Introduction
Previous studies have demonstrated that oxidative
stress has a significant role in the occurrence and progression of
hepatitis and hepatic fibrosis (1–4).
Oxidative stress results from the excessive production of reactive
oxygen species (ROS), and the inability of an organism to eliminate
them. Excessive ROS lead to lipid peroxidation, protein and DNA
damage, and injury to cellular structure and function (5). Numerous studies have reported the
important role of ROS in various types of hepatic injury (6–10).
In addition to ROS-induced inflammation, ROS may lead to loss of
normal regulatory functions, resulting in tissue injury and
excessive repair, and the development of hepatitis and hepatic
fibrosis (11,12). It has previously been demonstrated
that activation of hepatic stellate cells (HSCs) has a key role in
the progression of hepatic fibrosis; therefore, HSCs are considered
important target cells in hepatic fibrosis research (13). Furthermore, HSCs can be activated
by oxidative stress and transformed into myofibroblasts.
Myofibroblasts synthesize abundant extracellular matrix (ECM)
molecules, which may lead to hepatic fibrosis (14); therefore, how to inhibit the
activation of HSCs exposed to oxidative stress requires further
investigation. Nuclear factor-erythroid 2-related factor (Nrf2) is
a transcription factor that activates numerous antioxidant enzymes
and phase II detoxifying enzymes (15). In addition, Nrf2 has an important
role regulating oxidative stress (16,17);
however, the majority of studies regarding Nrf2 have focused on the
nervous and respiratory systems. The effects of Nrf2 on HSCs, and
the underlying molecular mechanisms, have seldom been reported.
The present study hypothesized that upregulation of
Nrf2 nuclear translocation would promote the expression of
antioxidant enzymes and phase II detoxifying enzymes, thus
protecting the liver against injury. The present study investigated
the alterations and regulatory mechanisms of the Nrf2 pathway,
which is of great significance for understanding the pathogenesis
of hepatic fibrosis and developing novel preventative strategies
and curative therapies.
Curcumin, which is an ingredient of the spice
turmeric, is present in the rhizomes of Curcuma longa Linn
(Zingiberaceae). Curcumin has been reported to exert antioxidant,
anti-inflammatory, anticancer and hepatoprotective effects
(18). Furthermore, curcumin
functions as an exogenous Nrf2 agonist, and can promote the nuclear
translocation and biological effects of Nrf2 (19). Therefore, the present study used
curcumin to upregulate Nrf2, and subsequently investigated the
effects of Nrf2 on HSCs.
To investigate the possible regulatory mechanisms
that underlie the Nrf2 pathway, the present study examined the
effects of curcumin on Nrf2. In addition, alterations in the levels
of ROS, malondialdehyde (MDA) and glutathione (GSH) were detected.
As an index of HSC activation, smooth muscle α-actin (α-SMA) and
desmin levels were measured. Furthermore, ECM-secreted proteins,
including type III procollagen (PCIII), type IV collagen (CIV),
laminin (LN) and hyaluronic acid (HA), were measured following
treatment of HSCs with curcumin. The results of the present study
indicated that curcumin was able to protect HSCs against oxidative
stress, and inhibit the activation of HSCs via induction of Nrf2
nuclear translocation.
Materials and methods
Materials
Curcumin and GO were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Anti-α-SMA (1:200; cat. no. sc-53142) and
anti-β-actin (1:500; cat. no. sc-47778) antibodies were obtained
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-Nrf2
(1:500; cat. no. BS6286) and anti-desmin (1:500; cat. no. BS1712)
antibodies were purchased from Bioworld Technology, Inc. (St. Louis
Park, MN, USA). Horseradish peroxidase-conjugated anti-rabbit
(1:10,000; cat. no. bs-0295G-HRP) and anti-mouse (1:10,000; cat.
no. bs-0296G-HRP) immunoglobulin (Ig)G, and fluorescein
isothiocyanate (FITC)-conjugated anti-rabbit (1:1,000; cat. no.
bs-0295G-FITC) and anti-mouse (1:1,000; cat. no. bs-0296G-FITC) IgG
secondary antibodies were obtained from Beijing Biosynthesis
Biotechnology Co., Ltd. (Beijing, China). Dihydroethidium (DHE) was
purchased from Beyotime Institute of Biotechnology (Jiangsu,
China). MDA and GSH kits were obtained from Nanjing Jiancheng
Biotechnology, Inc. (Nanjing, China). PCIII, CIV, LN and HA kits
were purchased from the Shanghai Naval Medical Institute (Shanghai,
China).
Cell culture
The HSC-T6 immortalized rat HSC line exhibits a
stable phenotype and biochemical characteristics (20). HSC-T6 cells were a generous gift
from Dr Ding of the Medical College of Xi'an Jiaotong University
(Xi'an, China). The cells were grown under standard conditions in a
normoxic atmosphere in high-glucose Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Sijiqing, Hangzhou,
China) in a humidified incubator containing 5% CO2 at
37°C. All subsequent experiments were conducted using cells at the
exponential stage of growth. Cells were seeded into a 25
cm2 plastic culture flask at a density of
1×106 cells. The cells were separated into three groups:
The negative control cells were incubated with 5 ml culture medium;
the oxidant-treated cells were incubated with 5 ml culture medium
supplemented with 100 mU/ml GO for 2 h before each experimental
manipulation; and the curcumin-treated cells were pre-treated with
5 ml culture medium containing 0.15 µmol curcumin for 3 h,
and then incubated in the same manner as the oxidant-treated
cells.
Western blot analysis
Total, cytoplasmic and nuclear proteins were
obtained from the cells using protein extraction kits (Beyotime
Institute of Biotechnology, Shanghai, China), according to the
manufacturer's protocol. The concentration of the protein samples
was quantified using a Bradford Protein Assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Subsequently, the protein samples (15 µg) were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and were transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
blocked in buffer containing skim milk for 2 h, and were then
incubated with anti-Nrf2 primary antibody at 4°C overnight, and
washed with phosphate-buffered saline (PBS). The membranes were
then incubated with a horseradish peroxidase-conjugated secondary
antibody at room temperature for 1 h. Pierce Enhanced
Chemiluminescence Western Blotting substrate (Thermo Fisher
Scientific, Inc.) was used to develop the blots, and
immunore-activity was visualized following exposure to X-OMAT BT
Film (Beyotime Institute of Biotechnology). β-actin was used as an
internal control. The results were quantified and normalized to
β-actin using Molecular Analyst software (version 1.4.1; Bio-Rad
Laboratories, Inc.)
