Introduction
Approximately 80% of the aging population is
suffering from lower back pain, an endemic problem that causes
substantial disability (1–3). Intervertebral disc (IVD) degeneration
is the leading cause of lower back pain (4). However, conservative treatment (such
as medication and physical therapy) and surgical treatments target
symptom reduction, rather than repairing or decelerating the
underlying degenerative process. Determining the mechanism
underlying the degeneration of the IVD may aid in developing
strategies to decelerate or reverse this process.
The IVD consists of two regions, namely, the inner
nucleus pulposus (NP) and the outer annulus fibrosus (AF). Normal
NP cells produce aggrecan and collagen type II. Aggrecans are
polyanionic due to their high content of carboxyl and sulfate
groups, and thus attract and bind water molecules, hydrating the
tissue in order to resist compressive loads (5). Since NP cells have an important role
in maintaining the integrity of IVD (6), NP cell loss resulting from necrosis
and apoptosis contributes to disc degeneration (7–10).
Therefore, the analysis of the apoptosis and proliferation of NP
cells may provide a novel therapeutic approach for the reversal of
the degenerative process.
Among the apoptosis signaling pathways, two
predominant caspase-dependent signaling pathways may be observed:
The intrinsic and the extrinsic signaling pathways, which are
mediated by the mitochondria and death receptor, respectively.
Numerous studies have demonstrated that the mechanism underlying
apoptosis in degenerated NP cells involves the two types of
signaling pathway, although the involvement varied among patients.
The two signaling pathways ultimately induce caspase-3 to initiate
apoptosis (11–14).
Survivin is a baculovirus inhibitor of apoptosis
repeat motif protein, which is located on the long arm of
chromosome 17 at position q25. It is abundantly expressed in
embryonic tissues and in the majority of tumors, however, survivin
is not present in normal differentiated cells (15–17).
The predominant function of survivin is the regulation of mitosis
progression and apoptosis inhibition (18). In embryonic tissues, survivin is
expressed in a strictly regulated manner during development, and
has an important role in the control of embryonic cell
mitosis/cytokinesis and apoptosis (19). In human cancer, survivin expression
detected by immunohistochemical staining is an important prognostic
parameter. High survivin expression levels in tumors correlates
with a more aggressive and invasive clinical phenotype, and poor
prognosis and low responsiveness to chemotherapeutic agents can be
expected (20,21). Survivin has been proposed as a
suitable molecular target for future anticancer therapies. However,
a recent study reported a role of survivin in non-malignant tissues
and normal cells (15). In
rheumatoid arthritis and osteoarthritis, high mRNA and protein
expression levels of survivin were observed, and results on the
proliferation and apoptosis of chondrocytes were consistent with
the findings of the present study (22–24).
The role of survivin in degenerated NP cells remains
to be elucidated. The present study investigated the expression
levels of survivin in human NP cells in vitro, and reported
selective survivin expression in degenerated human NP cells.
Further understanding of survivin expression and function in NP
cells may be an important step towards understanding the mechanism
underlying degeneration, and may prove useful in the development of
future gene therapies to decelerate the degeneration of NPs.
Materials and methods
Materials
Degenerative human NP cells were donated by ten
patients, which included six men and four women, with a mean age of
58 years (range, 47–66 years; Table
I). All patients were diagnosed with disk degeneration using
magnetic resonance imaging and had undergone spinal fusion to
relieve their chronic lower back pain. The degenerative condition
of intervertebral disk degeneration was evaluated according to the
Pfirrmann's grading system (25).
Normal NP cells were collected from two young male patients (aged
22 and 24 years) undergoing posterior nucleotomy in their left
ventricles, spinal fusion, and decompression, and were stable
within 24 h of trauma. The two men did not have any previous
documented clinical history of lower back pain, and their cells
were used as controls. The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China), and informed consent was signed by all patients
involved in the present study, in which they agreed to provide
their individual clinical information.
 | Table IPatient details and Pfirrmann
grade. |
Table I
Patient details and Pfirrmann
grade.
| Number | Gender | Age (years) | Diagnosis | Pfirrmann grade |
|---|
| 1 | Male | 47 | Disc degeneration
(L4/l5) | V |
| 2 | Male | 54 | Disc degeneration
(L5/S1) | IV |
| 3 | Male | 61 | Disc degeneration
(L4/l5) | V |
| 4 | Female | 59 | Disc degeneration
(L4/l5) | IV |
| 5 | Female | 54 | Disc degeneration
(L5/S1) | V |
| 6 | Male | 66 | Disc degeneration
(L4/l5) | IV |
| 7 | Female | 63 | Disc degeneration
(L4/l5) | V |
| 8 | Male | 55 | Disc degeneration
(L5/S1) | IV |
| 9 | Female | 63 | Disc degeneration
(L5/S1) | V |
| 10 | Male | 58 | Disc degeneration
(L5/S1) | V |
| 11 | Male | 22 | Lumber trauma
(L4/l5) | I |
| 12 | Male | 24 | Lumber trauma
(L4/l5) | II |
The tissue samples were harvested and collected
under sterile conditions at The Affiliated Hospital of Qingdao
University in 2014 between May and August. The patients with normal
cells had been involved in trauma accidents. Complete culture
medium [Dulbecco's modified Eagle's medium (DMEM)-F12, GE
Healthcare Life Science GE Healthcare Life Science, Logan, UT, USA]
supplemented with fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) maintained at 4°C was used as
transport medium. All biopsies were delivered to the laboratory
(the Medical Research Center at The Affiliated Hospital of Qingdao
University) for processing of cell culture on the day of
harvest.
