Introduction
By inhibiting bone resorption, bisphosphonates (BPs)
have been extensively used clinically for the treatment of
osteoporosis, Paget's disease and malignant diseases, including
multiple myeloma and metastasis to the bone (1,2).
Based on their chemical structure, BPs can be classified as either
nitrogen-containing or non-nitrogen-containing (3). As a bone metabolic regulator,
nitrogen-containing bisphosphonates (N-BPs) predominantly act on
osteoclasts. By inhibiting farnesyl diphosphate synthase, a key
enzyme in the mevalonic acid pathway, N-BPs inhibit the prenylation
of small GTPases, which maintain the functioning of osteoclasts.
The small GTPases accumulate in the cells, which erroneously
stimulates the downstream pathway, inhibiting the formation of
osteoclasts and inducing apoptosis of osteoclasts. Thus, the bone
resorption mediated by osteoclasts is reduced, lowering the bone
turnover rate and eventually inhibiting the bone mass loss
(4). Among the N-BPs, ZA exhibits
the most potent pharmacological action and affinity to bones,
particularly in sites of active bone metabolism (5).
In the last 30 years, with the continuing increase
in the clinical application of N-BPs, there has been increasing
awareness of the adverse reactions associated with their use,
including gastrointestinal symptoms, which develop with oral
administration, and severe esophagitis, vasculitis, pyrexia,
hypocalcemia and hypophosphatemia, which may be associated with
intravenous administration (6,7). In
2003, bisphosphonate-associated osteonecrosis of the jaw (BRONJ)
was reported for the first time, and relevant reports have been
constantly emerging since (8,9). The
American Association of Oral and Maxillofacial Surgeons defines
BRONJ as necrotic bone exposed in the maxillofacial region, lasting
for >8 weeks in patients treated with BPs who have not undergone
head and neck radiation therapy (10). Notably, a 0.8–12% cumulative
incidence of BRONJ in the USA was reported following the
intravenous injection of N-BPs for malignant disease in 2009
(10). This side effect is
predominantly observed with zoledronic acid (ZA) treatment due to
its high capacity for bone adhesion (11). To date, while the etiology of BRONJ
remains to be fully elucidated, a reduction in osteoblasts and
inhibition of osteoblast function have been observed in in
vivo studies (12,13), suggesting that the development of
BRONJ may be directly associated with the impact of ZA on
osteoblasts.
Bone morphogenetic proteins (BMPs), one of the
important extracellular signaling molecules regulating the
differentiation of osteoblast precursors into mature osteoblasts,
are a member of the transforming growth factor-β (TGF-β)
superfamily, which is a group of highly conservative functional
proteins with similar structures (14). BMPs induce the formation of bones,
cartilage and bone-associated connective tissues in organisms by
osteoblasts in an autocrine and paracrine-manner (15). BMP-2 is one of the most
investigated BMP family members, and has been identified as the
most potent inducer of osteogenesis. It acts by regulating the
Small mothers against decapentaplegic signaling pathway,
mitogen-activated protein kinase (MAPK) signaling pathway and
Runt-related transcription factor 2 (Runx2) signaling pathway
(16). Previous studies have
suggested that extracellular signal-regulated kinase (ERK) 1/2
activation stimulates osteoblast proliferation and differentiation,
activation of P38 is vital in osteoblast differentiation and Runx2
is an essential transcription factor for osteoblast differentiation
(17–21).
Although the in vivo and in vitro
actions of ZA on osteoclasts have been well-described, its role in
osteoblast function remains to be fully elucidated and remains
controversial at present. While Scheper et al claimed that
ZA concentrations of 0.4–4.5 µM were detected in the bone
tissues of patients with BRONJ (22), no well-recognized data are
available on the concentration of ZA to which osteoblasts are
exposed in organisms. In the past several decades, certain studies
have used specific concentrations of ZA to stimulate experimental
systems consisting of different types of osteoblasts, resulting in
varying conclusions (23–25). In addition, the impact of different
concentrations of ZA on the expression of BMP-2 e in osteoblasts
remains to be fully elucidated.
Therefore, the present study investigated the
effects of different concentrations of ZA on the viability and
functions of MC3T3-E1 cells, in order to reevaluate the effects of
ZA on osteoblasts in vitro and to examine the possible
etiopathogenesis of BRONJ.
Materials and methods
Cell culture
MC3T3-E1 cells, which are a well described as a
model for the osteoblastic phenotype (26), were obtained from the Cell Center
of the Chinese Academy of Medical Sciences (Beijing, China) and
were seeded at a density of 1×104 cells/cm2
for culture in regular growth culture media containing α-minimum
essential medium (α-MEM; GE Healthcare Life Sciences, Logan, UT,
USA) in a humidified atmosphere of 5% CO2 at 37°C. The
medium was supplemented with 10% fetal bovine serum (FBS; Gibco
Life Technologies, Grand Island, NY, USA), 100 U/l penicillin and
100 mg/l streptomycin (Gibco Life Technologies) in a humidified
atmosphere of 5% CO2 at 37°C. At 80% confluence, the
cells were cultured in osteoinductive medium, which was comprised
of α-MEM containing 10% FBS, 10 mM β-glycerophosphate
(Sigma-Aldrich, St. Louis, MO, USA), 50 µg/ml L-ascorbic
acid (Sigma-Aldrich) and 100 nM dexamethasone (Sigma-Aldrich). The
cells were then incubated with ZA (Sigma-Aldrich) at various
concentrations in a humidified atmosphere of 5% CO2 at
37°C. Cells in the control group were cultured in osteoinductive
medium without ZA. The medium was replaced every 3 days.
Cell Counting Kit (CCK)-8 assay
Cell viability was measured by the conversion of
Dojindo's highly water-soluble tetrazolium salt, WST-8, to a yellow
colored water-soluble formazan. The quantity of formazan dye
generated by the activity of mitochondrial dehydrogenases in the
cells is directly proportional to the cell viability. For the
assay, the MC3T3-E1 cells (1×104 cells/well) were
incubated in 96-well plates with osteoinductive medium in the
presence of various concentrations of ZA (0–100 µM) for 1,
3, 5 and 7 days in a humidified atmosphere of 5% CO2 at
37°C. Following treatment, 10 µl CCK-8 solution (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) was added to each
well and incubated at 37°C for 2 h. The optical density of each
well was measured using a microculture plate reader (Epoch; BioTek,
Winooski, VT, USA) at a wavelength of 450 nm.
Flow cytometric Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) assay
To assess apoptosis, an Annexin V/PI apoptosis kit
(MultiSciences Biotech, Co., Ltd., Hangzhou, China) was used,
according to the manufacturer's instructions. Briefly, following
incubation in 6-well plates with osteoinductive medium in the
presence of various concentrations of ZA (0, 0.01, 0.1, 1, 10 or
100 µM) for 1, 4 and 7 days, the cultured MC3T3-E1 cells
were gently resuspended in binding buffer and incubated for 5 min
at room temperature in the dark with 5 µl Annexin V-FITC and
10 µl PI. The AnnexinV-FITC and PI-labelled cells were
analyzed using a flow cytometer (FACSort; BD Biosciences,
Burlington, MA, USA). Using flow cytometry, dot plots of Annexin
V-FITC, on the X-axis, against PI, on the Y-axis, were used to
distinguish viable cells, which are negative for PI and Annexin
V-FITC, early apoptotic cells (Annexin V-positive/PI-negative) and
late apoptotic or necrotic cells
(AnnexinV-FITC-positive/PI-positive staining). The resultant data
was analyzed using CellQuest software version 3.1 (BD Biosciences,
San Jose, CA, USA).
