Introduction
Osteosarcoma is the most common type of malignant
disease in the bone and the third most common malignant tumor in
children (1). Although
osteosarcoma has a low incidence rate (<10% of all tumors)
(2), the disease exhibits
aggressive malignant phenotypes and is associated with a high
mortality rate (3). The most
common location of osteosarcoma development is in the long bones of
the limbs (3). Current treatment
for osteosarcoma is surgery combined with chemotherapy. However,
the 5-year survival rate for patients with metastasis remains
<20% (4,5). Although intensive efforts have been
made, little is known regarding the molecular mechanisms underling
this disease.
The RAS superfamily comprises a large number of
low-molecular-weight GTP-binding proteins (6). According to the degree of sequence
conservation, the superfamily can be divided into five distinct
families, including Ras, Rho, Rab, Sar1/Arf and Ran. Rab proteins
constitute the largest subfamily, and are key regulators of
membrane trafficking processes in eukaryotic cells (7). RBEL1, also termed RABL6, is a novel
Rab-like GTPase of unknown function. Recent studies suggest that
RBEL1 proteins are overexpressed in breast cancer cells (8) and are involved in cell growth and
survival (9). However, the
function of RBEL1 in the regulation of tumorigenesis and
development of human osteosarcoma is unclear.
The present study investigated the biological
function and underlying molecular mechanism of RBEL1 in
osteosarcoma. It was demonstrated that RBEL1 participates in the
regulation of osteosarcoma cell proliferation. Downregulation of
RBEL1 in osteosarcoma resulted in decreased colony formation and
cell proliferation rates. G1-S arrest was also observed in
RBEL1-depleted cells. Furthermore, it was shown that RBEL1
knockdown activated retinoblastoma 1 (Rb) and suppressed E2F
transcriptional activity. These findings demonstrate that RBEL1 is
a potential oncogene and a novel Rb inhibitor in osteosarcoma.
Materials and methods
Cell culture, small interfering (si)RNA
transfection and lentivirus infection
U2-OS cells and SAOS2 cells were purchased from the
American Type Culture Collection (Manassas, VA, USA) and maintained
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 15% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere with 5% CO2 at 37°C. For siRNA
transfection, siRNA targeting Rb (Cell Signaling Technology Inc.,
Danvers, MA, USA) and Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific Inc.) were mixed and incubated for 20 min at room
temperature. Then the mixture was added to cells plated in growth
medium without antibiotics. The medium was changed after 4–6 h. For
lentivirus infection, lentivirus targeting two different sequences
was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China):
KD1 targeting CGG CCT AAA GTA CCT TCA TAA and KD2 targeting CGG CCT
AAA GTA CCT TCA TAA. Cells were plated in growth medium without
antibiotics. At the time of infection, growth medium was replaced
by medium containing lentivirus and polybrene (8 µg/ml;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and incubated
for 12 h at 37°C.
Colony formation assay
Pretreated U2-OS cells and SAOS2 cells infected with
shRBEL1-1, shRBEL-2 or scramble lentivirus were plated in 6-well
plates (300 cells/well) and were maintained in DMEM supplemented
with 15% FBS in a humidified atmosphere of 5% CO2 at
37°C. Growth medium was changed every two days. After 12 days,
cultured cells were fixed in 4% paraformaldehyde (Santa Cruz
Biotechnology, Inc.) for 2 h and stained with 0.1% crystal violet
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) for 20 min. Colonies containing more than 50 individual
cells were then counted with the inverted microscope (CKX41;
Olympus Corporation, Beijing, China).
Bromodeoxyuridine (BrdU) incorporation
assay
Cell proliferation was determined using a Cell
Proliferation ELISA kit (containing BrdU labeling solution,
FixDenat and anti-BrdU-POD; Roche Diagnostics GmbH, Mannheim,
Germany). Briefly, pretreated U2-OS and SAOS2 cells were plated in
a 96-well plate. The following day, 10 µl/well BrdU labeling
solution was added and cells were incubated for 3 h. The medium was
replaced by 200 µl/well FixDenat and incubated for 30 min at
room temperature. Then FixDenat was removed using 100
µl/well anti-BrdU-POD. After washing twice with washing
buffer, 100 µl/well substrate solution was added. The
absorbance was measured at 450 nm with the ELx808 Absorbance Reader
(Bio-Tek Instruments, Inc., Winooski, VT, USA).
