Introduction
Due to the lack of vascularization in articular
cartilage and the low proliferative and migratory capacities of
chondrocytes, cartilage remains a challenging tissue to repair
(1–4). Current clinical treatment strategies
involve mosaicplasty, micro-fracture, periosteum or perichondrium
transplantation and fresh osteochondral allograft implantation
(5). These strategies appear
promising, however the long term outcomes are unsatisfactory
(3,6,7).
Tissue engineering has provided alternative possibilities for
hyaline cartilage repair using cell based therapy, utilizing
chondrocytes or adult stem cells combined with synthetic substrates
and bioactive factors to prepare for the functional replacement of
hyaline cartilage (8–10). Bioactive factors have been widely
utilized in cell based therapy to promote cell proliferation and
the production of the extracellular matrix, however current
therapeutic options are far from optimal and the anticipated
outcomes are rarely achieved (11). Overall, it remains to be fully
understood how improvements in chondrocyte amplification may be
achieved with the maintenance of the chondrocytic phenotype. The
aim is to generate active and phenotypically stabilized tissue
engineered cartilage in order to treat cartilage lesions.
Neuroleukin (NLK), a neurotrophic factor of spinal
and sensory neurons, is additionally a growth factor in mouse
salivary glands, and promotes the survival of peripheral and
central neurons in culture. NLK is additionally known as autocrine
motility factor (AMF) and phosphoglucose isomerase (PGI) (12,13),
where the peptide sequences of NLK, AMF and PGI indicate that they
are the same protein, with the differing names assigned based on
previous functional analyses. NLK is secreted by tumor cells and
functions as a cytokine to promote migration, invasion and
proliferation of tumor cells (14–16).
This paracrine function of NLK is further supported by studies on
the migration of vascular endothelial cells which propagate and
form new capillaries during tumor angiogenesis, in addition to the
proliferation and migration of fibroblasts treated with NLK
(17,18). Of note, Zhi et al (19) reported differential expression of
NLK in osseous tissue and during differentiation, with elevated
levels in superficial articular chondrocytes, proliferating
chondrocytes, fibroblasts and osteoblasts within the fracture
callus, however, NLF was absent in terminally differentiated
hypertrophic chondrocytes or osteocytes. These studies suggest that
NLK is involved in cartilage development and bone regeneration. In
the current study, the effect of NLK on the proliferation of
isolated rat articular chondrocytes was investigated in
vitro and the working concentrations of NLK were optimized
using a microfluidic device.
Materials and methods
Antibodies and reagents
The following antibodies were used in
immunofluorescent analyses: Mouse anti-collagen type II polyclonal
antibody (1:4,000; cat. no. BA0533; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), Alexa Fluor® 488 goat
anti-mouse secondary antibody (1:1,000; cat. no. A11001; Invitrogen
Life Technologies, Carlsbad, USA). Human recombinant NLK/PGI full
length protein was obtained from Abcam (Cambridge, UK; cat. no.
ab87625). Trypsin was purchased from Gibco Life Technologies
(Carlsbad, USA), collagenase type II from Sigma-Aldrich (St. Louis,
MO, USA), and the Cell Cycle Detection kit was from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China; cat. no. KGA511).
Chondrocyte isolation and cell
culture
All experimental procedures were approved by the
Institutional Committee of Animal Use and Protection (Dalian
Medical University, Dalian, China). A total of 12 Sprague-Dawley
rats were used in the present study, which were provided by the
Experimental Animal Center of the Dalian Medical University. The
rats were anesthetized with 3.6% chloral hydrate (1 ml/100 g;
Kermel, Tianjin, China) by intraperitoneal injection, prior to
sacrifice by cervical vertebra dislocation. The surrounding muscles
of the knee joints were removed to expose the articular cartilage,
which was collected using curved tissue scissors. Cartilage was
separated from the femoral heads and femoral condyles of 1
month-old male Sprague-Dawley rats and cut into 1.5 mm3
sections using a surgical blade in sterile phosphate-buffered
saline (PBS; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Primary chondrocytes were isolated by digestion
with 0.2% type II collagenase for 4 h at 37°C in an agitating water
bath and resuspended in Dulbecco's modified Eagle's medium/F-12 (GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (FBS; Gibco Life Technologies). Cells were cultured in
a humidified atmosphere at 37°C with 5% CO2.
Chondrocytes were trypsinized by 0.25% trypsin for subcultures.
