Introduction
Posterior capsule opacification (PCO) is the most
common postoperative complication of cataract surgery (1) and results from the proliferation and
migration of postoperative remnants of lens epithelial cells (LECs)
in the posterior lens capsule (2,3). It
has been reported that the residual postoperative LECs undergo the
epithelial-mesenchymal transition (EMT) process, enabling
pronounced migration and leading to PCO (4,5). EMT
is a transdifferentiation process, in which an epithelial cell
changes to exhibit a fibroblastic phenotype (4). EMT is activated during various
cellular processes, and is triggered by different signaling
molecules, including transforming growth factor β (TGF-β),
fibroblast growth factor and Notch (6). Among these, TGF-β is a major inducer
of EMT (7). TGF-β2 is
the predominant isoform of TGF-β in the aqueous humor of the eye,
the expression of which is elevated following cataract surgery
(8,9).
In our previous study, it was found that mammalian
target of rapamycin (mTOR) was activated during
TGF-β2-induced EMT in human lens epithelial cells
(HLECs), and inhibitors of mTOR impaired the EMT and reduced cell
motility (10). These results
suggest that mTOR is involved in the regulation of
TGF-β2-induced EMT. The large, serine/threonine protein
kinase mTOR is activated during various cellular processes,
including EMT. The mTOR signaling pathway forms a complex signaling
network, which integrates intracellular and extracellular signals
(10,11). The upstream regulators of the mTOR
signaling pathway include phosphatidylinositol 3-kinase
(PI3K)/protein kinase B(Akt), Ras and AMP-activated protein kinase
(11). One of the most important
sensors involved in the regulation of mTOR activity is PI3K, which
enhances the phosphorylation of Akt and subsequently activates mTOR
(12). Therefore, the present
study hypothesized that TGF-β2 induces the EMT of HLECs
through the PI3K/Akt/mTOR signaling pathway.
In the present study, whether the PI3K/Akt/mTOR
signaling pathway is involved in TGF-β2-induced EMT in
HLECs was investigated. Activation of the PI3K/Akt/mTOR signaling
pathway was investigated during the EMT process, and the PI3K
inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
(LY294002), was used to determine its effect on
TGF-β2-induced EMT in HLECs.
Materials and methods
Cell culture and treatment
Immortalized HLEB-3 cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), placed in a humidified atmosphere containing 5%
CO2 at 37°C. The cells were washed with
phosphate-buffered saline (PBS), and dissociated with 0.05% trypsin
and 0.02% EDTA. As in our previous experiments (7), when the cell cultures reached a
confluence of 80%, the cells were divided into three groups
(1×106 cells/ml each). In the first group, the cells
were stimulated with 10 ng/ml recombinant human TGF-β2
(Peprotech, Inc., Rocky Hill, NJ, USA) for 24 h in serum-free
medium to induce EMT. This was termed the TGF-β2 group.
To determine the effect of PI3K inhibition on EMT, the cells were
pretreated with LY294002 (Cell Signaling Technology, Inc., Danvers,
MA, USA) at an appropriate concentration for 1 h prior to being
co-treated with 10 ng/ml TGF-β2 for 24 h. This was
termed the LY294002+TGF-β2 group. The control group
consisted of cells, which were incubated under conventional
conditions without the presence of either TGF-β2 or
LY294002 in the medium. Following treatment, the cells were
collected for western blot analysis and confocal immunofluorescence
assays.
Analysis of cytotoxicity
A Cell Counting Kit 8 (CCK8) assay was used to
assess the cytotoxicity of LY294002 in the HLEB-3 cells. According
to the manufacturer's protocol, the cells were cultured in a 100
µl DMEM in 96-well plates at a density of 1×104
cells/well for 24 h, and were subsequently treated with LY294002 at
concentrations ranging between 0 and 80 µM (0, 10, 20, 30,
40, 50, 60, 70 and 80 µM) for another 24 h. Finally, 10
µl CCK8 solution (Sigma-Aldrich, St. Louis, MO, USA) was
added to each well for 2 h. A soluble orange formazan product was
produced, which was then quantified by light absorbance at 450 nm
using a Synergy™ HT Multi-Mode microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The quantity of dye
generated by the activity of mitochondrial dehydrogenases in the
cells is directly proportional to the number of living cells. This
assay was performed in triplicate. In addition, the morphological
changes of the HLEB-3 cells were observed and images were captured
under an inverted microscope (ECLIPSE TE2000-S; Nikon, Tokyo,
Japan).