Cell isolation and culture
Tissue samples were weighed and washed twice in
phosphate-buffered saline (PBS). The NP and AF were separated based
on their macroscopic morphologies by omitting the transitional
zone, as previously described (6).
Cells from each patient were isolated and cultured separately to
avoid interference. The NP tissue samples were sectioned (~1
mm2) prior to being digested with 0.25% trypsinase (GE
Healthcare Life Science) at 37°C under gentle agitation. After 20
min, digestion was stopped using DMEM-F12 supplemented with 15%
fetal calf serum, and the tissue samples were centrifuged at 560 ×
g for 5 min. Subsequently, 0.5% collagenase Type II (ICN
Biomedicals Inc., Costa Mesa, CA, USA) was used to treat the cells
at 37°C for ~4 h. The tissue samples were centrifuged at 560 × g
for 5 min and washed three times with DMEM-F12 supplemented with
15% fetal calf serum.
The cells were transferred to a 12.5-cm2
culture flask at a density of 105 cells/ml. The cells
were maintained in DMEM-F12 supplemented with 15% fetal calf serum
and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.), and cultured in a CO2 incubator
(SANYO Electric Co. Ltd., Moriguchi, Japan) at 37°C with humidity
(95%). The growth medium was changed every three days following
cell adherence. All experiments were conducted during passage two,
and sub-confluent cultures were used.
Cell culture in ischemic condition
NP cells were cultured in DMEM culture medium
(glucose deprivation) in a CO2 incubator at 37°C with 1%
oxygen and 95% humidity. These cells were subsequently used for
analyzing caspase-3 activity.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
mRNA survivin expression levels were assayed by
RT-qPCR. Total mRNA was extracted from the NP cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to a
one-step method in the manufacturer's instructions. A total of 1
µg mRNA was reverse transcribed into cDNA in 10 µl
reaction mixture using PrimeScript RT reagent (Takara Biotechnology
Co., Ltd., Dalian, China), and the reaction product was treated
with RNAse-free DNase I (Takara Biotechnology Co., Ltd.). The
absorbance was measured at 260 and 280 nm using a
Safire2 automatic microplate reader (Tecan Group Ltd.,
Männedorf, Switzerland) for quantification and quality control.
PCR was conducted with the following cycling
conditions in a LightCycler® 480 II (Roche Diagnostics,
Basel, Switzerland): 95°C for 5 min followed by 33 cycles of 94°C
for 45 sec, 56°C for 45 sec, 72°C for 45 sec, and a final extension
step at 72°C for 10 min. The probes were designed using the
PrimerExpress program (Applied Biosystems Life Technologies, Foster
City, CA, USA). Total gene specificity was confirmed through BLAST
searches (GenBank database sequences; www.ncbi.nlm.nih.gov/genbank/). The primers were
purchased from Sangon Biotech Co. Ltd. (Shanghai, China; Table II). Human GAPDH was used as the
internal control. In each experiment, the samples were analyzed in
duplicates. The values for survivin were associated with those of
their controls using the comparative Cq method (ΔΔCq method).
 | Table IINucleotide sequences of sense and
antisense primers. |
Table II
Nucleotide sequences of sense and
antisense primers.
| Gene | Primer | PCR product
(bp) |
|---|
| Survivin | F:
CAGATGACGACCCCATAGAGGA
R: CCTTTGCAATTTTGTTCTTGGC | 141 |
| GAPDH | F:
GGATTTGGTCGTATTGGG
R: GGAAGATGGTGATGGGATT | 205 |
Protein extraction and western blot
analysis
For the cell culture extracts, adherent cells were
washed with ice-cold PBS, and cell lysis was performed using 200
µl radioim-munoprecipitation assay buffer (Aidlab
Biotechnologies Co., Ltd, Beijing, China) with 2 µl
phenylmethanesulfonyl fluoride (Shanghai Macklin Biochemical
Co.,Ltd., Shanghai, China) on ice for 45 min. The cells were
removed by scraping. Subsequently, the lysis solution was
centrifuged at 6,700 × g for 5 min at 4°C. For western blot
analysis of survivin, GAPDH was used as an internal control, and
the proteins were separated by 10% SDS-PAGE (Beyotime Institute of
Biotechnology, Haimen, China) prior to being transferred onto
immobilon-P membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% fat-free dried milk and incubated
with monoclonal rabbit antibody (cat. no. ab76424; Abcam,
Cambridge, MA, USA) at 4°C for 8 h. The membranes were washed three
times with PBS (15 min per wash). The membranes were then incubated
with horseradish peroxidase-conjugated secondary antibody, goat
anti-rabbit IgG (dilution, 1:2,000; cat. no. CW0103; CW Bio,
Beijing, China) at room temperature for 2 h and the bands were
visualized using chemiluminescence (Pierce Biotechnology, Inc.,
Rockford, IL, USA). The images were analyzed using a Fusion FX7
(Vilber Lourmat, Marne-la-Vallée, France).
