Introduction
Gastric cancer (GC) is one of the most common causes
of cancer-associated mortality worldwide, and often progresses to
peritoneal metastasis (1).
Numerous genetic and epigenetic alterations occur in the process of
carcinogenesis and the progression of GC (2). The severity of GC may be associated
with activation of oncogenes, inactivation of tumor suppressor
genes, and deregulation of growth factors or their receptors. CDC42
is a small guanosine triphosphatase (GTPase) of the Rho family,
which regulates the cell migration of various types of human
cancer. At present, the role of cell division cycle 42 (CDC42) in
the development of GC remains unclear.
Previous studies have reported the incidence of
dysregulated expression of Rho GTPase family members and their
effector proteins in various types of cancer, thus suggesting that
these proteins may be involved in cancer initiation, progression
and metastasis (3,4). CDC42 is a member of the Rho family of
small GTPases, and is considered a molecular switch of cell
differentiation. CDC42 converts from its inactive GDP-bound form to
the active GTP-bound form in response to diverse stimuli (5). The exact role of CDC42 in GC has yet
to be elucidated; however, it is considered to positively regulate
cancer cell growth, migration and invasion (6). A previous study demonstrated that a
marked decrease in the levels of active CDC42 was correlated with
the highly invasive potential of cell lines established from
metastatic sites of colorectal adenocarcinoma (7). Furthermore, CDC42 has been shown to
exert negative regulatory effects on intrinsic migration/invasion,
and induce potentially relevant changes in the phosphorylation of
protein kinase C δ, extracellular signal-regulated kinases 1/2 and
protein kinase A in aggressive breast cancer cells (8). CDC42 has been implicated to have an
important role in colon and breast cancer; however, the regulatory
effects and the underlying mechanisms of CDC42 in GC remain
unknown. Based on previous studies (9,10),
the authors of the present study hypothesize that CDC42 may have an
important role in GC metastasis and participate in regulating the
migration and invasion of GC cells. In addition, CDC42 expression
may be correlated with GC metastasis. The present study aimed to
examine the association between CDC42 and the biological behavior
of GC. These results may improve understanding regarding the
mechanism underlying the invasion and metastasis of GC.
Materials and methods
Cell culture and reagents
The AGS and SGC7901 human GC cell lines were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100
IU/ml penicillin and 100 µg/ml streptomycin (Invitrogen) at
37°C in an atmosphere containing 5% CO2.
Small interfering RNA (siRNA) synthesis
and transient transfection
siRNA duplex oligonucleotides specifically targeting
the human CDC42 cDNA sequence were synthesized, as follows: Forward
5′-GAAACUUGCCAAGAACAAAUU-3′ and reverse 5′-UUUGUUCUUGGCAAGUUUCUU-3′
(siCDC42). As a control, the following random siRNA sequences were
used (Santa Cruz Biotechnology, Inc., Dallas, TX, USA): Forward
5′-UUCUCCGAACGUGUCACGU-3′ and reverse 5′-ACGUGACACGUUCGGAGAA-3′
(siCon). Transient transfection of siCDC42 or siCon was performed
using Lipofectamine® 2000 (Invitrogen), according to the
manufacturer's instructions. The cells were seeded at
1×105/dish and cultured for 48 h prior to transfection
with 100 nM siRNA for 48 h.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Prior to transfection (1 day), 1×105
cells in 100 µl growth medium were seeded in 96-well culture
plates. The cells were transfected with siCDC42 or siCon at a final
concentration of 100 nM. At 24, 48 or 72 h post-transfection, 100
µl sterile MTT stock solution (1 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) was added to each well. Following a 4 h incubation
at 37°C, the MTT solution was replaced with 200 µl dimethyl
sulfoxide, followed by incubation for 8 h. The absorbance was
measured at a wavelength of 570 nm using a micro-enzyme-linked
immunosorbent assay reader (Multiskan MK3; Thermo Labsystems,
Franklin, MA, USA). Each sample was evaluated in triplicate.