Microfluidic device design and
fabrication
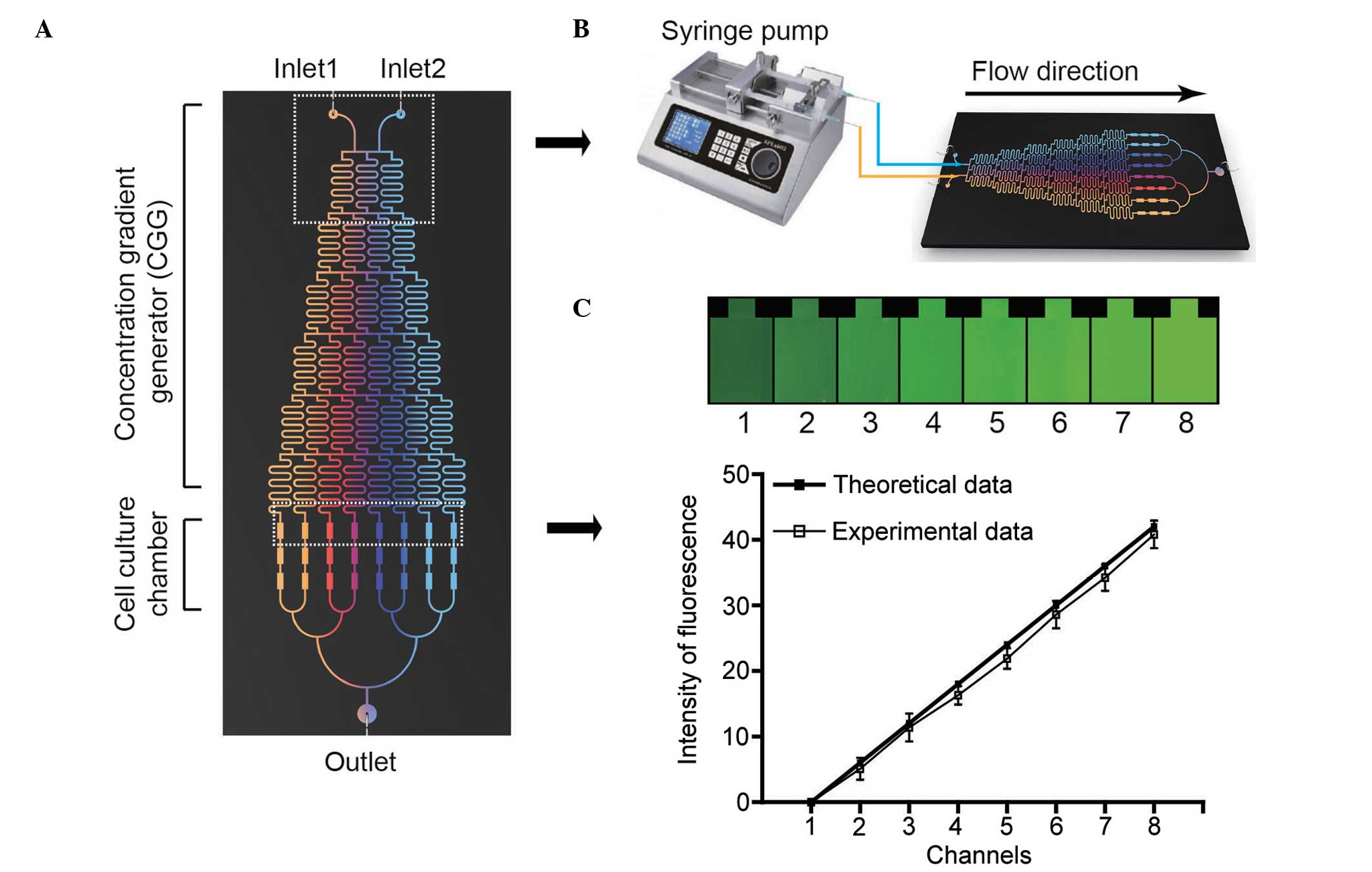
The microfluidic device in the current study
provided a pyramid shaped concentration gradient generator (CGG)
and a downstream cell culture module. The design and operation of
the microfluidic device are presented in Fig. 1. The device had two inlets, one
outlet and three cell culture chambers connected to resistance
channels. Each chamber was 100 µm in height, 400 µm
in width and 1,000 µm in length. A previous study observed
that the liquid exhibited laminar flow when it passed through the
serpentine channel of the microfluidic chip according to the
Reynolds effect when the capillary diameter was approximately 100
nm (20). All devices were
fabricated using conventional micro-fabrication techniques
involving SU-8 photolithography and polydimethylsiloxane (PDMS)
soft lithography. The transparency mask was designed by AutoCAD
2014 (Autodesk, Inc., San Rafael, CA, USA) and was used in 1:1
contact photolithography with an SU-8 photoresist consisting of
patterned photoresist on a silicon wafer. Positive replicas with
embossed channels were fabricated by molding PDMS against the
master. The PDMS replica and a clean glass substrate were
irreversibly sealed using oxygen plasma (2 Torr, 100 W) for 1
min.
Microfluidic chip operation
The inlets of the device were connected to a syringe
pump (model LSP02-1B; Baoding Longer Precision Pump Co., Ltd.,
Baoding, China) to drive fluid flow. The outlet of the device was
connected to a culture medium reservoir containing DMEM/F12.
Culture media with and without NLK were simultaneously infused into
the microfluidic device from the two inlets, with 2% serum
containing culture medium into the left and 2% serum medium with 30
ng/ml NLK into the right. The concentration gradient of NLK was
established within 30 sec. The flow speed was controlled at 0.1
ml/min. The device was stored in an incubator at 37°C with 5%
CO2 during the culture period.
CGG performance validation
By using this chip, solutions from inlets were
repeatedly split at the nodes, combined with neighboring streams in
a laminar fashion, and mixed by diffusion in serpentine channels.
As a result, the solutions were continuously diluted and a series
of concentrations was produced in the outlets of the CGG. In the
current study, solution A (concentration 0) and solution B
(concentration X) were injected into the CGG via the 2 inlets
simultaneously, and the concentrations in the 8 outlets were as
follows: 0, 1/7X, 2/7X, 3/7X, 4/7X, 5/7X, 6/7X and X. Fluorescein
isothiocyanate dextran (FITC-dextran) with a molecular weight of
20,000 Da (Sigma-Aldrich) was used as a probe for the CGG
performance validation according to a previous study (18). The fluorescence intensity of
FITC-dextran at the 8 outlets of the CGG were imaged by confocal
laser scanning microscopy (model TCSSP5; Leica Microsystems GmbH,
Wetzlar, Germany) and quantified using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA). The intensities were
compared with the theoretical values achieved by the equation, and
correlation factors were calculated. All the experiments were
repeated three times.
Cell cycle analysis
Cell cycle phase distribution was determined by flow
cytometry using propidium iodide (PI; Nanjing KeyGen Biotech Co.,
Ltd.) staining. For cell synchronization, cells were starved for 12
h prior to NLK treatment. Following 24 h culture in 2% FBS medium
and 24/48 h NLK treatment, cells were harvested, fixed in 70%
ice-cold ethanol for 12 h at 4°C, stained in 50 µg/ml PI
supplemented with 1 mg/ml RNase (Nanjing KeyGen Biotech Co., Ltd.)
and 0.1% Triton X-100 (Sigma-Aldrich), and analyzed by flow
cytometry (Accuri C6; BD Biosciences, Franklin Lakes, NJ, USA). The
histogram was used to present the percentage of cells in the
G1, S and G2/M phases. Cell cycle
distribution was analyzed by FlowJo 10.0.6 software (FlowJo, LLC.,
Ashland, OR, USA).
