Introduction
Liver transplantation is a serious surgical
procedure, which can result in complications to a number of other
organs (1,2). Multi-organ dysfunction (MODF) and
systemic inflammatory reaction syndrome (SIRS) are the two main
complications following liver transplantation, and contribute to a
high patient mortality rate; however, the underlying mechanisms of
these complications remain to be determined (3). During liver transplantation,
intestinal congestion is inevitable due to inferior vena cava (IVC)
and portal vein (PV) interruption, which results in intestinal
motility disorders and destruction of intestinal barriers (4). As previously reported, intestinal
barriers are a complex system and perform two important functions
in the body; nutrient absorption and defence against harmful
macromolecule penetration. Intestinal barriers are composed of
physical, chemical, biological and immunological elements. The
physical aspect includes a mucous layer, intestinal epithelial
cells and tight junctions located at the apical surface. The
chemical barrier involves gastric acid, digestive enzymes and bile.
The immunological barrier refers to lymphocytes and immunoglobulin
A (IgA) and the biological barrier is composed of normal intestinal
flora, and the important environmental factors for energy
absorption and storage. Destruction of the intestinal barriers
presents in a variety of ways, including flora shift, small
intestinal bacterial overgrowth, tight junction alterations and
increased gut permeability (5).
Enterogenous endotoxins are over-produced and there is increase in
bacterial translocation (6). Once
intestinal bacteria or endotoxins enter the venous or lymphatic
system, they translocate to other organs and result in remote organ
damage (7,8). Thus, intestinal epithelial cell
protection is important for patients undergoing liver
transplantation, and may be an effective strategy to protect
against MODF and SIRS. Therefore, there is an urgent requirement to
investigate the mechanisms underlying intestinal injury and to
develop effective strategies to protect against this damage.
Endotoxins are one of the most important
constituents of the outer membrane of Gram-negative bacteria (GNB)
and are key to the pathogenesis of GNB-associated MODF and SIRS
(9). Endotoxins that are
over-produced during liver transplantation bind to the primary
receptor, toll-like receptor 4 (TLR4), which is important in
post-liver transplantation intestinal injury (10,11).
TLR4 predominantly located on cell membranes recognizes
pathogen-associated molecular patterns. When activated during an
infection, it induces the transcription of certain immune genes and
results in activation of the nuclear factor (NF)-κB signaling
pathway and downstream inflammatory cascade that is activated by
inflammatory mediators, such as tumor necrosis factor (TNF)-α and
interleukin (IL)-6 (12).
NF-κB is the final effector molecule of the TLR4
signaling pathway and is pivotal in the translation and
transcription of inflammatory mediators and caspase expression,
which promotes the development of numerous intestinal diseases
(13). Overproduction of
pro-inflammatory cytokines is a characteristic of patients that
have undergone liver transplantation. In addition, apoptosis is
considered to be important in injuries of remote organs following
liver transplantation (14,15).
Thus, the present study aimed to determine whether apoptosis
mediated by the overproduction of inflammatory cytokines, via
TLR4/NF-κB signal pathway activation, may be the potential
mechanism underlying intestinal injury.
Materials and methods
Animals
All the experiments were conducted according to the
National Institutes of Health criteria for the care and use of
laboratory animals in research. The study was approved by the
Laboratory Animal Care Committee of Sun Yat-sen University
(Guangzhou, China). Male Sprague-Dawley rats (age, 8 weeks; weight,
200–220 g) were purchased from the Laboratory Animal Center of Sun
Yat-sen University and randomly assigned into five parallel groups
using a random number table and taking into consideration the
weight of the rats. The groups were as follows: Sham, and
reperfusion 4, 8, 16 and 24 h (AOLT model) groups (n=8 per group).
The rats (four per cage) were housed at room temperature, fed with
standard rat chow and had access to tap water ad libitum.
The room was well-ventilated with a 12-h light/dark cycle. The rats
were acclimated for one week prior to experiments. Food was
withheld 8 h prior to commencing the experiments, however, all
animals had free access to water.
Rat autologous orthotopic liver
transplantation (AOLT) model establishment
This model was established according to the methods
of previous studies (3,12). Briefly, an open face guard was used
to administer the ether inhalational anesthesia (Shanghai Baxter
Healthcare Co., Ltd., Shanghai, China) until the rats exhibited no
response to a needle stimulus. An incision was made to open the
abdominal cavity, the liver falciform ligament was ligated and the
blood vessel along the esophagus was severed. Then, the liver was
exposed. Following liberation of the supra hepatic vena cava (SVC),
the liver was replaced in its original position. The IVC was then
dissociated, until the upper region of the left renal vein was
completely liberated. The first hepatic portal (the H-shaped groove
on the surface of the liver, from which the portal vein, hepatic
duct and hepatic artery access the liver) was also dissected and
the PV was separated from the convergence of the inferior
mesenteric and splenic veins. The hepatic artery and biliary tract
were also successively detached, according to their anatomic
relationship, and the first hepatic portal was ligated
Microvascular clamps were used at the convergence of the inferior
mesenteric, splenic veins, hepatic artery, SVC and IVC, and the PV
was punctured with a 24-gauge needle in preparation for
reperfusion. Then, a 1-mm incision was made in the IVC wall as an
outflow tract and 2.5 ml/min pre-cold 4°C Ringer's lactate solution
(Shanghai Baxter Healthcare Co., Ltd.,) was injected until the
liver turned yellow. Finally, the incisions in the PV and IVC were
closed using 8–0 sutures (Hangzhou Huawei Medical Appliance Co.,
Ltd., Hangzhou, China). PV, SVC, IVC and the hepatic artery were
all unclamped. The duration of the anhepatic phase was 20±1 min
(1).