Cell cycle analysis
The cells (1×105 cells/dish) were plated
in 96-well plates and cultured for 24 h, before being transfected
with 100 nM siCDC42 or siCon for 48 h. For flow cytometry, the
cells were trypsinized (Amresco, Solon, OH, USA), pelleted by
centrifugation (100 × g for 5 min) and resuspended in 0.3 ml of
0.1% Triton X-100 (Sigma-Aldrich)/phosphate-buffered saline.
Subsequently, the cells were treated with RNase Type I-A
(Sigma-Aldrich) at 37°C for 15 min and stained with prop-idium
iodide (Invitrogen) for 10 min. Cellular DNA content was determined
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA). Cell cycle distribution was analyzed using ModFit LT (version
3.0; BD Biosciences) cell cycle analysis software. The experiment
was performed three times, with each sample evaluated in
triplicate.
Wound healing assay
The cells (5×105 cells/well) were
transfected with 100 nM siCDC42 or siCon for 24 h, and were plated
onto 6-well plates. Once the cells had reached 90% confluence, a
single wound was created by gently scratching the attached cells
with a sterile plastic pipette tip. The cells were then washed with
serum-free medium, and wounded cell monolayers were allowed to heal
for 24 h. Images were captured under an optical microsope (Nikon,
Tokyo, Japan) The experiment was performed three times, with each
sample evaluated in triplicate.
In vitro invasion assay
The cells (1×105) were transfected with
100 nM siCDC42 or siCon for 24 h, and were then plated onto the
upper side of BioCoat Matrigel Invasion Chambers (BD Biosciences).
RPMI-1640 medium supplemented with 10% FBS was added to the lower
chamber as a chemoattractant. Following a 24 h incubation, the
cells on the upper surface of the Matrigel membrane were removed,
and the invasive cells on the lower surface of the membrane were
stained with 0.2% crystal violet (Invitrogen) in 10% ethanol. Five
independent fields of invasive cells per well were observed under a
phase contrast microscope (TS100; Nikon), and images were captured.
The number of cells per field was counted and averaged. The
experiment was performed three times, with each sample evaluated in
triplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen). First-strand cDNA was
synthesized using 2.5 µg RNA and AMV retroviridase (Promega
Corporation, Madison, WI, USA). RT-qPCR was performed using the
Bio-Rad iCycler iQ™ Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the following primers:
CDC42, forward 5′-ACATCTGTTTGTGGATAACTCA-3′, reverse
5′-GGGAGCCATATACTCTTGGA-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-CCACCCATGGCAAATTCCATGGCA-3′, and
reverse 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. The PCR mixture was
prepared using SYBR Master mix (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's protocol. To ensure
that only the specific gene was amplified, a melting curve analysis
was conducted at the end of each PCR experiment. Expression levels
of each mRNA were determined using the ΔΔCq method, with
GAPDH used as an endogenous control.
Western blot analysis
For western blotting, the cells were lysed with
Tris-HCl for 30 min on ice. The protein concentrations were
determined using a bicinchoninic acid protein assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). The cell lysates (50
µg protein/lane) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were transferred to
nitrocellulose membranes (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA). The membranes were then blocked with 5% (v/v)
skimmed milk and probed with primary antibodies at 4°C overnight.
Following washing with phosphate-buffered saline, the membranes
were incubated with polyclonal rabbit anti-mouse horseradish
peroxidase-conjugated secondary antibodies (1:1,000 dilution;
sc-358943; Santa Cruz Biotechnology, Inc.) at room temperature for
1 h. The following primary antibodies, all polyclonal mouse
anti-human purchased from Santa Cruz Biotechnology, Inc., were used
in the present study: Anti-cyclin A (1:200 dilution; sc-271682),
anti-cyclin B1 (1:300 dilution; sc-245), anti-cyclin D1 (1:300
dilution; sc-70899), anti-cyclin E (1:300 dilution; sc-247),
anti-proliferating cell nuclear antigen (PCNA; 1:300 dilution;
sc-71858), anti-matrix metalloproteinase 9 (MMP9; 1:300 dilution;
sc-21733), anti-α-tubulin (1:300 dilution; sc-23950), anti-CDC42
(1:250 dilution; sc-390210) and anti-β-actin (1:1,000 dilution;
sc-8432). The bound antibodies were visualized using an enhanced
chemiluminescence system (GE Healthcare Life Sciences, Chalfont,
UK).