Alizarin Red S staining
To visualize mineralization of the extracellular
matrix as a marker of terminal differentiation, Alizarin Red S
staining was performed. The MC3T3-E1 cells were seeded into 24-well
plates at a density of 1×104 cells/cm2 and
incubated in the presence of various concentrations (0, 0.01, 0.1
or 1 µM) of ZA for 21 days in a humidified atmosphere of 5%
CO2 at 37°C. The cultured cells were washed with
phosphate-buffered saline (PBS) three times and fixed with 4%
paraformaldehyde (Wuhan Boster Biological Technology, Ltd.) for 15
min. The fixed cells were then stained at room temperature for 5
min with Alizarin Red S solution (Sigma-Aldrich). Following removal
of the dye, the cells were washed with distilled water and images
were captured with a digital camera (EOS 60D, Canon, Inc., Tokyo,
Japan). Calcified nodules appear bright red following Alizarin Red
S staining.
Measurement of ALP activity
The MC3T3-E1 cells were cultured in 24-well plates
at a density of 1×104 cells/cm2 with
osteoinductive medium in the presence or absence of ZA (0, 0.01,
0.1 or 1 µM) for 7 and 14 days in a humidified atmosphere of
5% CO2 at 37°C. The medium was removed, and the cell
monolayer was gently washed with ice-cold PBS three times and lysed
with radioimmunoprecipitation assay buffer (RIPA; Beyotime
Institute of Biotechnology, Haimen, China). The lysate was
centrifuged at 12,000 × g for 15 min, and the clear supernatant was
used for the measurement of ALP activity using an alkaline
phosphatase activity kit (Nanjing Jiancheng Biological Engineering
Institute, Nanjing, China). The ALP activity of each sample was
normalized to the total protein concentration.
ALP staining
The activity of ALP in the cells was measured using
ALP staining, to confirm the ALP activity in the ECM on cell layer
in the MC3T3-E1 cells. Following culture in 24-well plates at a
density of 1×104 cells/cm2 with
osteoinductive medium in the presence or absence of ZA (0, 0.01,
0.1 or 1 µM) for 10 days in a humidified atmosphere of 5%
CO2 at 37°C, the MC3T3-E1 cells were rinsed with PBS
three times and fixed in 4% paraformaldehyde for 15 min. The cells
were then stained using a Leukocyte Alkaline Phosphatase kit
(Sigma-Aldrich) for 30 min at 37°C. Following washing with PBS,
images of the cells were captured using the digital camera.
Enzyme-linked immunosorbent assay (ELISA)
for the detection of secreted BMP-2
A BMP-2 ELISA kit (R&D Systems Inc.,
Minneapolis, MN, USA) was used to detect the levels of BMP-2.
Briefly, the MC3T3-E1 cells were seeded into 24-well plates at a
density of 1×104 cells/cm2 were treated with
osteoinductive medium containing various concentrations (0, 0.01,
0.1 or 1 µM) of ZA. Following 7 days of incubation in a
humidified atmosphere of 5% CO2 at 37°C, the culture
medium from each well was transferred to individual microcentrifuge
tubes and centrifuged for 20 min at 1,000 × g and 4°C. The
resulting supernatant was then stored at −20°C until measurement
using the ELISA kit. The assay was performed, according to the
manufacturer's instructions.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the harvested cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Extracted total RNA (2–5µg) was used to
synthesize cDNA with the SuperScript II cDNA synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Gene expression analysis was performed
using qPCR (iQ5 system; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and normalized against 18S RNA. The PCR reactions were run in
a total volume of 20 µl, containing 10 µl
TransStart® Top Green qPCR SuperMix (concentration, 2×;
Beijing Transgen Biotech Co., Ltd., Beijing, China) and 0.2
µM of each primer. Subsequently, 1 µl template was
added to the reaction mix. The cycling conditions were as follows:
30 sec of polymerase activation at 94°C, followed by 45 cycles at
94°C for 5 sec and 60°C for 30 sec. In the present study, the
expression levels of collagen type I (Col I), ALP, osteocalcin
(OCN), runt-related transcription factor 2 (Runx2), BMP-2 in the
MC3T3-E1 cells were detected. The primer sequences were as follows:
Forward, 5′-TTCGAACGTCTGCCCTATCAA-3′ and reverse,
5′-ATGGTAGGCACGGGGACTA-3′ for 18S RNA; forward,
5′-ACGTCCTGGTGAAGTTG-3′ and reverse, 5′-CAGGGAAGCCTCTTTCTCCT-3′ for
Col I; forward, 5′-GCCTTACCAACTCTTTTGTGCC-3′ and reverse,
5′-GCTTGCTGTCGCCAGTAAC-3′ for ALP; forward,
5′-CTGACCTCACAGATCCCAAGC-3′ and reverse,
5′-TGGTCTGATAGCTCGTCACAAG-3′ for OCN; forward,
5′-GACTGTGGTTACCGTCATGGC-3′ and reverse,
5′-ACTTGGTTTTTCATAACAGCGGA-3′ for Runx2; and forward,
5′-GGGACCCGCTGTCTTCTAGT-3′ and reverse,
5′-TCAACTCAAATTCGCTGAGGAC-3′ for BMP-2. The 2−ΔΔCT
method was used to quantify the mRNA levels comparatively (27).
Western blot analysis
The MC3T3-E1 cells were seeded into 6-well dishes at
a density of 1×104 cells/cm2. Following
incubation with osteoinductive medium containing different
concentrations of ZA (0, 0.01, 0.1 or 1 µM) for 7 days, the
cells were washed with PBS three times and lysed for 30 min at 4°C
with RIPA buffer, according to the manufacturer's instructions.
Following centrifugation at 10,000 x g for 15 min, the soluble
fraction was used to perform western blotting. The total protein
concentrations for each sample were determined using a
Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology,
Rockford, IL, USA), and bovine serum albumin (BSA) was used as a
standard. Equal quantities of the proteins (30 µg) were
loaded onto 8–10% SDS-PAGE gels (Wuhan Boster Biological
Technology, Ltd.), separated and transferred onto nitrocellulose
membranes (Immobilon-P; EMD Millipore, Billerica, MA, USA). The
membranes were blocked at room temperature for 1 h with 5% BSA and
were then incubated overnight at 4°C with primary antibodies in
blocking solution. Primary antibodies against the following targets
were used: Monoclonal rabbit anti-p38 antibody (1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA; cat. no. 8690),
monoclonal rabbit anti-phosphorylated (p)-p38 antibody (1:1,000;
Cell Signaling Technology, Inc.; cat. no. 4511), monoclonal rabbit
anti-ERK 1/2 antibody (1:1,000; Cell Signaling Technology, Inc.;
cat. no. 4695), monoclonal rabbit anti-p ERK 1/2 antibody (1:1,000;
Cell Signaling Technology, Inc.; cat. no. 4370), polyclonal rabbit
anti-inactive caspase-3 antibody (1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; cat. no. SC-7148), polyclonal rabbit
anti-OCN antibody (1:500; Santa Cruz Biotechnology, Inc.; cat. no.
SC-30045), polyclonal rabbit anti-active caspase-3 antibody (1:200;
Abcam, Cambridge, UK; cat. no. ab2302), monoclonal rabbit anti-ALP
antibody (1:20,000; Abcam; cat. no. ab108337), polyclonal rabbit
anti-BMP-2 antibody (1:1,000; Abgent Biotech Co., Ltd., Suzhou,
China; cat. no. AP13858c), polyclonal rabbit anti-Runx2 antibody
(1:400; Wuhan Boster Biological Technology, Ltd.; cat. no.
BA3613-2), monoclonal mouse anti-glyceraldehyde phosphate
dehydrogenase (GAPDH) antibody (1:400; Wuhan Boster Biological
Technology, Ltd.; cat. no. BM1623), monoclonal mouse anti-β actin
antibody (1:400; Wuhan Boster Biological Technology, Ltd.; cat. no.
