Introduction
Myocardial ischemia is an important determinant of
survival in patients with coronary artery disease (CAD), which is a
prevalent cause of morbidity and mortality (1). Occlusion of an epicardial coronary
artery without revascularization inevitably results in necrosis of
the infarct myocardium (2). Timely
reperfusion therapy serves a key role in the initiation of a
molecular cascade leading to cardioprotection (3), and has been independently associated
with improved left ventricular function and prognosis (4).
Paradoxically, reperfusion of the previously
ischemic tissue may induce another form of myocardial injury,
ischemia reperfusion (I/R) injury (5). Compromised perfusion within the
supplied myocardium may result from events including the production
of oxygen free radicals and inflammatory mediators, in addition to
malfunction of the cell membrane transport system (6). The area of no-reflow has been
demonstrated to increase with increasing time following reperfusion
(7). Mechanisms of no-reflow are
complex however inflammation, plugging of the microcirculation with
neutrophils and vasoconstriction are suggested to serve a major
role in the process (8).
Neutrophils have been reported to serve a central role in the
inflammatory-like response to reperfusion through the generation of
oxygen free radicals, degranulation and release of proteases, the
release of arachidonic metabolites and additional proinflammatory
mediators, and these processes implicated as a primary mechanism
underlying I/R injury (9). The
no-reflow phenomenon was reported to occur in greater than 30% of
patients with acute myocardial infarction (10), and was suggested to be a strong
predictor of poor functional and clinical outcomes (11).
Growth differentiation factor-15 (GDF-15) is a
member of the transforming growth factor-β (TGF-β) cytokine
super-family and has been implicated as a novel biomarker in the
prognosis of cardiovascular diseases (12). It was demonstrated to be expressed
in certain types of normal tissue, such as that of the central
nervous system, and weakly expressed in cardiac tissue under
physiological conditions (13).
However, the expression was significantly upregulated in myocardial
I/R injury (14), and was
suggested to be induced in response to inflammation and heart
tissue injury (15). In addition,
a single measurement of GDF-15 on admission was suggested to
provide strong and independent prognostic information in cardiac
failure patients (16).
GDF-15 may be a myocardial protective cytokine and
act on multiple overlapping pathways, making it well suited for the
evaluation of myocardial I/R injury. Initial studies have indicated
that GDF-15 may aid with the understanding of the distinct
pathophysiological processes in acute myocardial infarction
(17,18). However, the potential
pathophysiological role of GDF-15 in the no-reflow phenomenon
remains to be fully elucidated. To gain insight into the
pathophysiology of GDF-15 in myocardial I/R injury, the current
study aimed to determine the expression of GDF-15 in a rat ischemia
model following different durations of reperfusion. In addition,
the present study aimed to determine whether GDF-15 is associated
with markers of myocardial damage (area of no-reflow, infarct area
and area at risk) in addition to certain established parameters in
the pathophysiology of acute I/R, including intercellular adhesion
molecule-1 (ICAM-1) and myeloperoxidase (MPO).
Materials and methods
Animals
A total of 144 male Wistar rats, weighing 290–340 g,
were purchased from the Animal Center of People's Liberation Army
Academy of Medical Sciences (Beijing, China. Animals were
maintained under conventional conditions (25°C, lights on from 7.00
am to 7.00 pm) with free access to water and food. All surgical
procedures and care administered to the animals were approved by
the Tianjin Medical University Animal Care and Use Committee
(Tianjin, China).
Myocardial I/R model
A total of 144 male Wistar rats were randomly
divided into two groups: The I/R group, I/R injury group and S
group, sham operation group (n=72), and underwent transient left
anterior descending (LAD) coronary artery ligation or a sham
operation, respectively. Mice were anesthetized with sodium
pentobarbital (100 mg/kg intraperitoneal, Tianjin Beike
Biotechnology Co., Ltd., Tianjin, China) and intubated. Artificial
respiration was maintained with a rodent ventilator (TKR-200C;
Jiangxi Teli Anaesthesia & Respiratory Equipment Co. Ltd.,
Jiangxi, China). A parasternal incision was made by cutting the
left third and fourth ribs and inter-costal muscles with a cautery
pen. A range of infarct sizes was obtained by passing a 7-0 silk
suture (Shanghai Medical Suture Needle Factory, Shanghai, China)
beneath the left anterior descending artery at points 1–2 mm
inferior to the left auricle, then tightening it over a length of
PE-20 tubing (Plastics One Inc., Roanoke, Va., USA) (which was
removed 60 min later to achieve reperfusion). The onset of ischemia
was confirmed by the development of cyanosis and typical elevation
of the ST segment (0.2 mV) in the electrocardiogram (Biopac Systems
Inc., Santa Barbara, CA, USA). At 1 h following occlusion, the
heart was reperfused by releasing the ligature. The ST segment was
gradually depressed thereafter, accompanied by the development of
cardiac arrhythmias, which indicated myocardial I/R injury. On
reperfusion, the chest was closed layer by layer. Animals
undergoing sham surgery underwent the same procedure except that
the suture was passed under the coronary artery without ligation.
The endotracheal tube was removed once spontaneous breathing
resumed. Animals in each group were further divided into 6
subgroups according to the reperfusion duration (2, 4, 6, 12, 24 h
and 7 days).
Infarct quantification
Assessment of ischemic risk area (RA), area of
infarct area (IA) and no-reflow (ANR) were performed as previously
described with some modifications (19). At the different times of
reperfusion, 0.5 ml 1% Thioflavin S (Sigma-Aldrich, St. Louis, MO,
USA) was injected into the carotid artery catheter of the rats in
each group. Following 20–30 sec, I/R injuries were delineated by
injection of 8 ml 2% phthalocyanine blue (Zhengzhou Diligent
Technology Co., Ltd., Zhengzhou, China) into the aorta following
re-ligation of the involved LAD coronary artery. Subsequently, the
heart was rapidly removed and sliced across the long axis of the
left ventricle, from apex to base, into 2 mm-thick transverse
sections (4–5 pieces). The area of no-reflow was visualized using a
Nikon Eclipse 80i Fluorescent Microscope (Nikon Corporation, Tokyo,
Japan). The sliced hearts were incubated in 1% triphenyl
tetrazolium chloride (TTC; Sigma-Aldrich) in normal saline (pH 7.0,
GenView Corp., Houston, TX, USA) at 37°C for 15 min. The
ventricular wall size (VWS), risk size (RS), infarct size (IS) and
size of no-reflow (SNR) were manually measured using Image-Pro Plus
software, version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA), and the values of ΣVWS, ΣRS, ΣIS and ΣSNR were obtained for
each rat myocardial biopsy. The AR was expressed as a percentage of
ΣRS for ΣVWS in each heart, while IA and ANR were calculated by
dividing ΣIS and ΣSNR, respectively, by ΣRS, as presented in
equations 1, 2, and 3.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of GDP-15 mRNA expression
Rats in each group were anesthetized by
intraperitoneal injection of 0.4% sodium pentobarbital (40 mg/kg),
and triplicate heart samples were obtained from the left ventricles
(below ligature) following perfusion with normal saline. One tissue
sample weighing approximately 70 mg was used for RT-qPCR analysis
of GDP-15 expression, and additional samples were stored in liquid
nitrogen for immunohistochemistry and enzyme-linked immunosorbent
assay (ELISA) analysis.