Statistical analysis
All experiments were performed three times, with
each sample evaluated in triplicate. SPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA) was used for all statistical analyses. The data
were analyzed by Student's t-test, and are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of siCDC42
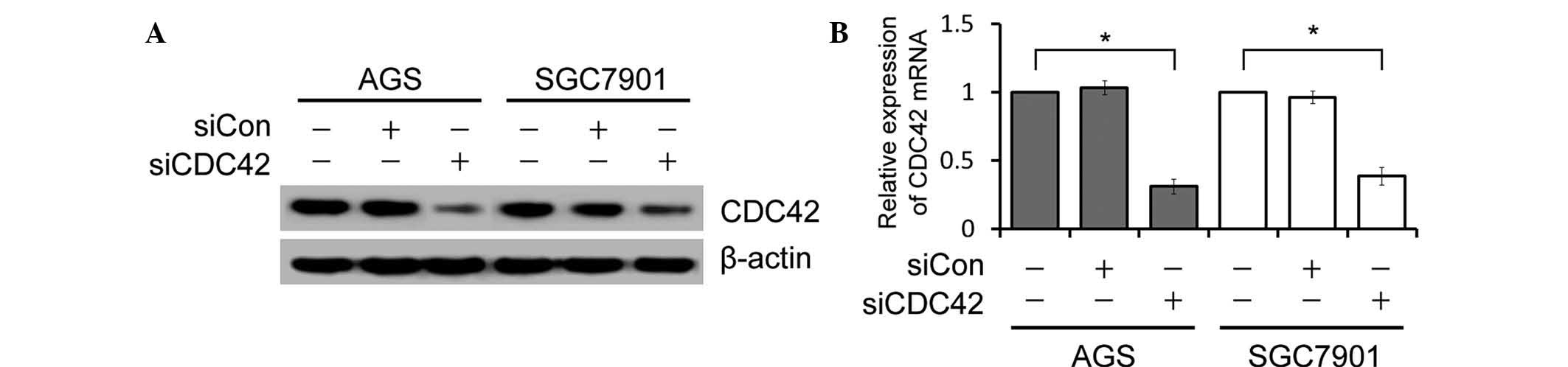
In order to elucidate the functional role of CDC42
in GC, the present study examined the effects of CDC42 expression
knockdown on GC cell growth in vitro. AGS and SGC7901 cells
were transfected with siCDC42 or siCon. Based on their high
expression of CDC42, the human AGS and SGC7901 cell lines were
selected for use as cell models in the present study. siCDC42
effectively suppressed the protein and mRNA expression levels of
CDC42 in the GC cells, as determined by western blotting and
RT-qPCR (Fig. 1).