Cell cycle analysis
Pretreated U2-OS and SAOS2 cells were harvested and
washed twice in phosphate-buffered saline (PBS). Then cells were
fixed in 70% ethanol at −20°C for 4 h. Cells were washed twice with
PBS and stained with propidium iodide (PI)/RNase Staining Buffer
(BD Biosciences, Franklin Lakes, NJ, USA) for 15 min. Cell cycle
distribution was then analyzed by flow cytometry with the
FACSCalibur flow cytometer (BD Biosciences).
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Reverse transcription was performed
using RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Real-time PCRs were conducted using an ABI Prism 7500 Detection
system (Applied Biosystems, Foster City, CA, USA) and SYBR Select
Master mix system (Applied Biosystems) according to the
manufacturer's instructions. Briefly, 10 µl 2X SYBR Select
Master Mix was mixed with primers (200 nM), cDNA template (100 ng)
and RNase free water made up to 20 µl. The cycling
conditions werw as follows: 50°C for 2 min and 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Immunoblot assay
Whole-cell extracts were prepared in
radioimmunoprecipitation assay buffer supplemented with 1 mM
phenylmethylsulfonyl fluoride (Beijing Solarbio Science &
Technology Co., Ltd.). Total protein concentration was resolved by
10% SDS-PAGE (Beijing Solarbio Science & Technology Co., Ltd.).
The proteins were then transfered to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Immunoblot analysis
was conducted with incubation overnight at 4°C with the following
antibodies: Anti-RBEL1 (1:1,000; ab111866) from Abcam (Cambridge,
MA, USA), anti-Rb (1:200; 554144) and anti-active Rb (1:200;
554164) from (BD Biosciences), anti-c-Myc (1:100; sc-789),
anti-cyclin D1 (1:200; sc-25765), anti-cyclin A2 (1:200; sc-53234)