Immunofluorescence
Cells were seeded (0.8×105 cells/well) on
sterile glass coverslips (0.8×0.8 cm) and cultured in 6-well plates
until 70% confluent. Cells were washed twice with PBS, fixed with
4% formaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) for 15 min and permeabilized with 0.1% Triton X-100 for 15
min, prior to blocking with 5% bovine serum albumin (Beijing
Solarbio Science & Technology Co., Ltd.) in PBS for 1 h at room
temperature. Cells were incubated with primary antibodies overnight
at 4°C. Subsequently, cells were incubated with fluorescent
secondary antibodies at room temperature for 1 h prior to staining
with 4′,6-diamidino-2-phenylindole and imaging using a fluorescence
microscope (model BX53; Olympus Corporation, Tokyo, Japan).
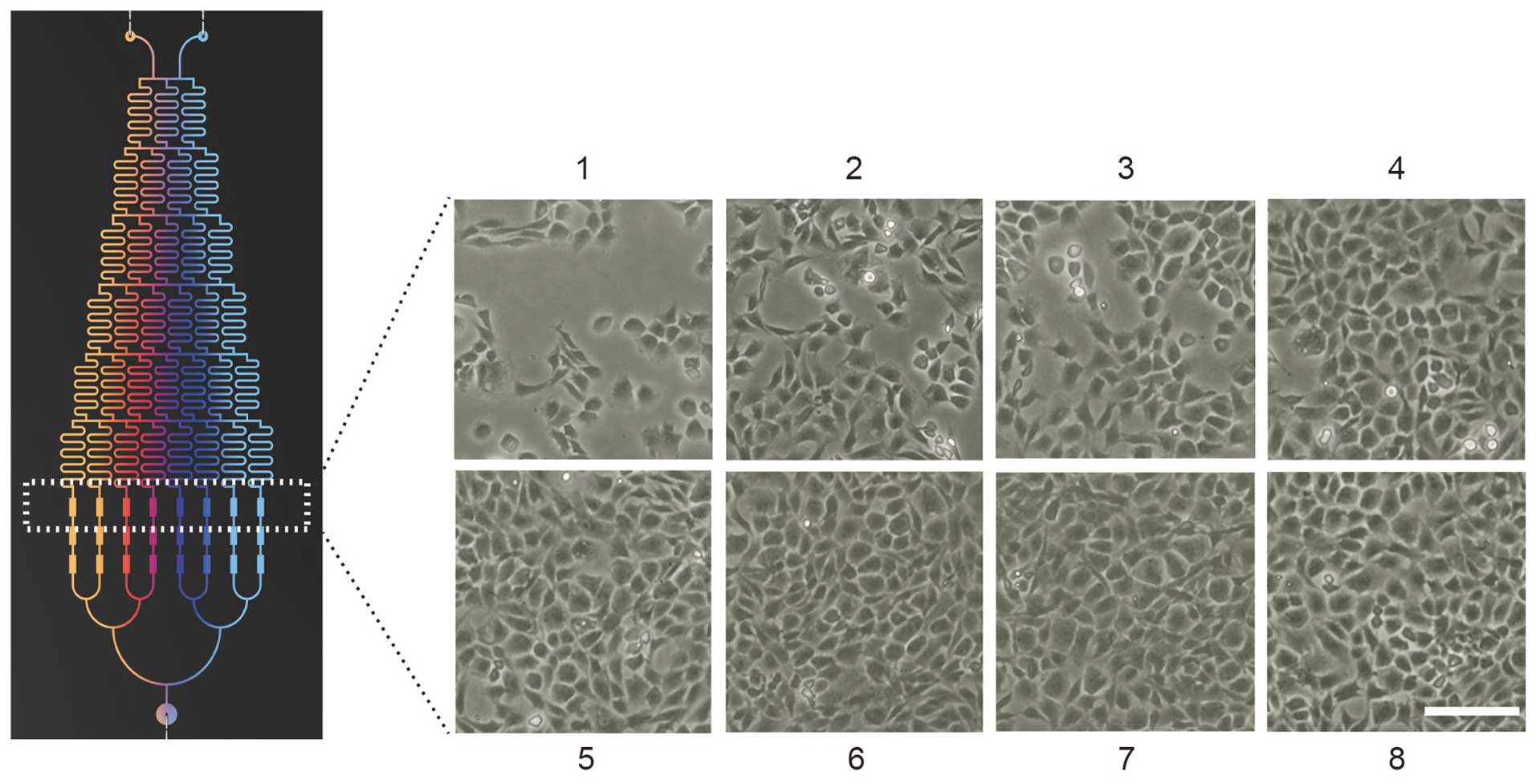
Observation of chondrocyte
morphology
After six days of culture in a microfluidic device
(designed and fabricated by Dalian University of Technology,
Dalian, China) with various concentrations of NLK (0, 4.28, 8.57,
12.85, 17.15, 21.42, 25.71 and 30 ng/ml), total chondrocyte numbers
were counted from several fields and morphological changes of the
chondrocytes were observed using a phase contrast microscope
(Olympus cx31; Olympus Corporation). Micrographs were captured from
the cell culture chambers of the eight groups.
Results
Microfluidic device design and gradient
generation
Dilution networks and micro-scale cell culture
chambers were incorporated into a single microfluidic device, which
is capable of producing multiple gradient concentrations for
on-chip cell culture assays. A series of solutions with different
concentrations was formed at the outlets of the CGG (0, 4.28, 8.57,
12.85, 17.15, 21.42, 25.71 and 30 ng/ml; Fig. 1A). The fluorescent probe
FITC-dextran was used as an indicator for estimating the gradient
produced by the CGG. From the two inlets, FITC-dextran was
simultaneously infused into the CGG by a syringe pump and the
resulting gradients (Fig. 1C) were
imaged and quantified. A total of eight FITC-dextran gradient
profiles were produced from gradient generators spanning the two
inlet concentrations (Fig. 1B).
The fluorescence intensities of FITC-dextran in the junctions
between the CGG and the cell culture chambers were quantified,
corrected by subtracting the background fluorescence and compared
with the theoretically obtained gradient. A good association
(correlation coefficient = 0.9953) was observed between the
experimental and theoretical data, as presented in Fig. 1C.