Total RNA was isolated using TRIzol reagent (Gibco
Life Technologies, Carlsbad, CA, USA) following the manufacturer's
instructions and quantified using a UV spectrophotometer (NanoDrop
2000c; Thermo Fisher Scientific, Wilmington, DE, USA). First-strand
cDNA was synthesized from 1 µg total RNA in 20 µl
final volume using a First Strand cDNA Synthesis kit (Invitrogen
Life Technologies, Carlsbad, CA, USA). Following reverse
transcription, amplification was conducted by PCR using FastStart
Universal SYBR Green Master kit (Roche Diagnostics, Basel,
Switzerland). RT-qPCR amplification was conducted using a
thermocycler (Applied Biosystems 7300; Foster City, CA, USA) under
the following conditions: 50°C for 2 min; 95°C for 10 min; 95°C for
15 sec, and 60°C for 1 min for 40 cycles. The mRNA levels of
β-actin were measured in each sample as an internal normalization
standard. The specific primers used for GDP-15 and β-actin are
presented in Table I.
 | Table IPrimers designed for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers designed for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Direction | Primer
sequence |
|---|
| GDP-15 | Forward |
5′-GACCTAGGTTGGAGCGACTG-3′ |
| Reverse |
5′-TAAGAACCACCGGGGTGTAG-3′ |
| β-actin | Forward |
5′-TACCACATCCAAGGAAGGCAGCA-3′ |
| Reverse |
5′-TGGAATTACCGCGGCTGCTGGCA-3′ |
Immunohistochemical analysis of GDP-15
protein expression
For immunohistochemistry, left ventricle sections
were incubated with hydrogen peroxide (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) for 20 min, washed
three times with phosphate-buffered saline (PBS), boiled in 10
mmol/l citric acid buffer (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 10 min for antigen retrieval, and then
blocked with goat serum (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) at 37°C for 20 min. The sections were
first incubated with rabbit anti-GDF-15/MIC-1 (1:300; cat. no.
A043818G; Shanghai LanJi Biology Co., Ltd, Shanghai, China) at 4°C
overnight and then washed three times with PBS. Subsequently, the
sections were incubated with biotin-labeled goat anti-rabbit IgG
antibody (1:300) and horseradish peroxidase-labeled streptavidin
(1:300) at 37°C for 20 min, respectively, and washed three times
with PBS using a Streptavidin-Alkaline Phosphatase (SAP)-Testing
kit (SAP-9101, Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The sections
were then counterstained with hematoxylin (Sigma-Aldrich). The
immunohistochemical results were analyzed by capturing images of
five randomly selected fields at a magnification of ×200 (Olympus
BX41 microscope; Olympus Optical Co., Ltd., Tokyo, Japan). A
semiquantitative score was given as the score of the percentage of
positive cells plus the score of the staining intensity of the
samples and was used to analyze the final results. For the
proportion of positive cells, 0–5% positive cells scored 0; 6–24%
positive cells scored 1; 25–50% positive cells scored 2; 51–75%
positive cells scored 3; and 76–100% positive cells scored 4. For
the staining intensity of positive cells, no color was scored as 0,
yellow was scored as 1, brown was scored as 2 and tan was scored as
3. Subsequently, staining results were divided into four grades as
follows: Negative (−) for total scores of 0, weakly positive (+)
for total scores of 1–2, positive (++) for total scores of 3–5 and
strongly positive (+++) for total scores of 6–7. The average scores
from 10 tissue samples from each rat were used for statistical
analysis.
ELISA of MPO activity
Myocardial tissue samples were collected following
the different reperfusion durations and were homogenized in order
to measure the activity of MPO, an indicator of neutrophil
infiltration. MPO activity was determined using a rat MPO ELISA kit
(Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China).
Absorbance measurements were performed bichromatically at a
wavelength of 450 nm with an ELISA reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and the MPO concentration was calculated
according to the standard curve.
Immunohistochemical analysis of ICAM-1
expression
Immunohistochemical staining was performed to
determine the time course of ICAM-1 expression in transient I/R
injury using the method as mentioned above. ICAM-1 staining was
performed on fresh frozen tissue using a rabbit anti-CD54/ICAM-1
antibody (1:300; cat. no. A046326I; Shanghai BlueGene Biotech Co.,
Ltd., Shanghai, China). The concentrated diaminobenzidine reagent
kit was purchased from Shanghai Jianglai Biotechnology Co.,
Ltd.
Hematoxylin and eosin (H&E)
staining
For the detection of histopathological damage,
sections were stained fixed in 10% formaldehyde and embedded in
paraffin. The sections (5–6 µm) were then stained with
H&E for pathomorphological analysis, as described previously
(20).