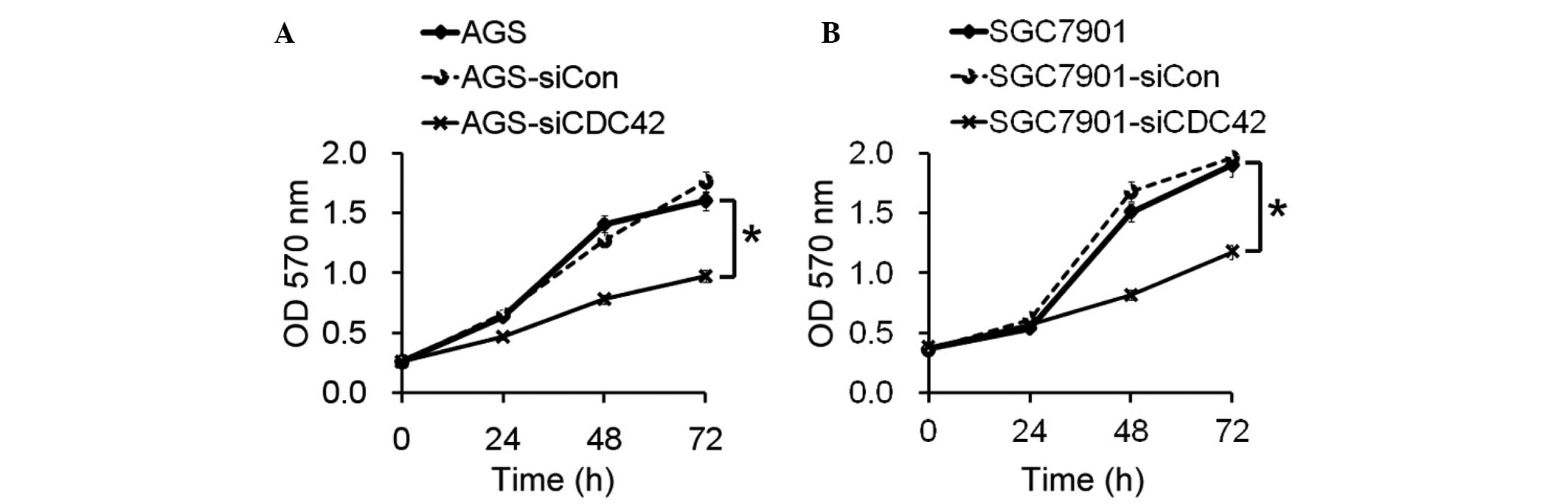
Role of CDC42 in growth of GC cells
The present study aimed to determine the biological
role of CDC42 in the growth of GC cells. The proliferative ability
of the AGS and SGC7901 cells transfected with siCDC42 was
significantly decreased, as compared with the siCon-transfected
cells (Fig. 2).
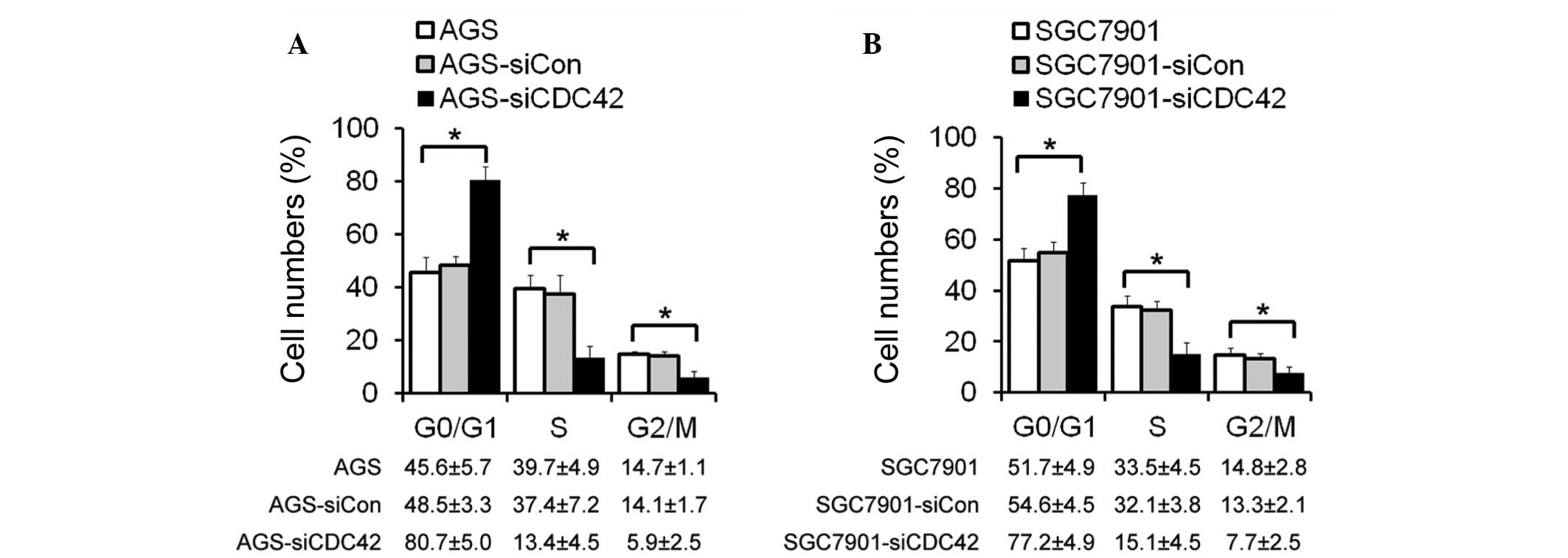
Cell cycle distribution following siCDC42
transfection
The mechanisms underlying the effects of CDC42 on
cell growth inhibition were also investigated. Cell cycle
distribution of the AGS and SGC7901 cells was determined by flow
cytometry. The number of cells in G0/G1 phase was increased, and
the proportion of cells in S phase and G2/M phase was decreased in