Western blotting
The cells were lysed in ice-cold
radioimmunoprecipitation buffer containing 20 mM Tris (pH 7.5), 150
mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA,
1% Na3VO4, 0.5 µg/ml leupeptin, 1 mM
phenylmethanesulfonylfluoride (Beyotime Institute of Biotechnology,
Shanghai, China) with phosphatase inhibitors (10 ml Phosstop
phosphatase inhibitor cocktail tablet; Roche Diagnostics, Basel,
Switzerland) at 4°C for 30 min. The cell lysates were then
centrifuged at 14,000 × g for 10 min to remove the insoluble
material. The proteins were quantified using a Pierce BCA Protein
Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Following being heated at 95°C for 5 min, 20 µg of each
protein sample was separated by 8% SDS-PAGE (Beyotime Institute of
Biotechnology), followed by transfer onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked in 5% non-fat milk for 3 h at room temperature and were
washed with Tris-buffered saline with 0.1% Tween 20 (TBST) for
three times for 5 mins. The membranes were subsequently incubated
with the following primary antibodies overnight at 4°C: Rabbit
anti-human monoclonal Akt (1:1,000; cat. no. 4691; Cell Signaling
Technology, Inc.), rabbit anti-human monoclonal phosphorylated
(p)-Akt (Ser473; 1:1,000; cat. no. 4060; Cell Signaling Technology,
Inc.), rabbit anti-human polyclonal mTOR (1:1,000; cat. no. 2972;
Cell Signaling Technology, Inc.), rabbit anti-human polyclonal
p-mTOR (Ser2448; 1:1,000; cat. no. 2971; Cell Signaling Technology,
Inc.), rabbit anti-human polyclonal connexin 43 (1:8,000; cat. no.
Ab11370; Abcam, Cambridge, UK), rabbit anti-human polyclonal
fibronectin (1:1,000; cat. no. Ab23750; Abcam) and mouse anti-human
monoclonal GAPDH (1:5,000; cat. no. M20006; Abmart, Shanghai,
China). The membranes were then incubated for 1 h in goat
anti-rabbit horseradish peroxidase (HRP)-conjugated secondary
antibody (cat. no. BA1054; Boster Systems, Inc., Pleasanton, CA,
USA) or goat anti-mouse IgG-HRP (cat. no. BA1050; Boster Systems,
Inc.) diluted at 1:5,000 in TBST. The membranes were washed three
times with TBST for 5 min and the immunoreactive protein bands were
detected using enhanced chemiluminescence (ECL) detection reagent
(Applygen Technologies, Inc., Beijing, China) and BioMax film
(Kodak, Rochester, NY, USA). The film was scanned and analyzed
using GEL-PRO Analyzer software 4.0 (Media Cybernetics, Inc.
Rockville, MD, USA).
Confocal cell immunofluorescence
The cells were placed on slides and fixed with 4%
paraformaldehyde for 5 min, followed by washing with PBS. To
inhibit the activity of endogenous peroxidase, the slides were
incubated in 3% hydrogen peroxide (Guangzhou Chemical Reagent
Factory, Guangzhou, China) at 37°C for 30 min, and were
subsequently blocked for another 30 min at 37°C with 5% bovine
serum albumin (Roche Diagnostics). Subsequently, the slides were
immunostained overnight with p-Akt (Ser473; 1:200; Cell Signaling
Technology, Inc.) at 4°C. Following washing with PBS, Alexa Fluor
555 goat anti-rabbit IgG (1:500; Invitrogen; Thermo Fisher
Scientific, Inc.) was added, and the slides were incubated in the
dark at 37°C for 30 min. Finally, the slides were embedded in
glycerine (Beyotime Institute of Biotechnology) and observed under
a confocal laser scanning microscope (Leica SP5-FCS; Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Values are presented as the mean ± standard
deviation from at least three independently performed experiments,
to account for possible variation in the cell cultures. For
statistical evaluation, one way analysis of variance was used.