BM0627). Subsequently, the membranes were washed thee times for 10
min each with tris-buffered saline (TBS) containing 0.1% Tween-20
(Wuhan Boster Biological Technology, Ltd.), and incubated with
anti-rabbit or anti-mouse horseradish peroxidase-conjugated
secondary antibodies (Wuhan Boster Biological Technology, Ltd.) for
1 h at room temperature. Finally, the membranes were washed three
times with TBS buffer, and immunoreactive bands were detected using
a BeyoECL Plus Western Blotting detection system (Beyotime
Institute of Biotechnology), according to the manufacturer's
instructions. For the blot densitometry assay, images of the bands
were captured using a Bio-Rad Gel Doc XR documentation system
(Bio-Rad Laboratories, Inc,) and the band density was determined
using Image Lab software version 5.1 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using one-way
analysis of variance to compare groups and experiments were
repeated three times. All statistical analyses were performed using
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) and P<0.05 was considered
to indicate a statistically significant difference.
Results
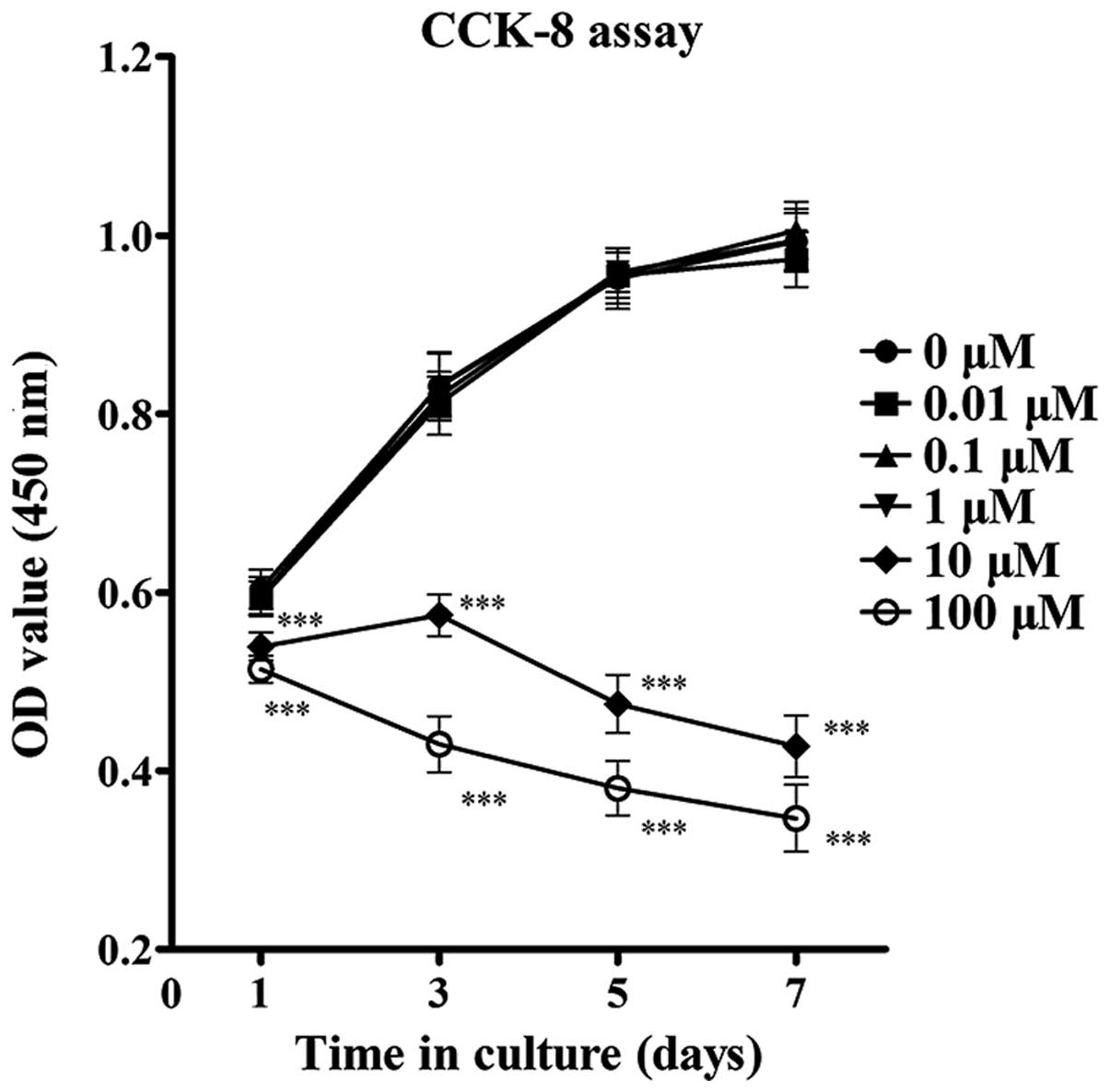
Effects of ZA on the viability of
MC3T3-E1 cells
The present study first determined the effect of ZA
on the viability of the MC3T3-E1 cells by performing a CCK-8 assay.
The results revealed that exposure of MC3T3-E1 cells to ZA at a
concentration between 0.01 and 1 µM for 1, 3, 5 and 7 days
did not affect cell viability significantly. However, significant
inhibition of cell viability was observed following treatment with
ZA at concentrations >10 µM after 1 day, which was
enhanced after 3, 5 and 7 days (Fig.
1). As the number and viability of MC3T3-E1 cells markedly
reduced at concentrations ≥10 µM, concentrations of ZA in
the range between 0.01 and 1 µM were used in the subsequent
assays to observe the differentiation and mineralization of
osteoblasts.