Statistical analysis
Data were presented as the mean ± standard
deviation. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used
for statistical analysis. Comparison between the two groups was
performed using Student's t-test, and a one-way analysis of
variance was used for multi-group comparison. Correlations were
assessed by the Pearson coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
Time-course of myocardial damage in
response to myocardial I/R
In order to define the time course of reperfusion
injury, anatomical no-reflow, area at risk and infarct area were
measured using Thioflavin S, phthalocyanine blue dye and TTC
staining following 1 h of LAD coronary occlusion and 2, 4, 6, 12,
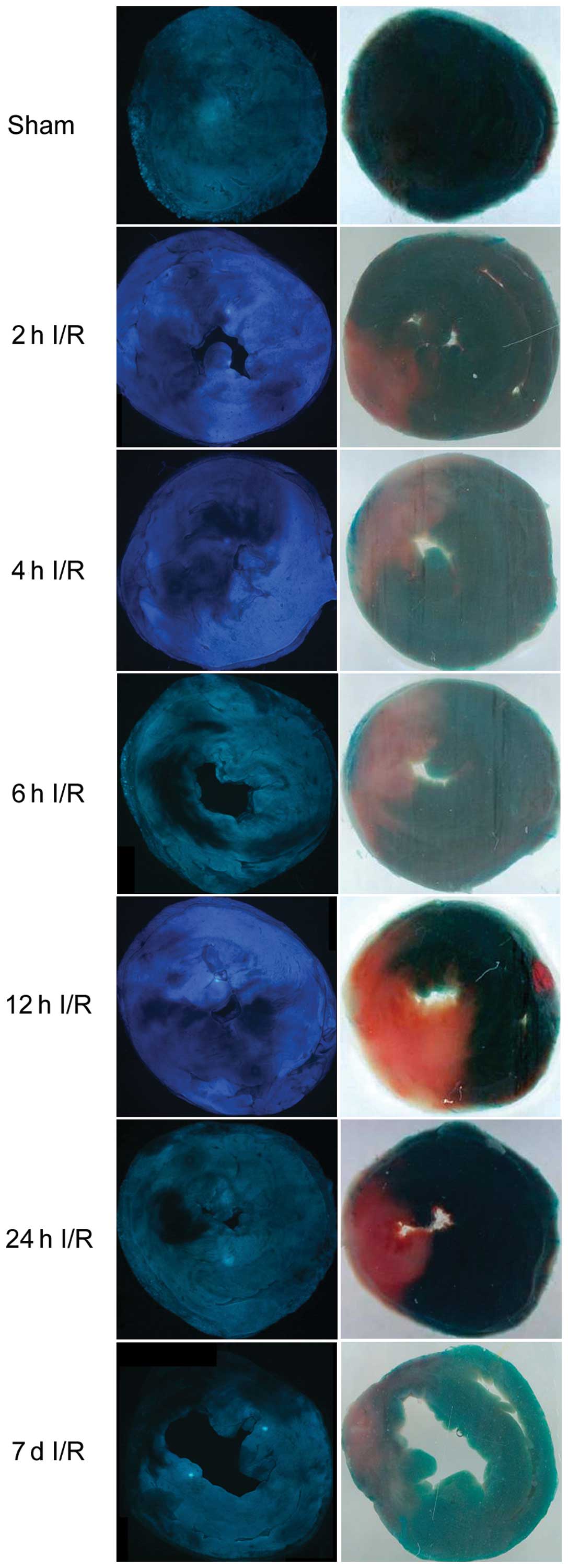
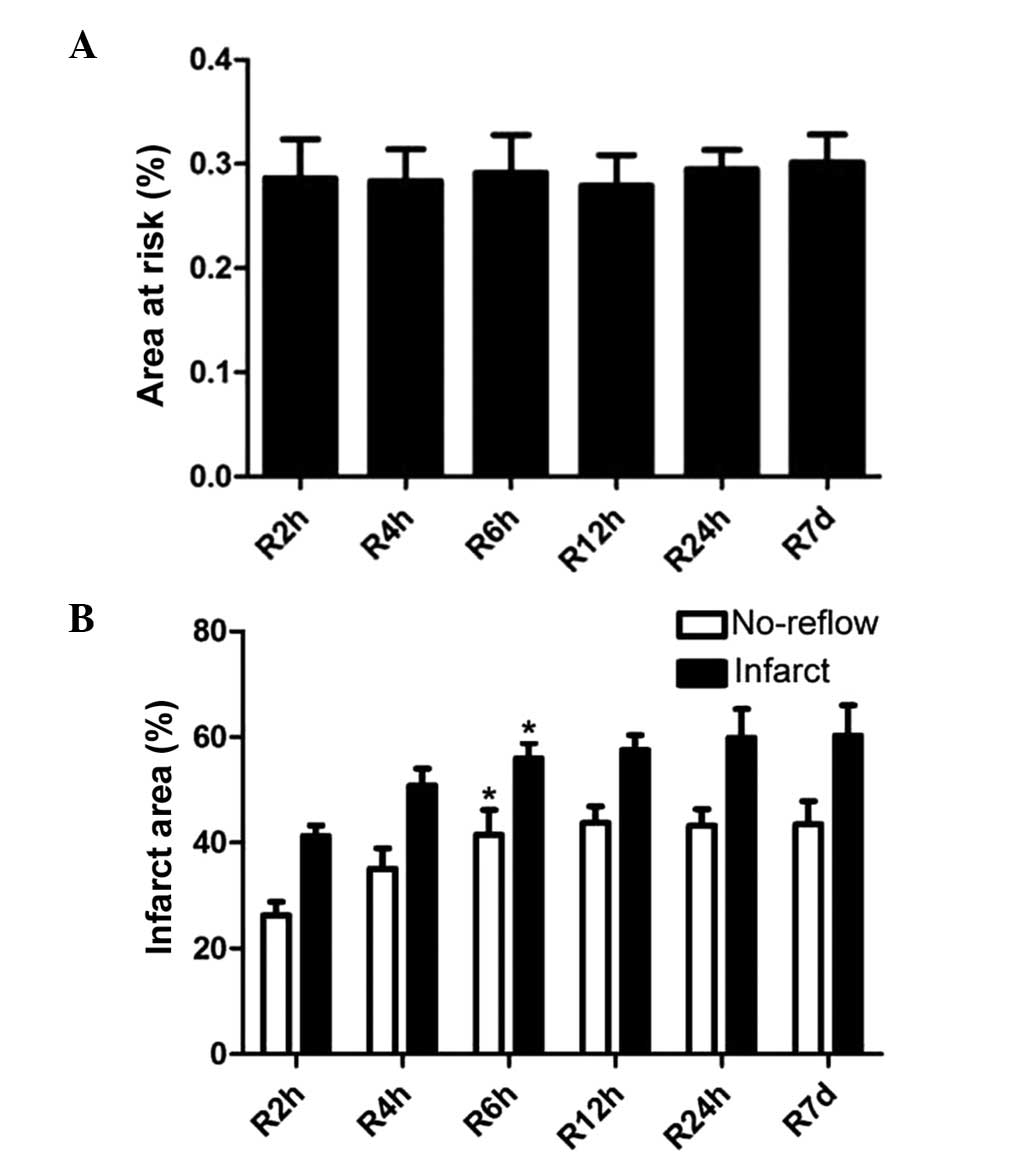
24 h and 7 days of reperfusion in rats (Fig. 1). The average area at risk was
28.60±9.20% in the 2 h reperfusion group, 28.35±7.55% in the 4 h
reperfusion group, 29.14±8.95% in the 6 h reperfusion group,
27.91±7.21% in the 12 h reperfusion group, 29.50±4.52% in the 24 h
reperfusion group and 30.14±6.53% in the 7 day reperfusion group,
with no significant differences among the six groups (Fig. 2A). However, the infarct area and
no-reflow area were significantly greater following 6 h of
reperfusion when compared with the 2 h reperfusion group
(P<0.05; Fig. 2B).