Statistical analyses were performed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TGF-β2 induces EMT in HLECs via the
PI3K/Akt/mTOR signaling pathway
To confirm whether TGF-β2 induces EMT in
HLECs, the present study investigated the expression levels of the
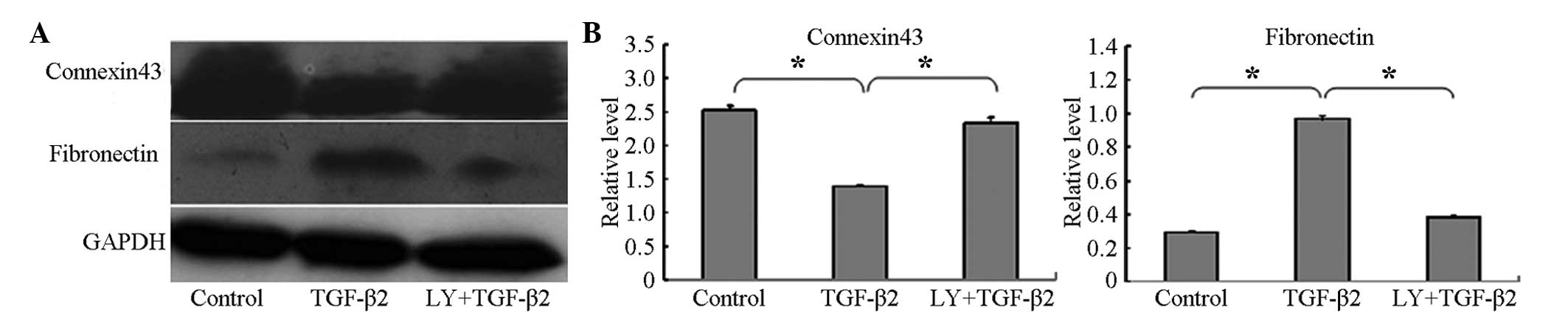
epithelial marker, connexin 43, and the mesenchymal marker,
fibronectin, using western blotting. The connexin 43 protein was
expressed at high levels in the HLEB-3 cells in the absence of
TGF-β2, and its level was significantly decreased
following treatment of the cells with 10 ng/ml TGF-β2
for 24 h. By contrast, compared with the control group, the
expression of fibronectin was significantly increased in the HLEB-3
cells following stimulation by TGF-β2, as shown in
Fig. 1A and B.
To determine whether the PI3K/Akt/mTOR signaling
pathway was activated during the TGF-β2-induced EMT, the
HLEB-3 cells were cultured with 10 ng/ml TGF-β2 for 24
h, and the phosphorylation levels of Akt and mTOR were then
examined using western blotting. The results showed that the
phosphorylation levels of Akt and mTOR were significantly
increased, compared with the untreated control cells (Fig. 2A and B). In addition, the confocal
cell immunofluorescence revealed that, the expression of p-Akt was
higher following treatment with TGF-β2, compared with
the untreated cells (Fig. 3). The
expression of p-Akt was shown as red fluorescence, which was
observed at low levels in the control cells (Fig. 3A–C). Following induction by
TGF-β2, the levels of fluorescence were markedly higher
(Fig. 3D–F). The confocal cell
immunofluorescence also demonstrated that the expression of p-Akt
was lower following co-treatment with LY294002 and
TGF-β2 (Fig. 3G–I).
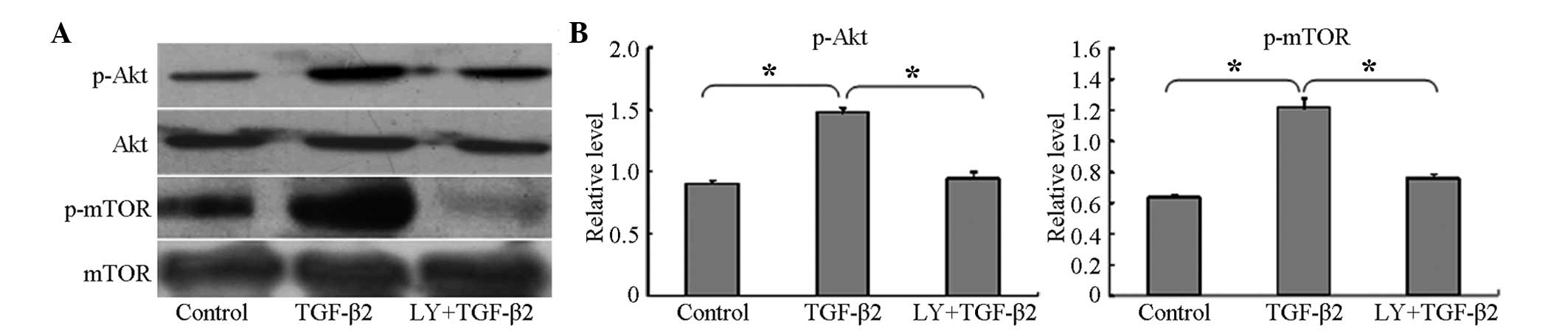 | Figure 2LY294002 inhibits the phosphorylation
of mTOR and Akt during TGF-β2-induced EMT. (A) Western
blot analyses revealed that TGF-β2 increased the
phosphorylation of Akt and mTOR, but did not alter the total
expression levels of Akt or mTOR. LY294002 inhibited the
phosphorylation of Akt and mTOR. Control group, cells were
incubated without TGF-β2 for 24 h; TGF-β2,
group, cells incubated with 10 ng/ml TGF-β2 for 24 h;
LY+TGF-β2 group, cells pretreated with 20 µM
LY294002 for 1 h, prior to incubation with TGF-β2 for 24
h. Representative blots of three independent experiments are shown.