and anti-cyclin-dependent kinase 2 (CDK2; 1:200; sc-748) from Santa
Cruz Biotechnology Inc., and polyclonal rabbit anti-human tubulin
(1:2,000; T2200) from (Sigma-Aldrich, St. Louis, MO, USA).
Membranes were then incubated with horseradish peroxidase
(HRP)-conjugated anti-rabbit immunoglobulin G secondary antibodies
(1:5,000; ZB-2301; Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) and was detected using the Western
Chemiluminescent HRP Substrate (EMD Millipore).
Statistical analysis
All data sets were analyzed by Student's t-test.
Data are presented as the mean ± standard deviation from three
independent experiments. Statistical analysis was conducted using
Graphpad Prism software, version 6 (GraphPad Software, Inc., San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
RBEL1 depletion in osteosarcoma cells
suppresses colony formation and cell proliferation
To explore the potential role of RBEL1 in
osteosarcoma, its expression in U2-OS and SAOS2 osteosarcoma cell
lines was inhibited by shRNA lentivirus targeting two different
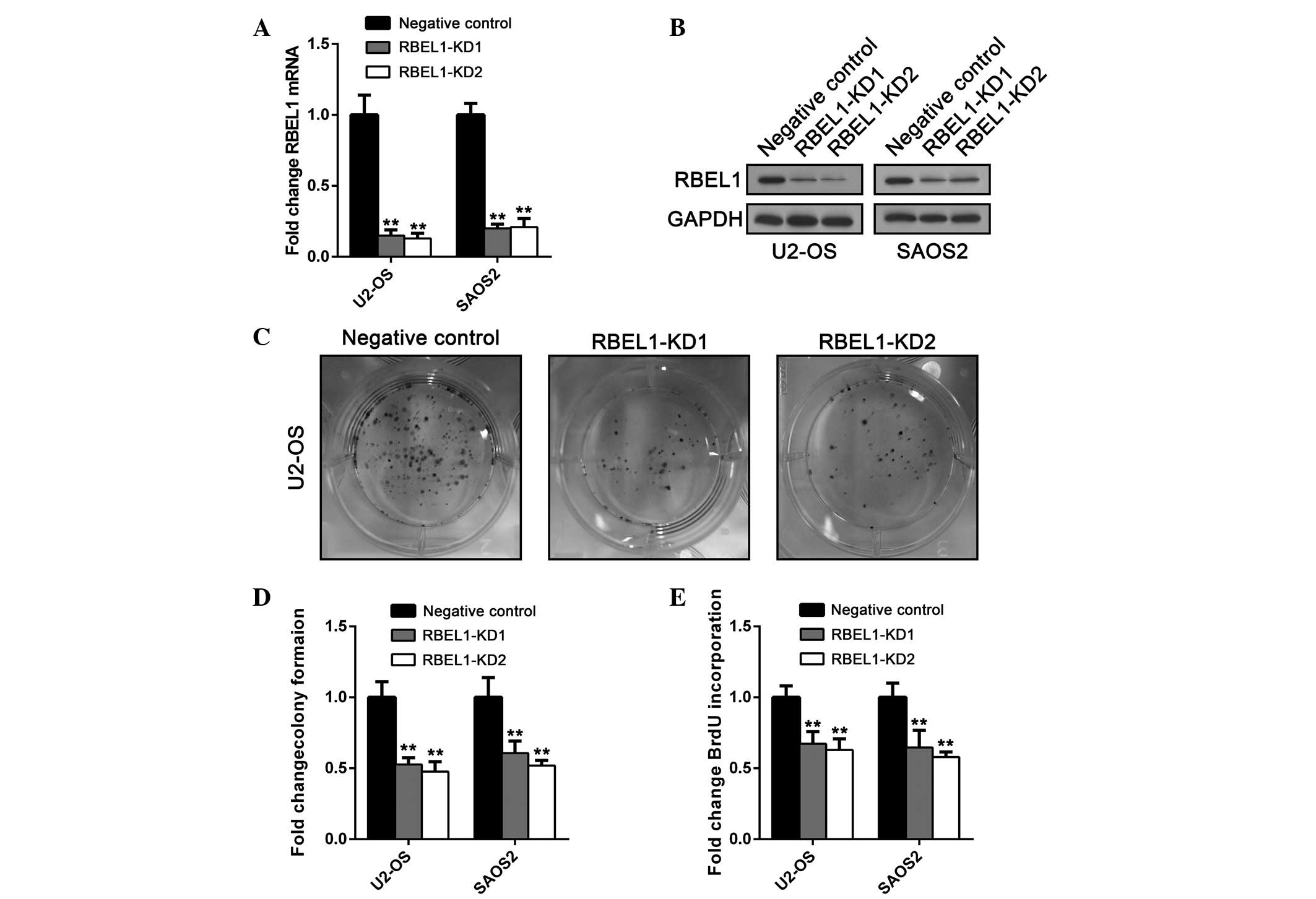
sequences. Knockdown efficiency was confirmed by RT-qPCR and
immunoblot analysis (Fig. 1A and
B). Then RBEL1 knockdown cells and negative control cells were
subjected to a colony formation assay. As shown in Fig. 1C, colony number and size were
suppressed following RBEL1 knockdown. Furthermore, a BrdU
incorporation assay was performed to determine whether RBEL1
depletion also had an impact on cell proliferation. In addtion, in
U2-OS and SAOS2 cells RBEL1 depletion significantly inhibited BrdU
incorporation suggesting suppressed proliferation (Fig. 1D). These data suggest that RBEL1
may act as a tumor suppressor in osteosarcoma.
