Introduction
Alzheimer's disease (AD) is a prevalent
neurodegenerative disease characterized by the progressive
deterioration of cognition and memory (1,2).
Pathological features of AD include the accumulation of
extracellular senile plaques predominantly composed of β-amyloid
(Aβ) peptide, intracellular neurofibrillary tangles and neuronal
loss, particularly in the hippocampus (3). Numerous previous studies have
indicated that brain-derived neurotrophic factor (BDNF) is
associated with the pathogenesis of AD. In 1991, Phillips et
al (4) demonstrated a
selective reduction in BDNF messenger (m)RNA expression levels in
the hippocampi of patients with AD. Subsequent studies indicated
that the precursor of BDNF (proBDNF), mature BDNF (mBDNF) and BDNF
mRNA expression levels were decreased in individuals with AD, and
the levels of BDNF were positively correlated with cognitive
measures, including the Global Cognitive Score and the Mini Mental
State Examination score (5,6). A
community-based, prospective cohort study, which involved 2,131
dementia-free participants (56% women) aged ≥60 years (age, 72±7
years; mean ± standard deviation), observed that increased serum
BDNF levels were associated with a reduced risk for dementia and AD
(7). These data suggest that BDNF
is important in the pathogenesis of AD.
BDNF is synthesized as a precursor protein, proBDNF,
which is post-translationally cleaved to produce mBDNF. This
processing of the BDNF protein appears to be key in regulating its
cellular function (8,9). ProBDNF preferentially binds to the
p75 neurotrophin receptor (p75NTR) leading to downstream
signaling, via signaling pathways involved in apoptosis, and
facilitating long-term depression in the hippocampus (10). By contrast, cleaved mBDNF binds to
the tropomyosin receptor kinase B (TrkB) receptor, promotes cell
survival and facilitates certain forms of long-term potentiation
(11). Previous studies have
demonstrated that BDNF signaling exerts neuroprotective effects
against Aβ peptide toxicity in vivo and in vitro
(12,13). However, it has also been reported
that proBDNF suppresses the proliferation of hippocampal neurons in
the dentate gyrus in AD rats and anti-proBDNF reverses the effects
(14). Therefore, the processing
of the BDNF protein may be important in AD.
Acupuncture at the Baihui (DU20) acupoint has long
been used in China to treat cognitive impairment. It is located on
a branch of the Du Meridian that runs over the head and is
associated with cognitive function in traditional Chinese medicine.
However, the efficacy of acupuncture at the Baihui (DU20) acupoint
in animal models of AD and its underlying mechanism of action are
not well understood. A previous study has demonstrated that
electroacupuncture (EA) at the Baihui (DU20) and Shenting (DU24)
acupoints ameliorates cognitive impairment in cerebral
ischemia-reperfusion injured rats (15). Therefore, in the present study, the
therapeutic efficacy of EA against cognitive deficits was evaluated
in APP/PS1 transgenic mice, and the effects of BDNF expression and
processing underlying the neuroprotective effects were
investigated.
Materials and methods
Materials and reagents
Terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) assay kits (DeadEnd Fluorometric TUNEL System)
were obtained from Promega Corporation (Madison, WI, USA) and
3,3′-diaminobenzidine (DAB) kits (Kit-0017) were purchased from
Fuzhou Maixin Biotech, Co., Ltd. (Fuzhou, China). Polyclonal rabbit
anti-Aβ(1-42) (1:200; cat. no. ab10148) and monoclonal rabbit
anti-BDNF (1:1,000; cat. no. ab108319) were obtained from Abcam
(Cambridge, UK). Polyclonal rabbit anti-proBDNF (1:400; cat. no.
ANT-006) was obtained from Alomone Labs, Ltd. (Jerusalem, Israel).
Monoclonal rabbit anti-TrKB (1:1,000; cat. no. 4603S), monoclonal
rat anti-phosphorylated (p)-TrKB (tyrosine816) (1:1,000;
cat. no. 4168S), monoclonal rabbit anti-p75NTR (1:1,000;
cat. no. 4201S) and monoclonal rabbit anti-β-actin (1:1,000; cat.
no. 4970S) primary antibodies were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The horseradish peroxidase
(HRP)-conjugated monoclonal goat secondary antibodies [anti-rabbit
IgG (1:5,000; cat. no. 7074P2) and anti-rat IgG (1:5,000; cat. no.
7077S)] were also obtained from Cell Signaling Technology, Inc. All
other chemicals used, unless otherwise stated, were obtained from
Beyotime Institute of Biotechnology (Haimen, China).
Animals
Thirty APP/PS1 double-transgenic mice
[B6C3-Tg(APPswe,PSEN1dE9)85Dbo/MmJNju], aged 3 months, and their 10
wild-type (WT) littermates (age, 3 months) were purchased from
Nanjing Biomedical Research Institute of Nanjing University
(Nanjing, China). The mice were housed five per cage in a
controlled environment (22–25°C; 55% relative humidity; 12-h
light/dark cycle) with access to food and water ad libitum.
They were maintained in a specific pathogen-free environment for
two months prior to being sacrificed. The experiments were approved
by the Institutional Animal Care and Use Committee of Fujian
University of Traditional Chinese Medicine, and were strictly in
accordance with international ethical guidelines and the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (16).