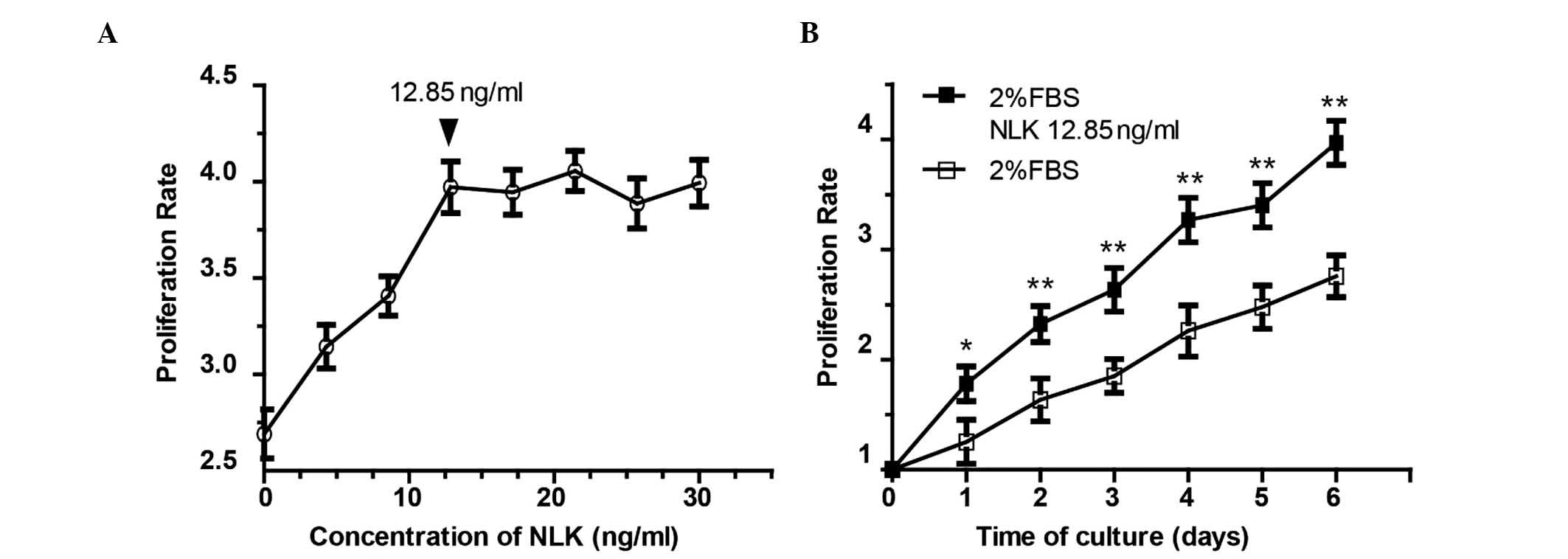
Microfluidic analyses of chondrocyte
proliferation with NLK
To evaluate the effect of NLK on chondrocyte
proliferation, isolated articular chondrocytes from Sprague-Dawley
rats were applied to the above-mentioned microfluidic chip with an
NLK concentration gradient. Following 6 days of culture, the
morphology of chondrocytes was imaged using phase contrast
microscopy (Fig. 2), and a
dose-dependent proliferative profile was observed following
treatment with NLK between 0 and 12.85 ng/ml (Fig. 3A). Culture medium with 2% serum was
used as the control. Among the eight concentrations (0, 4.28, 8.57,
12.85, 17.15, 21.42, 25.71 and 30 ng/ml), the proliferation rate
plateaued at 12.85 ng/ml. The 12.85 ng/ml NLK group exhibited a
3.96-fold increase in chondrocyte proliferation (Fig. 3A), with a statistically significant
difference compared with the control group (P<0.01). However,
there was no statistically significant difference between the 12.85
ng/ml NLK group and the higher concentrations (P>0.05). The
growth curve of the chondrocytes following 12.85 ng/ml NLK
stimulation is presented in Fig.
3B.
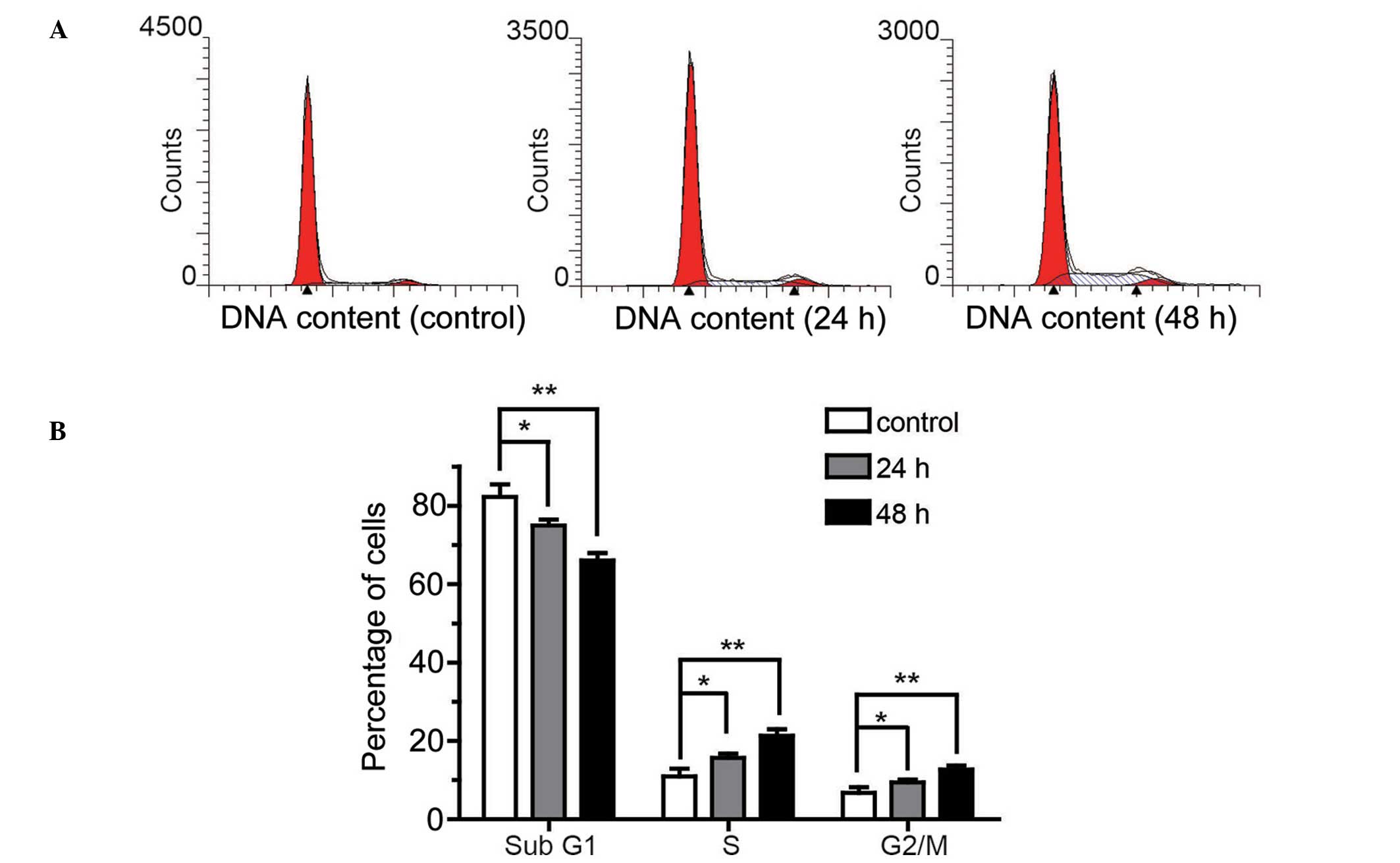
NLK increases the proportion of