Enhanced expression of mRNA and protein
levels of GDF-15 following myocardial I/R
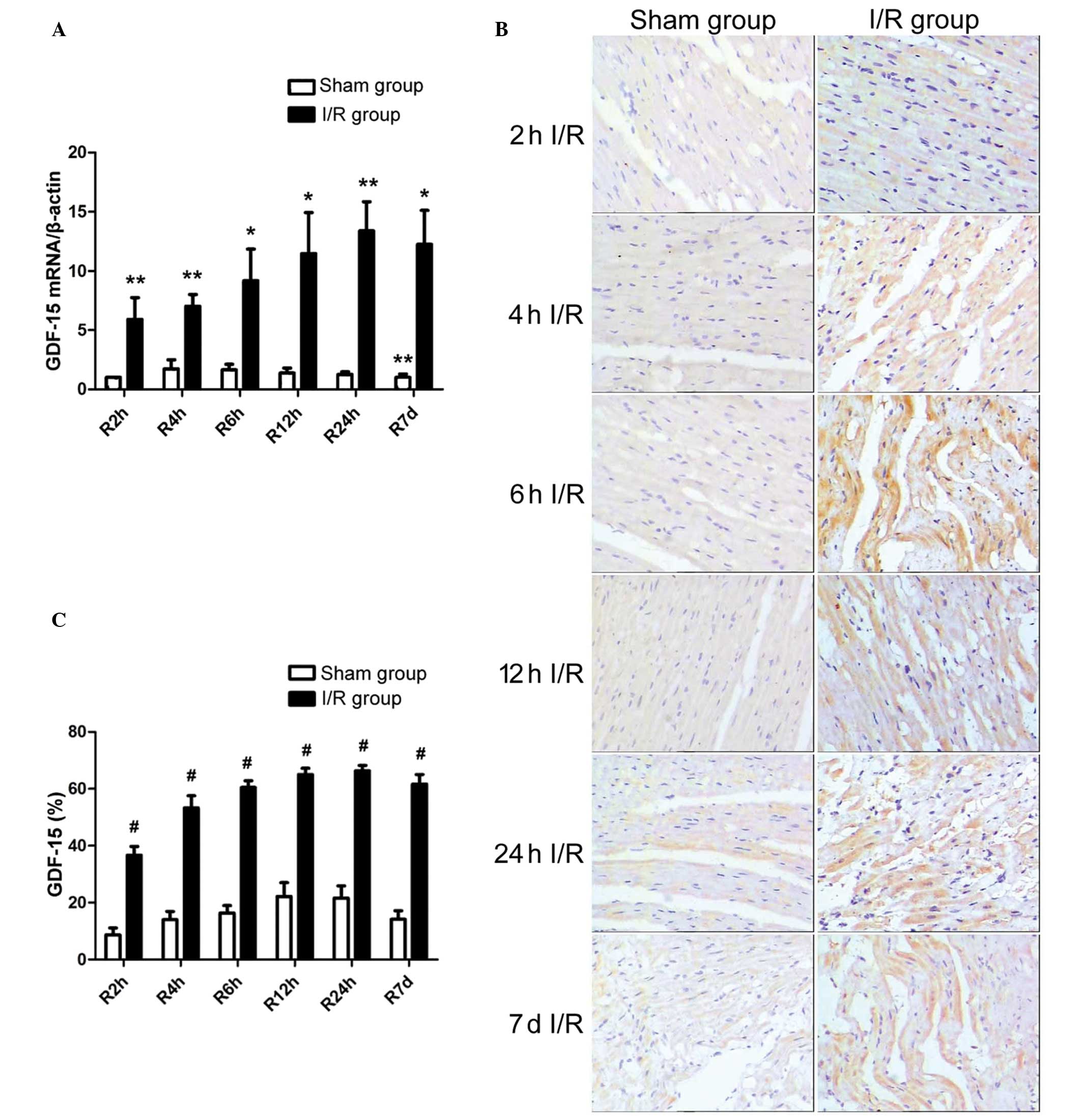
RT-qPCR demonstrated the increased left ventricular
mRNA expression of GDF-15 in I/R rats, with GDF-15 mRNA levels
significantly greater at the different reperfusion time points when
compared with that of the sham operation groups at the 2 h
reperfusion time point (Fig. 3A).
The enhanced expression of GDF-15 was confirmed by the
immunohistochemical staining, which indicated that the protein
expression levels of GDF-15 were upregulated following different
reperfusion times, as compared with that of the 2 h reperfusion
group in the sham operated rats (P<0.001; Fig. 3B and C). In addition, the mRNA and
protein expression levels of GDF-15 were increased with increasing
reperfusion time, and peaked at the reperfusion time of 24 h.
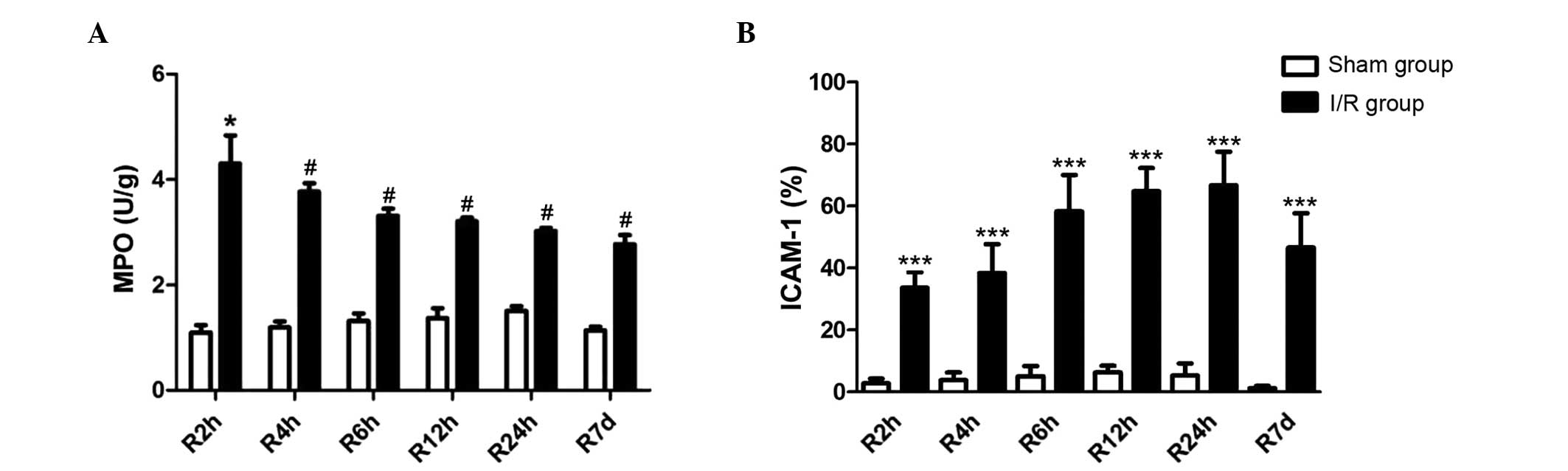
Increased MPO activity following
myocardial I/R
ELISA analysis indicated that MPO activities,
examined as an indicator of neutrophil infiltration and
accumulation in myocardial tissues, were significantly increased in
the I/R rats when compared with the sham operated rats, at all the
reperfusion time points investigated. However, MPO levels in the
I/R groups were attenuated with increasing reperfusion time, as
presented in Fig. 4A.
Enhanced ICAM-l expression following
myocardial I/R
Immunohistochemical staining indicated that the
expression of ICAM-1 was significantly enhanced following I/R, at
all reperfusion durations investigated. ICAM-1 levels were
increased with increasing durations of reperfusion, and the
greatest ICAM-1 level was observed in the 24 h reperfusion group
(Fig. 4B).
