Introduction
Rho-associated coiled-coil kinase (ROCK) is a
serine/threonine kinase comprising two isoforms, ROCK1 and ROCK2.
The small Rho G-protein activates ROCK, which phosphorylates
several downstream targets (1,2). The
Rho/ROCK signaling pathway has been shown to contribute to a
variety of cell functions, including smooth muscle contraction,
stress fiber formation, cell proliferation and cell migration
(3–5). The expression of ROCK1 is distributed
in the kidneys, liver, lungs, spleen and testes, whereas ROCK2 is
particularly expressed in the heart and brain (1,2,6). The
pharmacological inhibition of ROCK indicates that ROCK is important
in cardiovascular diseases, including hypertension, heart failure
and chronic kidney disease (7–14).
Several reports in the cardiovascular field have
focused on the association between ROCK and tissue fibrosis. In a
rat coronary artery occlusion model, the inhibition of ROCK by
fasudil, a non-selective ROCK1/2 inhibitor, reduced the expression
of inflammatory cytokines, including transforming growth factor-β1
(TGF-β1) and macrophage migration inhibitory factor, and prevented
cardiomyocyte hypertrophy and interstitial fibrosis (15). Furthermore, fasudil attenuated the
upregulation in the expression of profibrotic genes, including
collagen I and III, in the heart following pressure overload in
transverse aortic constriction (TAC) model mice, and ameliorated
myocardial remodeling and fibrosis (16). In terms of kidney diseases, fasudil
has been observed to inhibit the activation of ROCK and the
TGF-β-small mothers against decapentaplegic (Smad) pathway,
preventing glomerulosclerosis and tubulointerstitial fibrosis in an
aldosterone-induced renal injury model (17). In a unilateral ureteral obstruction
(UUO) model, the inhibition of ROCK by Y-27632 or fasudil inhibited
the activity of ROCK and the expression of fibrosis-associated
genes, including collagen, TGF-β1, and α-smooth muscle actin
(α-SMA), thereby preventing tubulointerstitial fibrosis (18,19).
These findings suggest that ROCK is important in the development of
tissue fibrosis.
Further investigations using ROCK1 or ROCK2 knockout
(KO) mice have been reported. The deletion of ROCK1 has been
demonstrated to suppress cardiac fibrosis and ventricular
remodeling following pressure overload by TAC (20) and protects the development of
albuminuria in a streptozotocin-induced diabetic kidney disease
model (21). Following
cardiac-specific deletion of ROCK2, a previous study reported that
angiotensin II-induced cardiac hypertrophy and fibrosis were
attenuated, compared with those in WT mice (22). These reports, as well as previous
studies involving ROCK inhibitors, suggest that ROCK1 and ROCK2
contribute to cardiac inflammation and fibrosis in the development
of heart failure. By contrast, the deletion of ROCK1 in a previous
study did not affect the expression levels of α-SMA, or collagen I
and III within the diseased kidney of a UUO model, nor did it
prevent UUO-induced renal fibrosis (23). Thus, the role and contribution of
ROCK2 in renal fibrosis remains to be fully elucidated. The present
study assessed whether ROCK2 is involved in the development of
renal fibrosis following UUO in ROCK2 HKO mice.
Materials and methods
Animals
This study was approved by the Experimental Animal
Care and Use Committee of Mitsubishi Tanabe Pharma Corporation
(Saitama, Japan). Male ROCK2 HKO (24) and WT mice were purchased from
Charles River Japan (Charles River Laboratories International,
Kanagawa, Japan) and were maintained at room temperature on a 12 h
light/dark cycle, and were allowed access to standard laboratory
chow (CRF-1; Oriental Yeast, Tokyo, Japan) and tap water ad
libitum. The animals were housed at the Animal Care Facility of
Mitsubishi Tanabe Pharma Corporation, in accordance with the
relevant protocols.
Unilateral ureteral obstruction
model
The mice were anesthetized with sevofrane (Maruishi
Pharmaceutical Co., Ltd., Osaka, Japan) and subjected to a left
flank incision. UUO was performed by complete ligation of the left
ureter at the ureteropelvic junction with a 4–0 silk suture (Niccho
Kogyo Co., Ltd., Tokyo, Japan). Sham-operated mice had their ureter
exposed without ligation. All mice used for experiments were 8–10
weeks of age following a 1 week acclimation period (WT, n=25; HKO,
n=38). The mice were sacrificed under anesthesia with sevofrane on
days 7 and 14 following surgery. For RNA and hydroxyproline
analyses, 42 mice [sham, WT (n=5) and HKO (n=8); day 7, WT (n=5)
and HKO (n=9); day 14, WT (n=5), HKO (n=10)], and for protein
analysis, 21 mice [sham, WT (n=7) and HKO (n=7); day 7, WT (n=3)
and HKO (n=4)] were sacrificed under anesthesia with sevofrane. The
kidneys were then removed and divided into two sections for the
subsequent analyses of RNA and hydroxyproline. For protein
analysis, the whole kidneys were used.