the siCDC42-transfected AGS and SGC7901 cells, as compared with the
control cells (Fig. 3). These
results suggest that the inhibitory effects of CDC42 knockdown on
GC cell growth may be mediated by cell cycle arrest at G1/S
phase.
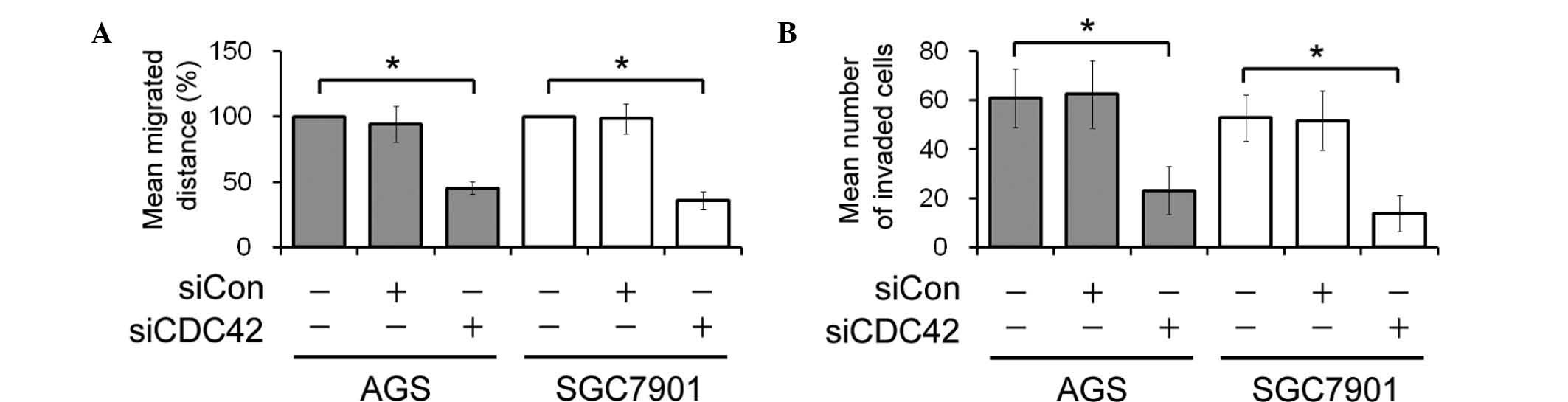
Migration and invasion of GC cells
following siCDC42 transfection
In vitro wound healing and invasive assays
were performed to determine the effects of CDC42 knockdown on GC
cell migration and invasion. The rate of wound closure in the
siCDC42-transfected AGS and SGC7901 cells was delayed, as compared
with the control cells (Fig. 4A).
In addition, the number of invasive siCDC42-transfected AGS and
SGC7901 cells was lower, as compared with the siCon-transfected AGS
and SGC7901 cells (Fig. 4B).