Time course of histopathological damage
following myocardial I/R
Results from the H&E staining indicated that in
the I/R group there was increasing neutrophil adherence to the
myocardial microvascular endothelium. In addition apoptosis and
necrosis (myocardial cell swelling, karyolysis, inflammatory cell
infiltration, and endothelial cell swelling and detachment) were
observed in numerous myocardial cells following I/R, in particular
at the reperfusion time points of 2, 4 and 6 h. The reperfusion for
24 h resulted in the death of myocardial cells, indicted by the
appearance of large tissue spaces, while in the sham operation
group marginal damage was observed in the threading sites (Fig. 5).
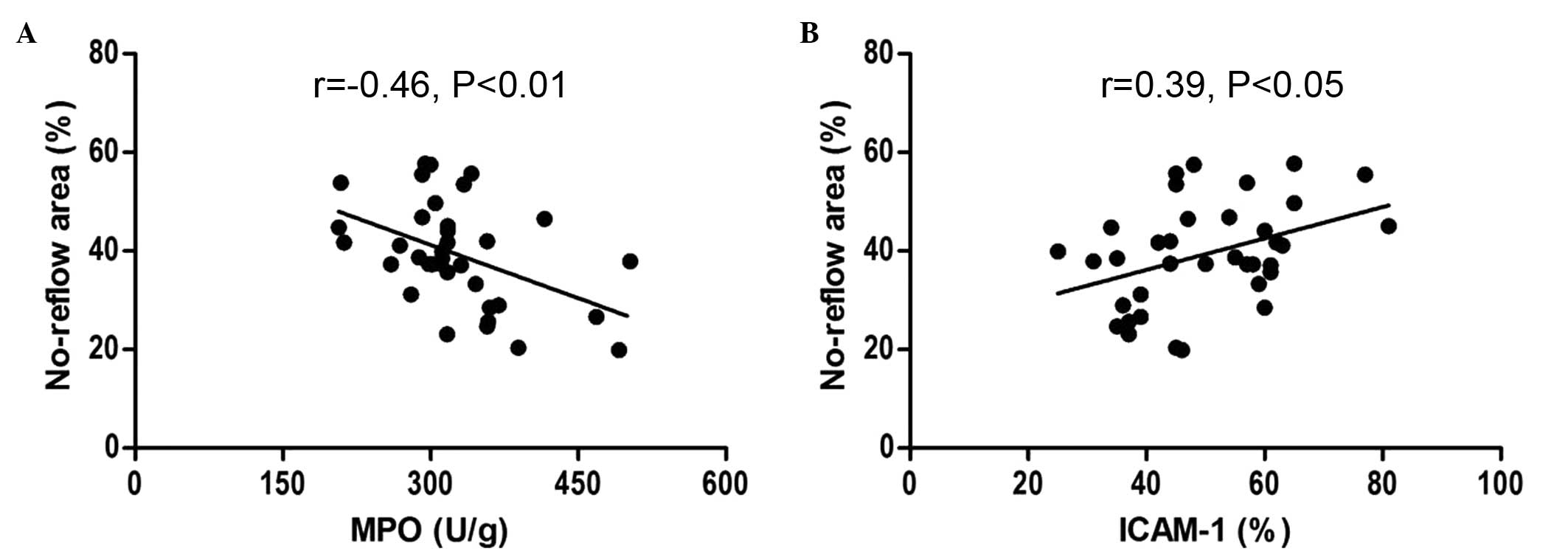
Correlation analysis of no-reflow area
with MPO and ICAM-1 levels following myocardial I/R
Correlation analysis indicated that in the I/R
group, the area of no-reflow was negatively correlated with MPO
activity (r=−0.46, P<0.01), however positively correlated with
ICAM-1 levels (r=0.39, P<0.05), as presented in Fig. 6.
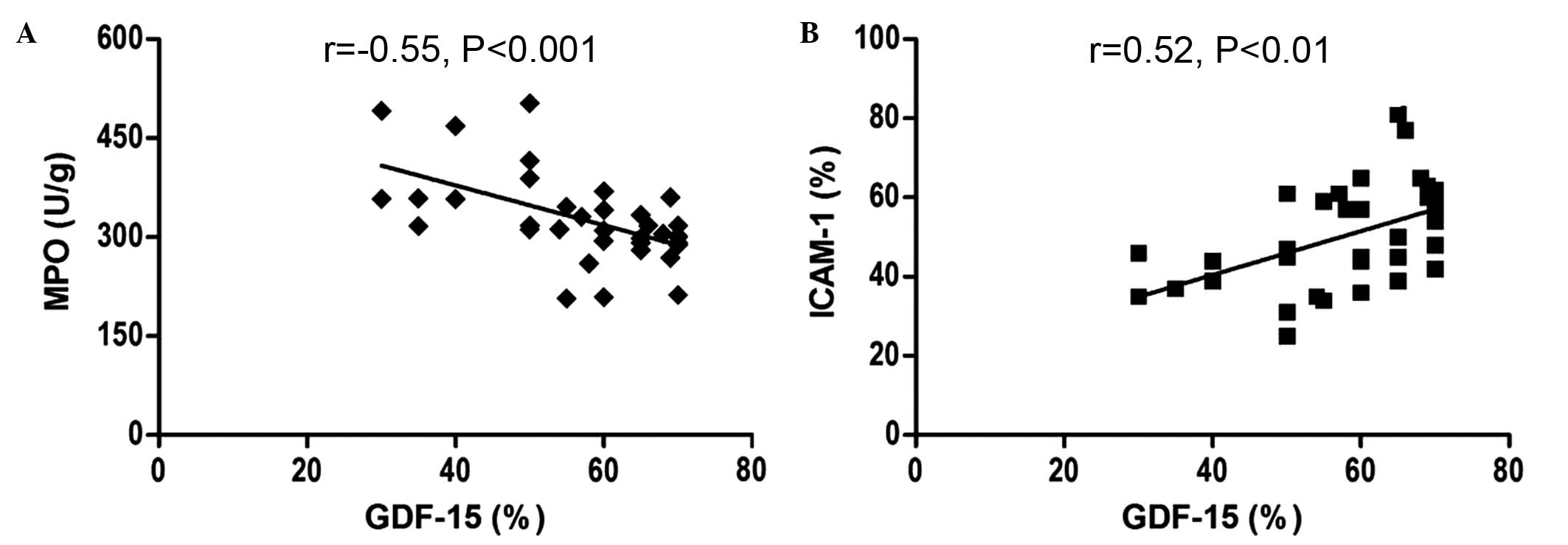
Correlation analysis of GDF-15 with MPO
and ICAM-1 levels following myocardial I/R
There was a negative association between the GDF-15
level and MPO activity (r=−0.55, P<0.001), while the ICAM-1
level was positively associated with the GDF-15 expression levels
following I/R (r= 0.52, P<0.01; Fig. 7).
Discussion
The current study demonstrated that the mRNA and
protein expression levels of GDF-15 were increased during the onset
and development of no-reflow, and peaked at 24 h of reperfusion.