Immunocytochemistry
Following a 24 h cell culture, in which the cells
became adherent, immunocytochemistry was conducted. Cell-coated
dishes (1×105 cells/ml) were fixed with 4%
paraformaldehyde for 30 min and washed with PBS. The cells were
permeabilized using 0.3% Triton X-100 (Sigma-Aldrich) for 15 min.
Endogenous peroxidases and biotins were quenched using 3%
H2O2. The cells were then blocked with FBS
and incubated with the indicated primary antibodies at 4°C
overnight. Sections stained for Nrf2 protein expression were washed
and incubated with a horseradish peroxidase-conjugated secondary
antibody at room temperature for 30 min. Bound secondary antibodies
were detected using Histostain-SP kits (ZSbio, Beijing, China),
according to the manufacturer's protocol. The reaction products
were visualized using diaminobenzidine tetrahydro-chloride (Tiangen
Biotech Co., Ltd., Beijing, China). Sections stained for α-SMA and
desmin protein expression were incubated with FITC-conjugated
secondary antibodies at room temperature for 30 min in the dark.
The nuclei were stained using Evans Blue (Sigma-Aldrich) in
sections stained for α-SMA. In the negative control group, primary
antibodies were substituted with PBS. Stained sections were viewed
under a Nikon Eclipse 800 fluorescent microscope (Nikon
Corporation, Tokyo, Japan).
Flow cytometry
HSC-T6 cells were maintained under standard
conditions, and were then transferred to culture dishes. Following
a 24 h culture, 1×106 cells from each group were placed
in tubes. The cells were washed with PBS and separated by
centrifugation at 432 x g for 10 min at 4°C. Serum-free media
supplemented with 2-µM DHE were added, and the tubes were
incubated at 4°C for 30 min in the dark. The remaining cells were
washed with PBS and subjected to further centrifugation at 432 x g
for 10 min at 4°C. Paraformaldehyde (2 ml; 4%) was added to each
tube and the tubes were incubated at 4°C for 30 min in the dark,
followed by centrifugation at 432 x g for 10 min at 4°C.
Subsequently, 300 µl PBS was added to the preparations and
mixed gently. DHE fluorescence was measured by flow cytometry
(FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA).
MDA and GSH assays
HSC-T6s were incubated in culture dishes and the
supernatants were collected for the detection of MDA and GSH by
spectrophotometry. Measurement of the product (MDA-TBA adduct) of a
reaction between MDA and 2-thiobarbituric acid, and of the product
(2-nitro-5-sulphur benzoic acid) of a reaction between GSH and
dithiodinitrobenzoic acid allow the levels of MDA and GSH to be
analyzed using colorimetric assays. MDA and GSH levels were
determined using kits, according to the manufacturers' protocols.
Absorbance was measured at 532 and 412 nm using a spectrophotometer
(UV-2450; Shimadzu Corporation, Kyoto, Japan) for MDA and GSH,
respectively. The concentrations of MDA and GSH were calculated
according to the equation provided in the kits.
Analysis of ECM secretion
Cells were initally separated into three groups and
cultured in serum-free medium overnight. Subsequently, the cells
were treated as mentioned previously (negative control,
oxidant-treated and curcumin-treated cells). Following
centrifugation at 1,000 x g for 20 min at 4°C, the supernatant was
collected and maintained at −80°C until further analysis. The
levels of PCIII, CIV, LN and HA secreted into the supernatant were
analyzed using commercially available radio-immunoassay kits,
according to the manufacturers' protocols.
Statistical analysis
The data are presented as the mean ± standard
deviation and significance was assessed using SPSS 12.0 software
(SPSS, Inc., Chicago, IL, USA). Statistical comparisons were
performed using one-way analysis of variance. Paired comparisons
were conducted using Student Newman Keuls-q test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin protects HSCs against GO-induced
oxidative stress injury
As a marker of oxidative stress, GO may react with
glucose in the culture media and subsequently promote the
generation of glucuronic acid and hydrogen peroxide
(H2O2). It is well known that DHE, which is a
superoxide radical-specific fluorescent probe, is able to enter
viable cells freely where it is oxidized by superoxide to form
ethidium, which binds to DNA and exhibits red fluorescence.
Therefore, the present study detected fluorescence intensity in
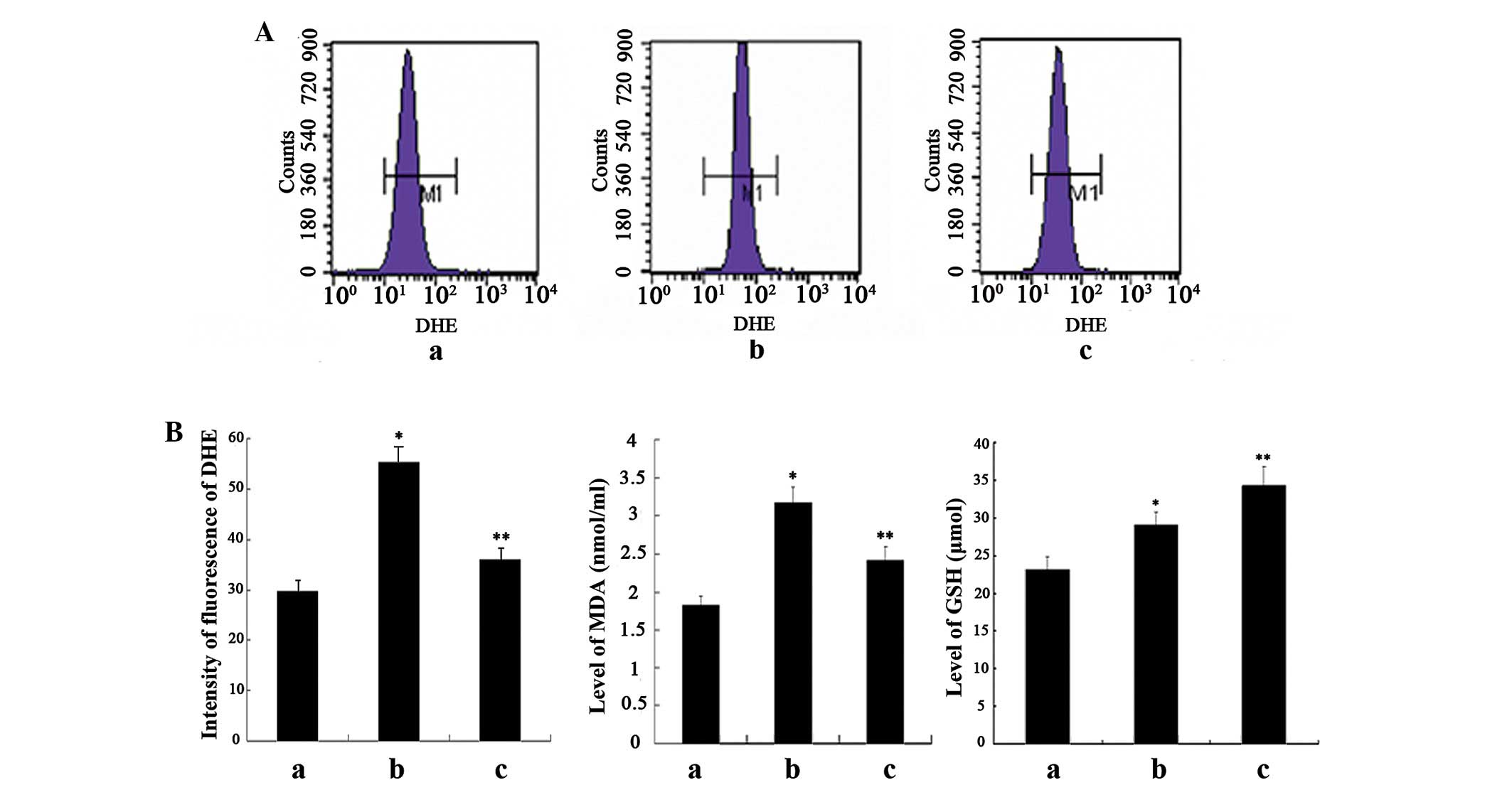
order to estimate the relative levels of ROS production. As shown
in Fig. 1A, compared with the
negative control cells, treatment of the cells with GO
significantly increased fluorescence intensity, whereas
pretreatment with curcumin significantly decreased fluorescence
intensity. However, the fluorescence intensity in the
curcumin-treated cells was stronger, as compared with in the
negative control cells.