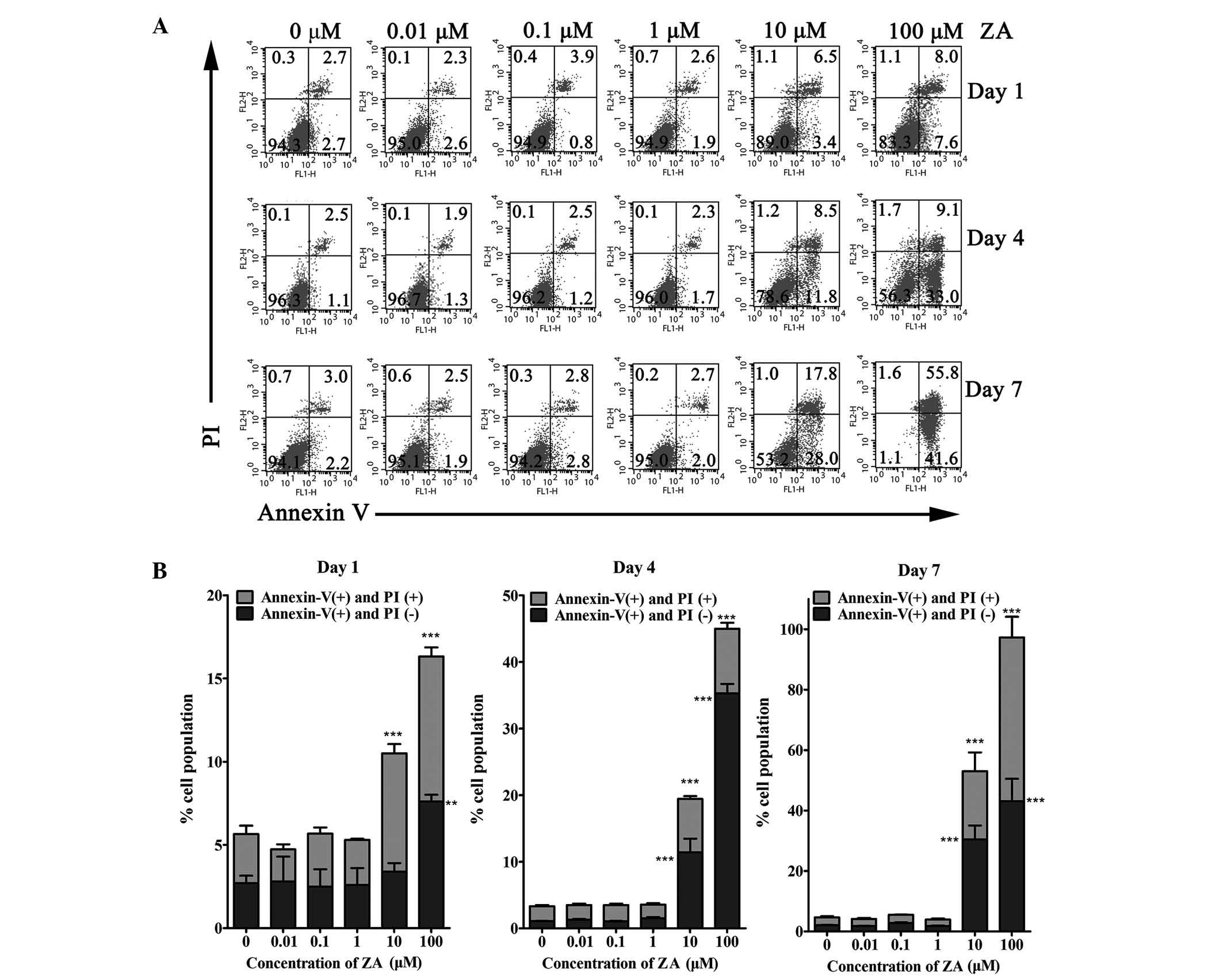
Effects of ZA on the apoptosis of
MC3T3-E1 cells
To determine whether the inhibition of cell
viability following ZA treatment was ascribed to apoptosis, Annexin
V/PI flow cytometric analyses were performed, to distinguish among
healthy cells, early apoptotic cells and late apoptotic or necrotic
cells. The results demonstrated that, in MC3T3-E1 cells treated
with 0.01, 0.1 and 1 µM ZA for 1, 4 and 7 days, the
percentage of viable cells was not significantly different to that
of the control group (0 µM ZA). By contrast, a
dose-dependent and time-dependent increase in the number of early
apoptotic cells and late apoptotic or necrotic cells were observed
following culture with higher concentrations of ZA. In the cells
cultured with 10 and 100 µM for 1 day, the percentage of
viable cells decreased, and the percentage of early apoptotic cells
and late apoptotic or necrotic cells increased marginally.
Following 4 days of incubation with 10 and 100 µM ZA, the
early apoptotic cells increased to 11.4±3.6 and 35.3±2.4%,
respectively, and the late apoptotic or necrotic cells increased to
8.0±0.7 and 9.7±1.5%, respectively. Following 7 days of incubation
with 10 or 100 µM ZA, the early apoptotic cells increased to
30.5±7.9 and 43.1±12.8%, and the late apoptotic or necrotic cells
increased to 22.6±10.7 and 30.5±7.9%, respectively. These data were
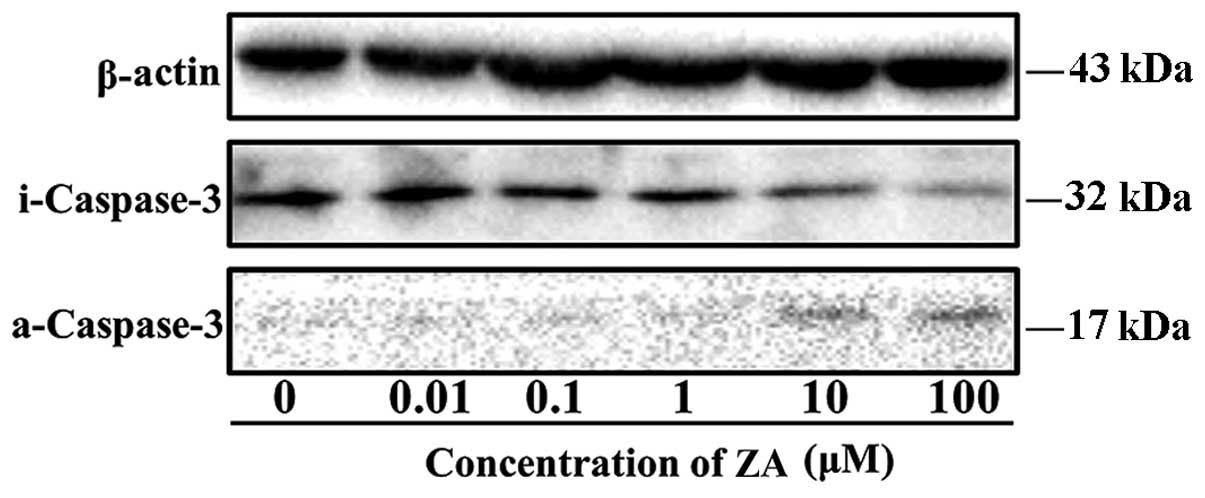
in accordance with the trend of cell viability (Fig. 2). Caspase-3 is a protease involved
in the initiation of the apoptotic pathway, at which the endogenous
and exogenous apoptotic pathways converge. The activation of
caspase-3 ultimately causes apoptosis (28). In order to assess the effects of ZA
on caspase-3 activation, total protein was extracted from the
MC3T3-E1 cells cultured with or without ZA (0.01–100 µM) for
3 days. Western blotting was then performed to detect the inactive
caspase-3 and active caspase-3, as indicators of the activation of
the apoptotic pathways. The protein level of inactive caspase-3 was
downregulated and the protein level of active caspase-3 was
upregulated at ZA concentrations of 10 and 100 µM, compared
with the other concentrations (Fig.
3). These results demonstrated that the inhibition of cell
viability by ZA was due to the induction of apoptosis.
Effects of ZA on the differentiation and
maturation of MC3T3-E1 cells
The formation of calcified nodule is one of the
markers of osteoblastic maturation (29). Although, ZA had no significant
effect on cell growth or apoptosis at concentrations of ≤1
µM in the present study, the formation of mineralized
nodules was significantly suppressed by ZA in a dose-dependent
manner (Fig. 4A). ALP is a
phenotypic marker for the early differentiation of osteoblasts. ALP
activity was examined using the alkaline phosphatase activity kit
and microplate reader, then confirmed with ALP staining to assess
the effect of ZA on the differentiation of MC3T3 E1 cells.
Following 7 and 14 days of ZA treatment, ZA decreased the ALP
activity of the MC3T3-E1 cells at concentrations between 0.01 and 1
µM, compared with the control, in a dose-dependent manner
(Fig. 4B and C). The effect of ZA
on the expression levels of the critical genes associated with
osteogenic differentiation, Col I, ALP, OCN and Runx2, were also
examined. At day 7, the cells treated with 0.01, 0.1 and 1
µM ZA exhibited downregulation in the expression levels of
the marker gene, compared with the control (0 µM), and this
downregulation was also concentration-dependent (Fig. 4D). The results of the western blot
analysis revealed that ZA had decreased the protein levels of ALP,
OCN and Runx2 in the MC3T3-E1 cells at day 7 of differentiation.
These results are consistent with those of the gene expression
levels (Fig. 4D and E). Taken
together, the data obtained suggested that ZA at concentrations
<1 µM exert inhibitory effects on the differentiation and
maturation of MC3T3-E1 cells.