(B) Data in the densitometric analyses of western blotting are
presented as the mean ± standard deviation of three independent
experiments (*P<0.05). LY294002/LY,
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Akt, protein
kinase B; mTOR, mammalian target of rapamycin; TFG-β2,
transforming growth factor β2; p-, phosphorylated. |
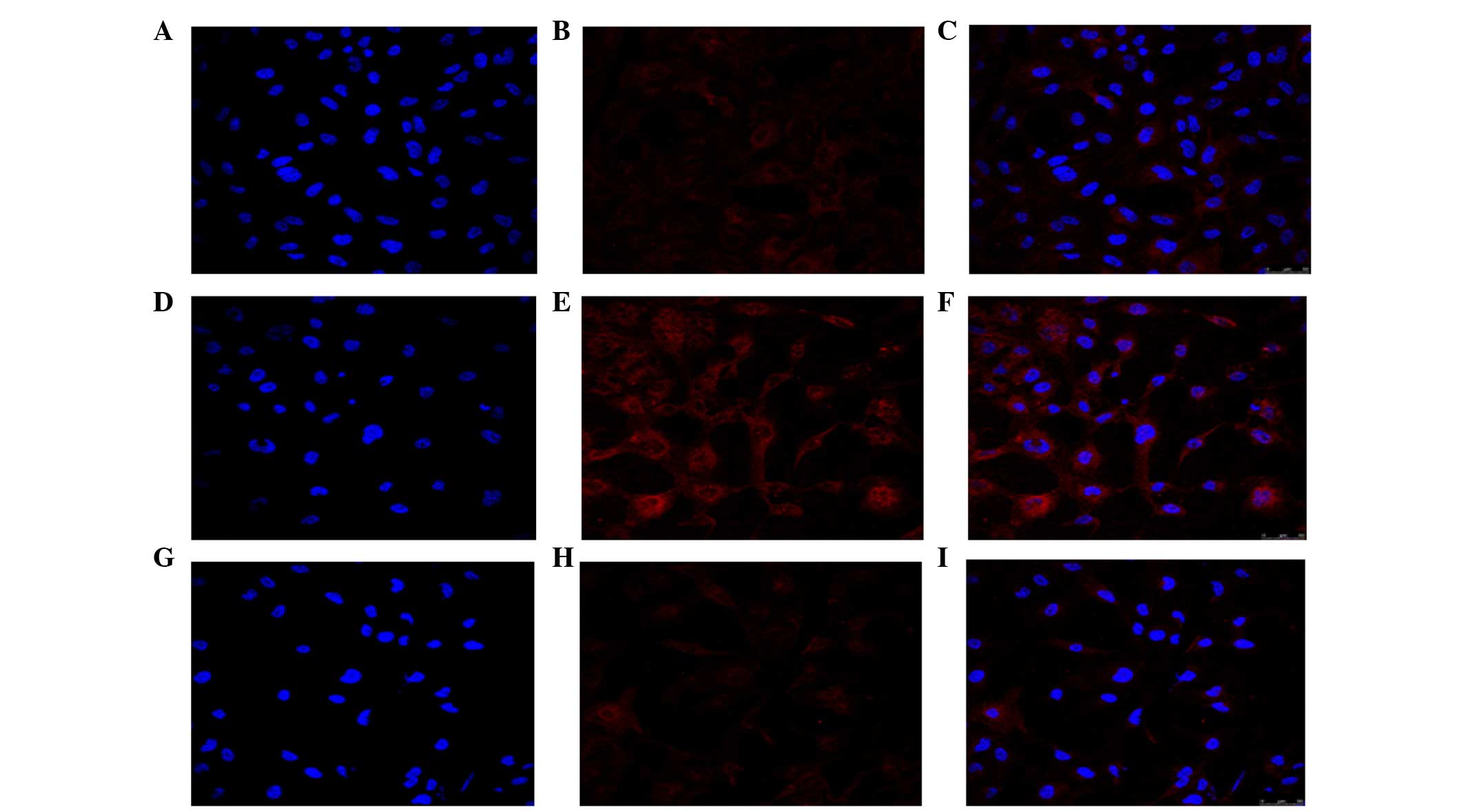 | Figure 3LY294002 inhibits the activation of
Akt during TGF-β2-induced EMT. Confocal cell
immunofluorescence revealed that, compared with the (A–C) control,
treatment with (D–F) TGF-β2 increased the expression of
p-Akt. (G–I) LY294002 decreased the expression of p-Akt, compared
with the control. Control group, cells incubated without
TGF-β2 for 24 h; TGF-β2 group, cells
incubated with 10 ng/ml TGF-β2 for 24 h;
LY+TGF-β2 group, cells pretreated with 20 µM
LY294002 for 1 h, prior to incubation with TGF-β2 for 24
h. The expression of p-Akt is shown as red fluorescence. Nuclei
stained with diamidinophenylindole appear blue. Images C, F, and I
show the merged images of A and B, D and E, and G and H,
respectively. Representative blots of three independent experiments
is shown (magnification, ×400). LY294002/LY,
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Akt, protein
kinase B; mTOR, mammalian target of rapamycin; TFG-β2,