GDF-15 was negatively correlated with the activity of MPO, an
indicator of neutrophil infiltration and accumulation in myocardial
tissue which contributes to the pathogenesis of myocardial
infarction, whilst MPO activity was negatively correlated with the
area of no-reflow. GDF-15 was significantly positively correlated
with ICAM-1 levels, which is known to be essential for the
transendothelial migration of neutrophils. Furthermore, ICAM-1
levels were observed to be significantly positively correlated with
the no-reflow area. Together these data suggest that GDF-15 may
serve a role in the pathophysiological process of no-reflow by
inhibiting neutrophil infiltration in an inflammatory-like response
to I/R.
No-reflow is a phenomenon defined as the inadequate
myocardial reperfusion of a given coronary segment without apparent
vessel obstruction. Increasing evidence suggests that no-reflow is
a strong and independent predictor of prognosis following I/R
(11,21), and is associated with a high
incidence of ventricular arrhythmias, heart failure and adverse
left ventricular remodeling following myocardial infarction
(22). No-reflow appears to
persist over an extended period of time rather than occurring as an
immediate event at the moment of reperfusion (23). However, the pathophysiology of
no-reflow is multifactorial and remains to be fully elucidated. I/R
injury may serve a central role in the pathophysiology of no-reflow
and the duration of reperfusion has been suggested to be one of the
major determinants of anatomical no-reflow, which is a form of
reperfusion injury at the microvascular level (24). An increased size of the no-reflow
zone has been reported to be associated with longer reperfusion
intervals in experimental infarction (19). In clinical settings, no-reflow was
reported to initiate while the patients are in hospital, however,
additionally has been demonstrated to occur in the months following
discharge (25,26), and to progress over time with large
interpatient variability (11,25).
In order to investigate the time course effect of myocardial I/R
injury in the current study, the area at risk, infarct area and
anatomical no-reflow were measured following 1 h of coronary artery
ligation and 2, 4, 6, 12, 24 h and 7 days of reperfusion in the
rat. These data demonstrated that in addition to the increase in
infarct area from 44.62±7.36 following 2 h reperfusion to
60.32±13.97 following 7 days of reperfusion, the anatomical zone of
no-reflow appeared to reach a plateau at 6 h without further
significant deterioration at 7 days. By contrast, there was no
significant difference in the area at risk during the different
reperfusion periods. A previous study in a canine myocardial
infarction model demonstrated that the zone of no-reflow was
continuously increased within the first 48 h of reperfusion
(27), and in a rabbit myocardial
infarction model the greatest significant increase in no-reflow
area occurred within the first 1–2 h of reperfusion (19). In addition, no-reflow was
established to persist for a minimum of 4 weeks within the
infarcted rat myocardial tissues (28). The precise time-course of no-reflow
differs between species and within the same species at various time
points of reperfusion, and the size of the no-reflow zone has been
demonstrated to be increased with time following reperfusion, as
demonstrated by the current study and previous studies (7,29,30).
Furthermore, the current study provided evidence to suggest that
the majority of no-reflow development occurs during the first hours
(6 h) following initiation of reperfusion, which may present a
potential time-window for therapeutic interventions (24). There is increasing evidence that
the myocardium adapts to I/R by synthesizing and responding to a
variety of stress-induced growth factors and cytokines, and that
identification of these endogenous homeostatic mechanisms may
provide novel targets for limiting I/R injury (31).
GDF-15, also known as macrophage inhibitory
cytokine-1, has been demonstrated to be a cardioprotective
cytokine, and is strongly and independently associated with the
prognosis of patients with acute myocardial infarction (32). GDF-15 has been used for risk
stratification in ST-elevation and non-ST-elevation acute coronary
syndrome, suggesting that GDF-15 may play a distinct role in the
pathophysiological process (33,34).
Increased expression of GDF-15 has been identified in patients with
myocardial I/R and in animal cardiomyocytes subjected to simulated
I/R (35). GDF-15 expression
levels were observed to be significantly increased within 12 h of
symptom onset and remained upregulated for a minimum of 2 weeks in
tissue from the infarcted myocardium of patients (14), while Eggers et al (35) reported a significant reduction in
GDF-15 levels during the first 6 months following acute coronary
syndrome. In a rat myocardial I/R model, Kempf et al
(14) demonstrated that a certain
period of ischemia was required for GDF-15 expression and secretion
during reperfusion, as indicated by the increased expression of
GDF-15 following 3 h ischemia and 3 h reperfusion however not
following 1 h ischemia and 5 h reperfusion. In contrast to the
results of these studies (15,16,36,37),
the current study demonstrated that expression of GDF-15 was
significantly enhanced by 1 h of ischemia followed by 2, 4, 6, 12,
24 h and 7 days of reperfusion. The mRNA and protein expression
levels of GDF-15 in myocardial tissue were significantly
upregulated by reperfusion and increased with the reperfusion time,
reaching a peak at 24 h of reperfusion. These differences may be
due to the different animals supplied and methods used. However,
and despite these differences, the current study provided evidence
suggesting that myocardial I/R induced a time-dependent increase in
the expression of GDF-15 within the first 24 h of the reperfusion
period, and that GDF-15 may act as a cardio-protective factor
immediately following I/R injury.
Reperfusion following ischemia is associated with
pathophysiological alterations that are reported to share numerous
characteristics with the myocardial inflammatory response, and
neutrophils may serve a major role in this inflammatory-like
response to I/R (9). In a previous
study, few neutrophils were observed to be present in normal or
acutely ischemic myocardium, whilst neutrophil accumulation and
infiltration was identified within the first hours following I/R
(38). Neutrophils may contribute
to myocardial necrosis, which in turn results in persistent
ischemia and the no-reflow phenomenon. In addition, neutrophil
depletion during the reperfusion period has been associated with
significant reductions in the area of the infarct and no-reflow
zones (39). The H&E staining
in the current study demonstrated significant induction of
myocardial neutrophil infiltration by I/R, in particular following
reperfusion for 2, 4 and 6 h. MPO is a well-established marker of
neutrophil infiltration and is required for the formation of
neutrophil extracellular traps, web-like structures consisting of
neutrophil DNA, granular proteins and several cytoplasmic proteins
that contribute to the inflammatory and immune responses following
injury (40), therefore MPO
activity was used as a marker of neutrophil accumulation and
infiltration (37). ICAM-1 is a
counter ligand for the β2-integrin cluster of differentiation
11b/18 on neutrophils and is required for the transendothelial
migration of neutrophils (41),
with ICAM-1 knockout associated with reduced I/R-induced myocardial
damage in a mouse model (42). The
current study demonstrated that I/R insult resulted in significant
enhancements of MPO activity and ICAM-1 expression, with MPO
activity markedly reduced with increasing reperfusion time.