proliferative state chondrocytes
To further characterize the pro-proliferative effect
of NLK on chondrocytes, flow cytometry was conducted to assess cell
cycle distribution. Chondrocytes were starved with serum-free
medium for 12 h to synchronize the cells, followed by treatment
with 2% FBS for 24 h and 12.85 ng/ml NLK with 2% FBS for 24 and 48
h. Cells were collected and analyzed post treatment. As presented
in Fig. 4A, an accumulation of
NLK-treated cells in the S phase of the cell cycle was observed
compared with the control cells at 24 and 48 h. In addition, the
G2/M phase distribution was increased following NLK
treatment. Quantification indicated that the percentage of sub
G1 phase cells was reduced from 82 to 61%, and the
proportion of cells in S and G2/M phases was
significantly greater in NLK-treated cells (P<0.01; Fig. 4B). These data further indicate that
NLK promotes proliferation of articular chondrocytes in
vitro.
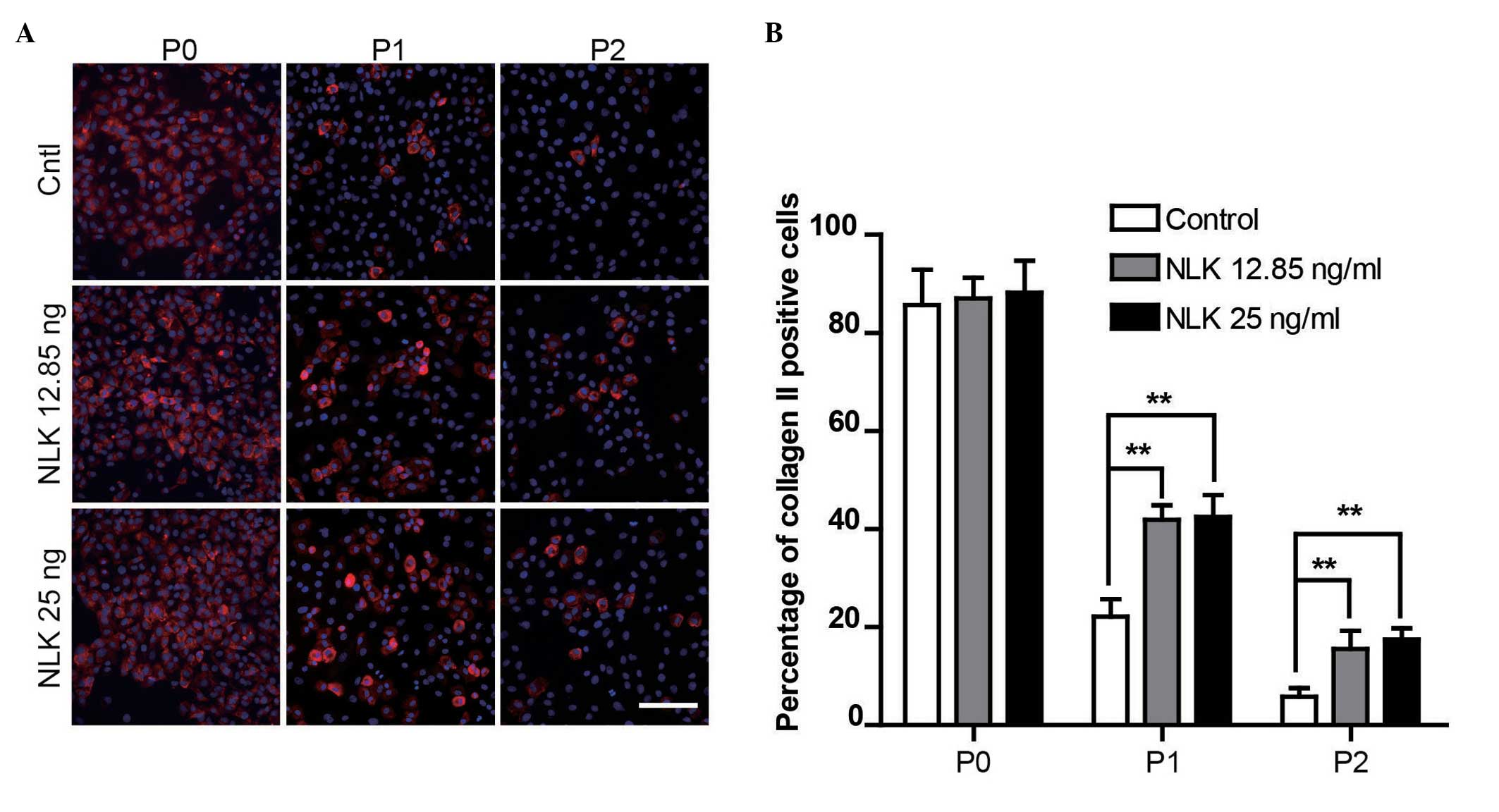
NLK stimulates type II collagen synthesis
in chondrocytes
As demonstrated above, NLK is able to serve as a
bioactive molecule for chondrocytes. Therefore, it was investigated
whether NLK is involved in phenotype maintenance of chondrocytes.