Effect of siCDC42 on cell cycle protein
levels
In order to investigate which signaling pathway
mediated the suppressive effects of CDC42 on cell cycle arrest,
cell cycle-related proteins, including cyclin A, cyclin D1, cyclin
E, PCNA and MMP9 were detected by western blotting. As shown in
Fig. 5, the protein expression
levels of cyclin A, cyclin D1, cyclin E, PCNA and MMP9 were
significantly reduced in the siCDC42-transfected cells, as compared
with the siCon-transfected cells. However, the expression levels of
cyclin B1 were not changed between the groups.
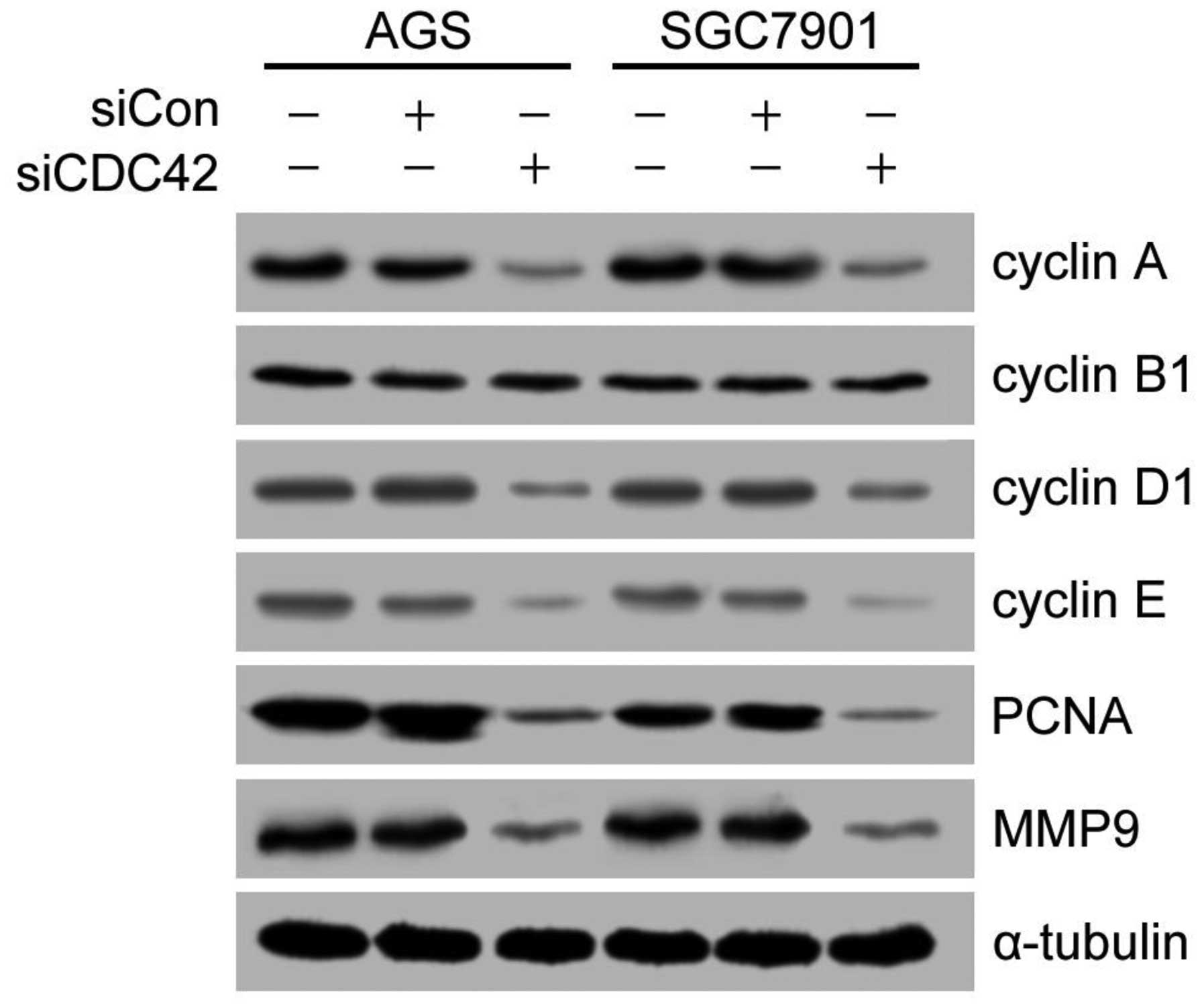 | Figure 5Effects of CDC42 knockdown on the
expression levels of cell cycle related-proteins and MMP9 in AGS
and SGC7901 human gastric cancer cells. The protein expression
levels of cyclin A, cyclin B1, cyclin D1, cyclin E, PCNA, MMP9 and
α-tubulin were detected by western blotting. α-tubulin was used as
the loading control. si, small interfering RNA; CDC42, cell
division cycle 42; Con, control; PCNA, proliferating cell nuclear
antigen; MMP9, matrix metalloproteinase 9. |
Discussion
Previous studies have demonstrated that CDC42 is
over-expressed in lung (11–13),
breast (14), testicular (15), colorectal (16) and esophageal (17) cancer. However, the effects and
underlying mechanisms of CDC42 on GC remain unclear.