RBEL1 depletion suppresses G1-S
transition in osteosarcoma cells
The cell cycle of eukaryotic cells is conventionally
divided into four phases and requires three switch-like transitions
at the onset of S phase and at entry and exit of mitosis. Among
them, G1-S transition is the most deregulated cell cycle control
mechanism in malignant diseases (10). Thus it was hypothesized that RBEL1
may modulate G1-S transition thereby promoting cell proliferation
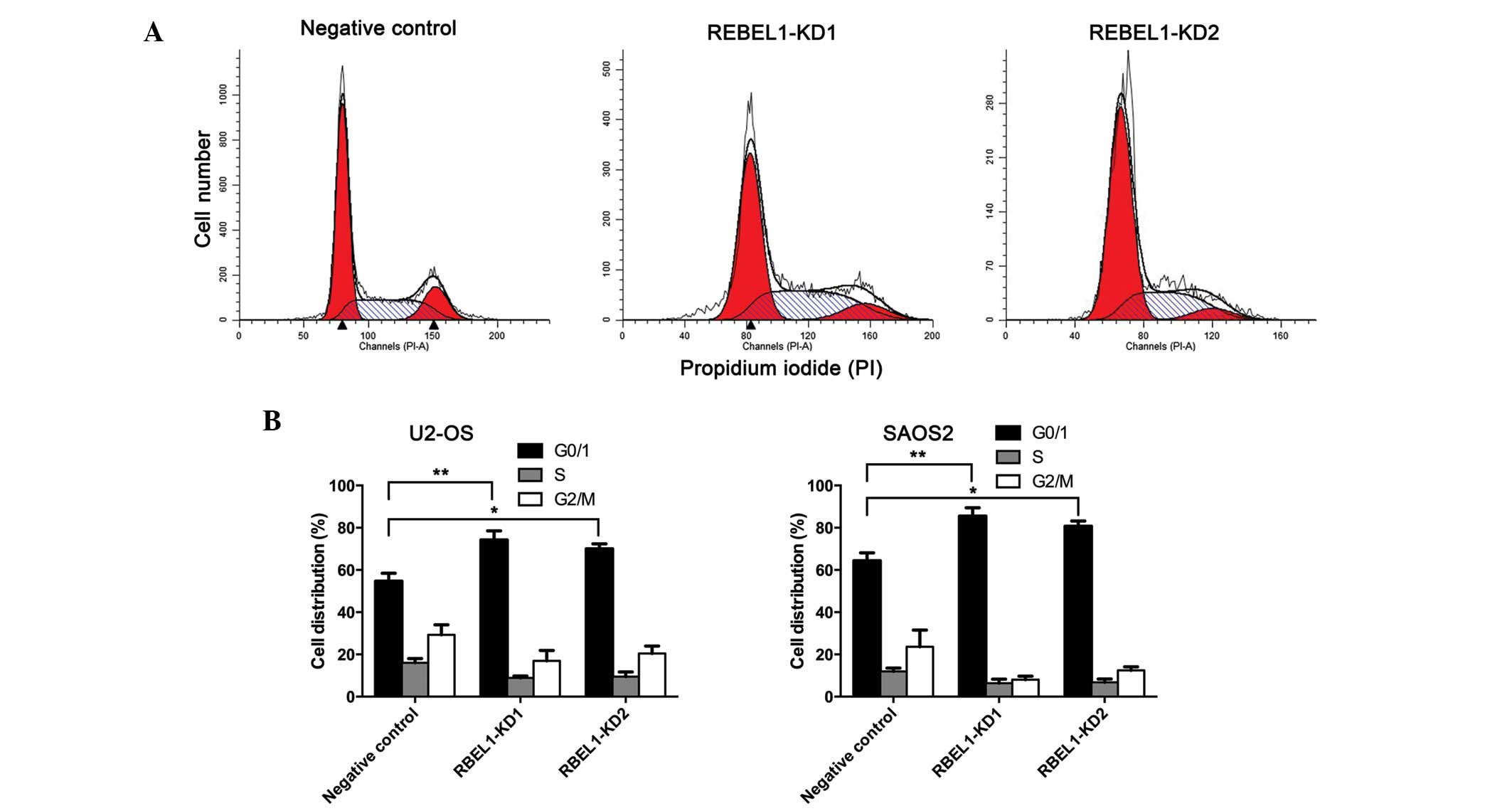
in osteosarcoma cells. Using flow cytometry, cell cycle
distribution of U2-OS and SAOS2 cells infected with RBEL1 shRNA or
negative control shRNA was examined. As shown in Fig. 2, RBEL1 depletion in U2-OS cells
resulted in enhanced G1-S arrest compared with negative control
cells. Similar results were also shown in SAOS2 cells. These
results indicate that RBEL1 may participate in G1-S transition
regulation in osteosarcoma.