The sham group underwent abdominal surgery and liver
dissection under ether inhalational anesthesia without cold
perfusion or reperfusion. The AOLT model rats were subjected to the
typical pathophysiological hepatic ischemia/reperfusion (I/R)
processes during liver transplantation. Compound lidocaine cream
(Beijing Ziguang Pharmaceutical Co., Ltd., Beijing, China) was
applied to the incisions for pain relief. Following surgery, the
rats were maintained in a temperature-controlled environment under
a 12-h light/dark cycle with free access to water. Rats in the sham
group were sacrificed by cervical dislocation 8 h after surgery and
the samples were subsequently obtained. Rats in the AOLT groups
were sacrificed by cervical dislocation 4, 8, 16 and 24 h after
reperfusion and the samples were obtained at the corresponding
time-points.
Histopathological examination
Intestinal specimens were obtained from 5 cm above
the terminal ileum and fixed in 10% buffered formalin (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), embedded in paraffin
(Nanjing Keygen Biotech Co., Ltd.), and processed for hematoxylin
and eosin staining (Nanjing Keygen Biotech Co., Ltd.). Samples were
visualized under an Eclipse E800 light microscope (Nikon
Corporation, Tokyo, Japan) and intestinal mucosal damage was graded
according to Chiu's criteria (3):
Grade 0, normal mucosa villi; grade 1, development of subepithelial
Gruenhagen's space at the tip of the villi; grade 2, extension of
the subepithelial space with moderate epithelial lifting; grade 3,
extensive epithelial lifting, possibly with a few denuded villi;
grade 4, denuded villi with dilated capillaries, and increased
cellularity of the lamina propria and exposed capillaries; and
grade 5, disintegration of the lamina propria, ulceration and
hemorrhage.
Assessment of fatty acid-binding protein
2 (FABP2), diamine oxidase (DAO), lipopolysaccharide (LPS), TNF-α
and IL-6
Plasma was harvested from the collected abdominal
aortic blood (2-ml blood samples were collected from each rat in
the sham group 8 h after surgery, and from the AOLT model groups at
4, 8, 16 and 24 h post-reperfusion) and maintained at −20°C. The
levels of DAO, FABP2, TNF-α and IL-6 were determined using their
corresponding enzyme-linked immunosorbent assay (ELISA) kits
according to the manufacturer's protocol (all kits were obtained
from R&D Systems, Minneapolis, MN, USA). LPS was detected using
an endotoxin detection assay kit (Lonza, Basel, Switzerland).
Western blotting
Western blotting was conducted as described
previously (3,12). Briefly, the membranes were blocked
for 30 min at room temperature with 5% non-fat dry milk
(Sigma-Aldrich, St. Louis, MO, USA) and immunoblotted using
anti-TLR4 (cat. no. sc-293072; monoclonal mouse anti-rat; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following several washes, the membranes were incubated for 1 h at
room temperature with horseradish peroxidase (HRP)-conjugated
polyclonal goat anti-mouse IgG (cat. no. sc-2005; 1:2,000; Santa
Cruz Biotechnology, Inc.) to detect TLR4 expression.
Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat. no.
sc-47724; monoclonal mouse anti-rat; 1:1,000; Santa Cruz
Biotechnology, Inc.) and its corresponding secondary antibody,
HRP-conjugated polyclonal goat anti-mouse IgG (1:2,000; Santa Cruz
Biotechnology, Inc.) served as a control. Protein-antibody
complexes were detected with an enhanced chemiluminescence system
(Keygen Biotech Co., Ltd.). Protein band sizes were estimated using
AlphaView 2.2.14407 software (ProteinSimple, Santa Clara, CA, USA).
The density was correlated to the protein expression and normalized
to GAPDH.
Immunofluorescence
Immunofluorescent staining was conducted according
to the appropriate protocol as previously described (1). Cryostat sections of OCT-embedded
intestinal samples (4-µm) were incubated with primary
antibodies against P65 (cat. no. sc-8008; monoclonal mouse
anti-rat; 1:100; Santa Cruz Biotechnology, Inc.) for one night at
4°C. Following incubation with goat anti-mouse IgG-fluorescein
isothiocyanate (FITC; cat. no. sc-2010; 1:100; Santa Cruz
Biotechnology, Inc.), the sections were observed and imaged under
×400 magnification using a fluorescence microscope (Olympus BX40;
Olympus Corporation, Tokyo, Japan). Nuclei were stained with
4′,6-diamidino-2-phenylindole (1 µg/ml; Nanjing KeyGen
Biotech Co., Ltd.).
Immunohistochemistry
Immunohistochemical staining was performed in
4-µm paraffinized sections, as described previously
(1). After being dewaxed and
dehydrated, the sections were incubated with
H2O2 (3%; Nanjing Keygen Biotech Co., Ltd.)
in order to inhibit endogenous peroxidase activity. The slides were
incubated with primary antibodies against caspase-3 (cat. no.
sc-7148; polyclonal rabbit anti-rat; 1:100; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Following incubation with
the corresponding secondary antibody, goat anti-rabbit IgG-FITC
(cat. no. sc-2012; 1:100; Santa Cruz Biotechnology, Inc.), the
samples were visualized under a light microscope (Eclipse E800).
Five photomicrographs were captured randomly (magnification, ×400).