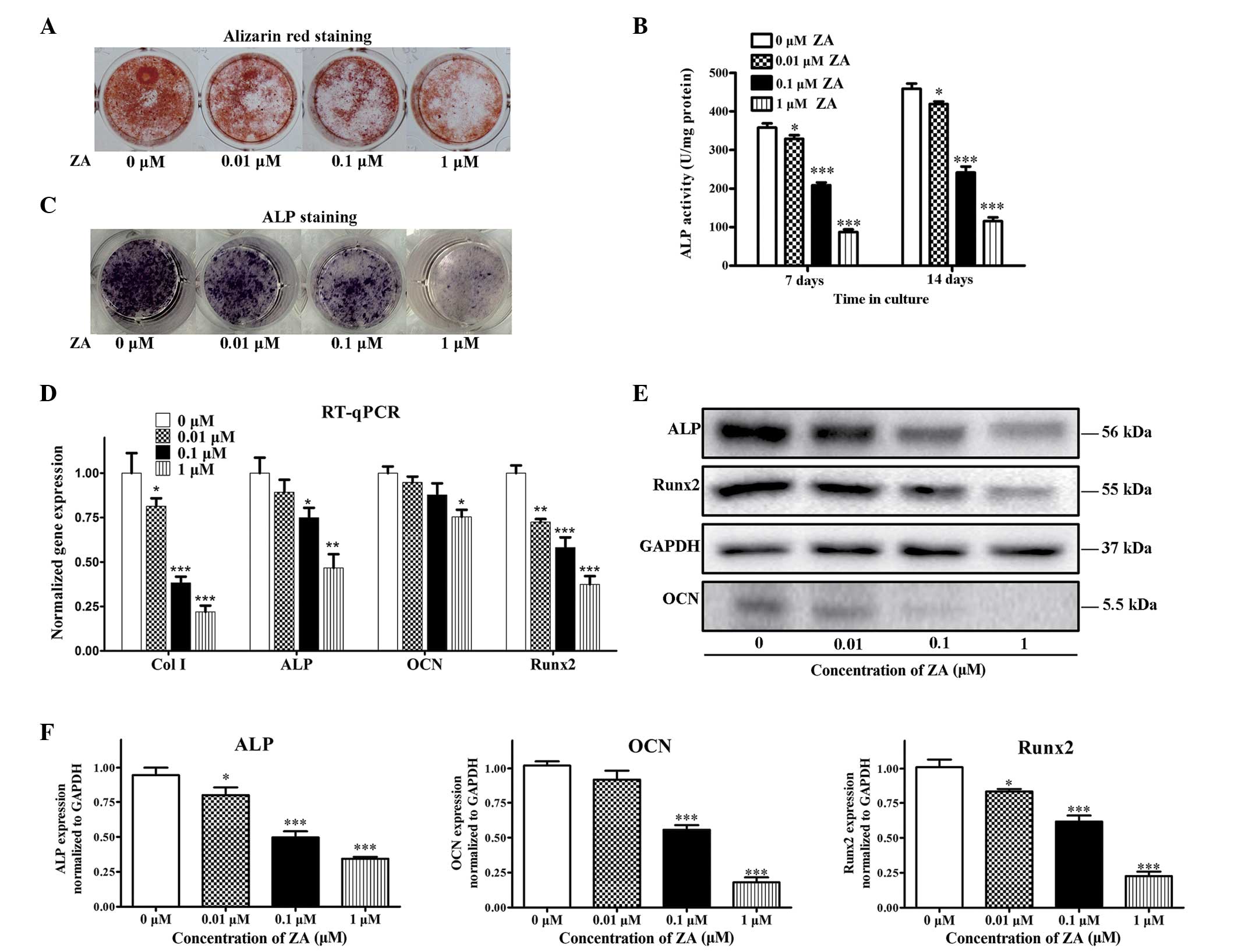 | Figure 4Effects of ZA on the differentiation
and maturation of MC3T3-E1 cells. The MC3T3-E1 cells were treated
with various concentrations of ZA (0–1 µM). (A) Alizarin red
S staining following 21 days of culture. (B) ALP activity on days 7
and 14. (C) ALP staining on day 7. (D) Gene expression levels of
Col I, ALP, OCN and Runx2 on day 7, determined using RT-qPCR
analysis normalized to 18S. (E) Protein levels of ALP, OCN and
Runx2 on day 7, determined using western blot analysis normalized
to GAPDH. (F) Quantitative analysis of the blots for the ALP, OCN
and Runx2 proteins. The results are expressed as the mean ±
standard error of the mean (n =3 for each group).
*P<0.05, **P<0.01 and
***P<0.001, compared with the 0 µM group. ZA,
zoledronic acid; Col I, collagen type I; ALP, alkaline phosphatase;
OCN, osteocalcin; Runx2, runt-related transcription factor 2;
GAPDH, glyceraldehyde phosphate dehydrogenase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Effects of ZA on the expression of BMP-2
and phosphorylation of the ERK 1/2 and p38 pathways in MC3T3-E1
cells
Given the importance of BMP-2 in osteoblastic
differentiation, the present study investigated whether ZA mediated
the alteration of osteoblast differentiation through regulation of
the expression of BMP-2. To confirm whether the expression levels
of BMP-2 were affected by the presence of ZA, a BMP-2 ELISA kit was
used. The results indicated that ZA (0.01, 0.1 and 1 µM)
significantly decreased the protein levels of BMP-2, in a
concentration-dependent manner, following 7 days of treatment
(Fig. 5A). For RT-qPCR and western
blot analyses, the cell extracts were collected 7 days following
treatment of the MC3T3-E1 cells with vehicle or various
concentrations of ZA. Dose-dependent decreases in the gene and
protein expression levels of BMP-2 were detected at concentrations
<1 µM, which were consistent with the results from the
ELISA described above (Fig. 5B–D).
Binding of BMP-2 to the BMP receptor induces receptor heterodimeric
complexes and subsequently activates MAPKs by phosphorylation
(14). The present study evaluated
the activation of p38 and ERK1/2 in ZA-treated cells. Treatment
with ZA did not affect the expression levels of unphosphorylated
p38 or ERK 1/2, however decreases in levels of p-p38 and p-ERK 1/2
were observed following 7 days exposure of the MC3T3-E1 cells to ZA
(Fig. 5C and D). Taken together,
these results indicated that ZA suppressed cell maturation and
differentiation of the MC3T3 cells in a BMP-2-dependent manner.