Determination of kidney hydroxyproline
content
The collagen content in the kidney was determined by
hydroxyproline using a modified version of a previously described
method (25,26). In brief, the kidneys were
homogenized in phosphate-buffered saline (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), at 700 µl/100 mg kidney
weight, and were completely hydrolyzed in 6 mol/l HCl (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) at 120°C for 6 h, and
filtered through a 0.45-µm Millex-HV filter (Merck
Millipore, Hessen, Germany). The samples were dried by vacuum
centrifugation using an EZ-2 plus (Genevac, Ltd., Suffolk, UK) for
16 h. The dried samples were solubilized in distilled water (2/3
volume of hydrolyzed sample). The samples were oxidized using
chloramine T solution, containing 1.4% sodium
p-toluenesulfonchloramide trihydrate (chloramine T; Wako
Pure Chemical Industries, Ltd.) and 10% n-propanol (Wako
Pure Chemical Industries, Ltd.) in citric acid buffer, which
consisted of 0.26 mol/l citric acid (Sigma-Aldrich, St. Louis, MO,
USA), 0.88 mol/l sodium acetate trihydrate (Wako Pure Chemical
Industries, Ltd.), 0.85 mol/l sodium hydroxide (Wako Pure Chemical
Industrie, Ltd.) and 1.2% acetic acid (Kanto Chemical Co., Inc.,
Tokyo, Japan). Following incubation at room temperature for 20 min,
Ehrlich's solution comprising 1 mol/l 4-dimethylaminobenzaldehyde
(Sigma-Aldrich), 18% perchloric acid (Sigma-Aldrich) and 60%
n-propanol, was added, and the samples were incubated at
65°C for 40 min. Subsequently, the absorbance was measured at 560
nm using a SpectraMax M5e microplate reader with SoftMax Pro ver.
5.4.1 (Molecular Devices; Thermo Fisher Scientific, Inc.). The
concentration of hydroxyproline was estimated using a standard
curve, using a pure solution of l-hydroxyproline (Wako Pure
Chemical Industries, Inc.), with the final results expressed as
hydroxyproline/mg protein. The kidney protein concentrations were
determined using a BCA Protein Assay (Pierce; Thermo Fisher
Scientific, Inc.) with bovine serum albumin as a standard.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses
The kidneys were homogenized and total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and purified according to the manufacturer's
protocol of an RNeasy Mini Kit (cat. no. 74106; Qiagen, Venlo,
Netherlands). The total RNA concentration was determined using a
NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Inc.).
cDNA was synthesized from 1 µg of total RNA using
SuperScript VILO Master mix (cat. no. 11755250; Invitrogen; Thermo
Fisher Scientific, Inc.) using an iCycler Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) under the following
conditions: 25°C for 10 min, 42°C for 60 min and 85°C for 5 min.
qPCR was performed with 1 µl cDNA in a total reaction volume
of 20 µl using a 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using TaqMan
technology. The cycling conditions were as follows: 50°C for 2 min,
95°C for 10 min and 40 cycles of 95°C for 15 sec and 60°C for 1
min. Data were analyzed using the standard curve method. The
results of each gene were normalized to that of 18SrRNA as an
internal control. The following TaqMan Gene Expression Assay
reagents were used: TGF-β1, Mm00441724_m1; α-SMA, Mm01546133_m1;
collagen 1a2, Mm00483888_m1 and 18SrRNA, 4308329 (all from Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Western blot analysis
The kidney tissues were homogenized in lysis buffer
containing 50 mmol/l Tris-HCl (pH 8.0; Nacalai Tesque, Inc., Kyoto,
Japan), 150 mmol/l NaCl (Wako Pure Chemical Industries, Inc.), 0.5%
sodium deoxycholate (Wako Pure Chemical Industries, Inc.), 0.1%
sodium dodecyl sulfate (Bio-Rad Laboratories, Inc.), 1% Triton
X-100 (Sigma-Aldrich), 1x protease inhibitors (complete; cat. no.
11697498001; Roche Diagnostics, Basel, Switzerland), and 1x
phosphatase inhibitors (P2850; Sigma-Aldrich).