The present study investigated the effects of CDC42
on the proliferation, migration and invasion of GC cells. The
results demonstrated that inhibition of CDC42 expression, via
siCDC42 transfection, inhibited the proliferation, migration and
invasion of AGS and SGC7901 GC cells. These findings suggested that
CDC42 may be a potential target for GC treatment.
The present study aimed to investigate the molecular
mechanisms underlying the effects of CDC42 knockdown on the
inhibition of GC cell proliferation. The results of the western
blot and MTT assays revealed that CDC42 knockdown inhibited the
cell proliferation. In addition, in order to confirm whether cell
cycle-associated proteins regulate the effects of CDC42 on GS-cell
proliferation, the expression levels of cell cycle-regulatory
proteins, including cyclin A, cyclin D1 and cyclin E, were
downregulated in the siCDC42-transfected cells.
PCNA is a homotrimeric protein, which is essential
for cell cycle progression, replication and DNA repair (18). A previous study indicated that PCNA
functions as a proliferation marker, since it is expressed in late
G1 phase and early S phase of the cell cycle (18). In the present study, knockdown of
CDC42 resulted in the downregulation of PCNA. Therefore, the
inhibitory effects of CDC42 knockdown may be mediated by cell cycle
arrest at G1/S phase.
Metastasis refers to the spread of cancer from the
original tumor site to other parts of the body, and is a
characteristic of malignant cancer (19). A previous study demonstrated that
CDC42 is an important regulator of metastasis in human cancer
(3). MMPs degrade components of
the extracellular matrix, and are involved in the regulation of
development, growth and spread of primary tumors (20). The expression levels of MMP9, an
important member of the MMP family, are significantly higher in GC
tissue (63.0%), as compared with in normal tissue (6.7%), and the
expression of MMP9 is associated with tumor size, depth of
invasion, lymph node metastasis, degree of histological
differentiation and pathological stage (21). In the present study, the expression
of MMP9 was attenuated following transfection with siCDC42. These
results indicated that the inhibitory effects of CDC42 knockdown on
GC cell migration and invasion may be mediated by MMP9.
In conclusion, the present study demonstrated that
siCDC42-induced suppression of CDC42 inhibited GC cell
proliferation by arresting cells at G1/S phase of the cell cycle,
and reducing the expression of cyclin A, cyclin D1, cyclin E and
PCNA. In addition, siCDC42 was shown to inhibit the migration and
invasion of GC cells via downregulation of MMP9. These data suggest
that CDC42 may be considered a promising target for the effective
treatment of GC.