Survivin knockdown by small interfering
(si)RNA
For transfection analysis, the cells were seeded
into six-well plates at 4×105 cells/well for 4 h prior
to knockdown being performed. For knockdown of survivin, an siRNA
with the following sequence: Sense, 5′-GCGCCUGCACCCCGGAGCG-3′ and
antisense, 5′-CGCUCCGGGGUGCAGGCGC-3′, was trans-fected as
previously described (24,26). An siRNA targeting green
fluorescence protein (GFP) with the following sequence: Sense,
5′-GUGUGCUGUUUGGAGGUCTT-3′, and antisense,
5′-GAACUCCAAACAGCACACCTT-3′, was transfected to serve as the
negative control. All siRNAs (Sangon Biotech Co., Ltd.) were used
at a concentration of 100 nmol/l, and transfected using X-treme
GENE siRNA transfection reagent (Roche Diagnostics).
Caspase-3 activity assay
Apoptosis levels were investigated by measuring
caspase-3 activity using a Caspase-3 Colorimetric Assay kit
(BioVision Inc., Milpitas, CA, USA). The NP cells were counted and
seeded at 1.5×106 cells for 24 or 48 h following
survivin-specific or control siRNA transfection. The cells were
then re-suspended in cell lysis buffer and 50 µl of 2X
reaction buffer (containing 10 mM DTT; BioVision, Inc.) and 5
µl DEVE-pNA were added. The samples were incubated for 90
min at 37°C, and their absorbancies were read at 405 nm using a
microplate reader (Sunrise; Tecan Group Ltd., Männedorf,
Switzerland).
Measurement of cell proliferation by
quantification of bromodeoxyuridine (BrdU) incorporation
A commercial ELISA cell proliferation assay (Roche
Diagnostics) was used according to the manufacturer's protocol. A
total of 48 h post-transfection with survivin-specific or control
siRNA, the cells were seeded at a density of 7×103 and
cultured in 96-well microtiter plates prior to being treated with
BrdU (final concentration, 10 mM; Roche Diagnostics) for 6 h.
Following treatment with a fixation solution (Roche Diagnostics),
the cells were labeled with anti-BrdU-POD Fab fragments (Roche
Diagnostics). The bound antibody was then quantified using a
peroxidase substrate (luminol/4-iodophenol; Roche Diagnostics), and
light emission was measured using a luminometer (Sunrise; Tecan
Group Ltd.).
Statistical analysis
All values are presented as the mean ± standard
error of the mean. Student's t-test and one-way analysis of
variance (ANOVA) and a post hoc Fisher's Least Significant
Difference (LSD) test were used to analyze the statistical
significance of the differences. Statistical analyses were
performed using SPSS 19 (IBM, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
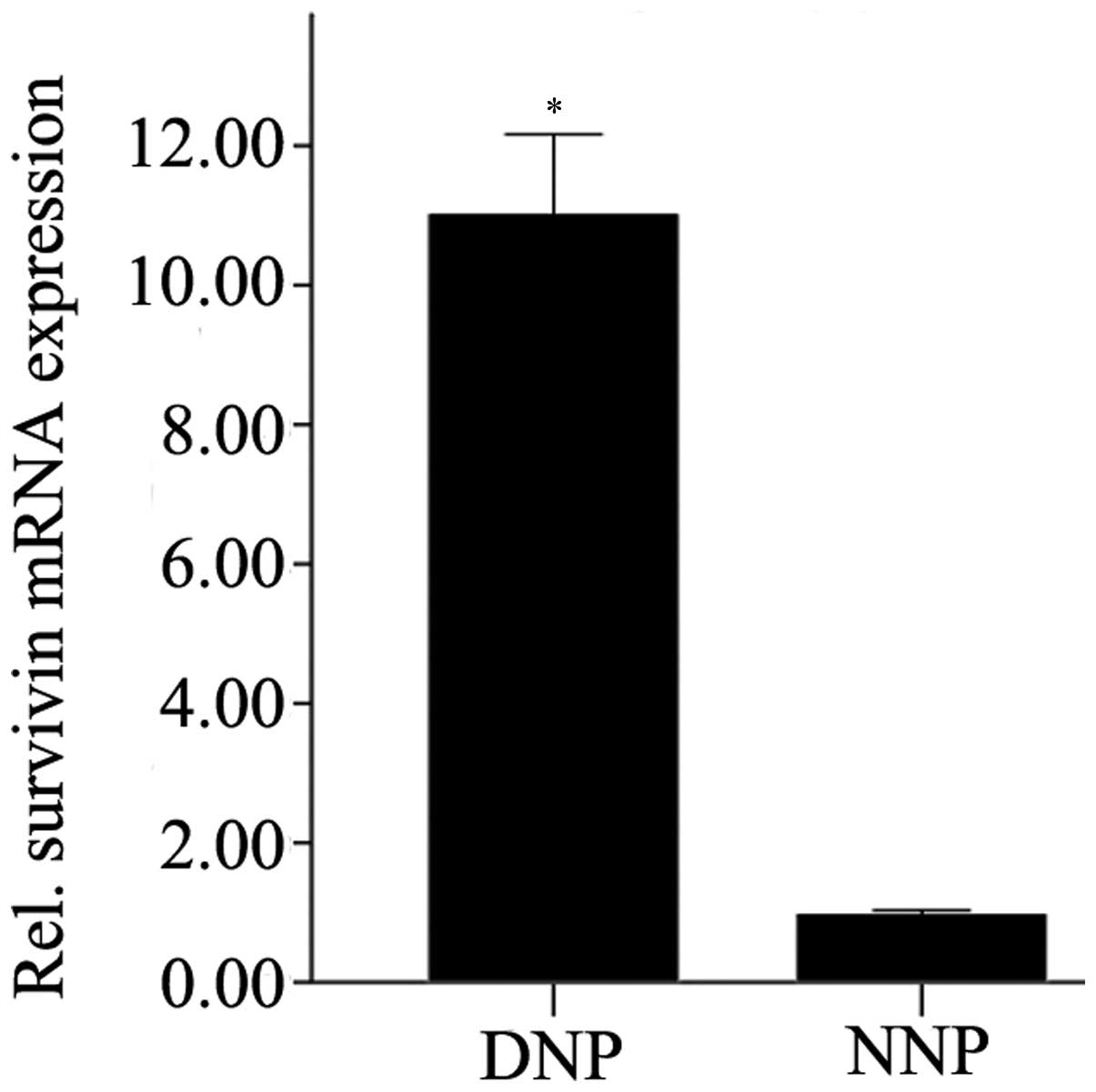
Survivin is expressed in degenerated NP
cells
Survivin expression in NP cells was analyzed by
RT-qPCR. The mRNA expression levels of survivin in degenerative NP
cells were significantly higher compared with that in normal NP
cells (P=0.01; Fig. 1).