transforming growth factor β2; p-, phosphorylated. |
Cytotoxicity of LY294002 in HLEB-3
cells
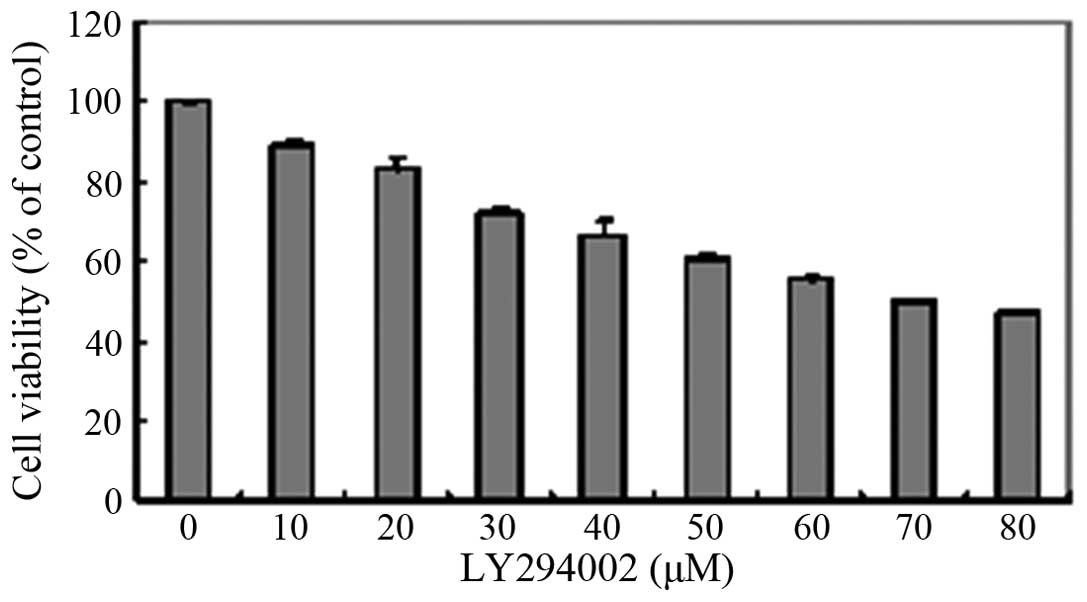
To determine an appropriate concentration of
LY294002 for use in the present study, a CCK8 assay was used for
the analysis of cytotoxicity. The results showed that LY294002
decreased cell viability in a concentration-dependent manner
(Fig. 4). When the cells were
treated with LY294002 at concentrations ≥30 µM, the cell
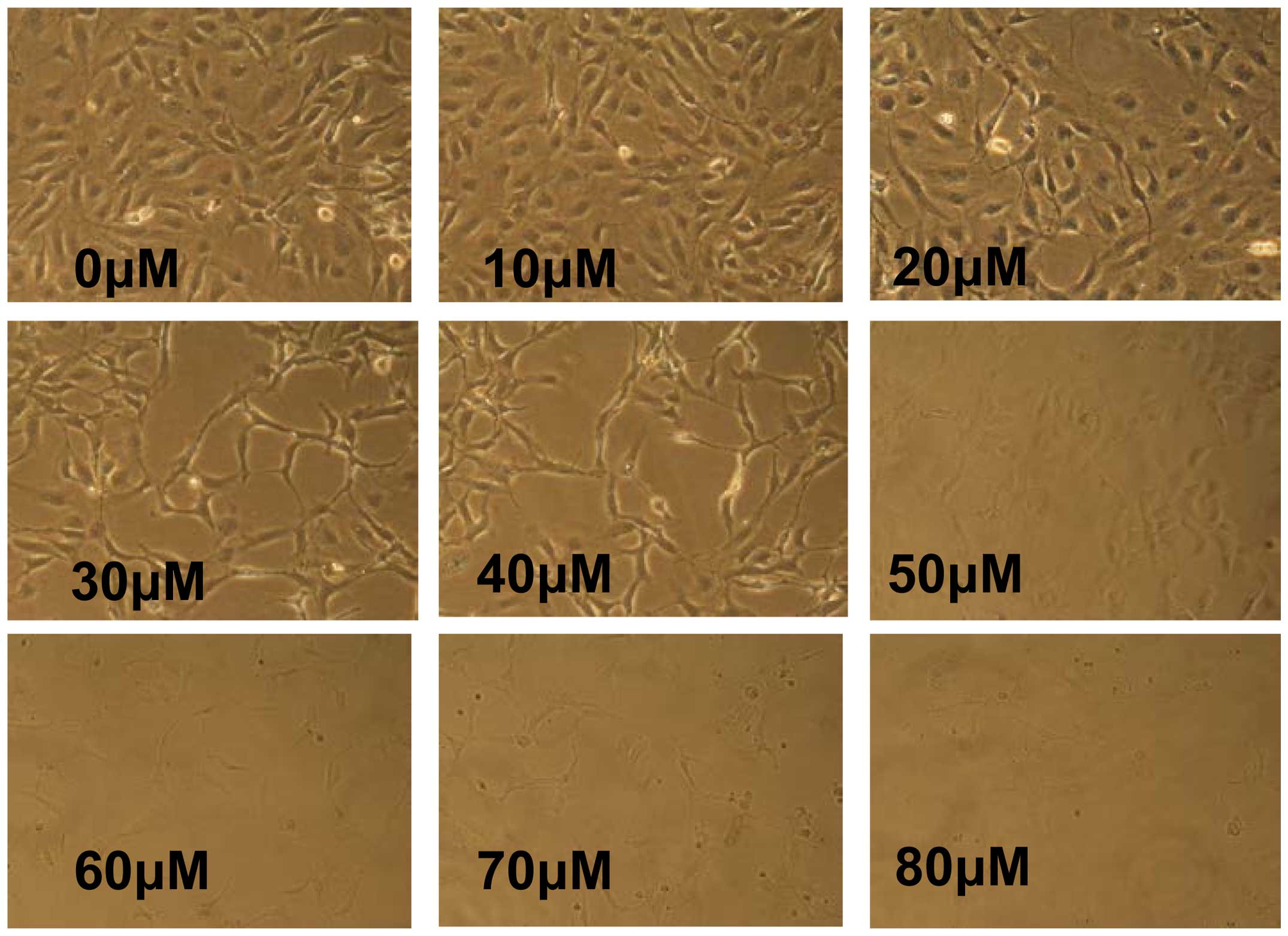
viability was ≤ 80%. In addition, the HLEB-3 cells exhibited a
change in morphology; with stretched, scattered and fragmented
shapes, decreased cell density and increased cell gaps, when the
concentration of LY294002 was ≥20 µM (Fig. 5). Therefore, 20 µM LY294002
was selected for use in the subsequent experiments.
LY294002 inhibits TGF-β2-induced EMT via
the PI3K/Akt/mTOR signaling pathway
To examine the inhibitory effect of LY294002 on the
PI3K/Akt/mTOR signaling pathway, the cells were pretreated with 20
µM LY294002 for 1 h prior to co-treatment with 10 ng/ml
TGF-β2 for 24 h. Western blotting demonstrated that the
levels of p-Akt and p-mTOR were significantly decreased, compared
with the cells cultured with TGF-β2 only for 24 h
(Fig. 2) and, as noted above, the