RBEL1 regulates Rb activity in
osteosarcoma cells
Rb is the major player in cell cycle control
(11,12). A recent study indicated that RBEL1
negatively regulates Rb activation (8). Therefore, it was hypothesized that
RBEL1 may regulate G1-S transition by inhibiting Rb activation. The
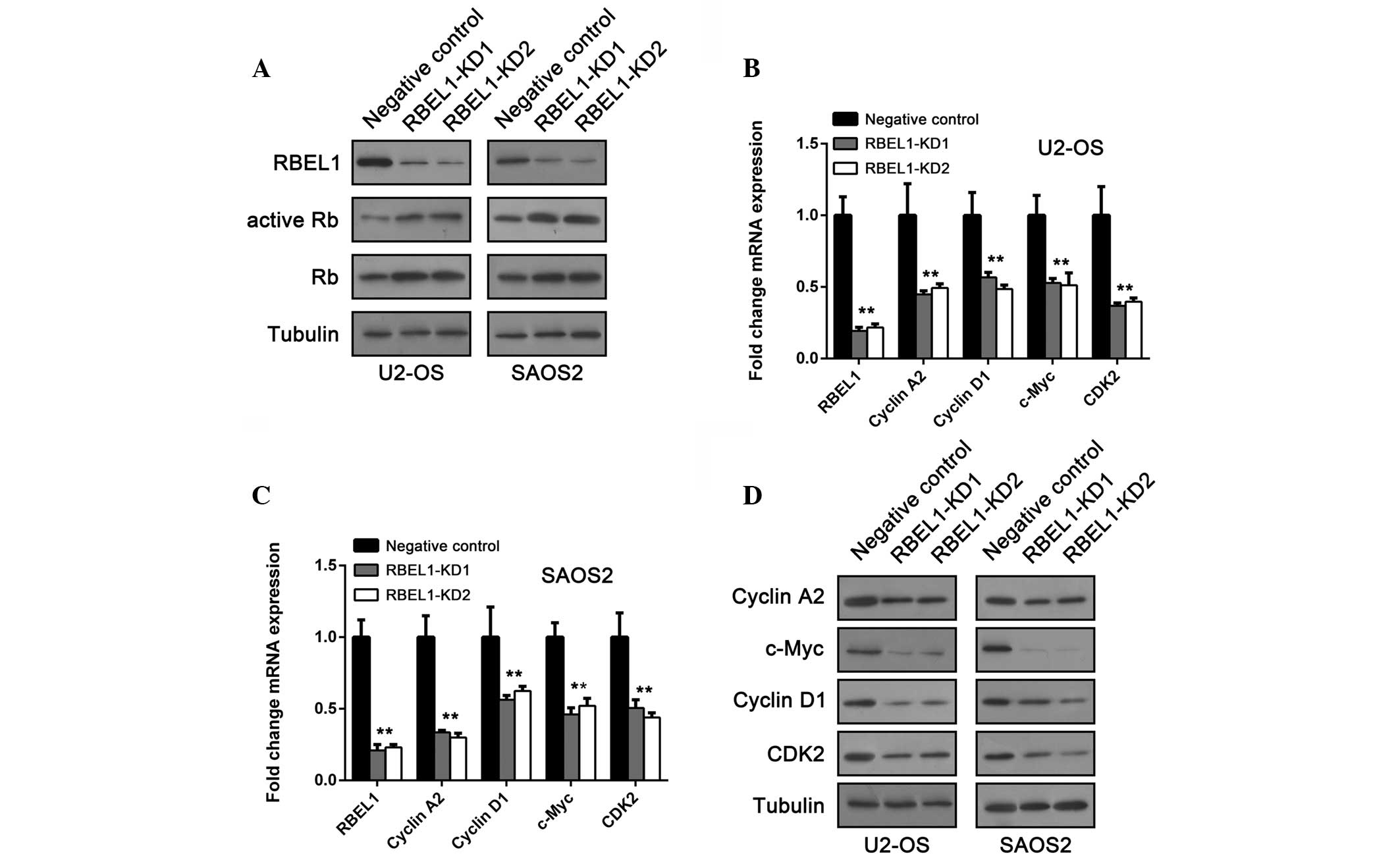
impact of RBEL1 depletion on Rb expression was analyzed. An
immunoblot assay showed that following RBEL1 knockdown,
osteosarcoma cells exhibited increased expression of Rb compared
with control cells (Fig. 3A). As
the hypophosphorylated form of Rb is the active form that
suppresses cell proliferation, it was examined whether
hypophosphorylated Rb was also upregulated. As expected, RBEL1
knockdown cells expressed more hypophosphorylated Rb compared with
the control (Fig. 3A). It is
well-known that Rb targets the E2F transcription factor to suppress
its downstream oncogene expression (11,12).