Animal grouping and treatment
Thirty APP/PS1 double-transgenic mice (that exhibit
early onset of Aβ deposition, followed by neuronal loss and
cognitive impairment) were randomly and evenly divided into three
groups (n=10) as follows: i) the APP/PS1 double-transgenic mice
control group (APP/PS1); ii) the EA at the Baihui (DU20) acupoint
group (APP/PS1 + DU20); and iii) the EA at non-acupoint group
(APP/PS1 + NA). Another 10 WT littermates (that do not exhibit
early onset of Aβ deposition, neuronal loss and cognitive
impairment) were designated as the aging control group (WT group).
In the APP/PS1 + DU20 group, mice were administered EA for 30 min
daily for 4 weeks. The acupuncture needles (diameter, 0.3 mm) were
inserted at a depth of 2–3 mm into the Baihui (DU20) acupoint,
which is located at the intersection of the sagittal midline and
the line linking the two ears. Stimulation was generated using EA
apparatus (model G6805; Suzhou Medical Appliance Factory, Shanghai,
China) and the stimulation parameters were set as disperse waves of
1 and 20 Hz. In the APP/PS1 + NA group, a non-acupoint (the area
below the costal region, 2 cm superior to the posterior superior
iliac spine and ~3 cm lateral to the spine) was punctured and
stimulated for 30 min daily for 4 weeks. The two groups (APP/PS1 +
DU20 and APP/PS1 + NA) received treatment with the same needles,
stimulation parameters and EA apparatus. The APP/PS1 and WT groups
did not receive any EA treatment.
Morris water maze test
All the mice were subjected to the Morris water maze
test, including orientation navigation and space exploration trials
following 4 weeks of treatment. This was conducted to investigate
spatial learning and memory ability, as previously described
(17). The water maze apparatus
(Chinese Academy of Sciences, Beijing, China) is a stainless steel
circular water tank (diameter, 120 cm; height, 50 cm) equipped with
a platform (diameter, 10 cm) placed in the third quadrant and
submerged 2 cm below the surface of the water. A video camera
attached to a computer was placed above the center of the tank to
record and analyze the trajectory of the mice. During the
orientation navigation trials, each mouse was placed in the water
at each of four equidistant locations to the platform. When a mouse
arrived at the platform within the 90 sec time restriction and
remained on it for 5 sec, it was considered to have found the
platform. If the mouse failed to find the platform within 90 sec,
it would be removed from the water and placed on the platform for
10 sec. The computer recorded the length of the route and the time
it took the mouse to find the platform. The orientation navigation
trials were repeated for four days. On the fifth day, the space
exploration test was performed to assess memory consolidation. In
this trial, the platform was removed from the tank, and the mice
were allowed to swim freely for 90 sec. The novel start position
was located 180° from the original platform position to ensure that
the spatial preference was a reflection of the memory of the goal
location, rather than for a specific swim path. The frequency that
each mouse crossed the center of the quadrant (where the platform
was previously located) was recorded.
TUNEL staining
The mice were anesthetized via injection of 10%
chloral hydrate (0.03 ml/100 g body weight; Shanghai Reagent
Factory, Shanghai, China) and transcardially perfused with a 0.9%
saline (NaCl) solution and 4% paraformaldehyde via the left
ventricle, and subsequently the brain was removed. The tissue
samples were fixed in cold 4% paraformaldehyde at 4°C overnight,
embedded in paraffin (Tianjin Fuchen Chemical Reagent Factory,
Tianjin, China) and cut into 5-μm sections. In situ
apoptosis was analyzed using a TUNEL assay kit according to the
manufacturer's protocols. The nuclei of all the cells were
visualized by DAPI staining and apoptotic cells were detected using
a confocal fluorescence microscope (LSM710; Carl Zeiss AG,
Oberkochen, Germany). Apoptotic cells were counted at four randomly
selected microscopic fields (magnification, ×400) and the apoptotic
rate was measured as a ratio of the TUNEL-positive cells to the
total number of cells.
Immunohistochemistry (IHC)
IHC was performed on the 5-μm paraffin
sections. Aβ(1-42) levels were examined with DAB kits according to
the manufacturer's protocols. The sections were incubated in 3%
hydrogen peroxide and normal serum at 37°C for 10 min to block the
non-specific protein binding. Sections were incubated with primary
anti-Aβ(1-42) antibody (1:200) at 4°C overnight and subsequently
incubated with secondary antibody. Aβ(1-42)-positive cells were
stained brown and hematoxylin was used to visualize the nuclei of
all cells. Images of Aβ(1-42) deposition in the hippocampus were
captured using an optic microscope (DFC310 FX; Leica Microsystems,
Inc., Buffalo Grove, IL, USA) and analyzed with an image analysis
system (Image-Pro Plus, version 6.0; Motic China Group Co., Ltd.,
Xiamen, China). The density of Aβ(1-42) deposition (the percentage
of positively-stained brown cells) was determined by subtracting
the background density and non-specific binding. The software was
used to perform the semi-quantitative evaluation.