confocal cell immunofluorescence confirmed that the expression of
p-Akt was lower following co-treatment with LY294002 and
TGF-β2 (Fig. 3G–I).
To further investigate whether LY294002 inhibited
the EMT induced by TGF-β2, the expression levels of the
associated protein markers, connexin 43 and fibronectin, were
determined following treatment of the HLEB-3 cells with 20
µM LY294002 for 1 h and subsequent co-treatment with 10
ng/ml TGF-β2 for 24 h. The results showed that the
protein level of connexin 43 increased, whereas the expression
level of fibronectin decreased in the cells pretreated with
LY294002, compared with the levels observed in the cells cultured
with 10 ng/ml TGF-β2 only for 24 h (Fig. 1).
Discussion
Our previous study demonstrated that
TGF-β2 can induce EMT in HLECs, and mTOR is activated
during TGF-β2-induced EMT (10). In the present study, the role of
the PI3K/Akt/mTOR signaling pathway during EMT was further
investigated. The results revealed that the PI3K/Akt/mTOR signaling
pathway was activated in the EMT induced by TGF-β2.
Treatment with LY294002, an inhibitor of PI3K, effectively
suppressed the activation of Akt and mTOR, and inhibited the
development of EMT. The results suggested that TGF-β2
induced EMT through activation of the PI3K/Akt/mTOR signaling
pathway in the cultured HLECs.