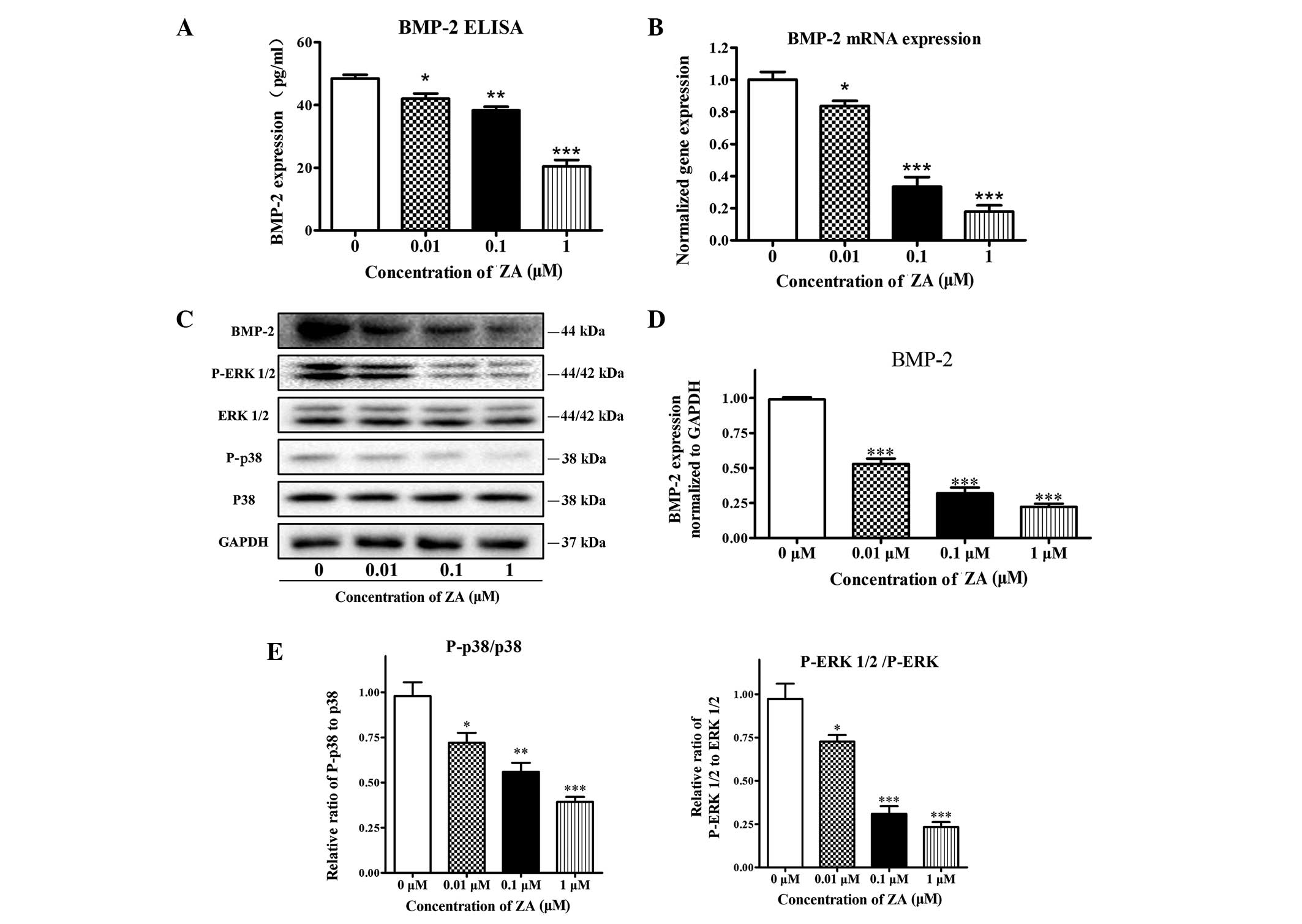 | Figure 5Effect of ZA on the expression of
BMP-2 and phosphorylation of the ERK 1/2 and p38 pathways in
MC3T3-E1 cells. The MC3T3-E1 cells were incubated with various
concentrations of ZA (0–1 µM) for 7 days. (A) Levels of
secreted BMP-2, measured using an ELISA. (B) mRNA expression levels
of BMP-2, determined using reverse transcription-quantitative
polymerase chain reaction analysis. (C) Protein levels of BMP-2,
p38, p-p38, ERK 1/2 and p-ERK 1/2, detected using western blot
analysis and normalized to GAPDH. (D) Quantitative analysis of the
blots for BMP-2. (E) Relative expression of p-ERK, normalized to
ERK, and relative expression of p-p38 normalized to p38. The
results are expressed as the mean ± standard error of the mean (n
=3 for each group). *P<0.05, **P<0.01
and ***P<0.001, compared with the 0 µM group.
BMP-2, bone morphogenetic protein 2; ERK 1/2, extracellular
signal-regulated kinase 1/2; p-, phosphorylated; ELISA,
enzyme-linked immunosorbent assay. |
Discussion
Although several hypotheses with persuasive data
have been put forward (30–32),
the mechanism underlying BRONJ remains to be fully elucidated.
Inhibition of osteoclasts, reduced angiogenesis and local infection
may be involved, at least in part, in BRONJ, but cannot entirely
explain the etiology (33). Trauma
of the jaw bone is considered to be the most common risk factor for
BRONJ, and the majority of the BRONJ cases reported have occurred
following trigger events, including dental extractions and
dentoalveolar surgery (34). For
this risk factor, a possible explanation is that the BPs, which are
accumulated on the bones, are locally released due to surgery or
other trauma, which directly affects the surrounding cells and
leads to the development of BRONJ. Previous studies have suggested
that patients receiving treatment with N-BPs may be at a higher
risk of BRONJ, compared with those treated with non-N-BPs (11). Considering that ZA is the N-BP with
the most potent pharmacological action, a high affinity for bone,
and is the most commonly used BP for malignant diseases,
ZA-associated BRONJ has attracted increasing attention. Raje et
al (35) observed that, in
multiple myeloma patients with BRONJ, intravenous injection of ZA
resulted in inhibition of the osteogenic markers at the gene and
protein levels. Compared with healthy subjects, downregulation of
the genes involved in osteoblast differentiation was observed,
regardless of the presence or absence of BRONJ, and was more marked
in patients with BRONJ (35).
Recker et al (36) reported
that annual ZA injections may lead to inhibition of osteogenic
markers. It can, therefore, be hypothesized that the direct effect
of ZA on osteoblasts contributes to the development of BRONJ. This
hypothesis is supported by the results of the present study, as
higher concentrations of ZA caused cytotoxicity to osteoblasts and
induced their apoptosis, and lower concentrations of ZA suppressed
osteoblast differentiation by downregulating the level of
BMP-2.
In the present study, it was observed that when ZA
concentrations were >10 µM, cell viability decreased
significantly and cell apoptosis increased significantly. At
concentrations <1 µM, ZA appeared to have no effect on
cell viability or apoptosis. This is consistent with the previous
observations of Pozzi et al (13), Peter et al (37) and Orriss et al (38). However, other studies reported
opposite conclusions. Bellido and Plotkin reported that, by
promoting the expression of connexin 43, BPs, including ZA,
preserve the viability of osteoblasts and osteocytes and inhibit
their apoptosis (39). Von Knoch
et al reported that 10 nM ZA stimulates human bone marrow
stromal cell proliferation and viability (23). In a study by Im et al on
alendronic acid, which is also a type of N-BP, it was found that
alendronic acid promotes osteoblasts proliferation at
concentrations <0.1 µM (40).
Co1 I, a major protein constituting the bone matrix,
is excreted by osteoblasts and provides the backbone for the
maturation and mineralization of the bone matrix (41). ALP is an early stage indicator for
bone differentiation as it is vital in the calcification of bone
matrix and is not expressed in undifferentiated precursor cells
(42). OCN is an intermediate-late
stage indicator of osteogenic differentiation, as it is excreted
from the cells during mineralization of bone matrix, being involved
in the formation of calcium hydroxyapatite (43). Runx2 is a transcription factor,
which is essential for osteoblast differentiation and regulates the
expression of bone matrix proteins, including OCN, Co1 I,
osteopontin and bone sialoprotein (21). Runx2 gene mutation in mice results
in dysosteogenesis of the clavicle and skull and significant
defects in bone formation (44).
Calcium deposition occurs in the late stage of osteogenic
differentiation, and the mineralization potentiality can be
evaluated by Alizarin Red S staining. In the present study, when
the ZA concentration was ≥10 µM, the number and viability of
osteoblasts were markedly reduced, making it not possible to
observe the differentiation and mineralization potentiality of
osteoblasts. In the present study, when the concentration of ZA was
≤1 µM, the expression levels of Co1 I, ALP, OCN and Runx2
were downregulated with increasing ZA concentrations, the ALP
activity was suppressed, and the formation of calcium nodules was
inhibited. It was concluded that ZA inhibits various levels of the
cell differentiation process between the early and terminal stages,
to inhibit the maturation and differentiation of MC3T3-E1 cells in
a dose-dependent manner. These results are supported by the studies
of Pozzi et al (13),
Schindeler and Little (25),
Orriss et al (38), and
Idris et al (45). By
contrast, Reinholz et al (46) suggested that, despite inhibiting
osteoblast proliferation, ZA may promote their differentiation.
Kellinsalmi et al (47)
observed that ZA reduces calcium deposition in a dose-dependent
manner, without interfering with osteoblast differentiation. Pan
et al (24) reported that
high concentrations (5–25 µM) of ZA increase mineral
deposition of human bone-derived osteoblast-like cells, despite
reductions in cell numbers due to cytotoxicity. These
contradictions on the effects on cell viability and functions may
be attributed to the experimental systems comprising different cell
types and culture conditions.