Following centrifugation at 15,000 × g for 20 min at
4°C (CF-15R; Hitachi, Ltd., Tokyo, Japan), the supernatant was
collected as lysate. The lysates were denatured for 5 min by
boiling. Protein concentration was determined using a BCA Protein
Assay (Pierce; Thermo Fisher Scientific, Inc.) with bovine serum
albumin as a standard. Equal quantities of lysate were separated on
a 4–15% TGX Precast Gel (Bio-Rad Laboratories, Inc.) and
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.) using the Trans-Blot Turbo Transfer System
(Bio-Rad Laboratories, Inc.). The blots were blocked in Starting
Block T20 (PBS) blocking buffer (Pierce; Thermo Fisher Scientific,
Inc.) at room temperature for 1 h. They were subsequently incubated
overnight at 4°C with the following primary antibodies: Anti-human
ROCK1 rabbit polyclonal antibody (1:200; H-85; cat. no. sc-5560,
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-chicken
β-actin mouse monoclonal antibody (1:1,000; C4; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.), anti-rat ROCK2 mouse monoclonal
antibody (1:5,000; cat. no. 610624; Transduction Laboratories; BD
Biosciences, Franklin Lakes, NJ, USA), anti-human myosin
phosphatase target subunit-1 (MYPT-1) rabbit polyclonal antibody
(1:1,000; cat. no. 2634; Cell Signaling Technology Inc., Danvers,
MA, USA) and anti-human phosphorylated (p)-MYPT-1 (Thr853) rabbit
polyclonal antibody (1:1,000; cat. no. 4563; Cell Signaling
Technology, Inc.) in Can Get Signal solution (Toyobo Co., Ltd.,
Osaka, Japan). The membranes were then incubated with the following
secondary polyclonal antibodies: ECL donkey anti-rabbit IgG
horseradish peroxidase (HRP)-conjugated species-specific whole
antibody (1:5,000; cat. no. NA934; GE Healthcare Life Sciences,
Buckinghamshire, UK) and ECL sheep anti-mouse IgG, HRP-conjugated
species-specific whole antibody (1:5,000; cat. no. NA931; GE
Healthcare Life Sciences) at room temperature for 1 h. The
membranes were developed using enhanced chemiluminescence methods
(LumiGLO Reserve Chemiluminescent Substrate kit; cat. no. 54-71-00;
KPL, Gaithersburg, MD, USA). The signal intensities of the specific
bands were detected using an LAS-3000 Luminescent Image Analyzer
(Fujifilm, Tokyo, Japan) and analyzed with Multi Gauge (ver. 3.0;
Fujifilm). For quantification, the signal intensities were
normalized to β-actin loaded in each well.
Statistical analyses
All data were expressed as the mean ± standard error
of the mean. Parameters between the sham- and UUO-operated mice, or
between the WT and ROCK2 HKO mice were compared using Student's
t-test. Statistical analyses were performed using SAS
system, version 8.0.0 (SAS Institute, Cary, NC, USA) in the
biometrics section. P<0.05 was considered to indicate a
statistically significant difference.
Results
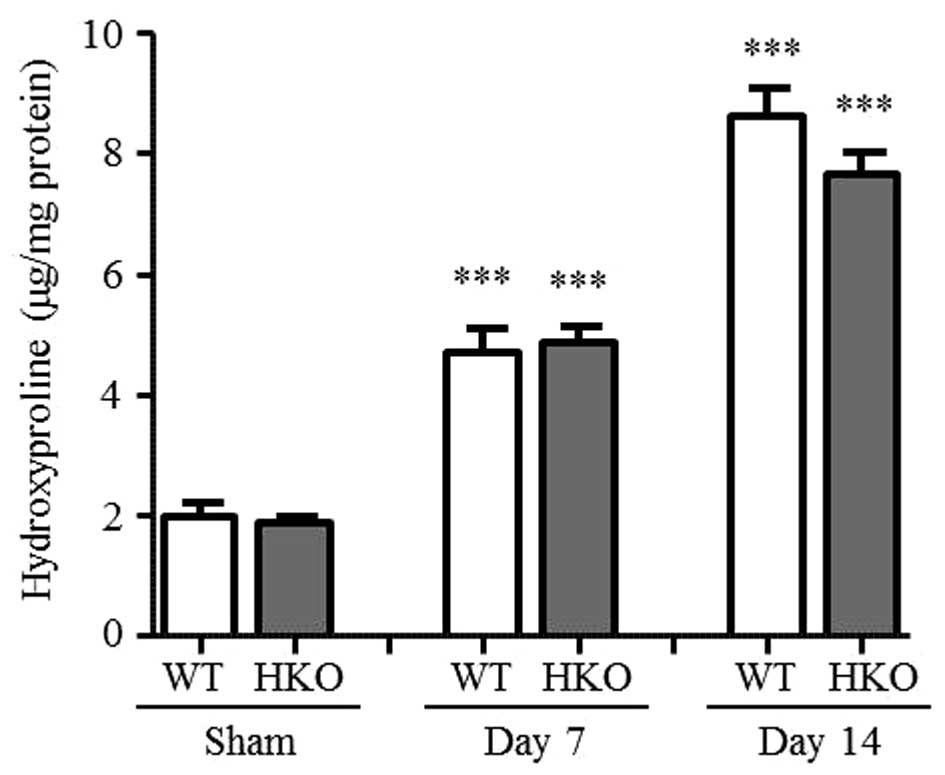
Hydroxyproline content in UUO
kidneys
To assess renal interstitial fibrosis, the present
study evaluated the accumulation of collagen in the UUO kidneys.
Total kidney collagen deposition was determined by measuring the
hydroxyproline content, which significantly increased in the
UUO-operated kidney, compared with that of the sham-operated
kidney.
Following 3 days of UUO, the hydroxyproline content
continued to increase (data not shown). On days 7 and 14 post-UUO,
the WT and HKO mice were compared. Compared with the sham-operated
group, the hydroxyproline content increased in the UUO-operated
kidney of the WT mice (2.4- and 4.4-fold on days 7 and 14 post-UUO,
respectively) and HKO mice (2.6- and 4.1-fold at days 7 and 14
post-UUO, respectively), as shown in Fig. 1. No significant differences were
identified.