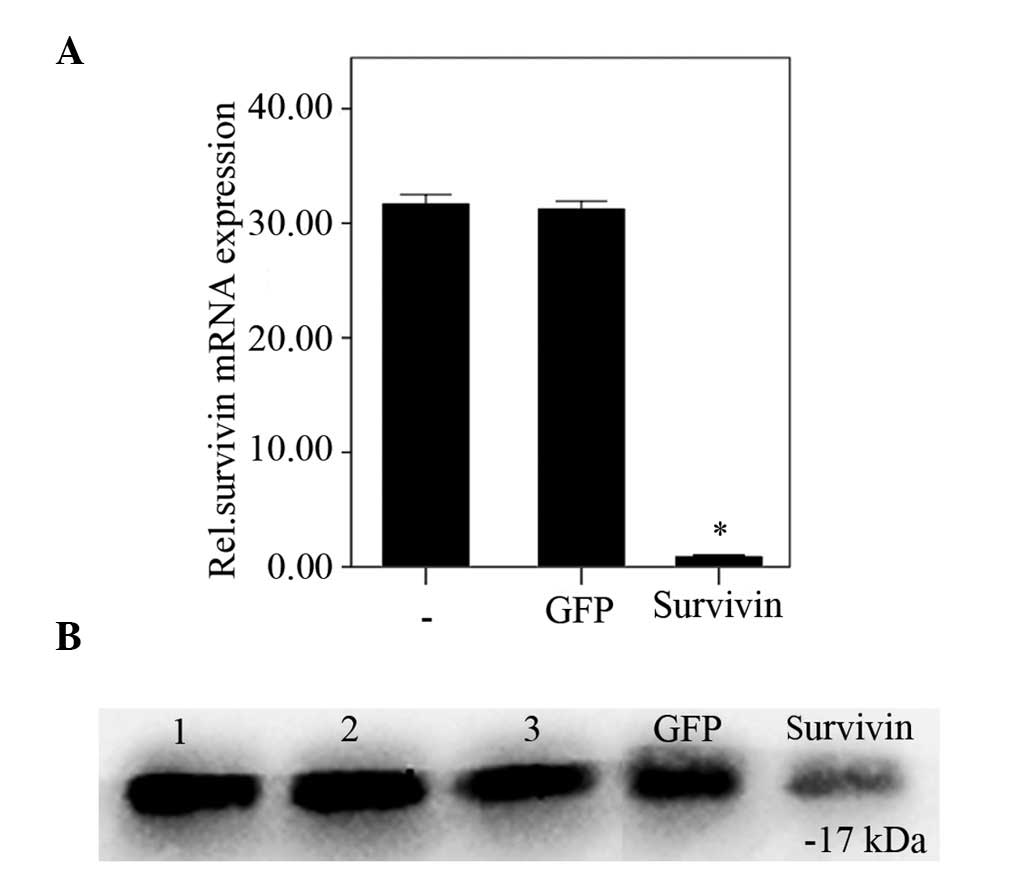
Expression levels of survivin
post-transfection with survivin-specific siRNA
Survivin expression levels in degenerative NP cells
were analyzed using RT-qPCR and western blot analysis following
transfection with survivin-specific siRNA. Protein and mRNA
survivin expression levels were significantly reduced 48 h
post-transfection with survivin-specific siRNA, compared with the
negative and blank control groups (P<0.01). No significant
difference was observed between the negative and blank control
groups (P=0.57; Fig. 2A and
B).