Western blot analysis
Protein was extracted from the hippo-campus and
separated by electrophoresis (90 V for 30 mins) on 12% SDS-PAGE
gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were
transferred onto polyvinylidene fluoride membranes (EMD Millipore
Billerica, MA, USA). The membranes were blocked for 2 h with 5%
nonfat dried milk at room temperature and incubated with antibodies
against BDNF (1:1,000), proBDNF (1:400), TrkB (1:1,000), p-TrkB
(1:1,000), p75NTR (1:1,000), and β-actin (1:5,000) at
4°C overnight and subsequently incubated with HRP-conjugated
secondary antibody (1:5,000) for 1 h. The protein bands were
visualized with enhanced chemiluminescence and imaged with the
Bio-Image Analysis system (Bio-Rad Laboratories, Inc.). The ratios
of protein band intensities to β-actin were determined and
subsequently normalized to the WT group.
Statistical analysis
All data were analyzed using the SPSS package,
version 18.0 (SPSS, Inc., Chicago, IL, USA). Quantitative data are
presented as the mean ± standard error. Student's t-tests and
one-way analysis of variance were used to assess statistical
differences among groups and P<0.05 was considered to indicate a
statistically significant difference.
Results
EA at the Baihui (DU20) acupoint
ameliorates cognitive impairment in APP/PS1 mice
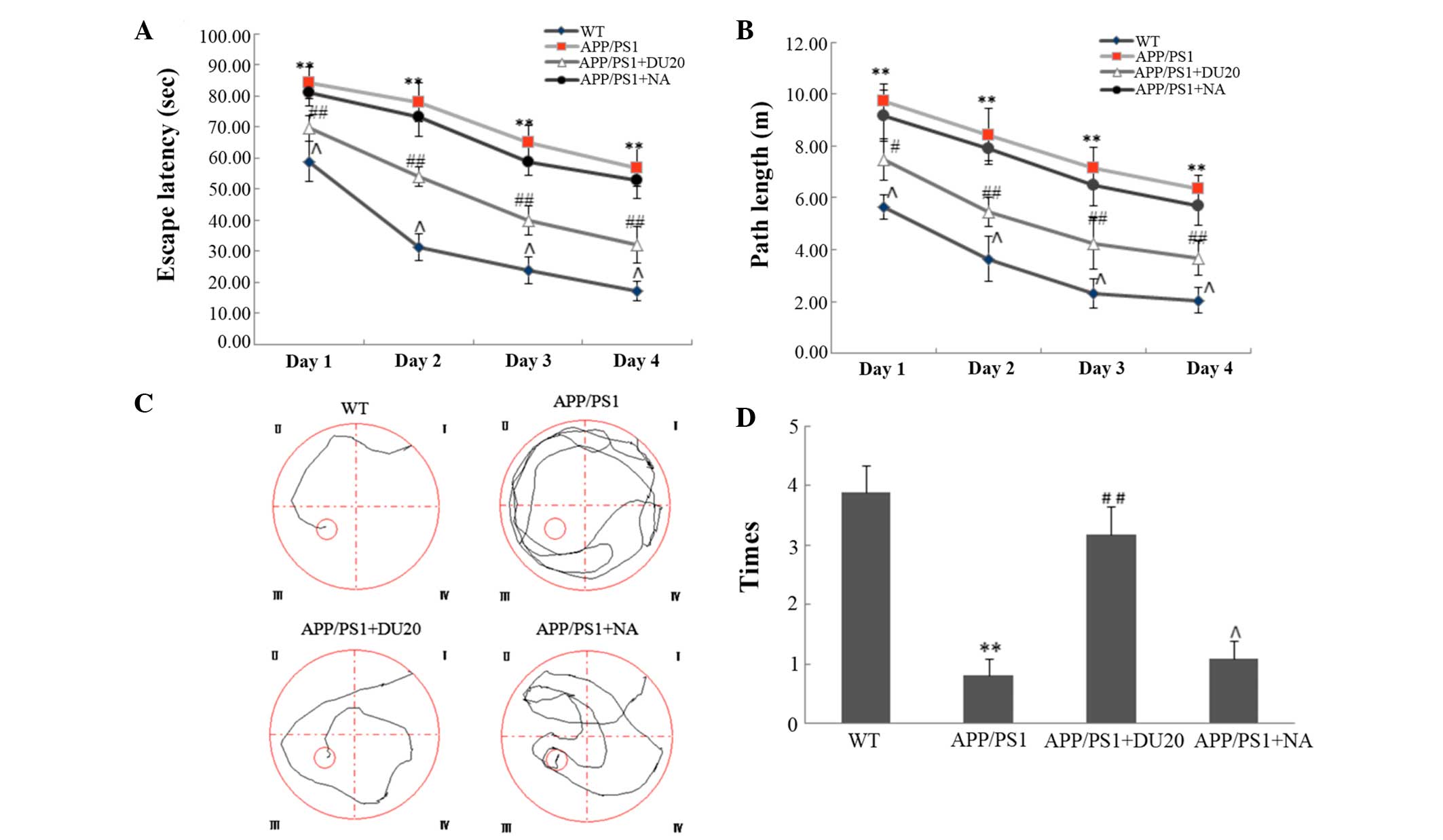
To evaluate the effect of EA at the Baihui (DU20)
acupoint on cognitive impairment in APP/PS1 mice, the Morris water
maze test was performed. In the orientation navigation test, the
APP/PS1 group demonstrated increased escape latency (the time it
took the mouse to find the platform) and longer path length
(Fig. 1A and B) compared with the
WT group (P<0.01). EA at the Baihui (DU20) acupoint treatment
significantly reduced the escape latency and the path length
compared with the APP/PS1 group (P<0.01; Fig. 1A and B). Furthermore, in the space
exploration test (where the platform was removed) the APP/PS1 mice
passed through the original position of the platform fewer times
than the WT group (P<0.01; Fig. 1C
and D). In the APP/PS1 + DU20 group, the frequency that the
mice crossed the position of the platform was significantly
increased compared with the APP/PS1 group (P<0.01; Fig. 1C and D). However, EA at a
non-acupoint (APP/PS1 + NA) resulted in similar findings for the
APP/PS1 mice (Fig. 1).