Furthermore, ROS may react with lipids in cell and
mitochondrial membranes, resulting in the production of MDA, which
can be used to measure lipid peroxidation. Due to its strong
cytotoxicity, MDA is able to alter membrane permeability or disrupt
membrane integrity, leading to oxidative injury. However, even in
the case of increased lipid peroxidation, oxidative stress only
occurs in cells that are unable to defend and protect against free
radical injury or chemically induced damage. GSH is an important
endogenous antioxidant, which reduces levels of ROS. GSH acts as a
free radical scavenger, a coenzyme for various antioxidant enzymes,
a regulator of thioldisulfide status, and is involved in the
detoxification of electrophilic xenobiotics via conjugation.
Therefore, the present study aimed to detect the levels of MDA and
GSH in the supernatant. Significantly enhanced levels of MDA were
detected in the oxidant-treated cells, as compared with the
negative control cells, which were correlated with the levels of
ROS. The MDA levels were markedly attenuated in response to
curcumin pretreatment, however they remained higher than in the
control cells. Furthermore, compared with the negative control
cells, the levels of GSH were slightly increased in the
oxidant-treated cells, whereas they were significantly elevated in
the curcumin-treated cells (Fig.
1B).
These results suggest that an oxidative stress model
of HSC-T6 was established with GO treatment, as demonstrated in the
increased levels of ROS and MDA. Furthermore, pretreatment with
curcumin was able to significantly suppress the degree of oxidative
stress, at least partially due to the induced expression of
endogenous GSH.
Curcumin promotes the nuclear
translocation of Nrf2
GSH synthesis is governed by Nrf2 via the regulation
of the rate-limiting enzymes glutamate cysteine ligase (GCL)
catalytic subunit (GCLC) and GCL modifier subunit (GCLM). To
elucidate the molecular mechanism by which curcumin protects HSCs
against oxidative stress injury, the present study detected the
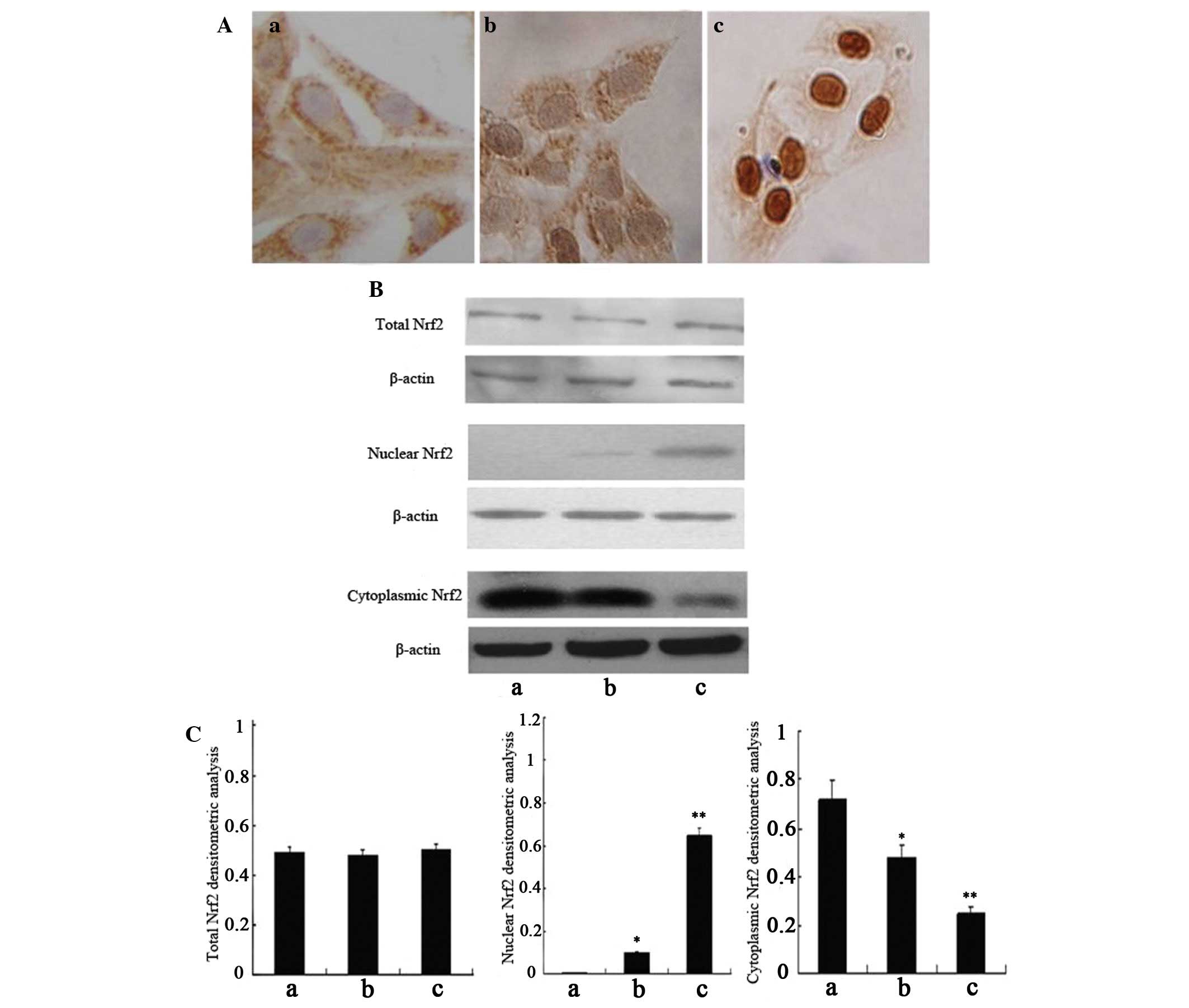
expression of Nrf2 in HSC-T6 cells by immunocytochemistry (Fig. 2A) and western blot analysis
(Fig. 2B and C). As expected, the
expression of total Nrf2 did not differ between the groups;
however, the expression levels of cytoplasmic Nrf2 were slightly
decreased in the oxidant-treated cells, and significantly decreased
in the curcumin-treated cells, as compared with the negative
control cells. Conversely, the expression of nuclear Nrf2 was