The average optical density (AOD) of the photomicrographs was
quantified using the high resolution pathological image analysis
system (HPIAS-1000; (Nanjing Keygen Biotech Co., Ltd.) to measure
expression levels of caspase-3.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The TUNEL assay (Nanjing Keygen Biotech Co., Ltd.)
was conducted to evaluate intestinal tissue apoptosis according to
the manufacturer's instructions. Briefly, 4-µm paraffinized
sections were dewaxed, rehydrated, and incubated with terminal
deoxynucleotidyl transferase enzyme at 37°C for 1 h. The reaction
was detected by incubation with anti-digoxigenin-peroxidase
(Nanjing Keygen Biotech Co., Ltd.) for 30 min at room temperature
and visualized in a buffer containing 3,3′diaminobenzidine. The
samples were counterstained by immersion in hematoxylin. The
stained tissue samples were observed using a fluorescence
microscope (EclipseE800) and five photomicrographs were obtained
randomly (magnification, ×400). The AOD from the photomicrographs
was quantified using the high-resolution pathological image
analysis system (HPIAS-1000) to measure TUNEL-positive cells.
Statistical analysis
Statistical analysis was performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Multiple comparisons among
groups were analyzed using one-way analysis of variance, followed
by Tukey's post hoc comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
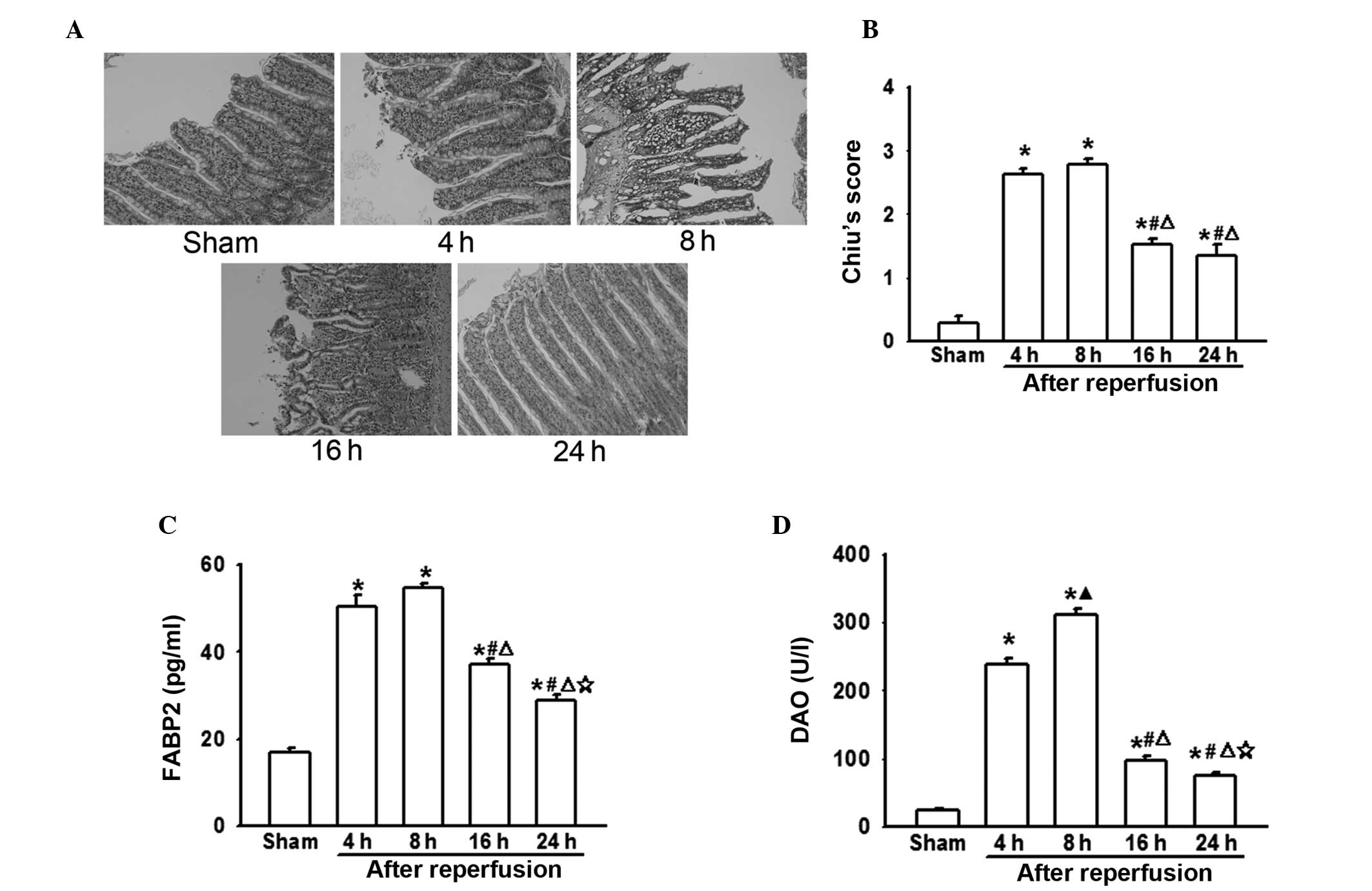
Intestinal injuries following AOLT at
different time-points
In the present study, rat AOLT models were
established to investigate the effect of liver transplantation on
the intestines. In this model, IVC and PV interruption during the
anhepatic phase leads to blood reflux disorder and serious
intestinal congestion, which result in significant intestinal
injuries (3). In the present
experiments, it was demonstrated that as the reperfusion time was
extended, pathological damage to the intestines increased. Four or
eight hours after reperfusion, intestinal damage peaked, resulting
in extensive epithelial lifting from the villi, which recovered
gradually (Fig. 1A and B). It was
also demonstrated that FABP2 and DAO levels were notably increased
8 h following reperfusion, which mirrored the pathological injury
of the intestine (Fig. 1C and
D).