To investigate whether RBEL1 depletion affects E2F transciptional
activity, the expression of its downstream targets, such as cyclin
A2, cyclin D1, c-Myc and CDK2, were examined. In agreement with
above results, the mRNA and protein expression of these oncogenes
was downregulated following RBEL1 knockdown in both cell types
(Fig. 3B–D). These results
indicate that RBEL1 may be a key regulator of Rb and its downstream
targets.
RBEL1 exerts its oncogenic effects by
inhibiting Rb
The results indicated that RBEL1 modulates cell
proliferation and G1-S transition, and can inhibit Rb activity.
Thus it was investigated whether RBEL1 exerts its oncogenic effects
by inhibiting Rb. To test this hypothesis, Rb expression was
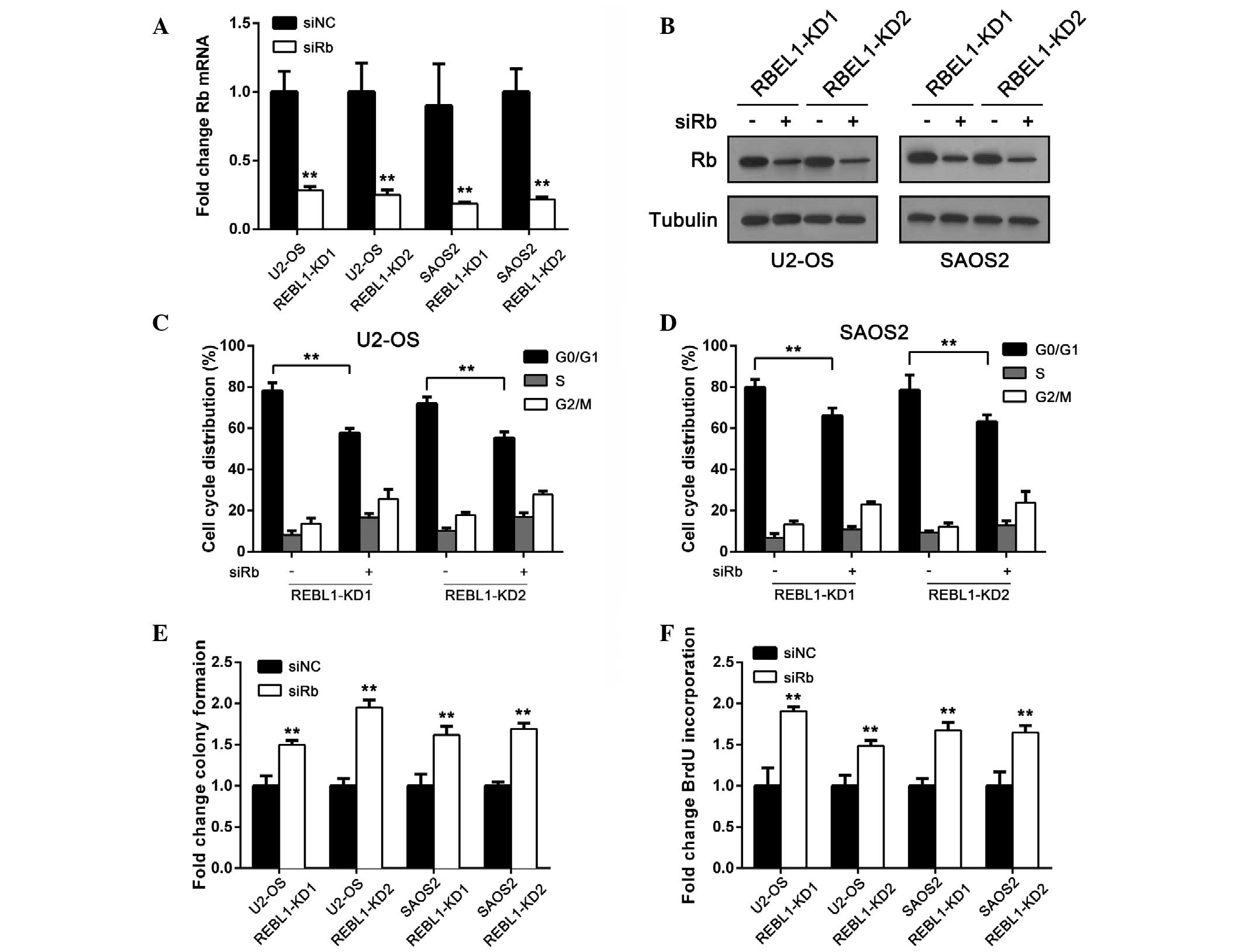
suppressed in RBEL1 knockdown cells to examine whether RBEL1
knockdown-induced tumor suppression can be rescued. The knockdown
efficiency was confirmed by RT-qPCR and an immunoblot assay
(Fig. 4A and B). A flow cytometry
assay showed that in U2-OS and SAOS2, RBEL1 knockdown-induced G1-S
arrest was decreased after Rb suppression (Fig. 4C and D). Furthermore, Rb
suppression reversed colony formation capability in cells with
RBEL1-knockdown (Fig. 4E). BrdU
incorporation was also increased following Rb suppression (Fig. 4F). These results suggest that RBEL1
exerts its oncogenic effects by inhibiting Rb.
Discussion
Numerous factors have been implicated in
tumorigenesis, such as mutations, chronic inflammation resulting
from bacterial or viral infection, and prolonged exposure to
radiation or oncogenic chemicals (13,14).
However, the etiology of osteosarcoma remains largely unknown.
Deregulated cell proliferation has been recognized as one of the
hallmarks of cancer (15,16). Intensive efforts were made and
numerous genetic or epigenetic events were attributed to
proliferation deregulation (17–22).
The present study demonstrates a novel regulatory mechanism of cell