absent in the negative control cells and was only slightly
expressed in the oxidant-treated cells; however, the expression
levels of nuclear Nrf2 were markedly increased in the
curcumin-treated cells, as compared with in the oxidant-treated
cells. Immunocytochemistry detected similar results to the western
blot analysis; only minimal positive brown staining was detected in
the nuclei of the oxidant-treated cells, as compared with the
control cells, in which no positive brown nuclear staining was
detected. However, in the curcumin-treated cells, the amount of
positive brown staining was abundant in the nucleus, as compared
with in the oxidant-treated cells.
These results indicate that oxidative stress may
activate the Nrf2 regulatory pathway, and pretreatment with
curcumin could protect HSCs against oxidative stress via promoting
the translocation of Nrf2 from the cytoplasm to the nucleus.
Curcumin blocks GO-induced α-SMA
expression and HSC activation
Desmin, which is a type of cytoskeletal intermediate
filament, has been widely used as a marker for distinguishing HSCs.
Desmin was expressed in all cell groups, as detected using
immunofluorescence (Fig. 3A), and
there were no differences in desmin expression between the groups,
as determined by western blot analysis (Fig. 3B). α-SMA is produced by activated
HSCs, and is a characteristic signal of HSC activation. As shown in
Fig. 3A, no fluorescence
expression of α-SMA was detected in the negative control cells,
whereas following treatment with GO for 2 h abundant fluorescence
expression of α-SMA was observed. Furthermore, the fluorescence
expression of α-SMA in the cells pretreated with curcumin was
markedly decreased, as compared with the oxidant-treated cells.
Consistent with the alterations in immunofluorescence, the protein
expression levels of α-SMA were significantly increased in the
oxidant-treated cells, as compared with the negative control cells,
whereas the expression levels of α-SMA were significantly decreased
in the curcumin-treated cells, as compared with the oxidant-treated
cells. However, the expression levels remained higher in the
curcumin-treated cells, as compared with in the negative control
cells (Fig. 3B and C).
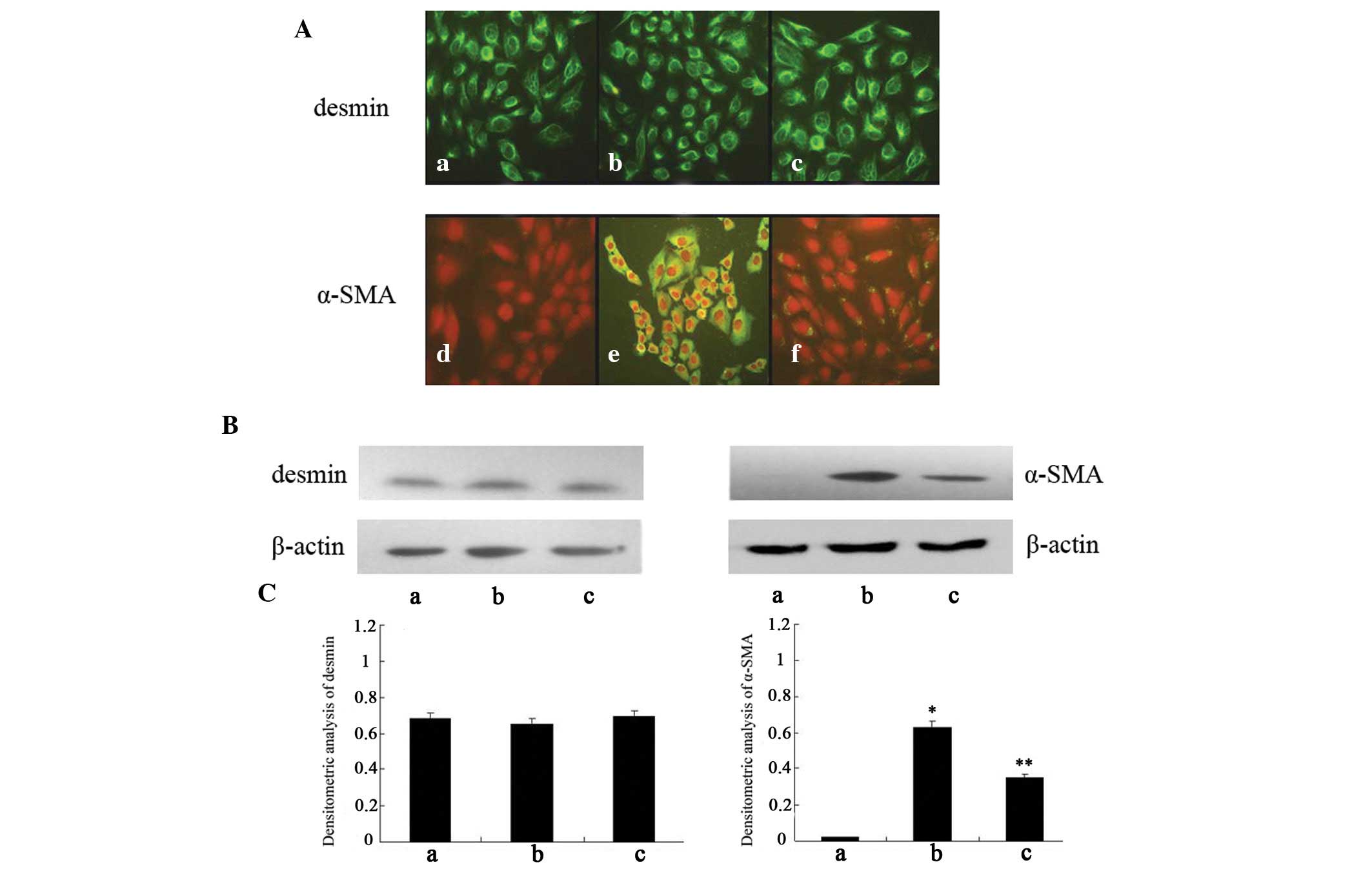 | Figure 3Expression of smooth muscle α-actin
(α-SMA) and desmin in various hepatic stellate cell (HSC)-T6
groups. (A) Activation of HSC-T6 in the various groups, as
determined by immunofluorescence (original magnification, ×400).