Collectively, these data suggest that EA at the Baihui (DU20)
acupoint ameliorates cognitive impairment in APP/PS1 mice.
EA at the Baihui (DU20) acupoint
decreases overexpression of Aβ(1-42) in the hippocampus of APP/PS1
mice
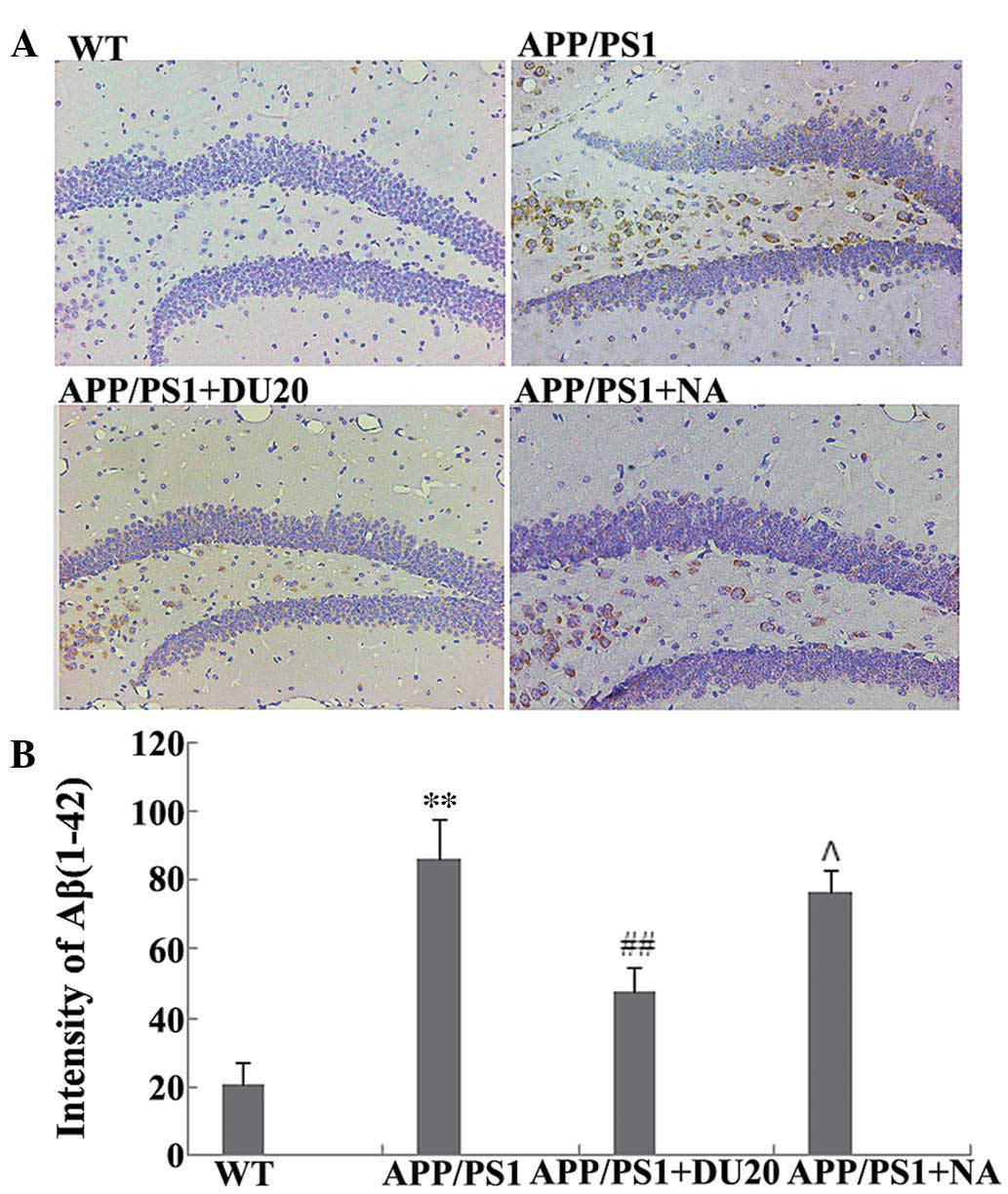
A typical pathological feature of AD is the
overexpression of Aβ(1-42). Therefore, the effect of EA at the
Baihui (DU20) acupoint on Aβ(1-42) deposition in the hippocampus of
APP/PS1 mice was investigated. As demonstrated in Fig. 2, the APP/PS1 group exhibited a
significant increase in Aβ(1-42) deposition (P<0.01 versus the
WT group), which was attenuated by EA at the Baihui (DU20) acupoint
(APP/PS1 + DU20), although not with stimulation of a non-acupoint
(APP/PS1 + NA). These results demonstrate that EA at the Baihui
(DU20) acupoint effectively decreases the deposition of Aβ(1-42) in
the hippocampus.
