Lee DH and Hong SS: The protective effect of
resveratrol on dimethylnitrosamine-induced liver fibrosis in rats.
Arch Pharm Res. 33:601–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sener G, Toklu HZ, Sehirli AO,
Velioğlu-Oğünç A, Cetinel S and Gedik N: Protective effects of
resveratrol against acetaminophen-induced toxicity in mice. Hepatol
Res. 35:62–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kasdallah-Grissa A, Mornagui B, Aouani E,
Hammami M, Gharbi N, Kamoun A and El-Fazaa S: Protective effect of
resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol
Alcohol. 41:236–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andrade JM, Paraíso AF, de Oliveira MV,
Martins AM, Neto JF, Guimarães AL, de Paula AM, Qureshi M and
Santos SH: Resveratrol attenuates hepatic steatosis in high-fat fed
mice by decreasing lipogenesis and inflammation. Nutrition.
30:915–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun B and Karin M: NF-kappaB signaling,
liver disease and hepatoprotective agents. Oncogene. 27:6228–6244.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abergel A, Sapin V, Dif N, Chassard C,
Darcha C, Marcand-Sauvant J, Gaillard-Martinie B, Rock E,
Dechelotte P and Sauvant P: Growth arrest and decrease of alpha-SMA
and type I collagen expression by palmitic acid in the rat hepatic
stellate cell line PAV-1. Dig Dis Sci. 51:986–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luedde T and Schwabe RF: NF-κB in the
liver-linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Liu S, Du T, Chen H, Li Z and Yan
J: NF-κB inhibition alleviates carbon tetrachloride-induced liver
fibrosis via suppression of activated hepatic stellate cells. Exp
Ther Med. 8:95–99. 2014.PubMed/NCBI
|
|
28
|
Matsuda S, Kobayashi M and Kitagishi Y:
Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic
fatty liver disease. ISRN Endocrinol. 2013:4724322013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weichhart T and Süemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67(Suppl 3): iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson LN, Larson SD, Silva SR, Rychahou
PG, Chen LA, Qiu S, Rajaraman S and Evers BM: PI3K/Akt activation
is critical for early hepatic regeneration after partial
hepatectomy. Am J Physiol Gastrointest Liver Physiol.
294:G1401–G1410. 2008. View Article : Google Scholar : PubMed/NCBI
|