(a–c) Staining assay of desmin in negative control cells,
oxidant-treated cells and curcumin-treated cells, respectively;
(d–f) staining assay of α-SMA in negative control cells,
oxidant-treated cells and curcumin-treated cells, respectively. Red
staining indicates nuclei, green staining indicates α-SMA
expression. (B) Expression of desmin and α-SMA, as determined by
western blot analysis. β-actin was used as a loading control. (C)
Densitometric analysis of western blotting. a, negative control
cells; b, oxidant-treated cells; c, curcumin-treated cells. Data
are presented as the mean ± standard deviation.
*P<0.01 vs. the negative control cells;
**P<0.01 vs. the oxidant-treated cells. |
These results suggest that GO-induced oxidative
stress may enhance α-SMA expression in HSCs, and the transformation
of HSCs to myofibroblast-like cells. Furthermore, treatment with
curcumin may activate the Nrf2 regulatory pathway and subsequently
suppress α-SMA expression and HSC activation, which may be
associated with its antifibrotic effects.
Curcumin inhibits the expression of ECM
molecules in GO-treated HSCs
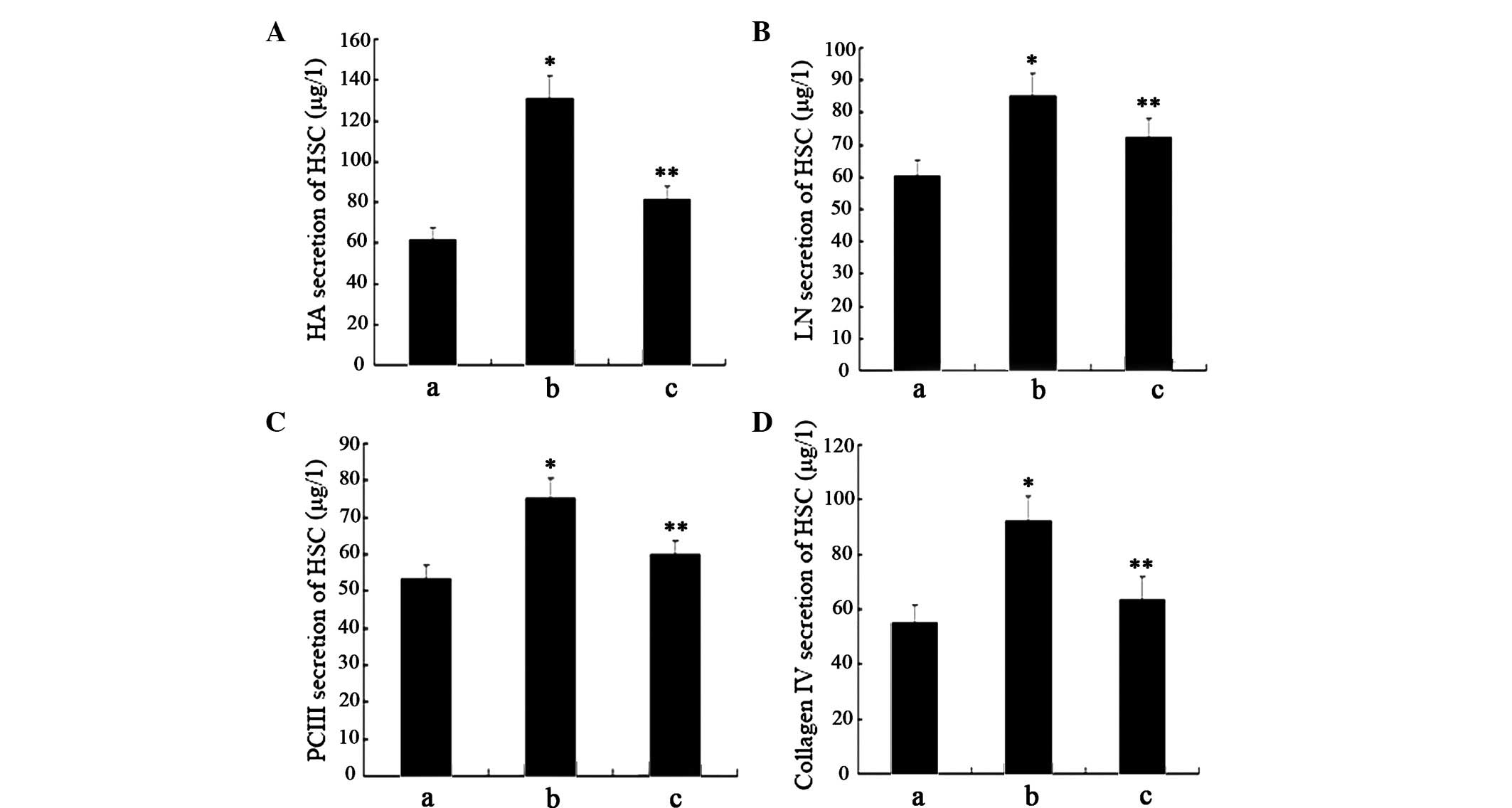
Serum levels of PCIII, CIV, LN and HA are often
considered indices of liver fibrosis; therefore, the present study
aimed to detect the levels of PCIII, CIV, LN and HA in the
supernatant (Fig. 4A–D). Compared
with the negative control cells, HSCs treated with GO for 2 h
exhibited significantly increased levels of ECM molecules.
Conversely, the levels of ECM molecules were markedly reduced
following pretreatment with curcumin; however, the levels remained
higher, as compared with in the negative control cells.
These results indicate that treatment with GO may
markedly increase the expression of ECM molecules in HSCs.