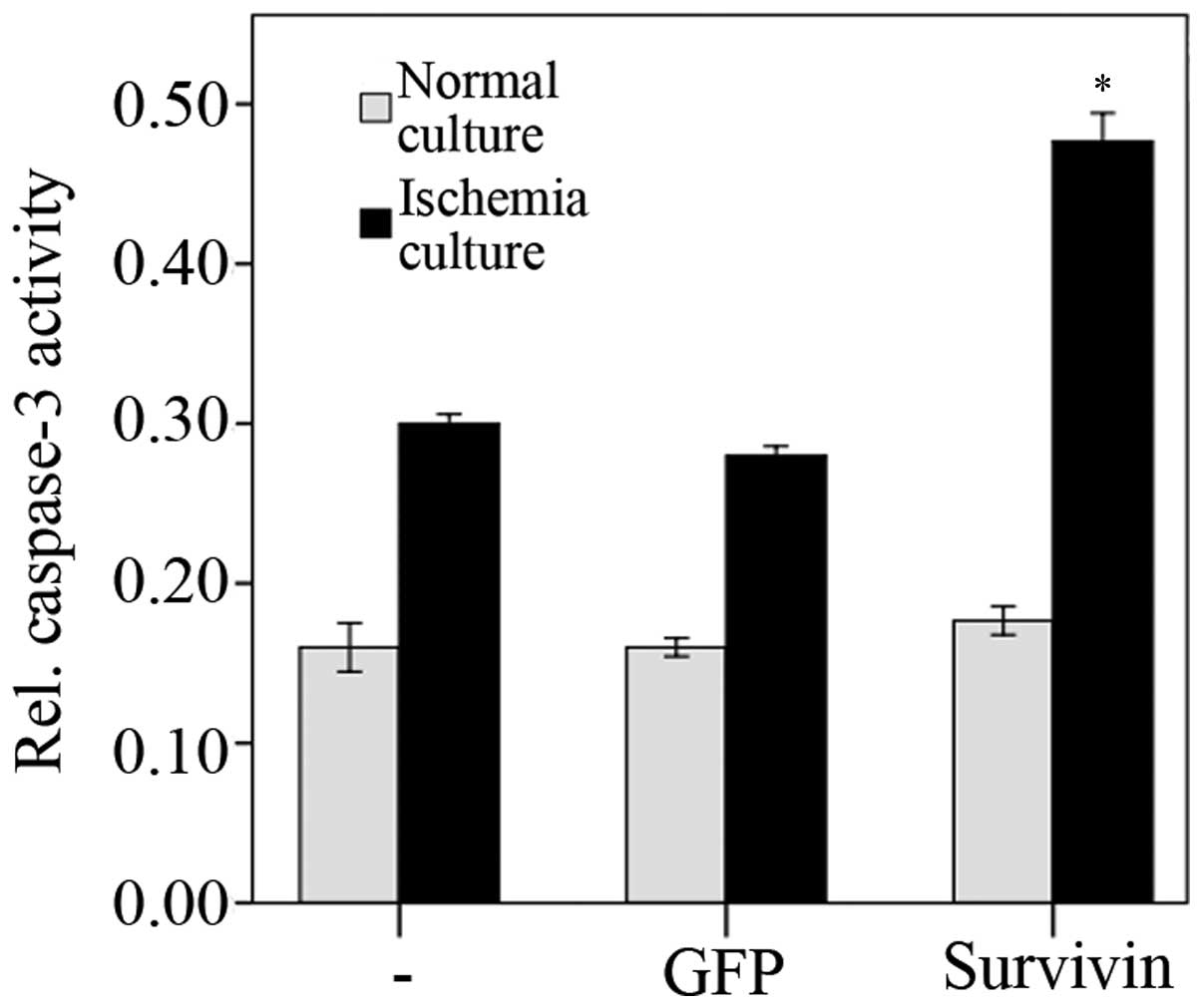
Survivin knockdown leads to an increased
sensitivity to pro-apoptotic stimuli
The activity levels of caspase-3 48 h
post-transfection with siRNA were assayed. Under normal culture
conditions (unstressed), transfection with survivin-specific siRNA
did not lead to significantly altered caspase-3 activity levels,
compared with the negative and blank control groups (P=0.49;
Fig. 3). However, when exposed to
ischemia in vitro (1% oxygen and glucose deprivation),
caspase-3 activity levels significantly increased 48 h after
transfection with siRNA (survivin siRNA + in vitro ischemia
compared with GFP siRNA + in vitro ischemia and
untransfected + in vitro ischemia, P<0.001;
Fig. 3). ANOVA and subsequent LSD
tests revealed increased apoptotic rates under all transfection
conditions (untransfected + unstressed compared with untransfected
+ in vitro ischemia, P<0.01; GFP siRNA + unstressed
compared with GFP siRNA + in vitro ischemia, P<0.01; and
survivin siRNA + unstressed compared with survivin siRNA + in
vitro ischemia, P<0.01) where unstressed refers to the NP
cells that were cultured in normal rather than ischemic conditions.
The transfection of GFP had no significant effect on apoptotic
levels (untransfected + unstressed compared with GFP siRNA +
unstressed, P=0.64; and untransfected + in vitro ischemia
compared with GFP siRNA + in vitro ischemia, P=0.17).