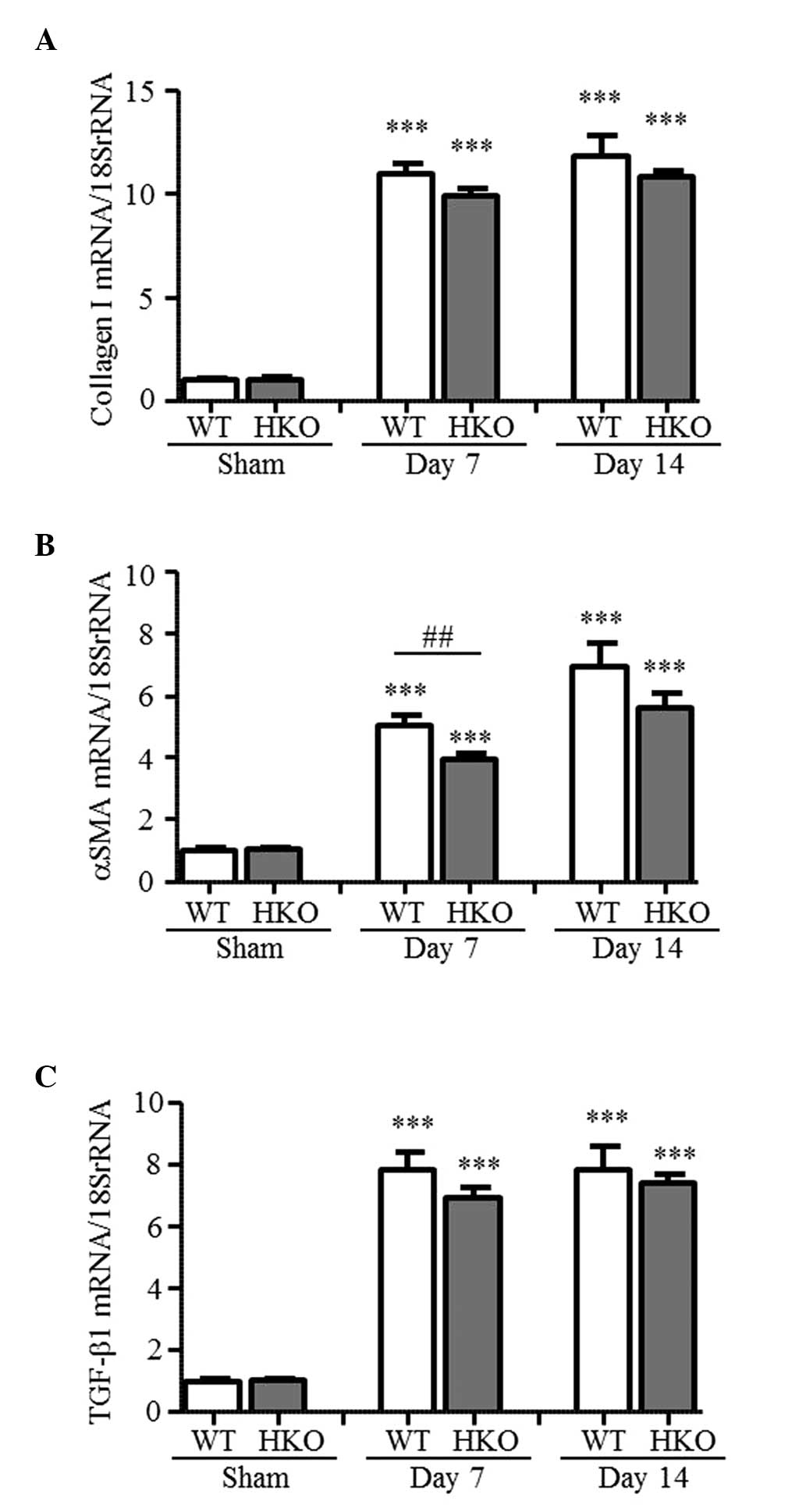
mRNA expression levels of collagen I,
α-SMA and TGF-β1 in UUO kidneys
The present study measured the expression levels of
fibrosis-associated genes to examine the effect of partial ROCK2
deletion. As shown in Fig. 2A–C,
the mRNA expression levels of collagen I, α-SMA and TGF-β1 in the
UUO-operated kidneys of the WT mice markedly increased, compared
with those in the sham-operated mice (collagen I, 11.0- and
11.8-fold; α-SMA, 5.1- and 6.9-fold; TGF-β1, 7.9- and 7.9-fold at
days 7 and 14 post-UUO, respectively). By contrast, the mRNA
expression level of α-SMA was markedly ameliorated in the HKO mice
at day 7 post-UUO, and was 78% of the level measured in the WT mice
(Fig. 2B). At day 14 post-UUO, no
statistically significant difference was observe in the mRNA
expression level of α-SMA in the HKO mice; however, the level of
expression was suppressed to 81% of that in the WT mice. In
addition, the mRNA expression levels of collagen I and TGF-β1
increased in the UUO-operated kidneys of the HKO mice (collagen I,
9.9- and 10.8-fold; TGF-β1, 6.9- and 7.4-fold at days 7 and 14
post-UUO, respectively); and their expression levels were almost
the same as those observed in the WT mice (Fig. 2A and C).