Furthermore, the activation of Nrf2 by curcumin may exert
inhibitory effects on the expression of ECM molecules in HSC, which
may be associated with its antifibrogenic effects.
Discussion
The Nrf2 pathway is regarded as the most important
pathway with regards to cellular protection against oxidative
stress (21). As a pivotal
modulator of the response to oxidative stress, the activation of
Nrf2 induces the expression of various protective antioxidant genes
(22,23). Under normal conditions, Nrf2 is
sequestered in the cytoplasm where it is bound to the
cytoskeleton-associated protein Keap1 (24). Oxidative stress promotes the
dissociation of the Nrf2-Keap1 complex, thus resulting in Nrf2
stabilization and translocation to the nucleus (25). In the nucleus, Nrf2 associates with
dimerization partners and binds antioxidant-response element
sequences, in order to induce the expression of various
detoxification and antioxidant enzyme genes that contribute to the
protective response (26).
Curcumin is a natural polyphenol product that is
derived from the rhizome of Curcuma longa. Numerous studies
have detected the various bioactivities of curcumin, including
antioxidant, anti-inflammatory, cell apoptosis-inducing and cell
proliferation-inhibiting activities (27–30).
In addition, it has been suggested that curcumin may be used
clinically against numerous types of cancer, inflammatory bowel
disease, irritable bowel syndrome (IBS), rheumatoid arthritis and
atherosclerosis (31). Cheng et
al (32) detected the effects
of curcumin on 25 patients with various types of high-risk or
premalignant lesions; following 3 months of treatment with
curcumin, some patients exhibited histological improvement in
premalignant lesions. Dhillon et al (33) studied the efficacy of curcumin on
patients with advanced pancreatic cancer, and demonstrated that
oral curcumin administration exerted biological activity in some
patients. Hanai et al (34)
conducted a double-blind, placebo-controlled trial in 89 patients
with ulcerative colitis (UC); curcumin was shown to reduce the
incidence of UC and may be considered a promising treatment for UC.
In addition, a blind pilot study demonstrated that abdominal pain
and the discomfort score of patients with IBS were significantly
reduced following treatment with curcumin (35).
Previous studies have detected various functions of
curcumin. Notably, curcumin has been shown to function as an
exogenous agonist of Nrf2 (19). A
previous study suggested that curcumin may alter the conformation
of Keap1, and promote the dissociation of Nrf2 from Keap1 and its
subsequent nuclear translocation (36). The present study evaluated the
effects of curcumin on Nrf2 regulation in HSCs; Nrf2 was localized
to the cytoplasm under normal conditions and little Nrf2 was
localized to the nucleus following the induction of oxidative
stress. However, pretreatment with curcumin induced a substantial
localization of Nrf2 to the nucleus in HSCs.
Increased levels of GSH are an index of Nrf2
activation, and increased GSH may be considered a major factor
underlying the protection associated with Nrf2 activation (37,38).
GSH not only produces reducing equivalents, which are necessary for
the conversion of H2O2 and lipid peroxides to
water and lipid alcohols (39),
but also has an important role in the protection of protein
sulfhydration against oxidation (40). The rate-limiting reaction in GSH
biosynthesis is catalyzed by GCL, which comprises two subunits:
GCLC and GCLM. Nrf2 is able to increase the expression of GCLC and
GCLM (41); therefore, the
preferential activation of Nrf2 leads to more efficient GSH
biosynthesis and improved antioxidant status (42). In the present study, the levels of
GSH were increased alongside increasing Nrf2 nuclear translocation
following curcumin treatment. GSH elevates the antioxidant ability
of cells against oxidative stress.
The pathogenesis of hepatic fibrosis has yet to be
completely clarified; however, it is generally accepted that the
activation of HSCs is central to the process (43). In the prophase of liver fibrosis,
quiescent HSCs transform into myofibroblasts, which are
characterized by the assembly of α-SMA stress fibers, loss of
cytosolic retinol and increased proliferation (44). This activation subsequently results
in the synthesis of cytokines and the accumulation of ECM molecules
(45). Considerable attention has
been focused on elucidating the mechanistic triggers of HSC
myofibroblast transformation. In addition, it is hypothesized that
oxidative stress may contribute to HSC activation (46).
In the present study, GO reacted with glucose in the
culture media resulting in the generation of glucuronic acid and
H2O2. These increased levels of ROS may
stimulate HSCs to undergo oxidative stress (47). The results of the present study
demonstrated that following treatment with GO, the levels of ROS in
the oxidant-treated cells were significantly increased, as compared
with in the negative control cells. The negative control cells were
quiescent, whereas the oxidant-treated cells were activated by
oxidative stress. In addition to the upregulation of Nrf2 nuclear
translocation in the curcumin-treated cells, the levels of ROS and
HSC activation were decreased, as compared with in the
oxidant-treated cells.
In-depth research regarding the mechanisms
underlying liver fibrosis has revealed the role of free radicals
and membrane lipid peroxidation in the process of liver injury
(48). Oxygen radicals attack
unsaturated fatty acids in cellular membranes and initiate lipid
peroxidation. Lipid peroxidation leads to alterations in the
permeability of cellular membranes, subsequently aggravating cell
dysfunction and promoting the secretion of ECM molecules (49,50).
Not only does oxidative stress stimulate HSC proliferation and
collagen synthesis, it also further damages cells by promoting
lipid peroxidation. MDA and other peroxidation products increase
collagen synthesis via HSC activation, and stimulate Kupffer cells
to release cytokines that promote fibrosis (51). Lipid peroxidation has an important
role in regulating collagen gene expression, and is associated with
cell injury and fibrosis. ROS and lipid peroxidation have been
implicated as profibrogenic mediators (52), whereas Nrf2 is effective at
suppressing cell damage resulting from lipid peroxidation (53). In the present study, the levels of
MDA were increased alongside ROS levels in the oxidant-treated
cells; however, following pretreatment with curcumin, the levels of
MDA were reduced, as compared with in the oxidant-treated
cells.
Liver fibrosis is the excessive deposition of ECM
molecules following liver injury. ECM accumulation is associated
with increased collagen synthesis and decreased matrix degradation,
contributing to liver fibrosis and remodeling (54). Active HSCs are the primary source
of the excessive production of ECM components (55), and the ECM enhanced density leads
to increased matrix stiffness, which is a significant stimulus for
the activation of HSCs (56). In
the present study, oxidative stress induced HSC activation, which
was followed by the enhanced synthesis of ECM components. Following
curcumin pretreatment, the activation of HSCs was inhibited, and
the secretion of ECM components was suppressed.