To determine the response of chondrocytes to NLK on type II
collagen synthesis during in vitro culture,
immunofluorescence analysis was conducted. Chondrocytes were
examined from passage 0 (P0; 24 h following isolation) to passage 2
(P2; 6–7 days following isolation) by staining with the type II
collagen antibodies. As presented in Fig. 5A, following 12 h serum-starvation,
P0, P1 and P2 chondrocytes were cultured with or without NLK (12.85
and 25 ng/ml), and cells with positive type II collagen stains were
counted. A reduction in the fluorescent signal of collagen II was
observed during prolonged culture in vitro due to the
dedifferentiation of primary chondrocytes, characterized by a
change from the expression and secretion of Collagen I instead of
Collagen II. A significant increase in collagen II positive cells
was observed following NLK treatment in P1 (22–41%) and P2 (5–17%)
groups compared with the control (without NLK). However, no
significant difference was observed in P0 or between NLK treatment
groups (Fig. 5B). A reduction in
the fluorescent signal was observed during prolonged culture in
vitro due to dedifferentiation of primary chondrocytes.
Discussion
Clinical management regarding cartilage repair may
be classified into three main categories: Marrow stimulation-based,
osteochondral transplantation, and cell-based repair techniques
(5). ACI is a cell-based tissue
regeneration technique, representing a novel biological approach
for the treatment of cartilage defects (21–23).
However, due to dedifferentiation of autologous chondrocytes during
expansion in vitro and following implantation, ACI may
subsequently result in scar tissue akin to fibrocartilage (22,24).
Therefore, studies have attempted to combine ACI with biomaterials
of natural or synthetic origin as scaffolds, together with the use
of bioactive factors providing proliferative stimuli, in order to
reconstruct a microenvironment in vitro which allows the
cells to grow as in their native tissue (25,26).
Bioactive factors function as key components in cell-based therapy.
Growth factors that exist in or are secreted by bone marrow
mesenchymal stem cells (BMSCs) and chondrocytes such as bone
morphogenic protein, vascular endothelial growth factor and
transforming growth factor-β do not only upregulate chondrogenic
markers, however, additionally are associated with ossification
(27–29). This may lead to the formation of
fibrocartilage with inferior mechanical properties and limited
durability (29,30).
NLK is a multifunctional protein with intra- and
extracellular functions (13,31,32).
It has been studied in neoplastic cells and their normal
counterpart cells for the expression, secretion and distribution,
suggesting that a wide range of normal and neoplastic cells express
and secrete NLK (33–35). Despite the extensive investigation
of the pro-metastatic effects in tumor cells, the role of NLK in
various normal tissues remains to be fully elucidated. The
expression of NLK has been reported to be associated with
cerebellar and hippocampal neurons, which are implicated in memory
and learning (36,37). NLK is reported to stimulate
migration, propagation and new capillary formation for human
umbilical vein endothelial cells (37,38).
In addition, fibroblasts gain increased proliferative and migratory
abilities following treatment with NLK (17). These results provide evidence that
NLK may act as a secreted factor to promote cell motility and
proliferation in normal tissues. In a previous study, differential
mRNA display was used and RNA populations isolated from osseous
tissues were compared, from which it was reported that NLK was
involved in cartilage development and bone regeneration (21). A high level of NLK expression was
identified in superficial articular chondrocytes in 1, 4 and
8-month-old normal mice, in addition to in proliferating
chondrocytes. However, in hypertrophic chondrocytes NLK is not
detected (19). Furthermore, NLK
expression was augmented during fracture healing. From day 3 post
fracture, strong NLK labeling was detected in osteoblasts of the
newly formed trabecular bone, proliferating chondrocytes of the
soft callus and fibrous periosteum. In the late stage of fracture
healing, immature osteocytes and proliferating chondrocytes had
differentiated towards mature osteocytes and hypertrophic
chondrocytes, when the expression of NLK was reduced (19). The spatial and temporal expression
of NLK in cartilage and osteoblast development indicates that NLK
serves a role during cartilage development.
Microfluidics, known as “lab-on-a-chip”, brings the
benefits of integration, miniaturization and automation to numerous
research areas (39,40). As a novel cell culture platform,
microfluidics provides spatial and temporal control of exogenous
stimuli via the integration of various functional units (41). Furthermore, microfluidic devices
have been successfully used for the long-term culture of cell lines
and primary cells (42,43). By using microfluidic chips,
experimental conditions are flexible and may be optimized by
changing either the type of growth factor or the concentration of
the input. Additionally, microfluidics make it possible to perform
parallel experiments to optimize conditions whilst using a limited
number of cells. A previous study reported data generated from a
microfluidic platform, which included the effects of fluid
mechanics on articular chondrocytes and BMSCs and the optimization
of biofactor concentrations (20,44).
As NLK is additionally implicated in tumor cell physiology, its
over-dosage during in vitro chondrocyte expansion may pose a
risk of adverse side effects. In order to maximize its positive
effects on chondrocyte proliferation while avoiding detrimental
side effects, the current study sought to use microfluidics to
optimize the concentration of NLK in chondrocyte in vitro
propagation. Using a microfluidic device, 8 concentrations of NLK
were generated precisely in 30 sec, and the effect of a range of
concentrations of NLK on chondrocyte proliferation was evaluated
over a 6 day period. A minimal concentration of 12.85 ng/ml NLK was
demonstrated to affect chondrocyte growth, leading to a ~4-fold
increase. This effect of NLK was further supported by the flow
cytometry results of cell cycle analysis (Fig. 4).