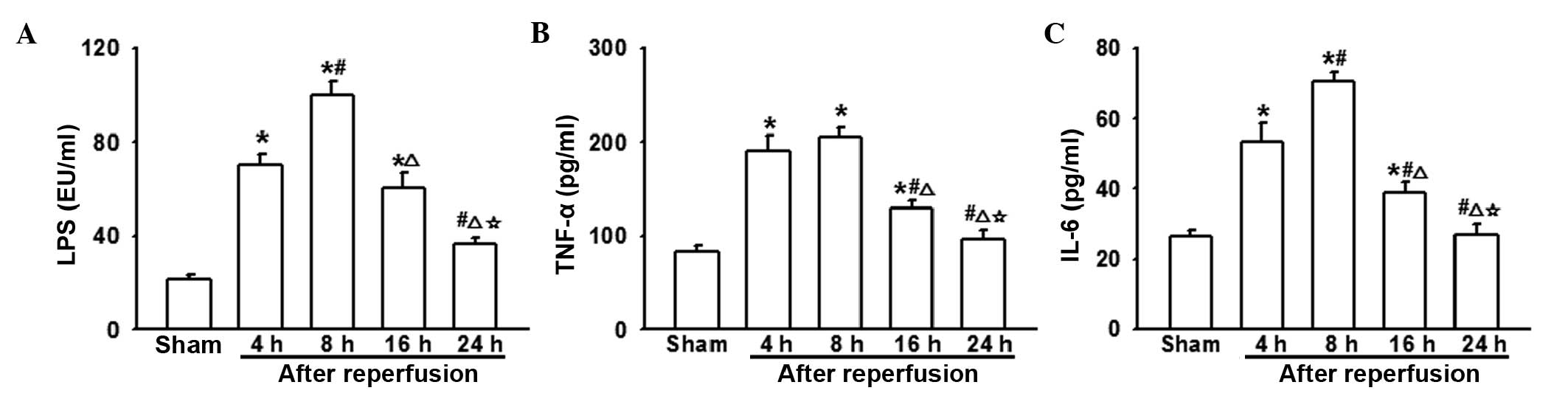
Changes in the levels of LPS, TNF-α and
IL-6 following AOLT at different time-points
Fig. 1 illustrated
the significance of AOLT-mediated intestinal injury. However, its
mechanism remains unclear. Intestinal motility and barriers have
been shown to be impaired following liver transplantation,
resulting in increased bacterial translocation and enterogenous
endotoxin levels (3,16). This was shown to initiate the
overproduction of pro-inflammatory cytokines, which may trigger
remote organ injury (3). The
present study demonstrated that the level of LPS increased
significantly 4–8 h following reperfusion and then decreased
gradually (Fig. 2A). TNF-α and
IL-6 are important inflammatory factors in liver transplantation
and are key in various inflammatory reactions. Thus, the levels of
TNF-α and IL-6 were determined in the present study. Fig. 2 shows that 4–8 h following
reperfusion, the levels of TNF-α and IL-6 were significantly
increased, compared with the sham group and then declined
gradually. After 24 h, no significant difference was identified
between sham group and the AOLT model groups (Fig. 2B and C).
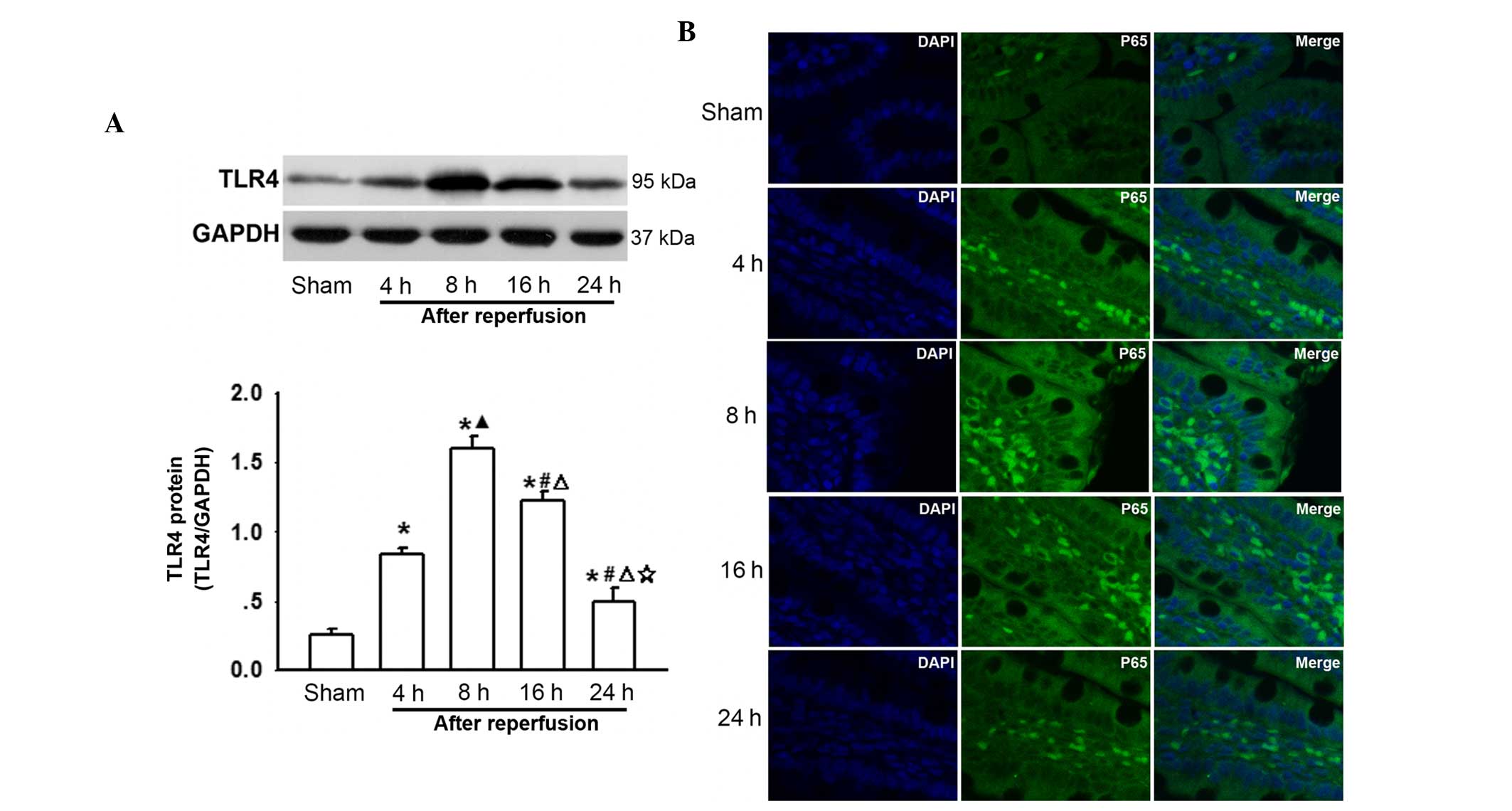
Changes in TLR4 and P65 expression on the
surface of intestine cells following AOLT at different
time-points
To the best of our knowledge, intestinal congestion