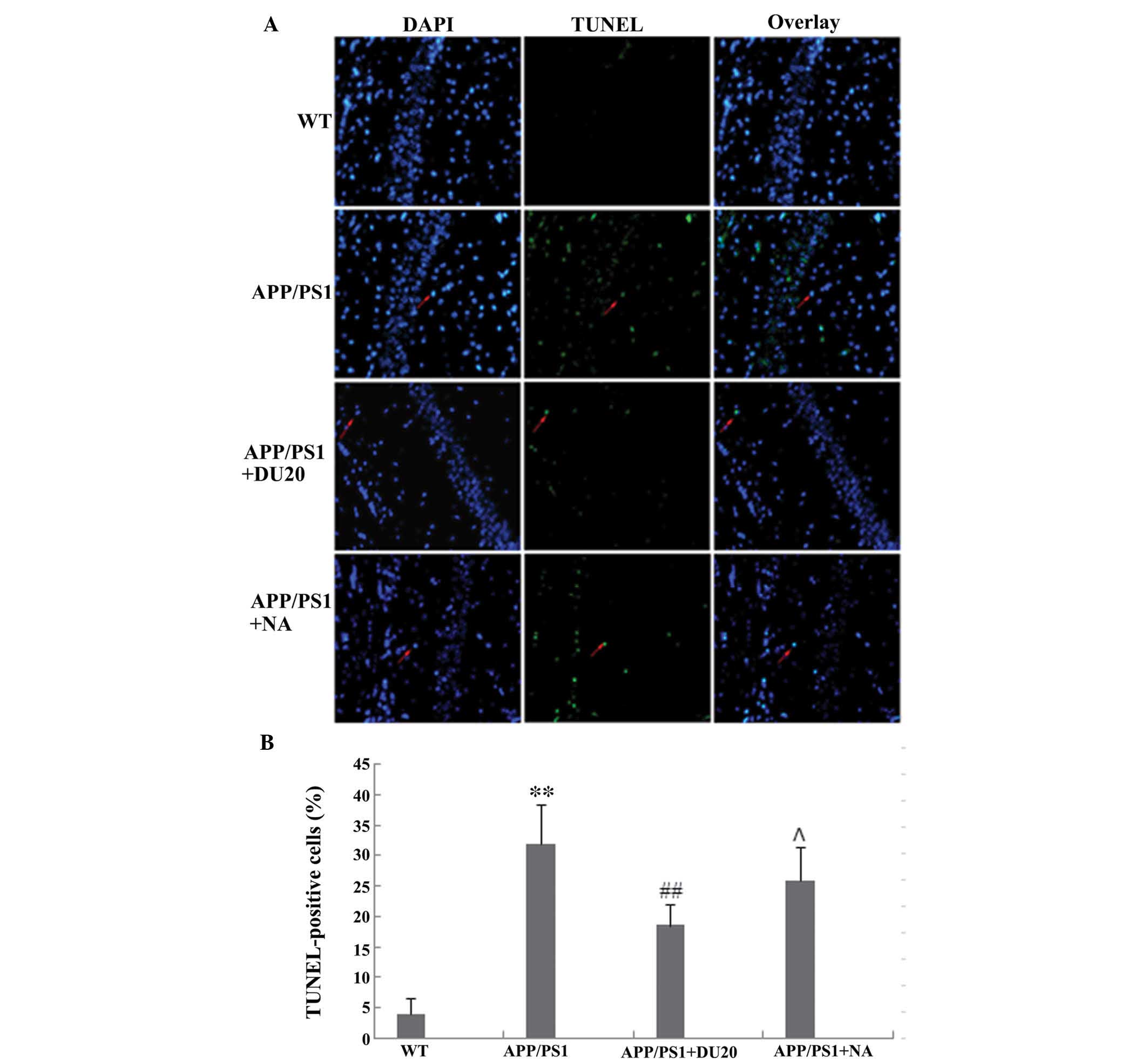
EA at the Baihui (DU20) acupoint inhibits
neuronal apoptosis in the hippocampus of APP/PS1 mice
Neuronal loss in the hippocampus is another
important pathological feature of AD. In order to investigate the
effects of EA at the Baihui (DU20) acupoint on neuronal apoptosis,
a TUNEL assay was conducted. The percentage of TUNEL-positive cells
was significantly increased in the hippocampus of the APP/PS1 group
(P<0.01 versus the WT group; Fig.
3).