In the present study, the effect of
TGF-β2 on EMT, and the activation of mTOR during
TGF-β2-induced EMT in HLECs was described. The mTOR
kinase integrates four major signals, including growth factors,
energy status, oxygen and amino acids, to regulate several
processes, and acts as a central regulator of cell metabolism,
growth, proliferation and survival (11). In general, growth factors stimulate
mTOR through the activation of Ras and PI3K/Akt (11). PI3K is an intracellular signaling
molecul and, when activated, it generates 3′-phosphoinositides,
which act as secondary messengers in the regulation of cell growth,
proliferation and migration (13,14).
As a downstream kinase of PI3K, Akt is phosphorylated at ser473 and
is recruited to the membrane (15). A previous study demonstrated that
platelet-derived growth factor can induce lenticular EMT through
the Akt pathway (16). Another
study reported that the inhibition of p-Akt reduced or prevented
the formation of PCO in an ex vivo canine lens capsule model
(17). In the present study,
confocal cell immunofluorescence showed higher expression levels of
p-Akt following treatment of the cells with TGF-β2.
Consistent with this result, western blotting revealed a marked
increase in the phosphorylation of the Akt protein. These results
suggested that Akt and mTOR were activated during the
TGF-β2-induced EMT process in the HLECs.
To further delineate whether the PI3K/Akt/mTOR
signaling pathway was involved in TGF-β2 induced EMT in
the HLECs, the present study used the PI3K inhibitor, LY294002.
PI3K is a dimeric enzyme consisting of an 85-kDa regulatory subunit
and a 110-kDa catalytic subunit (18). LY294002 acts on the P110 subunit to
specifically eliminate PI3K activity and subsequently inhibit the
PI3K signaling pathway (19).
Thus, LY294002 can potentially be used to provide a better
understanding of the function and regulatory mechanisms of the
enzyme and pathway mediated by PI3K. In the present study, the
results showed that the phosphorylation levels of Akt and mTOR were
significantly decreased following treatment of the cells with
LY294002, which suggested that LY294002 effectively inhibited the
activation of TGF-β2-induced PI3K/Akt/mTOR signaling. A
previous study reported similar effects when growth factor-induced
cells were treated with LY294002 in cultured rat hippocampal
neurons (20). In addition, the
present study found that the expression connexin 43 was
upregulated, and that of fibronectin was downregulated in the cells
pretreated with LY294002, indicating that the EMT induced by
TGF-β2 was prevented when LY294002 inhibited the
activation of the PI3K/Akt/mTOR pathway. Previous studies have
demonstrated that PI3K/Akt signaling is required for TGF-β-induced
EMT in mouse LECs and HLECs (21,22).
Our previous study demonstrated that mTOR is involved in the
regulation of TGF-β2-induced EMT. According to these
results, it was hypothesized that TGF-β2 induces EMT by
activating the PI3K/Akt/mTOR signaling pathway in HLECs.
In conclusion, the present study demonstrated that
Akt and mTOR were activated during TGF-β2-induced EMT.
In HLECs, treatment with 20 µM LY294002 inhibited the
activation of Akt and mTOR, thus preventing the induction of EMT by
TGF-β2. These results suggested that the PI3K/Akt/mTOR
signaling pathway is involved in the regulation of
TGF-β2-induced EMT in HLECs and may contribute to PCO.
At present, pharmacological PCO prophylaxis has not been achieved
(23). Although several approaches
have been developed to prevent PCO using chemicals, none of these
apporaches have been applied in a clinical setting (24–26).
The results of the present study demonstrated that LY294002 and
other PI3K/Akt/mTOR signaling pathway inhibitors, including
rapamycin and Ku-0063794, may be potential agents for the
prevention and treatment of PCO. However, further investigations
are required to investigate whether other signaling pathways,
including the Ras/mTOR pathway, also regulate EMT in HLECs.
Acknowledgments
This study was supported by funding provided by the
Guangzhou Pearl River Nova of Science and Technology (grant. no.
2011J2200050), the Guangdong Natural Science Foundation (grant. no.
S2011010000462) and the Guangzhou Science and Technology Commission
(grant. no. Z032012245).
References
|
1
|
Awasthi N, Guo S and Wagner BJ: Posterior
capsular opacification: A problem reduced but not yet eradicated.
Arch Ophthalmol. 127:555–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Apple DJ, Solomon KD, Tetz MR, Assia EI,
Holland EY, Legler UF, Tsai JC, Castaneda VE, Hoggatt JP and
Kostick AM: Posterior capsule opacification. Surv Ophthalmol.