in liver transplantation always results in enterogenous endotoxin
over-production and an increase in the levels of inflammatory
cytokines, which contribute to remote organ injury (Figs. 1 and 2). LPS activates TLR4 and its downstream
NF-κB, and TNF-α triggers translocation of NF-κB to the nucleus
(17), thus changes in TLR4 and
P65 expression were investigated. As shown in Fig. 3A, TLR4 protein levels were
increased after AOLT and peaked 8 h after reperfusion, which
coincided with the most severe intestinal pathological damage
(Fig. 1) and the increase in LPS,
TNF-α and IL-6 levels (Fig. 2). In
addition, P65 was activated at this time-point (Fig. 3B). Subsequently, the expression of
TLR4 and P65 gradually decreased as reperfusion time increased
(Fig. 3A and B). These results
suggested that the TLR4/NF-κB signaling pathway participated in
post-AOLT intestinal injury.
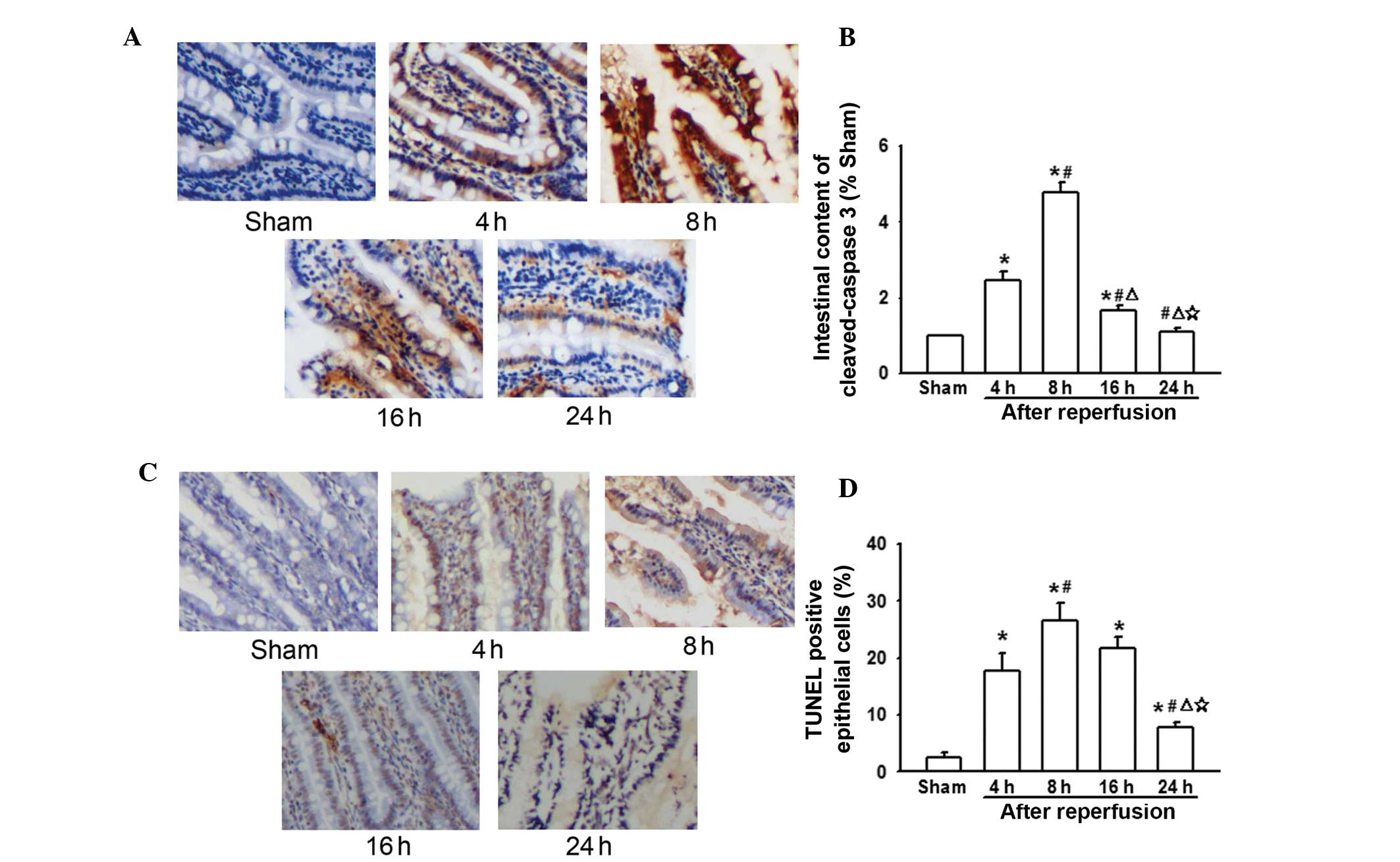
Changes in caspase-3 and apoptosis levels
in the intestine following AOLT at different time-points
During liver transplantation, intestinal congestion
was severe due to IVC and PV interruption. This lead to
enterogenous endotoxin and inflammatory cytokine over-production,
which could activate the TLR4/NF-κB signaling pathway (Figs. 2 and 3). Previous studies have demonstrated
that cytokine over-production and TLR4/NF-κB signal pathway
activation triggers caspase-3-independent cell apoptosis (18,19).