BMP-2 is one of the most important extracellular
signaling molecules stimulating bone formation and inducing
osteoblasts differentiation. By stimulating osteoblasts
differentiation, BMP-2 is important role in bone formation and bone
remolding (48,49). BMPs exert their biological effects
by binding to BMP receptors on the surface of cellular membrane.
Among transgenic mice, in which the expression of BMP receptors in
bone tissues was inhibited, the mice exhibited disorders of
physical development, short figure, skeletal maldevelopment and
reduced bone density. Bone morphometric investigation revealed
rarefaction of trabecular bone and mineralization disorder in these
mice (50). ERK 1/2 is important
in the proliferation and differentiation of osteoblasts. Previous
studies have suggested that ERK 1/2 is an important mediator in
inducing osteoblast differentiation, and that inhibiting the
activation of the ERK pathway may lead to the downregulation of
osteogenic markers (51,52). In the differentiation process of
osteoblasts, P38 contributes to the BMP-2-associated gene
expression of Col I and OCN, and the regulation of ALP activity. It
has been reported that BMP-2 may increase the activation and
activity of P38, and inhibition of P38 may attenuate the role of
BMP2 in stimulating osteogenic differentiation (17,18).
BMP2 controls the activity of Runx2 through the ERK 1/2 and P38
pathways (53). In the present
study, as ZA concentration increased, the expression of BMP-2
gradually reduced at the gene and protein levels and in the
exocrine culture. In addition, decreased phosphorylation of the
downstream ERK 1/2 and p38 pathways, and lower expression levels of
the key transcription factor, Runx2 were observed. These results
suggested that the dose-dependent inhibition of the expression of
BMP2 may be important in the process of ZA inhibiting osteoblast
differentiation.
The results of the present study led to the
hypothesis regarding the possible pathogenesis of BRONJ that,
following administration in vivo, ZA accumulates rapidly
within the bone and the cell viability and differentiation of
osteoblasts in the jaw are inhibited due to the continuous
exposure. The dead osteocytes fail to be replaced by fully
functioning osteoblasts, leading to impaired matrix mineralization
and bone formation, which eventually leads to sequestrum with empty
lacuna. The present study also hypothesized that, in the event of
dental surgery or other trauma, ZA adhering to the hydroxyapatite
is released either directly or due to enhanced bone resorption,
which increases the concentration of ZA that the osteoblasts are
exposed to. This change may aggravate suppression of osteoblasts
activities and increase the incidence of BRONJ. It is noteworthy
that the results of this in vitro investigation with ZA
requires careful interpretation, as no consensus has been reached
on the concentration at which ZA binds to bone matrix or the
concentration of ZA to which bone cells are exposed.
In conclusion, the investigations performed in the
present in vitro study demonstrated that ZA at higher
concentrations induced cytotoxicity towards osteoblasts, and ZA at
lower concentrations suppressed osteoblast differentiation by
downregulating the expression of BMP-2. These negative effects of
ZA on osteoblast activities may, at least partly, contribute
clinically to the development and evolution of BRONJ.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81070691 and 81171696).
Abbreviations:
|
BRONJ
|
bisphosphonate-associated
osteonecrosis of the jaw
|
|
ZA
|
zoledronic acid
|
|
ALP
|
alkaline phosphatase
|
|
BMP-2
|
bone morphogenetic protein-2
|
|
ERK 1/2
|
extracellular signal-regulated kinase
1/2
|
|
BPs
|
bisphosphonates
|
|
TGF-β
|
transforming growth factor-β
|
|
Col I
|
collagen type I
|
|
OCN
|
osteocalcin
|
|
Runx2
|
runt-related transcription factor
2
|
|
GAPDH
|
glyceraldehyde phosphate
dehydrogenase
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Bone HG, Hosking D, Devogelaer JP, Tucci
JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J,
Santora AC, et al: Ten years' experience with alendronate for
osteoporosis in postmenopausal women. N Engl J Med. 350:1189–1199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aapro M, Abrahamsson PA, Body JJ, Coleman
RE, Colomer R, Costa L, Crinò L, Dirix L, Gnant M, Gralow J, et al:
Guidance on the use of bisphosphonates in solid tumours:
recommendations of an international expert panel. Ann Oncol.
19:420–432. 2008. View Article : Google Scholar
|
|
3
|
Russell RG: Bisphosphonates: mode of
action and pharmacology. Pediatrics. 119(Suppl 2): S150–S162. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dunford JE, Rogers MJ, Ebetino FH, Phipps
RJ and Coxon FP: Inhibition of protein prenylation by
bisphosphonates causes sustained activation of Rac, Cdc42 and Rho
GTPases. J Bone Miner Res. 21:684–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawson MA, Xia Z, Barnett BL, Triffitt JT,
Phipps RJ, Dunford JE, Locklin RM, Ebetino FH and Russell RG:
Differences between bisphosphonates in binding affinities for
hydroxyapatite. J Biomed Mater Res B Appl Biomater. 92:149–155.
2010. View Article : Google Scholar
|
|
6
|
Vestergaard P, Schwartz K, Pinholt EM,
Rejnmark L and Mosekilde L: Gastric and esophagus events before and
during treatment of osteoporosis. Calcif Tissue Int. 86:110–115.
2010. View Article : Google Scholar
|
|
7
|
Coleman R, Burkinshaw R, Winter M,
Neville-Webbe H, Lestera J, Woodward E and Brown J: Zoledronic
acid. Expert Opin Drug Saf. 10:133–145. 2011. View Article : Google Scholar
|
|
8
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruggiero SL: Bisphosphonate-related
osteonecrosis of the jaw: an overview. Ann N Y Acad Sci.
1218:38–46. 2011. View Article : Google Scholar
|
|
10
|
Ruggiero SL, Dodson TB, Assael LA,
Landesberg R, Marx RE and Mehrotra B; American Association of Oral
and Maxillofacial Surgeons: American Association of Oral and
Maxillofacial Surgeons position paper on bisphosphonate-related
osteonecrosis of the jaws - 2009 update. J Oral Maxillofac Surg.
67:2–12. 2009.PubMed/NCBI
|
|
11
|
Diel IJ, Fogelman I, Al-Nawas B,
Hoffmeister B, Migliorati C, Gligorov J, Väänänen K, Pylkkänen L,
Pecherstorfer M and Aapro MS: Pathophysiology, risk factors and
management of bisphosphonate-associated osteonecrosis of the jaw:
Is there a diverse relationship of amino- and
non-aminobisphosphonates? Crit Rev Oncol Hematol. 64:198–207. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huja SS, Fernandez SA, Phillips C and Li
Y: Zoledronic acid decreases bone formation without causing
osteocyte death in mice. Arch Oral Biol. 54:851–856. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pozzi S, Vallet S, Mukherjee S, Cirstea D,
Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T and
Schoonmaker J: High-dose zoledronic acid impacts bone remodeling
with effects on osteoblastic lineage and bone mechanical
properties. Clin Cancer Res. 15:5829–5839. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: a critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar
|
|
15
|
Ten DP, Fu J, Schaap P and Roelen BA:
Signal transduction of bone morphogenetic proteins in osteoblast
differentiation. J Bone Joint Surg Am. 85-A(Suppl 3): 34–38.
2003.
|
|
16
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar
|
|
17
|
Hu Y, Chan E, Wang SX and Li B: Activation
of p38 mitogen-activated protein kinase is required for osteoblast
differentiation. Endocrinology. 144:2068–2074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their
implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jadlowiec J, Koch H, Zhang X, Campbell PG,
Seyedain M and Sfeir C: Phosphophoryn regulates the gene expression
and differentiation of NIH3T3, MC3T3-E1 and human mesenchymal stem
cells via the integrin/MAPK signaling pathway. J Biol Chem.