EA at the Baihui (DU20) acupoint, but not
at a non-acupoint, reverses the aberrant cell death observed in the
APP/PS1 mice
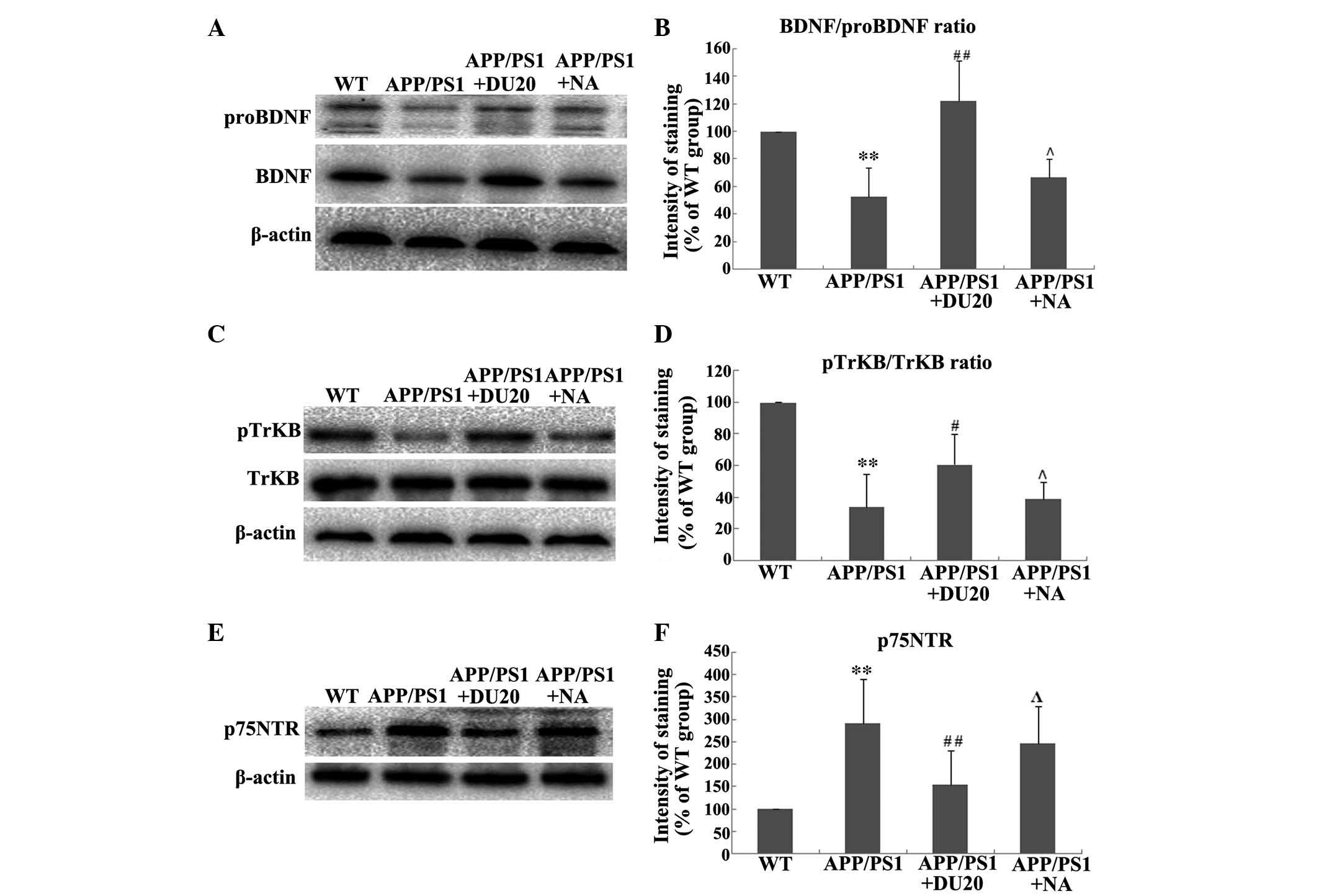
EA at the Baihui (DU20) acupoint altered the
expression and processing of BDNF in the hippocampus of APP/PS1
mice. As BDNF is important in the pathogenesis of AD, the effect of
EA at the Baihui (DU20) acupoint on the expression and processing
of BDNF in the hippocampus of APP/PS1 mice was investigated. EA at
the Baihui (DU20) acupoint was observed to significantly increase
the expression levels of BDNF and proBDNF (P<0.01 vs. the WT
group; Fig. 4A). Furthermore, EA
at the Baihui (DU20) acupoint significantly increased the
BDNF/proBDNF ratio in the APP/PS1 + DU20 group (P<0.01 vs. the
WT group; Fig. 4B). In addition,
p-TrkB was upregulated (Fig. 4C and
D), while the expression level of p75NTR was
decreased in the APP/PS1 + DU20 group (P<0.01 vs. the WT group;
Fig. 4D and E). These data suggest
that EA at the Baihui (DU20) acupoint may exert neuroprotective
effects via modulation of the expression and processing of
BDNF.
Discussion
AD is a prevalent neurodegenerative diseases
characterized by progressive deterioration of cognition and memory
(18). At present, the majority of
therapeutic agents used to clinically treat AD target the
cholinergic system, histaminergic system or 5-hydroxy tryptamine
receptor (19,20). These therapeutic agents improve the
symptoms associated with AD and delay progression to a certain
extent, however they do not stop or reverse the disease progression
(21). Acupuncture at the Baihui
(DU20) acupoint has long been used in China to treat cognitive
impairment and may be a promising treatment strategy for AD.