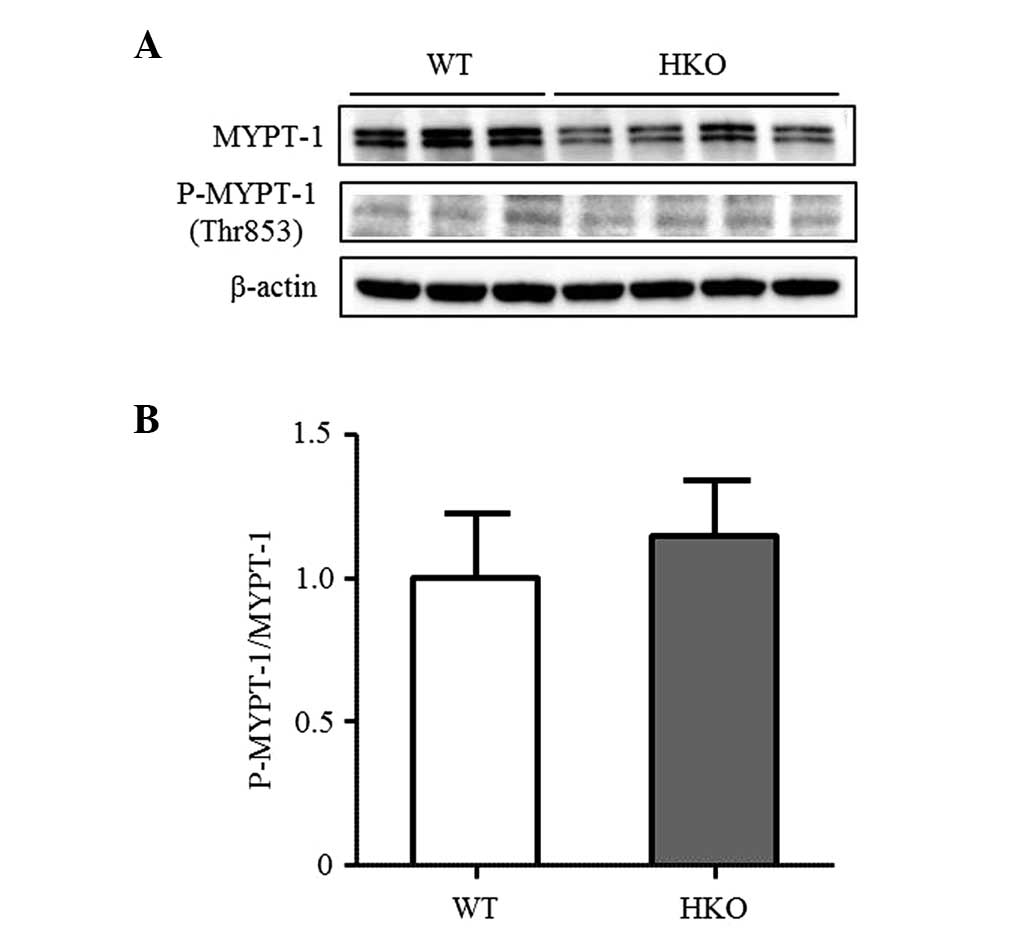
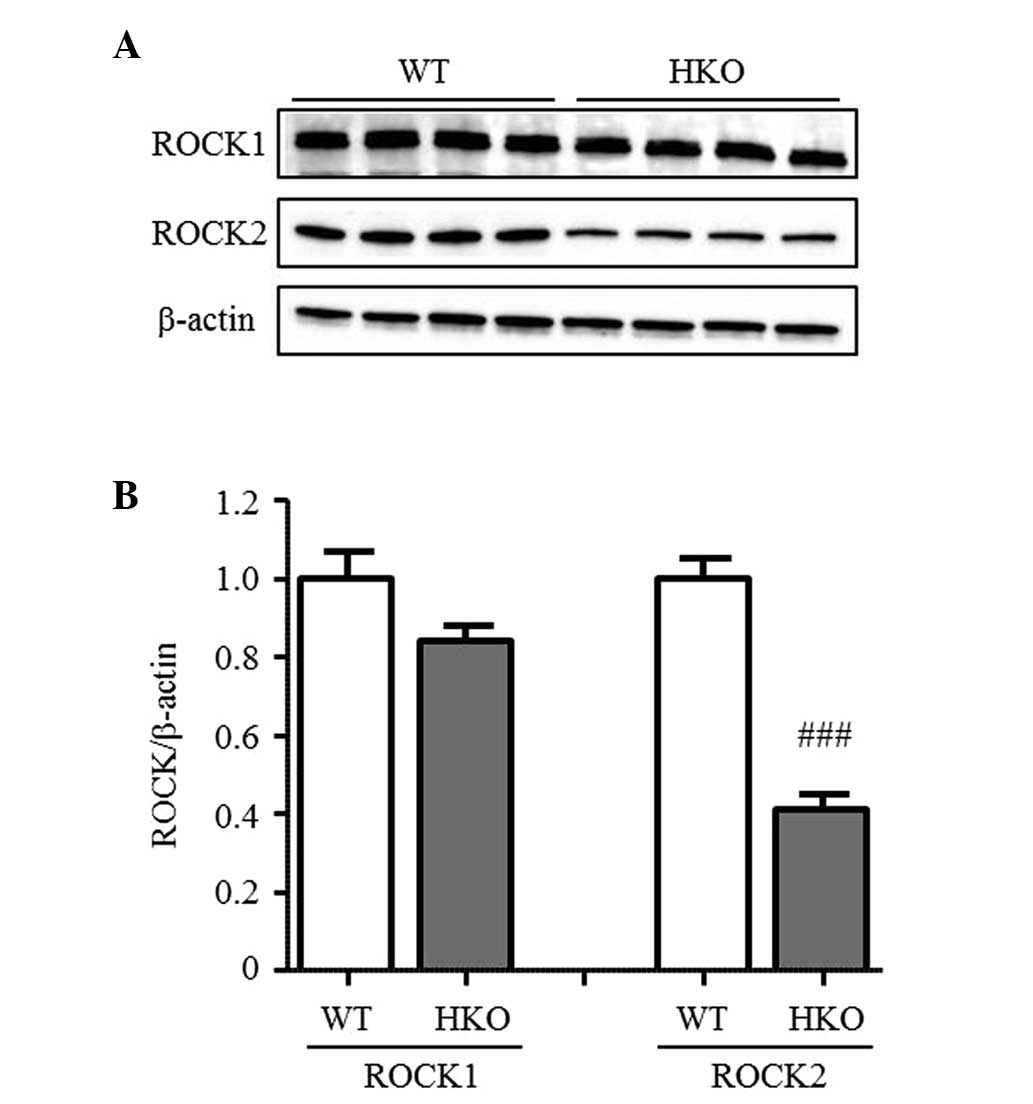
ROCK activity in UUO kidneys
To confirm the difference in ROCK activity between
the WT and HKO mice, the present study measured the phosphorylation
of MYPT-1, a target protein of ROCK in the UUO-operated kidney. The
phosphorylation of MYPT-1 was detected in the UUO-operated kidney
of the WT and HKO mice in a similar manner (Fig. 3A and B), indicating that the
activity of ROCK was not altered following the partial deletion of
ROCK2. In the normal kidneys, the protein expression level of ROCK2
in the HKO mice was 50% of that observed in the WT mice, however,
no differences were observed in the protein expression level of
ROCK1 between the WT and HKO mice kidneys (Fig. 4A and B). The expression ratio of
ROCK1 and ROCK2 was also unchanged in the UUO-operated kidney (data
not shown).
Discussion
In the present study, the effects of the partial
deletion of ROCK2 protein on the expression of fibrosis-associated
genes and renal interstitial fibrosis were investigated in a UUO
model in mice. In the WT mice, the hydroxyproline content and mRNA
expression of collagen I in the UUO-operated kidneys were
significantly increased at days 7 and 14 following UUO. The
fibrotic parameters in the UUO kidneys of the ROCK2 HKO mice also
increased. However, no statistical differences were identified
between the WT and ROCK2 HKO mice on the increment of these
fibrotic parameters in the UUO-operated kidneys, suggesting that
the partial deletion of the ROCK2 protein does not affect the level
of renal interstitial fibrosis induced by UUO. It has been reported
in normal mice that Y27632, a non-selective ROCK1/2 inhibitor,
suppresses the mRNA expression of collagen I and the deposition of
collagen in damaged kidneys 4 and 10 days following UUO (18). In addition, in rat UUO model,