proliferation in osteosarcoma cells. When RBEL1 was depleted, U2-OS
and SAOS2 cells generated fewer and smaller colonies. Cell
proliferation suppression and G1-S arrest was also observed in
RBEL1-depleted cells. These results suggest RBEL1 may act as an
oncogene in osteosarcoma.
The cell cycle is a tightly regulated process that
ensures specific events take place in an orderly manner. Any fault
in this regulatory network results in uncontrolled cell
proliferation or cell death (23,24).
The cell cycle is monitored by checkpoints that sense possible
defects during DNA synthesis and chromosome segregation. Cell cycle
arrest allows cells to properly repair these defects, thus
preventing their transmission to the resulting daughter cells
(25). Deregulated cell cycle
arrest has been observed in osteosarcoma (26,27).
However, the underlying molecular mechanism remains unclear. In the
present study, RBEL1 was observed to participate in cell cycle
control in osteosarcoma. G1-S transition is the most common
deregulated cell cycle transition in cancer (23) and in the present study RBEL1
downregulation induced G1-S arrest in U2-OS and SAO2 cells.
Rb is a tumor suppressor protein that is
dysfunctional in several types of cancer (28,29).
One function of Rb is to prevent excessive cell growth by
inhibiting cell cycle progression until a cell is ready to divide
(11,30). RBEL1 was found to negatively
regulate Rb activity. RBEL1 downregulation resulted in an increase
in Rb expression and an increase in the hypophosphorylated form of
the protein. Furthermore, E2F transcriptional activity was also
reduced. Cyclin A2, cyclin D1, c-Myc and CDK2, the downstream
target of E2F transcription factors, were upregulated in
RBEL1-depleted cells. These proteins are widely accepted as
oncogenes and cell cycle positive regulator in cancer cells. Thus,
RBEL1 may promote cell proliferation and G1-S transition by
inhibiting Rb activity.