Dedifferentiation commonly occurs during in
vitro expansion of chondrocytes, during which they gradually
lose their typical round shape and acquire a spindly
fibroblast-like form, with concurrent reductions in cartilaginous
protein synthesis such as type II collagen (29,45,46).
In the current study, immunofluorescence was conducted to examine
the effect of NLK on the synthesis of type II collagen by
chondrocytes. Treatment with NLK leads to a significant increase in
the number of positively stained cells relative to untreated cells
following in vitro passaging, indicating an inhibitory
effect of NLK on dedifferentiation. The optimized concentration of
12.85 ng/ml was not significantly different compared with a
saturated concentration of 25 ng/ml, confirming the data from the
microfluidic device.
In the present study, NLK was reported to act as an
exogenous factor to promote the proliferation of articular
chondrocytes during in vitro culture. A microfluidic device
was used to evaluate a range of concentrations of NLK on
chondrocyte proliferation, with a concentration of 12.85 ng/ml
demonstrated to be optimal. Furthermore, this concentration of NLK
may additionally prevent dedifferentiation of cultured chondrocytes
in vitro. Future studies will aim to provide mechanistic
insights into NLK regulation of chondrocyte growth, in addition to
assessing the application of NLK during ACI.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81371706 and
81201212) and the Education Department of Liaoning Province (grant
no. L2014360). Professor Han Liu is funded by the “Climbing
Scholar” and “Excellent Talents” schemes of Liaoning Province,
China.
References
|
1
|
Wang Q, Huang C, Xue M and Zhang X:
Expression of endogenous BMP-2 in periosteal progenitor cells is
essential for bone healing. Bone. 48:524–532. 2011. View Article : Google Scholar :
|
|
2
|
Wilusz RE, Sanchez-Adams J and Guilak F:
The structure and function of the pericellular matrix of articular
cartilage. Matrix Biol. 39:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demoor M, Ollitrault D, Gomez-Leduc T,
Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F,
Mallein-Gerin F, et al: Cartilage tissue engineering: Molecular
control of chondrocyte differentiation for proper cartilage matrix
reconstruction. Biochim Biophys Acta. 1840:2414–2440. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y and Tuan RS: Origin and function
of cartilage stem/progenitor cells in osteoarthritis. Nat Rev
Rheumatol. 11:206–212. 2015. View Article : Google Scholar
|
|
5
|
Rodrigues MT, Gomes ME and Reis RL:
Current strategies for osteochondral regeneration: From stem cells
to pre-clinical approaches. Curr Opin Biotechnol. 22:726–733. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kock L, van Donkelaar CC and Ito K: Tissue
engineering of functional articular cartilage: The current status.
Cell Tissue Res. 347:613–627. 2012. View Article : Google Scholar :
|
|
7
|
van Osch GJ, Brittberg M, Dennis JE,
Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT and Luyten FP:
Cartilage repair: Past and future–lessons for regenerative
medicine. J Cell Mol Med. 13:792–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edwards PK, Ackland T and Ebert JR:
Clinical rehabilitation guidelines for matrix-induced autologous
chondrocyte implantation on the tibiofemoral joint. J Orthop Sports
Phys Ther. 44:102–119. 2014. View Article : Google Scholar
|
|
9
|
Yu SM and Kim SJ: The thymoquinone-induced
production of reactive oxygen species promotes dedifferentiation
through the ERK pathway and inflammation through the p38 and PI3K
pathways in rabbit articular chondrocytes. Int J Mol Med.
35:325–332. 2015.
|
|
10
|
Goessler UR, Hörmann K and Riedel F:
Tissue engineering with chondrocytes and function of the
extracellular matrix (Review). Int J Mol Med. 13:505–513.
2004.PubMed/NCBI
|
|
11
|
Foldager CB: Advances in autologous
chondrocyte implantation and related techniques for cartilage
repair. Dan Med J. 60:B46002013.PubMed/NCBI
|
|
12
|
Yanagawa T, Funasaka T, Tsutsumi S,
Watanabe H and Raz A: Novel roles of the autocrine motility
factor/phosphoglucose isomerase in tumor malignancy. Endocr Relat
Cancer. 11:749–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Funasaka T and Raz A: The role of
autocrine motility factor in tumor and tumor microenvironment.
Cancer Metastasis Rev. 26:725–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsutsumi S, Hogan V, Nabi IR and Raz A:
Overexpression of the autocrine motility factor/phosphoglucose
isomerase induces transformation and survival of NIH-3T3
fibroblasts. Cancer Res. 63:242–249. 2003.PubMed/NCBI
|
|
15
|
Shih WL, Liao MH, Lin PY, Chang CI, Cheng
HL, Yu FL and Lee JW: PI 3-kinase/Akt and STAT3 are required for
the prevention of TGF-beta-induced Hep3B cell apoptosis by
autocrine motility factor/phosphoglucose isomerase. Cancer Lett.
290:223–237. 2010. View Article : Google Scholar
|
|
16
|
Kho DH, Nangia-Makker P, Balan V, Hogan V,
Tait L, Wang Y and Raz A: Autocrine motility factor promotes HER2
cleavage and signaling in breast cancer cells. Cancer Res.
73:1411–1419. 2013. View Article : Google Scholar :
|
|
17
|
Tsutsumi S, Yanagawa T, Shimura T,
Fukumori T, Hogan V, Kuwano H and Raz A: Regulation of cell
proliferation by autocrine motility factor/phosphoglucose isomerase
signaling. J Biol Chem. 278:32165–32172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silletti S and Raz A: Autocrine motility
factor is a growth factor. Biochem Biophys Res Commun. 194:446–457.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhi J, Sommerfeldt DW, Rubin CT and
Hadjiargyrou M: Differential expression of neuroleukin in osseous
tissues and its involvement in mineralization during osteoblast
differentiation. J Bone Miner Res. 16:1994–2004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Qin J, Lin B and Zhang W: The
effects of insulin-like growth factor-1 and basic fibroblast growth
factor on the proliferation of chondrocytes embedded in the
collagen gel using an integrated microfluidic device. Tissue Eng
Part C Methods. 16:1267–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spahn G, Kahl E, Muckley T, Hofmann GO and
Klinger HM: Arthroscopic knee chondroplasty using a bipolar
radiofrequency-based device compared to mechanical shaver: Results
of a prospective, randomized, controlled study. Knee Surg Sports
Traumatol Arthrosc. 16:565–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gelse K, von der Mark K, Aigner T, Park J
and Schneider H: Articular cartilage repair by gene therapy using
growth factor-producing mesenchymal cells. Arthritis Rheum.