fasudil was found to ameliorate collagen deposition in the damaged
kidney 16 days following UUO (19). These results suggest that the
pharmacological inhibition of ROCK can prevent the development of
UUO-induced renal interstitial fibrosis. By contrast, other studies
have demonstrated that, in ROCK1 KO mice, complete ROCK1 deletion
did not affect collagen accumulation or the mRNA expression levels
of collagen I in the UUO kidney 5 and 10 days following UUO. This
suggests that ROCK2, but not ROCK1, is involved in renal
interstitial fibrosis in UUO (23). However, in the present study,
partial ROCK2 deletion indicated a non-significant reduction in
renal interstitial fibrosis. Thus, it is likely that the effect of
the pharmacological inhibition of ROCK is inconsistent with that of
the deletion of ROCK protein on the development of renal
interstitial fibrosis following UUO.
It is evident that TGF-β1 signals and the Rho/ROCK
pathway are closely associated with renal interstitial fibrosis by
UUO (27). Y27632 is reported to
suppress the augmentation of the mRNA expression of TGF-β1 in UUO
mice (18). However, in ROCK1 KO
mice, the expression of TGF-β1 was found to be significantly
enhanced in the damaged kidney following UUO, compared with WT
mice, indicating that the absence of ROCK1 may not be able to
suppress the expression of TGF-β1 to protect against renal fibrosis
(23). Therefore, ROCK2 was
considered to be important in TGF-β1 signaling in renal
interstitial fibrosis following UUO. In the present study, partial
ROCK2 deletion did not suppress the mRNA expression of TGF-β1 or
ROCK1, and ROCK activity remained unaffected in the UUO-operated
kidney. These results suggested that the response to partial ROCK2
deletion was distinct from that to pharmacological ROCK
inhibition.
