Introduction
Signal transducer and activator of transcription 3
(STAT3) protein is a member of the STAT protein family. It acts as
a cytoplasmic transcription factor and is phosphorylated by
cytokines and growth factors, which results in its translocation
from the cell surface to the nucleus. Ligand-bound cell surface
receptors regulate tyrosine phosphorylation of the STAT3 protein
through Janus kinase (JAK) and growth factor receptor tyrosine
kinases (1). This process is rapid
and transient during STAT3 protein activation in normal cells.
However, abnormalities in the JAK/STAT signaling pathways have been
demonstrated to be involved in the development and progression of
several types of cancer (2,3).
STAT3 activation is observed in the majority of
human colon cancer cell lines (4,5).
However, the mechanisms underlying dysregulated STAT3 signaling
initiation in the progression of human colon cancer have yet to be
elucidated. STAT3 is known to promote the progression of cancer
following activation by various pathways; however, a tumor
suppressive role of STAT3 has recently been demonstrated, depending
on the mutational background of the tumor (6,7).
Colon cancer is the sixth most common cancer type and the fifth
leading cause of cancer-associated mortality in China (8). Since dietary characteristics have
changed in recent years, the mortality associated with colon cancer
has been estimated to be 608,000 people per year worldwide
(9). However, early screening,
diagnosis and development of chemotherapy may not be feasible in
elderly patients with human colon cancer with a poor prognosis, due
to its high toxicity and adverse reactions. Therefore, it is
necessary to identify novel treatment agents with less severe side
effects.
Numerous drugs used worldwide are extracted from
natural plant species, and are used for the clinical treatment of
patients with cancer (10,11). Eriocalyxin B is a natural
ent-kaurene diterpenoid compound extracted from Isodon
eriocalyx var. laxiflora, a herb of the Labiatae family,
distributed throughout Southwest China. It is reported to exhibit
anti-inflammatory and antibacterial functions in local folk
medicine, by modulating a variety of biological progresses via
multiple signaling pathways (11,12).
Furthermore, Eriocalyxin B inhibited proliferation and induced
apoptosis in several types of cancer cells, including ovarian
cancer (13), pancreatic
adenocarcinoma (14) and leukemia
(15) cells. Eriocalyxin B was
observed to arrest the cell cycle, induce apoptosis, and inhibit
cancer invasion. However, the mechanisms underlying these
anticancer effects remain under investigation. The present study
aimed to investigate the molecular mechanisms underlying
Eriocalyxin B induction of apoptosis and migration inhibition, as
well as invasion in SW1116 human colon cancer cell lines.
Materials and methods
Reagents
Eriocalyxin B was purchased from BioBioPha Co., Ltd.
(Kunming, China). Fetal bovine serum was purchased from Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou,
China), and Dulbecco's modified Eagle's medium and propidium iodide
(PI) were purchased from Sigma-Aldrich (St. Louis, USA). Primary
antibodies targeting STAT3 (rabbit polyclonal; cat. no. ab119352;
1:1,000), phosphorylated (p)-STAT3 (rabbit polyclonal; cat. no.
ab76315; 1:1,000, vascular endothelial growth factor (VEGF; mouse
polyclonal; cat. no. ab68334; 1:200), vascular endothelial growth
factor receptor 2 (VEGFR2; rabbit monoclonal; cat. no. ab131441;
1:500), proliferating cell nuclear antigen (PCNA; mouse polyclonal;
cat. no. ab92552; 1:5,000), matrix metalloproteinase (MMP)-2
(rabbit polyclonal; cat. no. ab110186; 1:1,000), and MMP-9 (mouse
polyclonal; cat. no. ab119906; 1:500) were purchased from Abcam
(Cambridge, MA, USA), and those targeting glyceraldehyde
3-phosphate dehydrogenase (GAPDH; rabbit monoclonal; cat. no. 5174;
1:1,500), JAK2 (rabbit monoclonal; cat. no. 3230; 1:1,000), and
p-JAK2 (rabbit monoclonal; cat. no. 3776; 1:800), were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase-conjugated goat anti-rabbit/anti-mouse
secondary antibodies (cat nos. A0208 and A0216; 1:1,000) were
purchased from Beyotime Institute of Biotechnology (Haimen,
China).