However, until now, the efficacy of acupuncture at the Baihui
(DU20) acupoint has only been evaluated in animal studies and its
underlying mechanism of action requires elucidation. In the present
study, EA at the Baihui (DU20) acupoint was observed to reverse the
cognitive impairments in APP/PS1 transgenic mice via modulation of
BDNF expression and processing.
The APP/PS1 transgenic mouse model mimics the
pathology of AD by combining two strategies to elevate Aβ levels,
overexpression of the gene encoding the human amyloid precursor
protein and the mutant presenilin-1 gene, which impairs amyloid
protein processing, resulting in elevated Aβ(1-42) levels (22). APP/PS1 transgenic mice exhibit
early onset and a high degree of Aβ deposition, followed by
neuronal loss and cognitive impairment (23). Thus, are considered to be reliable
and effective models that are widely used in AD research (24). In the current study, APP/PS1 mice
demonstrated increases in parameters assessed by the Morris water
maze test, including higher escape latency, longer path length, and
fewer times passing through the platform, as well as a notable
increase in Aβ(1-42) deposition and significant neuronal loss in
the hippocampus when compared with WT mice. Notably, EA at the
Baihui (DU20) acupoint (APP/PS1 + DU20) significantly reversed all
of these aberrations. However, EA at a non-acupoint (APP/PS1 + NA)
revealed similar results to the APP/PS1 mice. Therefore, EA at the
Baihui (DU20) acupoint is considered to be an effective method for
treatment of the cognitive impairments observed in APP/PS1
transgenic mice.
To further investigate the neuroprotective effects
of EA at the Baihui (DU20) acupoint in APP/PS1 mice, the expression
and processing of BDNF was investigated. BDNF is a major regulator
of synaptic plasticity, neuronal survival and differentiation, as
well as higher order processes, including learning, memory and
behavior (25). It is well
established that BDNF is important in the pathogenesis of AD
(26,27). Numerous studies have demonstrated
that BDNF administration exerts substantial protective effects on
crucial neuronal circuits involved in AD by preventing cell death
and neuronal atrophy, thereby ameliorating cognitive and behavioral
deficits (28,29). In the present study, EA at the
Baihui (DU20) acupoint was observed to significantly enhance the
expression of BDNF and proBDNF in APP/PS1 mice. These results
demonstrate that EA at the Baihui (DU20) acupoint may exert
neuroprotective effects by increasing expression levels of BDNF.
However, the mechanism by which EA increases the expression of BDNF
so as to exert neuroprotective effects remains to be elucidated.
BDNF is synthesized as a precursor protein, proBDNF that is
post-translationally cleaved to produce mBDNF. Notably, proBDNF
exerts opposing biological effects to BDNF signaling (30). ProBDNF binds to p75NTR
resulting in the activation of apoptosis-associated signaling
pathways and facilitating long-term depression in the hippocampus
(31,32). Therefore, processing of the BDNF
protein from proBDNF to mBDNF appears to be important in the
regulation of cellular functions. The outcomes of the processing
can be reflected by the relative expression level of BDNF and
proBDNF (the BDNF/proBDNF ratio), which is key in regulating
cellular functions. In the present study, EA at the Baihui (DU20)
acupoint increased the BDNF/proBDNF ratio. Furthermore, the
receptors of BDNF and proBDNF were investigated and it was observed
that EA at the Baihui (DU20) acupoint upregulated the expression of
p-TrkB and decreased the expression level of p75NTR in
the APP/PS1 mice. The results indicate that EA at the Baihui (DU20)
acupoint influenced the modulation and processing of the BDNF
protein from proBDNF to mBDNF, and eventually enhanced the
expression levels of mBDNF. Collectively, these data suggest that
EA at the Baihui (DU20) acupoint may exert neuroprotective effects
by adjusting the expression and processing of BDNF.
In conclusion, results from the present study
demonstrate the efficacy of EA at the Baihui (DU20) acupoint in
APP/PS1 transgenic mice, and indicate that EA at the Baihui (DU20)
acupoint may exert neuroprotective effects by modulating the
expression and processing of BDNF. These results suggest that EA at
the Baihui (DU20) acupoint may serve as a promising treatment
strategy for AD.
Acknowledgments
The authors would like to thank Clarity Manuscript
Consultants, LLC for their editorial assistance with this
manuscript.
References
|
1
|
Mayeux R: Clinical practice. Early
Alzheimer's disease. N Engl J Med. 362:2194–2201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. N Engl J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serrano-Pozo A, Frosch MP, Masliah E and
Hyman BT: Neuropathological alterations in Alzheimer disease. Cold
Spring Harb Perspect Med. 1:a0061892011. View Article : Google Scholar :
|
|
4
|
Phillips HS, Hains JM, Armanini M, Laramee
GR, Johnson SA and Winslow JW: BDNF mRNA is decreased in the
hippocampus of individuals with Alzheimer's disease. Neuron.
7:695–702. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee J, Fukumoto H, Orne J, Klucken J, Raju
S, Vanderburg CR, Irizarry MC, Hyman BT and Ingelsson M: Decreased
levels of BDNF protein in Alzheimer temporal cortex are independent
of BDNF polymorphisms. Exp Neurol. 194:91–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng S, Wuu J, Mufson EJ and Fahnestock M:
Precursor form of brain-derived neurotrophic factor and mature
brain-derived neurotrophic factor are decreased in the pre-clinical
stages of Alzheimer's disease. J Neurochem. 93:1412–1421. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinstein G, Beiser AS, Choi SH, Preis SR,
Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, et al:
Serum brain-derived neurotrophic factor and the risk for dementia:
The Framingham Heart Study. JAMA Neurol. 71:55–61. 2014. View Article : Google Scholar :
|
|
8
|
Barker PA: Whither proBDNF? Nat Neurosci.