In renal interstitial fibrosis, the transformation
to myofibroblasts, which are considered to be the dominant collagen
producing cells, is a crucial step toward collagen synthesis and
deposition. In kidney fibrosis, the resident fibroblast,
epithelial, endothelial and bone marrow-derived cells can acquire
the phenotype of myofibroblasts and express the characteristic
proteins, including α-SMA (27–29).
Furthermore, the ROCK signaling pathway is reported to be important
in the transformation of cells into activated myofibroblasts
(30,31). In the present study, the expression
level of α-SMA was markedly decreased in the UUO kidney tissues of
the ROCK2 HKO mice, compared with that of the WT mice. In the ROCK1
KO mice, the augmentation of mRNA and protein expression levels of
α-SMA caused by UUO were not decreased (23). Thus, it is likely that ROCK2 may be
implicated in UUO-induced transformation via a signal cascade
independent of TGF-β1. In addition, the present study found in the
mice UUO model, that the effect of ROCK2 HKO was inconsistent with
the effect of the pharmacological inhibition of ROCK. With respect
to this inconsistency, ROCK inhibitors are considered to be poor in
isoform selectivity and other target specificity. Y-27632 and
fasudil equivalently inhibit ROCK1 and ROCK2 activity; and fasudil
also inhibits protein kinase (PK)N, PKC, myosin light chain kinase
and mitogen-activated protein kinase kinase 1 (32). Therefore, it can was suggested that
the partial deletion of ROCK2 may be insufficient in suppressing
UUO-induced fibrotic responses. In addition, it is possible that
the results depend on the experimental procedures and
pathophysiological conditions in the UUO model. In order to address
these problems, further investigations are required using
kidney-specific ROCK1 or ROCK2 deletion, and using more specific
inhibitors for ROCK1 or ROCK2. In conclusion, using ROCK2 HKO mice,
the present study demonstrated that the partial deletion of ROCK2,
as with ROCK1 inhibition, is insufficient for effectively
preventing renal interstitial fibrosis.
Acknowledgments
The authors would like to thank Dr Shuh Narumiya
(Medical Innovation Center, Graduate School of Medicine, Kyoto
University, Kyoto, Japan) for their permission to use the ROCK2 HKO
mice. The authors would also like to thank Dr Akiyoshi Fukamizu and
Dr Junji Ishida (Life Science Center, Tsukuba Advanced Research
Alliance, Tsukuba University, Ibaraki, Japan) for their guidance
and support, and would like to thank Dr Kenji Arakawa, Dr Rikako
Yamauchi and Dr Taku Sato (Mitsubishi Tanabe Pharma Corporation)
for their support.
Abbreviations:
|
ROCK
|
Rho-associated coiled-coil kinase
|
|
UUO
|
unilateral ureteral obstruction
|
|
HKO
|
heterozygous knockout
|
References
|
1
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amin E, Dubey BN, Zhang SC, Gremer L,
Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV
and Ahmadian MR: Rho-kinase: Regulation, (dys) function and
inhibition. Biol Chem. 394:1399–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satoh K, Fukumoto Y and Shimokawa H:
Rho-kinase: Important new therapeutic target in cardio-vascular
diseases. Am J Physiol Heart Circ Physiol. 301:H287–H296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hahmann C and Schroeter T: Rho-kinase
inhibitors as therapeutics: From pan inhibition to isoform
selectivity. Cell Mol Life Sci. 67:171–177. 2010. View Article : Google Scholar
|
|
5
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi J and Wei L: Rho kinase in the
regulation of cell death and survival. Arch Immunol Ther Exp
(Warsz). 55:61–75. 2007. View Article : Google Scholar
|
|
7
|
Ming D, Yan BP, Liao JK, Lam YY, Yip GW
and Yu CM: Rho-kinase inhibition: A novel therapeutic target for
the treatment of cardiovascular diseases. Drug Discov Today.
15:622–629. 2010. View Article : Google Scholar
|
|
8
|
Budzyn K, Marley PD and Sobey CG:
Targeting Rho and Rho-kinase in the treatment of cardiovascular
disease. Trends Pharmacol Sci. 27:97–104. 2006. View Article : Google Scholar
|
|
9
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushiyama T, Oda T, Yamamoto K, Higashi K,
Watanabe A, Takechi H, Uchida T, Oshima N, Sakurai Y, Miura S and
Kumagai H: Protective effects of Rho kinase inhibitor fasudil on
rats with chronic kidney disease. Am J Physiol Renal Physiol.