Abbreviations:
|
CDC42
|
cell division cycle 42
|
|
GC
|
gastric cancer
|
|
PCNA
|
proliferating cell nuclear antigen,
siRNA, small interfering RNA
|
|
FBS
|
fetal bovine serum
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
ECL
|
enhanced chemiluminescence
|
|
MMP9
|
matrix metalloproteinase 9
|
Acknowledgments
The present study was supported by the Chinese
National Natural Science Foundation (grant nos. 31100838 and
31571171), the Nanjing Medical University Science and Technology
Development Program (grant no. 09NJMUZ30), the Shanghai Natural
Science Foundation (grant no. 15ZR1414900) and the Young Teachers
of Shanghai Universities Training Program.
References
|
1
|
Krejs GJ: Gastric cancer: Epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: Research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
4
|
Van der Meel R, Symons MH, Kudernatsch R,
Kok RJ, Schiffelers RM, Storm G, Gallagher WM and Byrne AT: The
VEGF/Rho GTPase signalling pathway: A promising target for
anti-angiogenic/anti-invasion therapy. Drug Discov Today.
16:219–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sinha S and Yang W: Cellular signaling for
activation of Rho GTPase Cdc42. Cell Signal. 20:1927–1934. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stengel K and Zheng Y: Cdc42 in oncogenic
transformation, invasion, and tumorigenesis. Cell Signal.
23:1415–1423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Toledo M, Anguille C, Roger L, Roux P
and Gadea G: Cooperative anti-invasive effect of Cdc42/Rac1
activation and ROCK inhibition in SW620 colorectal cancer cells
with elevated blebbing activity. PLoS One. 7:e483442012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuo Y, Wu Y and Chakraborty C: Cdc42
negatively regulates intrinsic migration of highly aggressive
breast cancer cells. J Cell Physiol. 227:1399–1407. 2012.
View Article : Google Scholar
|
|
9
|
Lee YC, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011. View Article : Google Scholar
|
|
10
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen QY, Jiao DM, Yao QH, Yan J, Song J,
Chen FY, Lu GH and Zhou JY: Expression analysis of Cdc42 in lung
cancer and modulation of its expression by curcumin in lung cancer
cell lines. Int J Oncol. 40:1561–1568. 2012.PubMed/NCBI
|
|
12
|
Zhang JY, Zhang D and Wang EH:
Overexpression of small GTPases directly correlates with expression
of δ-catenin and their coexpression predicts a poor clinical
outcome in nonsmall cell lung cancer. Mol Carcinog. 52:338–347.
2013. View
Article : Google Scholar
|
|
13
|
Liu Y, Xu H, Liu N, Wang L and Wang E:
Correlation of expression of p120ctn, RhoA, and Cdc42 and their
significance in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi.
8:304–308. 2005.In Chinese. PubMed/NCBI
|
|
14
|
Jiang LC, Zhang Y and Qu XC: Effects of
Cdc42 overexpression on the estrogen-enhanced multidrug resistance
in breast cancer cells. Zhonghua Zhong Liu Za Zhi. 33:489–493.
2011.In Chinese. PubMed/NCBI
|
|
15
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gómez Del Pulgar T, Valdés-Mora F, Bandrés
E, Pérez-Palacios R, Espina C, Cejas P, García-Cabezas MA, Nistal
M, Casado E, González-Barón M, et al: Cdc42 is highly expressed in
colorectal adenocarcinoma and downregulates ID4 through an
epigenetic mechanism. Int J Oncol. 33:185–193. 2008.PubMed/NCBI
|
|
17
|
Liu Z, Feng JG, Tuersun A, Liu T, Liu H,
Liu Q, Zheng ST, Huang CG, Lv GD, Sheyhidin I and Lu XM: Proteomic
identification of differentially-expressed proteins in esophageal
cancer in three ethnic groups in Xinjiang. Mol Biol Rep.
38:3261–3269. 2011. View Article : Google Scholar
|
|
18
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar :
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
21
|
Yang S, Zhao Z, Wu R, Lu H, Zhang X, Huan
C, Wang C, Wu X and Guan G: Expression and biological relationship
of vascular endothelial growth factor-A and matrix
metalloproteinase-9 in gastric carcinoma. J Int Med Res.
39:2076–2085. 2011. View Article : Google Scholar
|