In conclusion, the present study demonstrated that
curcumin-induced Nrf2 activation may protect HSCs against oxidative
stress-induced injury, and this effect was characterized by
enhanced Nrf2 nuclear translocation and antioxidant capacity. The
underlying mechanism remains to be elucidated; however, the present
study proposes a broader application for Nrf2 in the prevention and
treatment of hepatic damage.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30800515, 81270485
and 81170376) and the Natural Science Foundation of Shaanxi
Province (grant no. 2013JM4021).
Abbreviations:
|
Nrf2
|
nuclear factor-erythroid 2-related
factor
|
|
HSC
|
hepatic stellate cell
|
|
GO
|
glucose oxidase
|
|
ROS
|
reactive oxygen species
|
|
MDA
|
malondialdehyde
|
|
GSH
|
glutathione
|
|
α-SMA
|
smooth muscle α-actin
|
|
ECM
|
extracellular matrix
|
|
PCIII
|
type III procollagen
|
|
C IV
|
type IV collagen
|
|
LN
|
laminin
|
|
HA
|
hyaluronic acid
|
|
DHE
|
dihydroethidium
|
References
|
1
|
Parola M and Pinzani M: Hepatic wound
repair. Fibrogenesis Tissue Repair. 2:42009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loguercio C and Federico A: Oxidative
stress in viral and alcoholic hepatitis. Free Radic Biol Med.
34:1–10. 2003. View Article : Google Scholar
|
|
3
|
Lin X, Zhang S, Huang R, Wei L, Tan S,
Liang S, Tian Y, Wu X, Lu Z and Huang Q: Helenalin attenuates
alcohol-induced hepatic fibrosis by enhancing ethanol metabolism,
inhibiting oxidative stress and suppressing HSC activation.
Fitoterapia. 95:203–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee BH, Hsu WH, Hsu YW and Pan TM:
Suppression of dimerumic acid on hepatic fibrosis caused from
carboxymethyllysine (CML) by attenuating oxidative stress depends
on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem
Toxicol. 62:413–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bisbal C, Lambert K and Avignion A:
Antioxidants and glucose metabolism disorders. Curr Opin Clin Nutr
Metab Care. 13:439–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anoush M, Eghbal MA, Fathiazad F, Hamzeiy
H and Kouzehkonani NS: The protective effects of garlic extract
against acetaminophen-induced oxidative stress and glutathione
depletion. Park J Biol Sci. 12:765–771. 2009. View Article : Google Scholar
|
|
7
|
Sun Q, Long Z, Wu H, Liu Y, Wang L, Zhang
X, Wang X and Hai C: Effect of alcohol on
diethylnitrosamine-induced hepatic toxicity: Critical role of ROS,
lipid accumulation, and mitochondrial dysfunction. Exp Toxicol
Pathol. 67:491–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takemoto K, Hatano E, Iwaisako K, Takeiri
M, Noma N, Ohmae S, Toriguchi K, Tanabe K, Tanaka H, Seo S, et al:
Necrostatin-1 protects against reactive oxygen species
(ROS)-induced hepatotoxicity in acetaminophen-induced acute liver
failure. FEBS Open Bio. 4:777–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Browning JD and Horton JD: Molecular
mediators of hepatic steatosis and liver injury. J Clin Invest.
114:147–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh S, Vrishni S, Singh BK, Rahman I and
Kakkar P: Nrf2-ARE stress response mechanism: A control point in
oxidative stress-mediated dysfunctions and chronic inflammatory
diseases. Free Radic Rec. 44:1267–1288. 2010. View Article : Google Scholar
|
|
12
|
Matsunami T, Sato Y, Ariga S, Sato T,
Kashimura H, Haseqawa Y and Yukawa M: Regulation of oxidative
stress and inflammation by hepatic adiponectin receptor 2 in an
animal model of nonalcoholic steatohepatitis. Int J Clin Exp
Pathol. 3:472–481. 2010.PubMed/NCBI
|
|
13
|
De Minicis S, Candelaresi C, Agostinelli
L, Taffetani S, Saccomanno S, Rychlicki C, Trozzi L, Marzioni M,
Benedetti A and Svegliati-Baroni G: Endoplasmic Reticulum stress
induces hepatic stellate cell apoptosis and contributes to fibrosis
resolution. Liver Int. 32:1574–1584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tacke F and Weiskirchen R: Liver fibrosis
- pathogenesis and novel therapeutic approaches. Internist (Berl).
51:21–29. 2010.In German. View Article : Google Scholar
|
|
15
|
Vargas MR and Johnson JA: The Nrf2-ARE
cytoprotective pathway in astrocytes. Expert Rev Mol Med.
11:e172009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klaassen CD and Reisman SA: Nrf2 the
rescue: Effects of the antioxidative/electrophilic response on the
liver. Toxicol Appl Pharmacol. 244:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamlé J, Marhenke S, Borlak J, von
Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M and
Vogel A: Nuclear factor-eythroid 2-related factor 2 prevents
alcohol-induced fulminant liver injury. Gastrornterology.
134:1159–1168. 2008. View Article : Google Scholar
|
|
18
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anti-cancer agent: Review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahman I: Antioxidant therapeutic advances
in COPD. Ther Adv Respir Dis. 2:351–374. 2008. View Article : Google Scholar
|
|
20
|
Ishiqaki N, Yamamoto N, Jin H, Uchida K,
Terai S and Sakaida I: Continuos intravenous infusion of atrial
natriuretic peptide (ANP) prevented liver fibrosis in rat. Biochem
Biophys Res Commun. 378:354–359. 2009. View Article : Google Scholar
|
|
21
|
Copple IM, Goldring CE, Kitteringham NR
and Park BK: The Nrf2-Keap1 defense pathway: Role in protection
against drug-induced toxicity. Toxicology. 246:24–33. 2008.
View Article : Google Scholar
|
|
22
|
Mazur W, Lindholm P, Vuorinen K,
Myllärniemi M, Salmenkivi K and Kinnula VL: Cell-specific elevation
of NRF2 and sulfiredoxin-1 as markers of oxidative stress in the
lungs of idiopathic pulmonary fibrosis and non-specific
interstitial pneumonia. APMIS. 118:703–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Churchman AT, Anwar AA, Li FY, Sato H,
Ishii T, Mann GE and Siow RC: Transforming growth factor-beta1
elicits Nrf2-mediated antioxidant responses in aortic smooth muscle
cells. J Cell Mol Med. 13:2282–2292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z,
Fang D and Zhang DD: Direct interaction between Nrf2 and p21
(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol
Cell. 34:663–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaspar JW, Niture SK and Jaiswal AK: Nrf2:
INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med.