Thus, it was hypothesized that this may be the reason for liver
transplantation-mediated intestinal injury. The present results
indicated that expression of caspase-3 also peaked 8 h following
reperfusion, which was in line with the levels of LPS, TNF-α, IL-6,
TLR4/P65, and also decreased in line with the reduction in the
levels of LPS, TNF-α, IL-6 and TLR4/P65 (Fig. 4A). In addition, the levels of
TUNEL-positive epithelial cells coincided with caspase-3 expression
(Fig. 4B). This suggests that
caspase-3-induced apoptosis mediated by cytokines and the
TLR4/NF-κB signaling pathway may be important in post-liver
transplantation intestinal injury.
Discussion
Liver transplantation is considered to be the most
effective strategy to cure final-stage liver disease; however, it
is associated with a number of serious complications that affect
patient survival (1,2). It has previously been demonstrated
that intestinal motility and barriers are impaired following liver
transplantation (20). Findings
from the present study (Fig. 1)
demonstrated that pathological injury of the intestine was most
marked 8 h after reperfusion, which was coincident with the changes
in FABP2 and DAO levels (well-known markers of intestinal mucosa
barrier destruction). Bacterial translocation and enterogenous
endotoxemia are notable, and may lead to post-operative MODF and
SIRS (21). These contribute to
the high mortality rate of patients undergoing liver
transplantation; however, the mechanism underlying these effects
remains unclear. Thus, in the present study, rat AOLT models were
established to investigate the possible mechanisms underlying liver
transplantation-induced intestinal injury. The rat AOLT model has
advantages compared with allogeneic orthotopic liver
transplantation, such as avoiding obvious rejection reactions and
complex conditions of recipients, in addition there is
repeatability of experiments and a high survival rate of rats.
Compared with a pure hepatic I/R injury model, rat AOLT models
undergo the predominant procedures involved in liver
transplantation, including blood reflux disorder (1). Thus, the rat AOLT model was used in
the present study.
During liver transplantation, IVC and PV
interruption result in severe intestinal congestion. In addition,
intestinal motility and barriers have been shown to be impaired
following liver transplantation (20). Bacterial trans-location and
enterogenous endotoxin increase, which result in enterogenous LPS
over-production, inflammatory cytokine production (TNF-α and IL-6)
and TLR4/NF-κB signaling pathway activation (17,21,22).
The present study determined that the TLR4/NF-κB signal pathway
activation triggered caspase-3-independent cell apoptosis.
Caspase-3 expression and intestinal epithelial cell apoptosis were
notably increased following AOLT. These changes coincided with
pathological damage of the intestine following liver
transplantation. This indicated that post-liver transplantation
intestinal injury was mediated by endotoxin over-production,
cytokine production and TLR4/NF-κB signaling pathway-induced
apoptosis.
Bacterial translocation and enterogenous endotoxin
are important in remote organ damage (23). LPS is one of the most important
constituents of the outer membrane of GNB and is recognized to be
key in the pathogenesis of GNB-associated sepsis (24,25).
The present study demonstrated that the levels of enterogenous
endotoxin peaked at 8 h after reperfusion, the point at which the
intestine exhibited the most notable damage (Fig. 2A). Macromolecular LPS binds to TLR4
(considered to be the primary receptor), which results in myeloid
differentiation primary response gene 88-dependent signaling and
activation of the downstream inflammatory cascade. Furthermore,
activation of IκB kinase-β and mitogen-activated protein kinase
phosphorylation were important in this process (18,26).
This mediates NF-κB-dependent nuclear transcription and TNF-α
production.
During liver transplantation, IVC and PV
interruption lead to liver I/R injury, which initiates the
over-production of pro-inflammatory cytokines (3,22).
TNF-α is considered to be an important pro-inflammatory cytokine,
which mediates the degradation of IκBα and promotes translocation
of NF-κB to the nucleus (27).
Simultaneous over-production of LPS/TLR4/NF-κB-induced inflammatory
cytokine along with over-production of pro-inflammatory cytokines
triggered remote organ injuries. TNF-α is not a direct
chemoattractant, however, following liver transplantation, it
induced inflammation via the NF-κB signaling pathway. TNF-α is
considered to be an important pro-inflammatory cytokine, which
mediates the degradation of IκBα and promotes translocation of
NF-κB to the nucleus (27).
Translocation of NF-κB activates the transcription of various genes
that are involved in cell proliferation, including IL-6 and
caspase-3 (28), which were
demonstrated to mediate post-liver transplantation intestinal
injury amplification and cell apoptosis. Thus, TNF-α, IL-6, and
caspase-3 were important in the development of intestinal injury
following liver transplantation in the present study. This was
demonstrated to be correlated with pathological injury of the
intestine. These results suggested that pro-inflammatory cytokine
overproduction induced apoptosis following liver transplantation
and were critical in post-liver transplantation intestinal
injury.