279:53323–53330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nohe A, Keating E, Knaus P and Petersen
NO: Signal transduction of bone morphogenetic protein receptors.
Cell Signal. 16:291–299. 2004. View Article : Google Scholar
|
|
21
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheper MA, Badros A, Salama AR, Warburton
G, Cullen KJ, Weikel DS and Meiller TF: A novel bioassay model to
determine clinically significant bisphosphonate levels. Support
Care Cancer. 17:1553–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Knoch F, Jaquiery C, Kowalsky M,
Schaeren S, Alabre C, Martin I, Rubash HE and Shanbhag AS: Effects
of bisphos-phonates on proliferation and osteoblast differentiation
of human bone marrow stromal cells. Biomaterials. 26:6941–6949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan B, Farrugia AN, To LB, Findlay DM,
Green J, Lynch K and Zannettino AC: The nitrogen-containing
bisphosphonate, zoledronic acid, influences RANKL expression in
human osteoblast-like cells by activating TNF-alpha converting
enzyme (TACE). J Bone Miner Res. 19:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schindeler A and Little DG: Osteoclasts
but not osteoblasts are affected by a calcified surface treated
with zoledronic acid. in vitro Biochem Biophys Res Commun.
338:710–716. 2005. View Article : Google Scholar
|
|
26
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding WX, Ni HM, DiFrancesca D, Stolz DB
and Yin XM: Bid-dependent generation of oxygen radicals promotes
death receptor activation-induced apoptosis in murine hepatocytes.
Hepatology. 40:403–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu YB, Li YH, Kong XH, Zhang R, Sun Y, Li
Q, Li C, Liu L, Wang J and Mei QB: The beneficial effect of Radix
Dipsaci total saponins on bone metabolism in vitro and in vivo and
the possible mechanisms of action. Osteoporos Int. 23:2649–2660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allen MR and Burr DB: Mandible matrix
necrosis in beagle dogs after 3 years of daily oral bisphosphonate
treatment. J Oral Maxillofac Surg. 66:987–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stresing V, Fournier PG, Bellahcene A,
Benzaïd I, Mönkkönen H, Colombel M, Ebetino FH, Castronovo V and
Clézardin P: Nitrogen-containing bisphosphonates can inhibit
angiogenesis in vivo without the involvement of farnesyl
pyrophosphate synthase. Bone. 48:259–266. 2011. View Article : Google Scholar
|
|
32
|
Naik NH and Russo TA:
Bisphosphonate-related osteonecrosis of the jaw: the role of
actinomyces. Clin Infect Dis. 49:1729–1732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuhl S, Walter C, Acham S, Pfeffer R and
Lambrecht JT: Bisphosphonate-related osteonecrosis of the jaws-a
review. Oral Oncol. 48:938–947. 2012. View Article : Google Scholar
|
|
34
|
Hoff AO, Toth BB, Altundag K, Johnson MM,
Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, et
al: Frequency and risk factors associated with osteonecrosis of the
jaw in cancer patients treated with intravenous bisphosphonates. J
Bone Miner Res. 23:826–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raje N, Woo SB, Hande K, Yap JT,
Richardson PG, Vallet S, Treister N, Hideshima T, Sheehy N, Chhetri
S, et al: Clinical, radiographic and biochemical characterization
of multiple myeloma patients with osteonecrosis of the jaw. Clin
Cancer Res. 14:2387–2395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Recker RR, Delmas PD, Halse J, Reid IR,
Boonen S, García-Hernandez PA, Supronik J, Lewiecki EM, Ochoa L,
Miller P, et al: Effects of intravenous zoledronic acid once yearly
on bone remodeling and bone structure. J Bone Miner Res. 23:6–16.
2008. View Article : Google Scholar
|
|
37
|
Peter B, Zambelli PY, Guicheux J and
Pioletti DP: The effect of bisphosphonates and titanium particles
on osteoblasts: An in vitro study. J Bone Joint Surg Br.
87:1157–1163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Orriss IR, Key ML, Colston KW and Arnett
TR: Inhibition of osteoblast function in vitro by
aminobisphosphonates. J Cell Biochem. 106:109–118. 2009. View Article : Google Scholar
|
|
39
|
Bellido T and Plotkin LI: Novel actions of
bisphosphonates in bone: Preservation of osteoblast and osteocyte
viability. Bone. 49:50–55. 2011. View Article : Google Scholar
|
|
40
|
Im GI, Qureshi SA, Kenney J, Rubash HE and
Shanbhag AS: Osteoblast proliferation and maturation by
bisphosphonates. Biomaterials. 25:4105–4115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van der Rest M and Garrone R: Collagen
family of proteins. FASEB J. 5:2814–2823. 1991.PubMed/NCBI
|
|
42
|
Owen TA, Holthuis J, Markose E, van Wijnen
AJ, Wolfe SA, Grimes SR, Lian JB and Stein GS: Modifications of
protein-DNA interactions in the proximal promoter of a
cell-growth-regulated histone gene during onset and progression of
osteoblast differentiation. Proc Natl Acad Sci USA. 87:5129–5133.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Delany AM, Amling M, Priemel M, Howe C,
Baron R and Canalis E: Osteopenia and decreased bone formation in
osteo-nectin-deficient mice. J Clin Invest. 105:13252000.
View Article : Google Scholar
|
|
44
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Idris AI, Rojas J, Greig IR, Van't Hof RJ
and Ralston SH: Aminobisphosphonates cause osteoblast apoptosis and
inhibit bone nodule formation in vitro. Calcif Tissue Int.
82:191–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reinholz GG, Getz B, Pederson L, Sanders
ES, Subramaniam M, Ingle JN and Spelsberg TC: Bisphosphonates
directly regulate cell proliferation, differentiation and gene
expression in human osteoblasts. Cancer Res. 60:6001–6007.
2000.PubMed/NCBI
|
|
47
|
Kellinsalmi M, Mönkkönen H, Mönkkönen J,
Leskelä HV, Parikka V, Hämäläinen M and Lehenkari P: In vitro
comparison of clodronate, pamidronate and zoledronic acid effects
on rat osteoclasts and human stem cell-derived osteoblasts. Basic
Clin Pharmacol Toxicol. 97:382–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sykaras N and Opperman LA: Bone
morphogenetic proteins (BMPs): how do they function and what can
they offer the clinician? J Oral Sci. 45:57–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiao Y, Haase H, Young WG and Bartold PM:
Development and transplantation of a mineralized matrix formed by
osteo-blasts in vitro for bone regeneration. Cell Transplant.
13:15–25. 2004. View Article : Google Scholar
|
|
50
|
Zhao M, Harris SE, Horn D, Geng Z,
Nishimura R, Mundy GR and Chen D: Bone morphogenetic protein
receptor signaling is necessary for normal murine postnatal bone
formation. J Cell Biol. 157:1049–1060. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jaiswal RK, Jaiswal N, Bruder SP,
Mbalaviele G, Marshak DR and Pittenger MF: Adult human mesenchymal
stem cell differentiation to the osteogenic or adipogenic lineage
is regulated by mitogen-activated protein kinase. J Biol Chem.
275:9645–9652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cortizo AM, Lettieri MG, Barrio DA, Mercer
N, Etcheverry SB and McCarthy AD: Advanced glycation end-products
(AGEs) induce concerted changes in the osteoblastic expression of
their receptor RAGE and in the activation of extracellular
signal-regulated kinases (ERK). Mol Cell Biochem. 250:1–10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gallea S, Lallemand F, Atfi A, Rawadi G,
Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, et
al: Activation of mitogen-activated protein kinase cascades is
involved in regulation of bone morphogenetic protein-2-induced
osteoblast differentiation in pluripotent C2C12 cells. Bone.
28:491–498. 2001. View Article : Google Scholar : PubMed/NCBI
|