47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katsuoka F, Motohashi H, Ishii T,
Aburatani H, Engel JD and Yamamoto M: Genetic evidence that small
maf proteins are essential for the activation of antioxidant
response element-dependent genes. Mol Cell Biol. 25:8044–8051.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumor and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joe B, Rao UJ and Lokesh BR: Presence of
acidic glycoprotein in the serum of arthritic rats: Modulation by
capsaicin and curcumin. Mol Cell Biochem. 169:125–134. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YX, Pindolina KR, Janakiraman N, Noth
C, Chapman RA and Gautam SC: Curcumin, a compound with
anti-inflammatory and anti-oxidant properties, down-regulates
chemokine expression in bone marrow stromal cell. Exp Hematol.
25:413–422. 1997.PubMed/NCBI
|
|
30
|
Dujic J, Kippenberger S, Ramirez-Bosca A,
Diaz-Alperi J, Bereiter-Hahn J, Kaufmann R, Bernd A and Hofmann M:
Curcumin in combination with visible light inhibits tumor growth in
a xenograft tumor model. Int J Cancer. 124:1422–1428. 2009.
View Article : Google Scholar
|
|
31
|
Fan X, Zhang C, Liu DB, Yan J and Liang
HP: The clinical applications of curcumin: Current state and the
future. Curr Pharm Des. 19:2011–2031. 2013.
|
|
32
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
33
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanai H, Iida T, Takeuchi K, Watanabe F,
Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M,
et al: Curcumin maintenance therapy for ulcerative colitis:
Randomized, multi-center, double-blind, placebo-controlled trial.
Clin Gastroenterol Hepatol. 4:1502–1506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bundy R, Walker AF, Middleton RW and Booth
J: Turmeric extract may improve irritable bowel syndrome
symptomology in otherwise healthy adults: A pilot study. J Altern
Complement Med. 10:1015–1018. 2004. View Article : Google Scholar
|
|
36
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the haem oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma ZC, Hong Q, Wang YG, Tan HL, Xiao CR,
Liang QD, Zhang BL and Gao Y: Ferulic acid protects human umbilical
vein endothelial cells from radiation induced oxidative stress by
phosphatidylinositol 3-kinase and extracelluar signal-regulated
kinase pathways. Biol Pharm Bull. 33:29–34. 2010. View Article : Google Scholar
|
|
38
|
Stridh MH, Correa F, Nodin C, Weber SG,
Blomstrand F, Nilsson M and Sandberg M: Enhanced glutathione efflux
from astrocytes in culture by low extracellular Ca2+ and
curcumin. Neurochem Res. 35:1231–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ballatori N, Krance SM, Notenboom S, Shi
S, Tieu K and Hammond CL: Glutathione dysregulation and the
etiology and progression of human disease. Biol Chem. 390:191–214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bindoli A, Fukuto JM and Forman HJ: Thiol
chemistry in peroxidase catalysis and redox signaling. Antioxid
Redox Signal. 10:1549–1564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson JA, Johnson DA, Kraft AD, Calkins
MJ, Jakel RJ, Vargas MR and Chen PC: The Nrf2-ARE pathway: An
indicator and modulator of oxidative stress in neurodegeneration.
Ann N Y Acad Sci. 1147:61–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kraft AD, Johnson DA and Johnson JA:
Nuclear factor E2-related factor 2-dependent antioxidant response
element activation by tert-butylhydroquinone and sulforaphane
occurring preferentially in astrocytes conditions neurons against
oxidative insult. J Neurosci. 24:1101–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Reichard JF and Petersen DR: Hepatic
stellate cells lack AP-1 responsiveness to electrophiles and
phorbol 12-myristate-13-acetate. Biochem Biophys Res Commun.
322:842–853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maeda K, Koda M, Matono T, Sugihara T,
Yamamoto S, Ueki M, Murawaki Y, Yamashita N and Nishiyama S:
Preventive effects of ME3738 on hepatic fibrosis induced by bile
duct ligation in rats. Hepatol Res. 38:727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Urtasun R, Conde de la Rosa L and Nieto N:
Oxidative and nitrosative stress and fibrogenic response. Clin
Liver Dis. 12:769–790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Leclercq I, Brymora JM, Xu N,
Ramezani-Moghadam M, London RM, Brigstock D and George J: Kupffer
cells mediate leptin-induced liver fibrosis. Gastroenterology.
137:713–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beyer TA, Xu W, Teupser D, auf dem Keller
U, Bugnon P, Hildt E, Thiery J, Kan YW and Werner S: Impaired liver
regeneration in Nrf2 knockout mice: Role of ROS-mediated
insulin/IGF-1 resistance. EMBO J. 27:212–223. 2008. View Article : Google Scholar
|
|
48
|
Qin Y and Tian YP: Preventive effects of
chronic exogenous growth hormone levels on diet-induced hepatic
steatosis in rats. Lipids Health Dis. 9:782010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kulbacka J, Saczko J and Chwiłkowska A:
Oxidative stress in cells damage processes. Pol Merkur Lekarski.
27:44–47. 2009.In Polish. PubMed/NCBI
|
|
50
|
Moselhy SS and Ali HK: Hepatoprotective
effect of cinnamon extracts against carbon tetrachloride induced
oxidative stress and liver injury in rats. Biol Res. 42:93–98.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sener G, Kabasakal L, Yüksel M, Gedik N
and Alican Y: Hepatic fibrosis in biliary-obstructed rats is
prevented by Ginkgo biloba treatment. World J Gastroenterol.
11:5444–5449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim J, Seok YM, Jung KJ and Park KM:
Reactive oxygen species/oxidative stress contributes to progression
of kidney fibrosis following transient ischemic injury in mice. Am
J Physiol Renal Physoil. 297:F461–F470. 2009. View Article : Google Scholar
|
|
53
|
Osburn WO, Wakabayashi N, Misra V, Nilles
T, Biswal S, Trush MA and Kensler TW: Nrf2 regulates an adaptive
response protecting against oxidative damage following
diquat-mediated formation of superoxide anion. Arch Biochem
Biophys. 454:7–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Snyder JC, Zemke AC and Stripp BR:
Reparative capacity of airway epithelium impacts deposition and
remodeling of extracellular matrix. Am J Respir Cell Mol Biol.
40:633–642. 2009. View Article : Google Scholar :
|
|
55
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wells RG: The role of matrix stiffness in
regulating cell behavior. Hepatology. 47:1394–1400. 2008.
View Article : Google Scholar : PubMed/NCBI
|