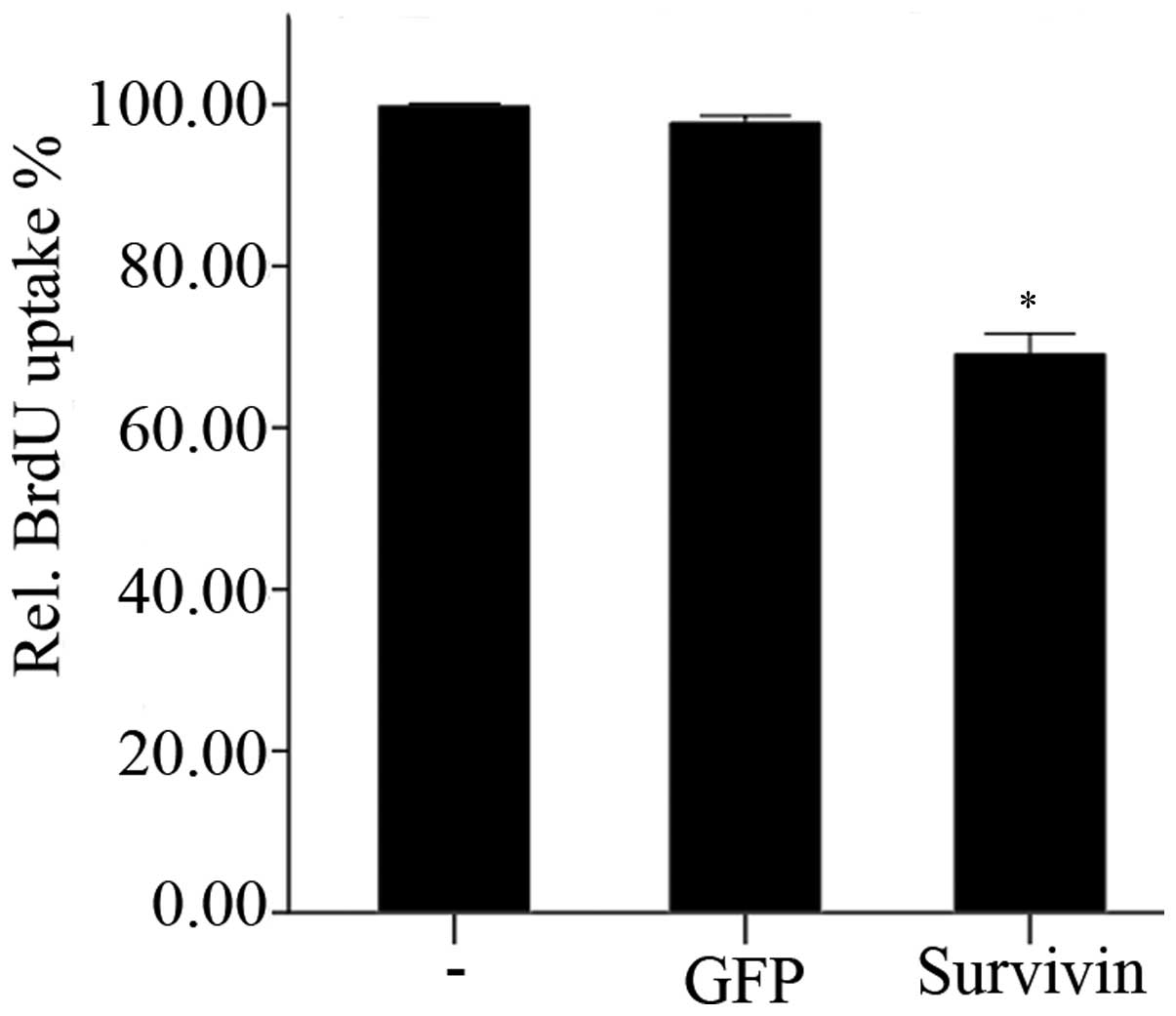
Survivin knockdown leads to reduced
proliferation rates
The effects of transfection with survivin-specific
siRNA on the proliferation of NP cells is shown in Fig. 4. BrdU uptake was significantly
(P<0.01) reduced 48 h following knockdown of survivin, compared
with the negative and blank control groups. However, transfection
with GFP-siRNA did not lead to any significant alterations in BrdU
uptake after 48 h, compared with the blank control group
(P=0.347).
Discussion
Degenerative disc disease is a serious and common
health care problem, and therapeutic strategies have traditionally
focused primarily on treating the symptoms. Therefore, novel
methods that would stimulate the regeneration of disc tissues or
decelerate the progress of age-associated disc degeneration are
required. Thus, it is important to understand the changes that
occur with aging, the causes of these changes, and the mechanism
underlying degeneration.
Several studies have investigated the mechanisms
underlying disc degeneration, including a decrease in cellular
concentration, cell senescence, cell apoptosis, decreasing
extracellular matrix anabolism and increasing extracellular matrix
catabolism (27,28). The role of oncofetal gene survivin
has been extensively investigated in cell proliferation and
apoptosis in tumor cells (29,30).
However, limited data is available regarding its expression in
degenerative NP cells. Yang et al (31) reported that survivin was expressed
in fetal disc tissue samples and was differentially expressed
between degenerated NP tissue samples and normal NP tissue samples
(31,32). Immunohistochemical staining
demonstrated that survivin expression was present in 20-, 26- and
28-week fetal age intervertebral discs, and the differences in
expression levels between samples were not statistically
significant. Survivin expression levels were detectable in
degenerated NP tissue samples, whereas they were significantly
downregulated in normal NP tissue (P=0.048). These results
demonstrated that survivin has an important role in fetal
intervertebral disc growth, and is likely to be involved in the
regulation of apoptosis and cell proliferation during the
degeneration of NP tissue (31,32).