However, the expression of ICAM-1 was observed to increase with
increasing reperfusion duration and peaked in the 24 h reperfusion
group, suggesting an increase in neutrophil accumulation and
infiltration occurred following myocardial I/R. Furthermore, there
was a significant correlation between the no-reflow area and MPO
activity, and between expression of ICAM-1 and the no-reflow area.
In addition, MPO and ICAM-1 levels were significantly correlated
with the myocardial expression levels of GDF-15 following I/R,
consistent with a previous study, which indicated that GDF-15 was
markedly associated with the levels of ICAM-1 (43). In a previous study, the interaction
of activated integrin with ICAM-1 was reported to lead to
neutrophil arrest on the endothelium, initiate transendothelial
migration and was required for neutrophil adherence to sites of
sterile inflammation (44).
Activation of β2-integrin exposed the ICAM-1 binding domain and
increased the affinity for ICAM-1 (45).
GDF-15 has been identified as an endogenous
inhibitor that counteracts β2-integrin activation. A previous study
demonstrated that GDF-15 is the first cytokine identified to
contribute to the inhibition of leukocyte recruitment, adhesion and
transendothelial migration, via blocking activation of β2-integrin
affinity and clustering, thereby preventing neutrophil adhesion to
ICAM-1 and transendothelial migration (17). Thus, this suggests that GDF-15
protects against myocardial no-reflow in a mouse model of
myocardial infarction at the early stage of reperfusion. This
effect may be through the inhibition of MPO activity, in addition
to integrin activation and integrin-ICAM-1 interaction that
contributes to the promotion of inflammatory neutrophil recruitment
and neutrophil arrest on the endothelium, initiating
transendothelial migration, which in turn results in reduced
neutrophil infiltration and myocardial tissue injury. However,
further studies are required to clarify the potential
mechanisms.
In conclusion, the current study demonstrates the
time course effect of reperfusion on the expression of GDF-15 in
the myocardial I/R rat model. The expression of GDF-15 was
demonstrated to increase with increasing reperfusion time in the
onset and development no-reflow. Reperfusion for 6 h resulted in
significant re-flow in ischemic myocardium. Therefore, GDF-15 may
protect the I/R myocardium from no-reflow by inhibiting the
inflammatory response that predominantly involves neutrophil
infiltration and trans-endothelial migration.
Acknowledgments
The current study was supported by the Tianjin
Natural Science Foundation (grant nos. 12JCYBJC15900 and
09JCYBJC11900) and the Science Foundation of Chinese People's Armed
Police Force Logistics University (grant no. WHTD201301-3).
References
|
1
|
Lee G, Sou SM, Twerenbold R, Reichlin T,
Oshima S, Hochgruber T, Zürcher S, Matter D, Tanglay Y, Freese M,
et al: B-type natriuretic peptide and clinical judgment in the
detection of exercise-induced myocardial ischemia. Am J Med.
127:427–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bell RM and Yellon DM: Conditioning the
whole heart - not just the cardiomyocyte. J Mol Cell Cardiol.
53:24–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ, Tsang A, Mocanu MM and
Yellon DM: Ischemic preconditioning protects by activating
prosurvival kinases at reperfusion. Am J Physiol Heart Circ
Physiol. 288:H971–H976. 2005. View Article : Google Scholar
|
|
4
|
Zeymer U, Bauer T, Gersh BJ, Zahn R, Gitt
A, Jünger C and Senges J: Beneficial effect of reperfusion therapy
beyond the preservation of left ventricular function in patients
with acute ST-segment elevation myocardial infarction. Int J
Cardiol. 146:177–180. 2011. View Article : Google Scholar
|
|
5
|
Braunwald E and Kloner RA: Myocardial
reperfusion: A double-edged sword? J Clin Invest. 76:1713–1719.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin J-Y, Hung L-M, Lai LY and Wei F-C:
Kappa-opioid receptor agonist protects the microcirculation of
skeletal muscle from ischemia reperfusion injury. Ann Plast Surg.
61:330–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nallamothu BK, Bradley EH and Krumholz HM:
Time to treatment in primary percutaneous coronary intervention. N
Engl J Med. 357:1631–1638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niccoli G, Burzotta F, Galiuto L and Crea
F: Myocardial no-reflow in humans. J Am Coll Cardiol. 54:281–292.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordan JE, Zhao ZQ and Vinten-Johansen J:
The role of neutrophils in myocardial ischemia-reperfusion injury.
Cardiovasc Res. 43:860–878. 1999. View Article : Google Scholar
|
|
10
|
Ito H, Maruyama A, Iwakura K, Takiuchi S,
Masuyama T, Hori M, Higashino Y, Fujii K and Minamino T: Clinical
implications of the 'no reflow' phenomenon. A predictor of
complications and left ventricular remodeling in reperfused
anterior wall myocardial infarction. Circulation. 93:223–228. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ndrepepa G, Tiroch K, Fusaro M, Keta D,
Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A, et
al: 5-year prognostic value of no-reflow phenomenon after
percutaneous coronary intervention in patients with acute
myocardial infarction. J Am Coll Cardiol. 55:2383–2389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Li Z and Gao W: Growth
differentiation factor 15 in cardiovascular diseases: From bench to
bedside. Biomarkers. 16:466–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao EC, Koniaris LG, Zimmers-Koniaris T,
Sebald SM, Huynh TV and Lee SJ: Characterization of
growth-differentiation factor 15, a transforming growth factor β
superfamily member induced following liver injury. Mol Cell Biol.
20:3742–3751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kempf T, Eden M, Strelau J, Naguib M,
Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin
JD, et al: The transforming growth factor-β superfamily member
growth-differentiation factor-15 protects the heart from
ischemia/reperfusion injury. Circ Res. 98:351–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonaca MP, Morrow DA, Braunwald E, Cannon
CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L and
Wollert KC: Growth differentiation factor-15 and risk of recurrent
events in patients stabilized after acute coronary syndrome:
Observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol.
31:203–210. 2011. View Article : Google Scholar
|
|
16
|
Anand IS, Kempf T, Rector TS, Tapken H,
Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H and Wollert
KC: Serial measurement of growth-differentiation factor-15 in heart
failure: Relation to disease severity and prognosis in the
Valsartan Heart Failure Trial. Circulation. 122:1387–1395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kempf T, Zarbock A, Widera C, Butz S,
Stadtmann A, Rossaint J, Bolomin-Vittori M, Korf-Klingebiel M, Napp
LC, Hansen B, et al: GDF-15 is an inhibitor of leukocyte integrin
activation required for survival after myocardial infarction in
mice. Nat Med. 17:581–588. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eitel I, Blase P, Adams V, Hildebrand L,
Desch Sm, Schuler G and Thiele H: Growth-differentiation factor 15
as predictor of mortality in acute reperfused ST-elevation
myocardial infarction: insights from cardiovascular magnetic
resonance. Heart. 97:632–640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reffelmann T and Kloner RA: Microvascular
reperfusion injury: Rapid expansion of anatomic no reflow during
reperfusion in the rabbit. Am J Physiol Heart Circ Physiol.