304:F1325–F1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishikimi T, Koshikawa S, Ishikawa Y,
Akimoto K, Inaba C, Ishimura K, Ono H and Matsuoka H: Inhibition of
Rho-kinase attenuates nephrosclerosis and improves survival in
salt-loaded spontaneously hypertensive stroke-prone rats. J
Hypertens. 25:1053–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanda T, Wakino S, Hayashi K, Homma K,
Ozawa Y and Saruta T: Effect of fasudil on Rho-kinase andv
nephropathy in subtotally nephrectomized spontaneously hypertensive
rats. Kidney Int. 64:2009–2019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie X, Peng J, Chang X, Huang K, Huang J,
Wang S, Shen X, Liu P and Huang H: Activation of RhoA/ROCK
regulates NF-κB signaling pathway in experimental diabetic
nephropathy. Mol Cell Endocrinol. 369:86–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou H, Li YJ, Wang M, Zhang LH, Guo BY,
Zhao ZS, Meng FL, Deng YG and Wang RY: Involvement of RhoA/ROCK in
myocardial fibrosis in a rat model of type 2 diabetes. Acta
Pharmacol Sin. 32:999–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rikitake Y, Oyama N, Wang CY, Noma K,
Satoh M, Kim HH and Liao JK: Decreased perivascular fibrosis but
not cardiac hypertrophy in ROCK1+/-haploinsufficient mice.
Circulation. 112:2959–2965. 2005.PubMed/NCBI
|
|
16
|
Li Q, Xu Y, Li X, Guo Y and Liu G:
Inhibition of Rho-kinase ameliorates myocardial remodeling and
fibrosis in pressure overload and myocardial infarction: Role of
TGF-β1-TAK1. Toxicol Lett. 211:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun GP, Kohno M, Guo P, Nagai Y, Miyata K,
Fan YY, Kimura S, Kiyomoto H, Ohmori K, Li DT, et al: Involvements
of Rho-kinase and TGF-beta pathways in aldosterone-induced renal
injury. J Am Soc Nephrol. 17:2193–2201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagatoya K, Moriyama T, Kawada N, Takeji
M, Oseto S, Murozono T, Ando A, Imai E and Hori M: Y-27632 prevents
tubulointerstitial fibrosis in mouse kidneys with unilateral
ureteral obstruction. Kidney Int. 61:1684–1695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh S, Yamaguchi T, Hitomi A, Sato N,
Shiraiwa K, Ikegaki I, Asano T and Shimokawa H: Fasudil attenuates
interstitial fibrosis in rat kidneys with unilateral ureteral
obstruction. Eur J Pharmacol. 455:169–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YM, Bo J, Taffet GE, Chang J, Shi J,
Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ and Wei
L: Targeted deletion of ROCK1 protects the heart against pressure
overload by inhibiting reactive fibrosis. FASEB J. 20:916–925.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou L, Liu F, Huang XR, Chen H, Chung AC,
Shi J, Wei L, Lan HY and Fu P: Amelioration of albuminuria in ROCK1
knockout mice with streptozotocin-induced diabetic kidney disease.
Am J Nephrol. 34:468–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okamoto R, Li Y, Noma K, Hiroi Y, Liu PY,
Taniguchi M, Ito M and Liao JK: FHL2 prevents cardiac hypertrophy
in mice with cardiac-specific deletion of ROCK2. FASEB J.
27:1439–1449. 2013. View Article : Google Scholar :
|
|
23
|
Fu P, Liu F, Su S, Wang W, Huang XR,
Entman ML, Schwartz RJ, Wei L and Lan HY: Signaling mechanism of
renal fibrosis in unilateral ureteral obstructive kidney disease in
ROCK1 knockout mice. J Am Soc Nephrol. 17:3105–3114. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thumkeo D, Keel J, Ishizaki T, Hirose M,
Nonomura K, Oshima H, Oshima M, Taketo MM and Narumiya S: Targeted
disruption of the mouse Rho-associated kinase 2 gene results in
intrauterine growth retardation and fetal death. Mol Cell Biol.
23:5043–5055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woessner JF: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this imino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kivirikko KI, Laitinen O and Prockop DJ:
Modifications of a specific assay for hydroxyproline in urine. Anal
Biochem. 19:249–255. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: Pathologic significance,
molecular mechanism and therapeutic intervention. J Am Soc Nephrol.
15:1–12. 2004. View Article : Google Scholar
|
|
28
|
Piera-Velazquez S, Li Z and Jimenez SA:
Role of endothelial-mesenchymal transition (EndoMT) in the
pathogenesis of fibrotic disorders. Am J Pathol. 179:1074–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duffield JS: Cellular and molecular
mechanisms in kidney fibrosis. J Clin Invest. 124:2299–2306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel S, Takagi KI, Suzuki J, Imaizumi A,
Kimura T, Mason RM, Kamimura T and Zhang Z: RhoGTPase activation is
a key step in renal epithelial mesenchymal transdifferentiation. J
Am Soc Nephrol. 16:1977–1984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodrigues-Díez R, Carvajal-González G,
Sánchez-López E, Rodríguez-Vita J, Rodrigues Díez R, Selgas R,
Ortiz A, Egido J, Mezzano S and Ruiz-Ortega M: Pharmacological
modulation of epithelial mesenchymal transition caused by
angiotensin II. Role of ROCK and MAPK pathways. Pharm Res.
25:2447–2461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamura M, Nakao H, Yoshizaki H,
Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J and Hidaka H:
Development of specific Rho-kinase inhibitors and their clinical
application. Biochim Biovphys Acta. 1754:245–252. 2005. View Article : Google Scholar
|