In the present study, it was demonstrated that
caspase-3 expression and TUNEL-positive epithelial cells in rats
undergoing AOLT were markedly increased compared with that in the
sham group (Fig. 4). This
suggested that apoptosis was important in AOLT-induced intestinal
injury. Caspase-3 was considered to be the executor of apoptosis in
the caspase cascade and resulted in apoptosis in various types of
tissue (29). Many different
factors induce apoptosis, such as intestinal congestion-mediated
I/R injury, bacterial trans-location, oxidative stress or
inflammatory reaction (30–32).
However, until now, intestinal cell apoptosis resulting from liver
transplantation has not been well investigated. Thus, to the best
of our knowledge, the present study was the first to investigate
possible mechanisms of AOLT-induced intestinal injury. It was
demonstrated that intestinal injury was associated with cell
apoptosis mediated by endotoxin and cytokine over-production
triggered by NF-κB signaling pathway activation.
Liver transplantation exhibits serious complications
on a number of different systems. The present study, predominantly
focused on intestinal injuries and investigated the possible
underlying mechanisms. During surgery, IVC and the PV interruption
during the anhepatic phrase, resulted in blood reflux disorder,
which led to intestinal congestion (3). This impaired intestinal motility and
barriers, resulting in an increase of bacterial translocation and
overproduction of enterogenous endotoxin (4). Endotoxins may activate the TLR4/NF-κB
signaling pathway, and induce TNF-α production and cell apoptosis.
In addition, TNF-α promoted translocation of NF-κB to the nucleus
mediated by the degradation of IκBα, resulting in intestinal cell
apoptosis and injury (33). Thus,
the cascade was amplified intensively and intestinal injury
increased.
In conclusion, post-liver transplantation intestinal
injury was shown to be associated with TLR4/NF-κB signaling pathway
activation-induced cell apoptosis. These findings may aid in the
development of effective strategies for intestine protection, as
well as protection of other organs, during liver
transplantation.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81471892 and
81401628); the Natural Science Foundation of Guangdong Province,
China (grant no. S2012010008930); the Science and Technology
Planning Project of Guangdong Province, China (grant nos.
2013B021800181 and 2008B030301053); and the Fundamental Research
Funds for the Central Universities of China (grant no.
14ykpy24).
References
|
1
|
Luo C, Yuan D, Li X, Yao W, Luo G, Chi X,
Li H, Irwin MG, Xia Z and Hei Z: Propofol attenuated acute kidney
injury after orthotopic liver transplantation via inhibiting gap
junction composed of connexin 32. Anesthesiology. 122:72–86. 2015.
View Article : Google Scholar
|
|
2
|
Ohkohchi N: Mechanisms of preservation and
ischemic/reperfusion injury in liver transplantation. Transplant
Proc. 34:2670–2673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ge M, Chi X, Zhang A, Luo G, Sun G, Xie H
and Hei Z: Intestinal NF-E2-related factor-2 expression and
antioxidant activity changes in rats undergoing orthotopic liver
autotransplantation. Oncol Lett. 6:1307–1312. 2013.PubMed/NCBI
|
|
4
|
Goto S, Kamada N, Moore T, Ware F, Lord R,
Kobayashi E and Kim YI: The influence of intestinal congestion on
survival of preserved liver grafts in rat liver transplantation.
Transplantation. 58:974–977. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai X and Wang B: Role of gut barrier
function in the pathogenesis of nonalcoholic fatty liver disease.
Gastroenterol Res Pract. 2015:2873482015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanada Y, Mizuta K, Urahashi T, Ihara Y,
Wakiya T, Okada N, Yamada N, Ushijima K, Otomo S, Sakamoto K, et
al: Impact of endotoxin measured by an endotoxin activity assay
during liver transplantation. J Surg Res. 180:349–355. 2013.
View Article : Google Scholar
|
|
7
|
Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ,
Liang SY, Sun K, Liao WY and Gong JP: Protective effect of
glutamine-enriched early enteral nutrition on intestinal mucosal
barrier injury after liver transplantation in rats. Am J Surg.
199:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo H, Guo P and Zhou Q: Role of
TLR4/NF-κB in damage to intestinal mucosa barrier function and
bacterial translocation in rats exposed to hypoxia. PloS One.
7:e462912012. View Article : Google Scholar
|
|
9
|
Fang WF, Douglas IS, Wang CC, Kao HC,
Chang YT, Tseng CC, Huang KT, Chang HC and Lin MC: 5-Lipoxygenase
activating protein (FLAP) dependent leukotriene biosynthesis
inhibition (MK591) attenuates lipid A endotoxin-induced
inflammation. PloS One. 9:e1026222014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarmiento D, Montorfano I, Cáceres M,
Echeverría C, Fernández R, Cabello-Verrugio C, Cerda O, Tapia P and
Simon F: Endotoxin-induced vascular endothelial cell migration is
dependent on TLR4/NF-κB pathway, NAD(P)H oxidase activation and
transient receptor potential melastatin 7 calcium channel activity.
Int J Biochem Cell Biol. 55:11–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riehl TE, Foster L and Stenson WF:
Hyaluronic acid is radio-protective in the intestine through a TLR4
and COX-2-mediated mechanism. Am J Physiol Gastrointest Liver
Physiol. 302:G309–G316. 2012. View Article : Google Scholar
|
|
12
|
Zhang A, Chi X, Luo G, Hei Z, Xia H, Luo
C, Wang Y, Mao X and Xia Z: Mast cell stabilization alleviates
acute lung injury after orthotopic autologous liver transplantation
in rats by down-regulating inflammation. PloS One. 8:e752622013.