12:105–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greenberg ME, Xu B, Lu B and Hempstead BL:
New insights in the biology of BDNF synthesis and release:
Implications in CNS function. J Neurosci. 29:12764–12767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Harte-Hargrove LC, Siao CJ,
Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W,
et al: proBDNF negatively regulates neuronal remodeling, synaptic
transmission, and synaptic plasticity in hippocampus. Cell Reports.
7:796–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minichiello L: TrkB signalling pathways in
LTP and learning. Nat Rev Neurosci. 10:850–860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arancibia S, Silhol M, Moulière F, Meffre
J, Höllinger I, Maurice T and Tapia-Arancibia L: Protective effect
of BDNF against beta-amyloid induced neurotoxicity in vitro and in
vivo in rats. Neurobiol Dis. 31:316–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kemppainen S, Rantamäki T, Jerónimo-Santos
A, Lavasseur G, Autio H, Karpova N, Kärkkäinen E, Stavén S, Vicente
Miranda H, Outeiro TF, et al: Impaired TrkB receptor signaling
contributes to memory impairment in APP/PS1 mice. Neurobiol Aging.
33:2012.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu ZQ, Li J, Deng J, Jiang XJ and Zhou HD:
Effects of proBDNF on cell proliferation and differentiation in
hippocampal dentate gyrus in Alzheimer's disease rat model. Chin
Med J. 90:1353–1356. 2010.In Chinese.
|
|
15
|
Feng X, Yang S, Liu J, Huang J, Peng J,
Lin J, Tao J and Chen L: Electroacupuncture ameliorates cognitive
impairment through inhibition of NF-κB-mediated neuronal cell
apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med
Rep. 7:1516–1522. 2013.PubMed/NCBI
|
|
16
|
International Ethical Guidelines for
Biomedical Research Involving Human Subjects. WHO/CIOMS; Geneva:
1993
|
|
17
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar
|
|
18
|
Selkoe DJ: Alzheimer's disease is a
synaptic failure. Science. 298:789–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haas HL, Sergeeva OA and Selbach O:
Histamine in the nervous system. Physiol Rev. 88:1183–1241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brioni JD, Esbenshade TA, Garrison TR,
Bitner SR and Cowart MD: Discovery of histamine H3 antagonists for
the treatment of cognitive disorders and Alzheimer's disease. J
Pharmacol Exp Ther. 336:38–46. 2011. View Article : Google Scholar
|
|
21
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Radde R, Bolmont T, Kaeser SA,
Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg
H, Gengler S, et al: Abeta42-driven cerebral amyloidosis in
transgenic mice reveals early and robust pathology. EMBO Rep.
7:940–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borchelt DR, Ratovitski T, van Lare J, Lee
MK, Gonzales V, Jenkins NA, Copeland NG, Price DL and Sisodia SS:
Accelerated amyloid deposition in the brains of transgenic mice
coexpressing mutant presenilin 1 and amyloid precursor proteins.
Neuron. 19:939–945. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilkei-Gorzo A: Genetic mouse models of
brain ageing and Alzheimer's disease. Pharmacol Ther. 142:244–257.
2014. View Article : Google Scholar
|
|
25
|
Binder DK and Scharfman HE: Brain-derived
neurotrophic factor. Growth Factors. 22:123–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cosker KE, Courchesne SL and Segal RA:
Action in the axon: generation and transport of signaling
endosomes. Curr Opin Neurobiol. 18:270–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schindowski K, Belarbi K and Buée L:
Neurotrophic factors in Alzheimer's disease: role of axonal
transport. Genes, Brain Behav. 7(suppl 1): 43–56. 2008. View Article : Google Scholar
|
|
28
|
Tapia-Arancibia L, Aliaga E, Silhol M and
Arancibia S: New insights into brain BDNF function in normal aging
and Alzheimer disease. Brain Res Rev. 59:201–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castello NA, Nguyen MH, Tran JD, Cheng D,
Green KN and LaFerla FM: 7,8-Dihydroxyflavone, a small molecule
TrkB agonist, improves spatial memory and increases thin spine
density in a mouse model of Alzheimer disease-like neuronal loss.
PLoS One. 9:e914532014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu B, Pang PT and Woo NH: The yin and yang
of neurotrophin action. Nat Rev Neurosci. 6:603–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woo NH, Teng HK, Siao CJ, Chiaruttini C,
Pang PT, Milner TA, Hempstead BL and Lu B: Activation of p75NTR by
proBDNF facilitates hippocampal long-term depression. Nat Neurosci.
8:1069–1077. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teng HK, Teng KK, Lee R, Wright S, Tevar
S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, et al: ProBDNF
induces neuronal apoptosis via activation of a receptor complex of
p75NTR and sortilin. J Neurosci. 25:5455–5463. 2005. View Article : Google Scholar : PubMed/NCBI
|