Based on the above-mentioned results (31,32),
the difference between the expression levels of survivin in
degenerative NP cells and normal NP cells was investigated in the
present study. The mRNA expression levels of survivin were
significantly increased in degenerative NP cells, whereas absent or
low expression levels were reported in normal NP cells, which was
concordant with the survivin expression levels reported in the
corresponding tissue samples of a previous study (32). The results indicated the expression
of survivin gene transcription and translation in degenerative NP
cells, and suggested that survivin may have a role in the
degeneration of NP cells. Therefore, based on the effects and
function of survivin, alterations in cell proliferation and
apoptosis were measured following survivin knockdown by siRNA. The
results revealed the role of survivin in the degeneration of NP
cells.
siRNA is an effective tool for silencing the
expression of survivin, and GFP-siRNA was used as a negative
control in the present study. At the RNA level, survivin knockdown
48 h after transfection led to a marked reduction in survivin mRNA
expression levels. The protein expression level of survivin was
also significantly decreased 48 h post transfection.
Cell proliferation was measured through the
quantification of BrdU incorporation. BrdU uptake in the
degenerative NP cells was reduced following survivin knockdown and
suppression. These results suggested a role for survivin in cell
proliferation. Numerous studies have demonstrated that survivin is
essential for mitosis and cell division, and knockdown of survivin
function has been associated with cell division defects,
demonstrated by the presence of supernumerary centrosomes, the
formation of multipolar mitotic spindles and the failure of
cytokinesis (33–35). Therefore, in degenerative NP cells,
the re-expression of survivin may be useful to increase cell
proliferation in order to compensate for the decreasing cellular
concentration during the degenerative process.
Survivin also has an anti-apoptotic function. No
alteration between the activity levels of caspase-3 in the positive
and control groups were demonstrated in the unstressed NP cells.
These results may be attributable to the culture conditions
(sufficient oxygen and glucose). However, the spinal disc is the
largest avascular organ in the body and does not directly supply
blood to NP cells (36,37), therefore material and gas exchange
predominantly depends on diffusion from the nearest blood supply.
Owing to the progressive age-associated degeneration and
calcification of the cartilage endplate (27), the number of arteries that supply
the periphery of the disc decreases. This decrease impairs the
diffusion function of the disc such that nutrition and oxygen
supply deteriorate. Therefore, degenerative NP cells are under
relatively ischemic conditions. Following in vitro ischemic
culture (1% oxygen, glucose deprivation), caspase-3 activity levels
in the NP cells were significantly increased compared with
unstressed NP cells, and after transfection of survivin-siRNA,
caspase-3 activity levels significantly increased. The results
demonstrated that ischemic culture conditions induced NP cell
apoptosis, and expression of survivin may contribute to the
reduction of caspase-3 activity exhibiting an anti-apoptotic
function in the degeneration process.
In the present study, siRNA was used to investigate
the role of survivin in degenerative NP cells, and the results
revealed the effects of survivin-siRNA transfection on the
proliferation and apoptosis of NP cells under ischemic conditions
in vitro. The signaling pathway of survivin in cell division
and apoptosis requires further investigation in order to provide a
greater understanding of the mechanism underlying survivin
regulation. In addition, the construction of lentiviral-survivin
for transfection of the degenerative NP cells, and examination of
the effects of long-term expression of survivin in degenerative NP
cells will be a further research direction.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that survivin is
expressed in degenerative NP cells, and analyzed the general
functions of survivin, including in cell proliferation and
apoptosis. The results of the present study demonstrated that the
expression of survivin had an important role in degenerative NP
cells. The data suggested that survivin was a potential target for
gene therapy, and supplied fundamental information regarding
decreasing NP cell apoptosis by increasing survivin expression
levels in order to decelerate the degeneration of NP cells.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81171758).
References
|
1
|
Hoy D, Brooks P, Blyth F and Buchbinder R:
The Epidemiology of low back pain. Best Pract Res Clin Rheumatol.
24:769–781. 2010. View Article : Google Scholar
|
|
2
|
Takahashi K, Aoki Y and Ohtori S:
Resolving discogenic pain. Eur Spine J. 17(Suppl 4): S428–S431.
2008. View Article : Google Scholar
|
|
3
|
Hillman M, Wright A, Rajaratnam G, Tennant
A and Chamberlain MA: Prevalence of low back pain in the community:
Implications for service provision in Bradford UK. J Epidemiol
Community Health. 50:347–352. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen WH, Liu HY, Lo WC, Wu SC, Chi CH,
Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ and Deng WP:
Intervertebral disc regeneration in an ex vivo culture system using
mesenchymal stem cells and platelet-rich plasma. Biomaterials.
30:5523–5533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metallo-proteinases 3 during human intervertebral disc
degeneration. Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vonk LA, Kroeze RJ, Doulabi BZ,
Hoogendoorn RJ, Huang C, Helder MN, Everts V and Bank RA: Caprine
articular, meniscus and intervertebral disc cartilage: An integral
analysis of collagen network and chondrocytes. Matrix Biol.
29:209–218. 2010. View Article : Google Scholar
|
|
8
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in inter-vertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao CQ, Liu D, Li H, Jiang LS and Dai LY:
Interleukin-1beta enhances the effect of serum deprivation on rat
annular cell apoptosis. Apoptosis. 12:2155–2161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gruber HE and Hanley EN Jr: Analysis of
aging and degeneration of the human intervertebral disc. Comparison
of surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998. View Article : Google Scholar
|
|
11
|
Ha KY, Kim BG, Kim KW, Oh IS and Seo JY:
Apoptosis in the sequestrated nucleus pulposus compared to the
remaining nucleus pulposus in the same patient. Spine (Phila Pa
1976). 36:683–689. 2011. View Article : Google Scholar
|
|
12
|
Kaneyama S, Nishida K, Takada T, Suzuki T,
Shimomura T, Maeno K, Kurosaka M and Doita M: Fas ligand expression
on human nucleus pulposus cells decreases with disc degeneration
processes. J Orthop Sci. 13:130–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo YJ, Wu LC, Sun JS, Chen MH, Sun MG and
Tsuang YH: Mechanical stress-induced apoptosis of nucleus pulposus
cells: An in vitro and in vivo rat model. J Orthop Sci. 19:313–322.