283:H1099–H1107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Chen P, Yu H, Luo W, Pi M, Wu Y,
Wang L, Yang F and Gou Y: Combinatorial effects of conception and
governor vessel electroacupuncture and human umbilical cord
blood-derived mesenchymal stem cells on pathomorphologic lesion and
cellular apoptosis in rats with cerebral ischemia/reperfusion. J
Tradit Chin Med. 33:779–786. 2013. View Article : Google Scholar
|
|
21
|
Chan W, Stub D, Clark DJ, Ajani AE,
Andrianopoulos N, Brennan AL, New G, Black A, Shaw JA, Reid CM, et
al Melbourne Interventional Group Investigators: Usefulness of
transient and persistent no reflow to predict adverse clinical
outcomes following percutaneous coronary intervention. Am J
Cardiol. 109:478–485. 2012. View Article : Google Scholar
|
|
22
|
Galasso G, Schiekofer S, D'Anna C, Gioia
GD, Piccolo R, Niglio T, Rosa RD, Strisciuglio T, Cirillo P,
Piscione F, et al: No-reflow phenomenon: Pathophysiology,
diagnosis, prevention, and treatment. A review of the current
literature and future perspectives. Angiology. 65:180–189. 2014.
View Article : Google Scholar
|
|
23
|
Rezkalla SH and Kloner RA: No-reflow
phenomenon. Circulation. 105:656–662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reffelmann T and Kloner RA: The no-reflow
phenomenon: A basic mechanism of myocardial ischemia and
reperfusion. Basic Res Cardiol. 101:359–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niccoli G, Cosentino N, Lombardo A,
Sgueglia GA, Spaziani C, Fracassi F, Cataneo L, Minelli S, Burzotta
F, Maria Leone A, et al: Angiographic patterns of myocardial
reperfusion after primary angioplasty and ventricular remodeling.
Coron Artery Dis. 22:507–514. 2011.PubMed/NCBI
|
|
26
|
Poli A, Fetiveau R, Vandoni P, del Rosso
G, D'Urbano M, Seveso G, Cafiero F and De Servi S: Integrated
analysis of myocardial blush and ST-segment elevation recovery
after successful primary angioplasty: Real-time grading of
micro-vascular reperfusion and prediction of early and late
recovery of left ventricular function. Circulation. 106:313–318.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rochitte CE, Lima JA, Bluemke DA, Reeder
SB, McVeigh ER, Furuta T, Becker LC and Melin JA: Magnitude and
time course of microvascular obstruction and tissue injury after
acute myocardial infarction. Circulation. 98:1006–1014. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reffelmann T, Hale SL, Dow JS and Kloner
RA: No-reflow phenomenon persists long-term after
ischemia/reperfusion in the rat and predicts infarct expansion.
Circulation. 108:2911–2917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurrelmeyer KM, Michael LH, Baumgarten G,
Taffet GE, Peschon JJ, Sivasubramanian N, Entman ML and Mann DL:
Endogenous tumor necrosis factor protects the adult cardiac myocyte
against ischemic-induced apoptosis in a murine model of acute
myocardial infarction. Proc Natl Acad Sci USA. 97:5456–5461. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Croisille P, Revel D and Saeed M: Contrast
agents and cardiac MR imaging of myocardial ischemia: from bench to
bedside. Eur Radiol. 16:1951–1963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reffelmann T, Hale SL, Li G and Kloner RA:
Relationship between no reflow and infarct size as influenced by
the duration of ischemia and reperfusion. Am J Physiol Heart Circ
Physiol. 282:H766–H772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan SQ, Ng K, Dhillon O, Kelly D, Quinn
P, Squire IB, Davies JE and Ng LL: Growth differentiation factor-15
as a prognostic marker in patients with acute myocardial
infarction. Eur Heart J. 30:1057–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wollert KC, Kempf T, Lagerqvist B, Lindahl
B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H,
et al: Growth differentiation factor 15 for risk stratification and
selection of an invasive treatment strategy in non ST-elevation
acute coronary syndrome. Circulation. 116:1540–1548. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kempf T, Björklund E, Olofsson S, Lindahl
B, Allhoff T, Peter T, Tongers J, Wollert KC and Wallentin L:
Growth-differentiation factor-15 improves risk stratification in
ST-segment elevation myocardial infarction. Eur Heart J.
28:2858–2865. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eggers KM, Kempf T, Lagerqvist B, Lindahl
B, Olofsson S, Jantzen F, Peter T, Allhoff T, Siegbahn A, Venge P,
et al: Growth-differentiation factor-15 for long-term risk
prediction in patients stabilized after an episode of
non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc
Genet. 3:88–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wollert KC: Growth-differentiation
factor-15 in cardiovascular disease: From bench to bedside, and
back. Basic Res Cardiol. 102:412–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Go LO, Murry CE, Richard VJ, Weischedel
GR, Jennings RB and Reimer KA: Myocardial neutrophil accumulation
during reperfusion after reversible or irreversible ischemic
injury. Am J Physiol. 255:H1188–H1198. 1988.PubMed/NCBI
|
|
38
|
Litt MR, Jeremy RW, Weisman HF,
Winkelstein JA and Becker LC: Neutrophil depletion limited to
reperfusion reduces myocardial infarct size after 90 minutes of
ischemia. Evidence for neutrophil-mediated reperfusion injury.
Circulation. 80:1816–1827. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu FC, Chuang YH, Tsai YF and Yu HP: Role
of neutrophil extracellular traps following injury. Shock.
41:491–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang C, Li R, Zeng Q, Ding Y, Zou Y, Mao
X, Hu W, Xiong R and Li M: Effect of minocycline postconditioning
and ischemic postconditioning on myocardial ischemia-reperfusion
injury in atherosclerosis rabbits. J Huazhong Univ Sci Technolog
Med Sci. 32:524–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Issekutz AC, Rowter D and Springer TA:
Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in
neutrophil transendothelial migration. J Leukoc Biol. 65:117–126.
1999.PubMed/NCBI
|
|
42
|
Metzler B, Mair J, Lercher A, Schaber C,
Hintringer F, Pachinger O and Xu Q: Mouse model of myocardial
remodelling after ischemia: Role of intercellular adhesion
molecule-1. Cardiovasc Res. 49:399–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eggers KM, Kempf T, Lind L, Sundström J,
Wallentin L, Wollert KC and Siegbahn A: Relations of
growth-differentiation factor-15 to biomarkers reflecting vascular
pathologies in a population-based sample of elderly subjects. Scand
J Clin Lab Invest. 72:45–51. 2012. View Article : Google Scholar
|
|
44
|
Wang J and Arase H: Regulation of immune
responses by neutrophils. Ann NY Acad Sci. 1319:66–81. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Froio RM, Sciuto TE, Dvorak AM,
Alon R and Luscinskas FW: ICAM-1 regulates neutrophil adhesion and
transcellular migration of TNF-α-activated vascular endothelium
under flow. Blood. 106:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|