37:73–116. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonnell PJ, Krause W and Glaser BM: In
vitro inhibition of lens epithelial cell proliferation and
migration. Ophthalmic Surg. 19:25–30. 1988.PubMed/NCBI
|
|
4
|
de Iongh RU, Wederell E, Lovicu FJ and
McAvoy JW: Transforming growth factor-beta-induced
epithelial-mesenchymal transition in the lens: A model for cataract
formation. Cells Tissues Organs. 179:43–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saika S, Yamanaka O, Flanders KC, Okada Y,
Miyamoto T, Sumioka T, Shirai K, Kitano A, Miyazaki K, Tanaka S and
Ikeda K: Epithelial-mesenchymal transition as a therapeutic target
for prevention of ocular tissue fibrosis. Endocr Metab Immune
Disord Drug Targets. 8:69–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barrallo-Gimeno A and Nieto MA: The snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dawes LJ, Elliott RM, Reddan JR, Wormstone
YM and Wormstone IM: Oligonucleotide microarray analysis of human
lens epithelial cells: TGFbeta regulated gene expression. Mol Vis.
13:1181–1197. 2007.PubMed/NCBI
|
|
9
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta signaling through the smad pathway: Role in
extracellular matrix gene expression and regulation. J Invest
Dermatol. 118:211–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng Q, Guo H, Xiao L, Cui Y, Guo R, Xiao
D and Huang Y: mTOR regulates TGF-β2 -induced
epithelial-mesenchymal transition in cultured human lens epithelial
cells. Graefes Arch Clin Exp Ophthalmol. 251:2363–2370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laplante M and Sabatini DM: mTOR signaling
at a glance. J Cell Sci. 122:3589–3594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Downward J: Mechanisms and consequences of
activation of protein kinase B/Akt. Curr Opin Cell Biol.
10:262–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su CH, Wang CY, Lan KH, Li CP, Chao Y, Lin
HC, Lee SD and Lee WP: Akt phosphorylation at Thr308 and Ser473 is
required for CHIP-mediated ubiquitination of the kinase. Cell
Signal. 23:1824–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong W, Cheng BH, Jia SB and Tang LS:
Involvement of the PI3K/Akt signaling pathway in platelet-derived
growth factor-induced migration of human lens epithelial cells.
Curr Eye Res. 35:389–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandler HL, Webb TR, Barden CA,
Thangavelu M, Kulp SK, Chen CS and Colitz CM: The effect of
phosphorylated Akt inhibition on posterior capsule opacification in
an ex vivo canine model. Mol Vis. 16:2202–2214. 2010.PubMed/NCBI
|
|
18
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vlahos CJ, Matter WF, Hui KY and Brown RF:
A specific inhibitor of phosphatidylinositol 3-kinase,
2-(4-morpholinyl)-8-phenyl- 4H-1-benzopyran-4-one (LY294002). J
Biol Chem. 269:5241–5248. 1994.PubMed/NCBI
|
|
20
|
Yang XP, Liu TY, Qin XY and Yu LC:
Potential protection of
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside against
staurosporine-induced toxicity on cultured rat hippocampus neurons.
Neurosci Lett. 576:79–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho HJ, Baek KE, Saika S, Jeong MJ and Yoo
J: Snail is required for transforming growth factor-beta-induced
epithelial-mesenchymal transition by activating PI3 kinase/Akt
signal pathway. Biochem Biophys Res Commun. 353:337–343. 2007.
View Article : Google Scholar
|
|
22
|
Yao K, Ye PP, Tan J, Tang XJ and Shen Tu
XC: Involvement of PI3K/Akt pathway in TGF-beta2-mediated
epithelial mesenchylmal transition in human lens epithelial cells.
Ophthalmic Res. 40:69–76. 2008. View Article : Google Scholar
|
|
23
|
Findl O, Buehl W, Bauer P and Sycha T:
Interventions for preventing posterior capsule opacification.
Cochrane Database Syst Rev. (2): CD0037382010.PubMed/NCBI
|
|
24
|
Biswas NR, Mongre PK, Das GK, Sen S, Angra
SK and Vajpayee RB: Animal study on the effects of catalin on after
cataract and posterior capsule opacification. Ophthalmic Res.
31:140–142. 1999. View Article : Google Scholar
|
|
25
|
Chandler HL, Barden CA, Lu P, Kusewitt DF
and Colitz CM: Prevention of posterior capsular opacification
through cyclooxy-genase-2 inhibition. Mol Vis. 13:677–691.
2007.PubMed/NCBI
|
|
26
|
Rabsilber TM and Auffarth GU:
Pharmacological means to prevent secondary cataract. Klin Monbl
Augenheilkd. 223:559–567. 2006.In German. View Article : Google Scholar : PubMed/NCBI
|