In conclusion, these findings suggest a novel
mechanism underlying osteosarcoma progression and cell cycle
regulation. RBEL1 inhibits Rb activity to upregulated oncogenic
factors, such as cyclin A2, cyclin D1, c-Myc and CDK2, therefore
promoting G1-S transition and cell proliferation in osteosarcoma
cells. These results suggest that RBEL1 may be a novel therapeutic
target and potential biomarker for osteosarcoma. However, whether
RBEL1 is upregulated in human osteosarcoma samples and how RBEL1
exerts its Rb inhibitory function remain unclear and require
further investigation.
References
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura T, Matsumine A, Matsubara T,
Asamuma K, Niimi R, Uchida A and Sudo A: Retrospective analysis of
metastatic sarcoma patients. Oncol Lett. 2:315–318. 2011.PubMed/NCBI
|
|
5
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stenmark H: Rab GTPases as coordinators of
vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montalbano J, Jin W, Sheikh MS and Huang
Y: RBEL1 is a novel gene that encodes a nucleocytoplasmic Ras
superfamily GTP-binding protein and is overexpressed in breast
cancer. J Biol Chem. 282:37640–37649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montalbano J, Lui K, Sheikh MS and Huang
Y: Identification and characterization of RBEL1 subfamily of
GTPases in the Ras superfamily involved in cell growth regulation.
J Biol Chem. 284:18129–18142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hochegger H, Takeda S and Hunt T:
Cyclin-dependent kinases and cell-cycle transitions: Does one fit
all? Nat Rev Mol Cell Biol. 9:910–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burkhart DL and Sage J: Cellular
mechanisms of tumour suppression by the retinoblastoma gene. Nat
Rev Cancer. 8:671–682. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Classon M and Harlow E: The retinoblastoma
tumour suppressor in development and cancer. Nat Rev Cancer.
2:910–917. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Travis LB, Demark Wahnefried W, Allan JM,
Wood ME and Ng AK: Aetiology, genetics and prevention of secondary
neoplasms in adult cancer survivors. Nat Rev Clin Oncol.
10:289–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barcellos-Hoff MH: Does microenvironment
contribute to the etiology of estrogen receptor-negative breast
cancer? Clin Cancer Res. 19:541–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta SC, Kim JH, Prasad S and Aggarwal
BB: Regulation of survival, proliferation, invasion, angiogenesis
and metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fritz V and Fajas L: Metabolism and
proliferation share common regulatory pathways in cancer cells.
Oncogene. 29:4369–4377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Whitfield ML, George LK, Grant GD and
Perou CM: Common markers of proliferation. Nat Rev Cancer.
6:99–106. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng H, Cheng Y, Guo Z, Zhang F, Lu X,
Feng L, Wang X and Xu Z: Overexpression of CyclinA2 ameliorates
hypoxia-impaired proliferation of cardiomyocytes. Exp Ther Med.
8:1513–1517. 2014.PubMed/NCBI
|
|
22
|
Chen G, Wang H, Xie S, Ma J and Wang G:
STAT1 negatively regulates hepatocellular carcinoma cell
proliferation. Oncol Rep. 29:2303–2310. 2013.PubMed/NCBI
|
|
23
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian Y, Wan H and Tan G: Cell
cycle-related kinase in carcinogenesis. Oncol Lett. 4:601–606.
2012.PubMed/NCBI
|
|
25
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Zhou Q, Zhang N, Li W, Ying M,
Ding W, Yang B and He Q: E2F1 impairs all-trans retinoic
acid-induced osteogenic differentiation of osteosarcoma via
promoting ubiquitination-mediated degradation of RARα. Cell Cycle.
13:1277–1287. 2014. View
Article : Google Scholar :
|
|
27
|
Wang XH, Cai P, Wang MH and Wang Z:
MicroRNA-25 promotes osteosarcoma cell proliferation by targeting
the cell cycle inhibitor p27. Mol Med Rep. 10:855–859.
2014.PubMed/NCBI
|
|
28
|
Dick FA and Rubin SM: Molecular mechanisms
underlying RB protein function. Nat Rev Mol Cell Biol. 14:297–306.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: An exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prior IA, Lewis PD and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012. View Article : Google Scholar : PubMed/NCBI
|