View Article : Google Scholar
|
|
13
|
Ohkawara H, Ishibashi T, Sugimoto K, Ikeda
K, Ogawa K and Takeishi Y: Membrane type 1-matrix
metalloproteinase/Akt signaling axis modulates TNF-alpha-induced
procoagulant activity and apoptosis in endothelial cells. PloS One.
9:e1056972014. View Article : Google Scholar
|
|
14
|
Mosbah IB, Zaouali MA, Martel C, Bjaoui M,
Abdennebi HB, Hotter G, Brenner C and Roselló-Catafau J: IGL-1
solution reduces endoplasmic reticulum stress and apoptosis in rat
liver transplantation. Cell Death Dis. 3:e2792012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biancofiore G, Bindi L, Miccoli M, Metelli
MR, Panicucci E, Baggiani A and Filipponi F: Balance of pro- and
anti-inflammatory cytokines in cirrhotic patients undergoing liver
transplantation. Transpl Immunol. 28:193–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liboredo JC, Vilela EG, Ferrari MD, Lima
AS and Correia MI: Nutrition status and intestinal permeability in
patients eligible for liver transplantation. JPEN J Parenter
Enteral Nutr. 39:163–170. 2015. View Article : Google Scholar
|
|
17
|
Yang S, Li R, Qu X, Tang L, Ge G, Fang W,
Qiao Z, Ma J, Hou Y and Liu H: Fosinoprilat alleviates
lipopolysaccharide (LPS)-induced inflammation by inhibiting
TLR4/NF-κB signaling in monocytes. Cell Immunol. 284:182–186. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li PM, Li YL, Liu B, Wang WJ, Wang YZ and
Li Z: Curcumin inhibits MHCC97H liver cancer cells by activating
OS/TLR-4/caspase signaling pathway. Asian Pac J Cancer Prev.
15:2329–2334. 2014. View Article : Google Scholar
|
|
19
|
Jung DY, Lee H, Jung BY, Ock J, Lee MS,
Lee WH and Suk K: TLR4, but not TLR2, signals autoregulatory
apoptosis of cultured microglia: A critical role of IFN-beta as a
decision maker. J Immunol. 174:6467–6476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheyu C and Lunan Y: Early changes of
small intestine function in rats after liver transplantation.
Transplant Proc. 38:1564–1568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swank GM and Deitch EA: Role of the gut in
multiple organ failure: Bacterial translocation and permeability
changes. World J Surg. 20:411–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bedirli A, Sakrak O, Soyuer I and
Muhtaroglu S: Portosystemic shunt prevents apoptosis in rat
intestinal mucosa caused by total hepatic ischemia. Eur Surg Res.
36:293–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY
and Wang GS: Over-starvation aggravates intestinal injury and
promotes bacterial and endotoxin translocation under high-altitude
hypoxic environment. World J Gastroenterol. 17:1584–1593. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka KA, Kurihara S, Shibakusa T, Chiba
Y and Mikami T: Cystine improves survival rates in a LPS-induced
sepsis mouse model. Clin Nutr. pii:S0261–S5614. 2014.
|
|
25
|
Guo L, Zheng Z, Ai J, Huang B and Li XA:
Hepatic scavenger receptor BI protects against
polymicrobial-induced sepsis through promoting LPS clearance in
mice. J Biol Chem. 289:14666–14673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang RL, Yuan Y, Zou GM, Liu G, Tu J and
Li Q: LPS-stimulated inflammatory environment inhibits
BMP-2-induced osteoblastic differentiation through crosstalk
between TLR4/MyD88/NF-κB and BMP/Smad signaling. Stem Cells Dev.
23:277–289. 2014. View Article : Google Scholar :
|
|
27
|
Ren Z, Cui J, Huo Z, Xue J, Cui H, Luo B,
Jiang L and Yang R: Cordycepin suppresses TNF-α-induced NF-κB
activation by reducing p65 transcriptional activity, inhibiting
IκBα phosphorylation and blocking IKKγ ubiquitination. Int
Immunopharmacol. 14:698–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang L, Tang Y, Qin J, Peng Y, Yuan Q,
Zhang F and Tao L: Vasoactive intestinal peptide enhances
TNF-α-induced IL-6 and IL-8 synthesis in human proximal renal
tubular epithelial cells by NF-κB-dependent mechanism.
Inflammation. 35:1154–1160. 2012. View Article : Google Scholar
|
|
29
|
Zhang Z, Chen J, Chen L, Yang X, Zhong H,
Qi X, Bi Y and Xu K: Low frequency and intensity ultrasound induces
apoptosis of brain glioma in rats mediated by caspase-3, Bcl-2 and
survivin. Brain Res. 1473:25–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X
and Zhang YM: Activation of BV2 microglia by lipopolysaccharide
triggers an inflammatory reaction in PC12 cell apoptosis through a
toll-like receptor 4-dependent pathway. Cell Stress Chaperones.
20:321–331. 2015. View Article : Google Scholar :
|
|
31
|
Zhou Y, Wang Q, Mark Evers B and Chung DH:
Oxidative stress-induced intestinal epithelial cell apoptosis is
mediated by p38 MAPK. Biochem Biophys Res Commun. 350:860–865.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JM, Eckmann L, Savidge TC, Lowe DC,
Witthöft T and Kagnoff MF: Apoptosis of human intestinal epithelial
cells after bacterial invasion. J Clin Invest. 102:1815–1823. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn KS, Sethi G and Aggarwal BB:
Simvastatin potentiates TNF-alpha-induced apoptosis through the
down-regulation of NF-kappaB-dependent antiapoptotic gene products:
Role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J
Immunol. 178:2507–2516. 2007. View Article : Google Scholar : PubMed/NCBI
|