2014. View Article : Google Scholar
|
|
14
|
Park JB, Lee JK, Park EY and Riew KD:
Fas/FasL interaction of nucleus pulposus and cancer cells with the
activation of caspases. Int Orthop. 32:835–840. 2008. View Article : Google Scholar
|
|
15
|
Li F and Brattain MG: Role of the Survivin
gene in pathophysiology. Am J Pathol. 169:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wheatley SP and McNeish IA: Survivin: A
protein with dual roles in mitosis and apoptosis. Int Rev Cytol.
247:35–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F and Ling X: Survivin study: An update
of 'what is the next wave'? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, de Bruin A, Caldas H, Fangusaro
J, Hayes J, Conway EM, Robinson ML and Altura RA: Essential role
for survivin in early brain development. J Neurosci. 25:6962–6970.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Tenev T, Martins LM, Downward J
and Lemoine NR: The ubiquitin-proteasome pathway regulates survivin
degradation in a cell cycle-dependent manner. J Cell Sci. 113(Pt
23): 4363–4371. 2000.PubMed/NCBI
|
|
21
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
22
|
Lechler P, Balakrishnan S, Schaumburger J,
Grässel S, Baier C, Grifka J, Straub RH and Renkawitz T: The
oncofetal gene survivin is re-expressed in osteoarthritis and is
required for chondrocyte proliferation in vitro. BMC Musculoskelet
Disord. 12:1502011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baran M, Möllers LN, Andersson S, Jonsson
IM, Ekwall AK, Bjersing J, Tarkowski A and Bokarewa M: Survivin is
an essential mediator of arthritis interacting with urokinase
signalling. J Cell Mol Med. 13:3797–3808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bokarewa M, Tarkowski A and Magnusson M:
Pathological survivin expression links viral infections with
pathogenesis of erosive rheumatoid arthritis. Scand J Immunol.
66:192–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar
|
|
26
|
Warnecke C, Zaborowska Z, Kurreck J,
Erdmann VA, Frei U, Wiesener M and Eckardt KU: Differentiating the
functional role of hypoxia-inducible factor (HIF)-1alpha and
HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin
is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J.
18:1462–1464. 2004.PubMed/NCBI
|
|
27
|
Buckwalter JA: Aging and degeneration of
the human intervertebral disc. Spine (Phila Pa 1976). 20:1307–1314.
1995.
|
|
28
|
Buckwalter JA, Woo SL, Goldberg VM, Hadley
EC, Booth F, Oegema TR and Eyre DR: Soft-tissue aging and
musculoskeletal function. J Bone Joint Surg Am. 75:1533–1548.
1993.PubMed/NCBI
|
|
29
|
Ghadimi MP, Young ED, Belousov R, Zhang Y,
Lopez G, Lusby K, Kivlin C, Demicco EG, Creighton CJ, Lazar AJ, et
al: Survivin is a viable target for the treatment of malignant
peripheral nerve sheath tumors. Clin Cancer Res. 18:2545–2557.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romagnoli M, Séveno C, Bataille R and
Barillé-Nion S: Survivin in cancerology: Molecular aspects and
therapeutic applications. Med Sci (Paris). 24:821–827. 2008.In
French. View Article : Google Scholar
|
|
31
|
Yang KS, Yue B and Ma XX: The expression
of survivin and its significance in fetal intervertebral disc.
Qingdao Daxue Yixueyuan Xuebao. 49:205–206. 2013.
|
|
32
|
Yang KS: The expression of survivin and
its significance ininter-vertebral disc. Dissertation. Qingdao
University; 2013
|
|
33
|
Chen J, Wu W, Tahir SK, Kroeger PE,
Rosenberg SH, Cowsert LM, Bennett F, Krajewski S, Krajewska M,
Welsh K, et al: Down-regulation of survivin by antisense
oligonucleotides increases apoptosis, inhibits cytokinesis and
anchorage-independent growth. Neoplasia. 2:235–241. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reed JC and Bischoff JR: BIRinging
chromosomes through cell division-and survivin' the experience.
Cell. 102:545–548. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Skoufias DA, Mollinari C, Lacroix FB and
Margolis RL: Human survivin is a kinetochore-associated passenger
protein. J Cell Biol. 151:1575–1582. 2000. View Article : Google Scholar
|
|
36
|
Anderson DG and Tannoury C: Molecular
pathogenic factors in symptomatic disc degeneration. Spine J. 5(6
Suppl): S260–S266. 2005. View Article : Google Scholar
|
|
37
|
Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K
and An HS: The origin of chondrocytes in the nucleus pulposus and
histologic findings associated with the transition of a notochordal
nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact
rabbit intervertebral discs. Spine (Phila Pa 1976). 28:982–990.
2003. View Article : Google Scholar
|