48:430–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haene R, Qamirani E, Story RA, Pinsker E
and Daniels TR: Intermediate outcomes of fresh talar osteochondral
allografts for treatment of large osteochondral lesions of the
talus. J Bone Joint Surg Am. 94:1105–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nukavarapu SP and Dorcemus DL:
Osteochondral tissue engineering: Current strategies and
challenges. Biotechnol Adv. 31:706–721. 2013. View Article : Google Scholar
|
|
25
|
Chan BP and Leong KW: Scaffolding in
tissue engineering: General approaches and tissue-specific
considerations. Eur Spine J. 17(Suppl 4): 467–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng CW, Solorio LD and Alsberg E:
Decellularized tissue and cell-derived extracellular matrices as
scaffolds for orthopaedic tissue engineering. Biotechnol Adv.
32:462–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krejci P, Masri B, Fontaine V, Mekikian
PB, Weis M, Prats H and Wilcox WR: Interaction of fibroblast growth
factor and C-natriuretic peptide signaling in regulation of
chondrocyte proliferation and extracellular matrix homeostasis. J
Cell Sci. 118:5089–5100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phornphutkul C, Wu KY, Yang X, Chen Q and
Gruppuso PA: Insulin-like growth factor-I signaling is modified
during chon-drocyte differentiation. J Endocrinol. 183:477–486.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bobick BE and Kulyk WM: The MEK-ERK
signaling pathway is a negative regulator of cartilage-specific
gene expression in embryonic limb mesenchyme. J Biol Chem.
279:4588–4595. 2004. View Article : Google Scholar
|
|
30
|
Gikas PD, Bayliss L, Bentley G and Briggs
TW: An overview of autologous chondrocyte implantation. J Bone
Joint Surg Br. 91:997–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Araki K, Shimura T, Yajima T, Tsutsumi S,
Suzuki H, Okada K, Kobayashi T, Raz A and Kuwano H: Phosphoglucose
isomerase/autocrine motility factor promotes melanoma cell
migration through ERK activation dependent on autocrine production
of interleukin-8. J Biol Chem. 284:32305–32311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niizeki H, Kobayashi M, Horiuchi I,
Akakura N, Chen J, Wang J, Hamada JI, Seth P, Katoh H, Watanabe H,
et al: Hypoxia enhances the expression of autocrine motility factor
and the motility of human pancreatic cancer cells. Br J Cancer.
86:1914–1919. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Timar J, Trikha M, Szekeres K, Bazaz R,
Tovari J, Silletti S, Raz A and Honn KV: Autocrine motility factor
signals integrin-mediated metastatic melanoma cell adhesion and
invasion. Cancer Res. 56:1902–1908. 1996.PubMed/NCBI
|
|
34
|
Niinaka Y, Paku S, Haga A, Watanabe H and
Raz A: Expression and secretion of neuroleukin/phosphohexose
isomerase/maturation factor as autocrine motility factor by tumor
cells. Cancer Res. 58:2667–2674. 1998.PubMed/NCBI
|
|
35
|
Dobashi Y, Watanabe H, Matsubara M,
Yanagawa T, Raz A, Shimamiya T and Ooi A: Autocrine motility
factor/glucose-6-phosphate isomerase is a possible predictor of
metastasis in bone and soft tissue tumours. J Pathol. 208:44–53.
2006. View Article : Google Scholar
|
|
36
|
Luo Y, Long JM, Lu C, Chan SL, Spangler
EL, Mascarucci P, Raz A, Longo DL, Mattson MP, Ingram DK and Weng
NP: A link between maze learning and hippocampal expression of
neuroleukin and its receptor gp78. J Neurochem. 80:354–361. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Cheng XR, Zhang GR, Zhou WX and
Zhang YX: Autocrine motility factor receptor is involved in the
process of learning and memory in the central nervous system. Behav
Brain Res. 229:412–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Funasaka T, Haga A, Raz A and Nagase H:
Tumor autocrine motility factor is an angiogenic factor that
stimulates endothelial cell motility. Biochem Biophys Res Commun.
285:118–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhise NS, Ribas J, Manoharan V, Zhang YS,
Polini A, Massa S, Dokmeci MR and Khademhosseini A: Organ-on-a-chip
platforms for studying drug delivery systems. J Control Release.
190:82–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Björnmalm M, Yan Y and Caruso F:
Engineering and evaluating drug delivery particles in microfluidic
devices. J Control Release. 190:139–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye N, Qin J, Shi W, Liu X and Lin B:
Cell-based high content screening using an integrated microfluidic
device. Lab Chip. 7:1696–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kane BJ, Zinner MJ, Yarmush ML and Toner
M: Liver-specific functional studies in a microfluidic array of
primary mammalian hepatocytes. Anal Chem. 78:4291–4298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tourovskaia A, Figueroa-Masot X and Folch
A: Differentiation-on-a-chip: A microfluidic platform for long-term
cell culture studies. Lab Chip. 5:14–19. 2005. View Article : Google Scholar
|
|
44
|
Zhong W, Tian K, Zheng X, Li L, Zhang W,
Wang S and Qin J: Mesenchymal stem cell and chondrocyte fates in a
multishear microdevice are regulated by Yes-associated protein.
Stem Cells Dev. 22:2083–2093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sanz-Ramos P, Duart J, Rodríguez-Goñi MV,
Vicente-Pascual M, Dotor J, Mora G and Izal-Azcárate I: Improved
Chondrogenic Capacity of Collagen Hydrogel-Expanded Chondrocytes:
In Vitro and in Vivo Analyses. J Bone Joint Surg Am. 96:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bobick BE, Chen FH, Le AM and Tuan RS:
Regulation of the chondrogenic phenotype in culture. Birth Defects
Res C Embryo Today. 87:351–371. 2009. View Article : Google Scholar : PubMed/NCBI
|