Cell culture
Human SW1116 colon cancer cell lines were obtained
from the Academia Sinica Cell Bank (Shanghai, China), and cultured
to 80% confluence in low-glucose DMEM supplemented with 10% (v/v)
fetal bovine serum, 100 IU/ml penicillin and 10 mg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cell cultures were incubated in an atmosphere containing 5%
CO2 at 37°C for 72 h.
Cell proliferation assay
The proliferative effects of Eriocalyxin B on the
SW1116 cells were determined using a Cell Counting kit-8 assay
(Dojindo Molecular Technologies, Inc., Rockville, MD, USA) as
previously described (16).
Briefly, the SW1116 cells in logarithmic growth-phase were
harvested and cultured in 96-well plates in 100 µl volumes.
The cells were then treated with various concentrations (0.2, 0.5
and 1 µmol/l) of Eriocalyxin B, and incubated for 0, 6, 12,
24 and 48 h at 37°C. Cells that were not treated with Eriocalyxin B
served as a control group. Absorbance at a wavelength of 450 nm was
measured using a Multiskan EX plate reader (Thermo Fisher
Scientific, Inc.).
Cell cycle analysis by flow
cytometry
For cell cycle analysis, the SW1116 cells were
seeded in 12-well plates at a density of 1×103
cells/well, prior to being treated with various concentrations of
Eriocalyxin B (0.2, 0.5 and 1 µmol/l) for 48 h. Following
treatment, the number of cells in the various phases of cell cycle
was determined by PI staining, in order to ascertain the DNA
content (17). Cells that were not
treated with Eriocalyxin B served as a control group. Data
acquisition was performed using an EPICSXLMCL flow cytometer
(Beckman Coulter, Inc. Brea, CA, USA) using Cell Quest software BD
Accuri C6 version 1.0.264.21 (BD Biosciences, Franklin Lakes, NJ,
USA).
Migration and invasion assay
The migration and invasion of the SW1116 cells were
measured using a Transwell assay as previously described (18). Briefly, Transwell membranes
(Corning Incorporated, Corning, NY, USA) coated with or without
Matrigel (BD Biosciences) were used to measure cell migration and
invasion ability. The SW1116 cells were treated with various
concentrations of Eriocalyxin B (0.2, 0.5 and 1 µmol/l). The
chambers were subsequently placed in a 37°C incubator for 48 h. The
filters were fixed with 4% methanol and stained with 0.5%
methylrosanilnium chloride solution (JRDUN Biotechnology Co., Ltd.,
Shanghai, China) for 30 min. Untreated cells served as a control
group. Evaluation of the number of migratory and invasive cells was
performed under a microscope (x200; CX41RF; Olympus Corporation,
Tokyo, Japan).
Western blot analysis
Following treatment with Eriocalyxin B at the
desired concentrations for the appropriate times, the expression
levels of proliferation proteins (PCNA), migration and invasion
proteins (MMP-2 and MMP-9) and angiogenesis-associated proteins
(VEGF and VEGFR2) were detected by western blotting according to
the manufacturer's instructions. Cells untreated with Eriocalyxin B
served as a control group. GAPDH antibody was used as an internal
control. The experiment was repeated three times independently.
Briefly, the SW1116 cell lysates were harvested and protein was
extracted using radioimmunoprecipitation buffer (JRDUN
Biotechnology Co., Ltd.). Total protein (50 µg) was
separated using 10 or 15% SDS-PAGE (JRDUN Biotechnology Co., Ltd.)
prior to being transferred to polyvinylidene difluoride membranes
(Sigma-Aldrich). The membranes were blocked with fat-free milk for
1 h at room temperature (25°C). The membranes were subsequently
incubated with the primary antibodies for 2 h at 25°C, prior to
being incubated with secondary antibodies for 1 h at 37°C, and were
subsequently washed three times with Tris-buffered saline with
Tween 20. The blots were visualized using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA), and the
signals were quantified by densitometry using Quantity One software
version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance followed by Dunnett's test
was used to analyze significant differences between results.
Statistical analyses were performed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
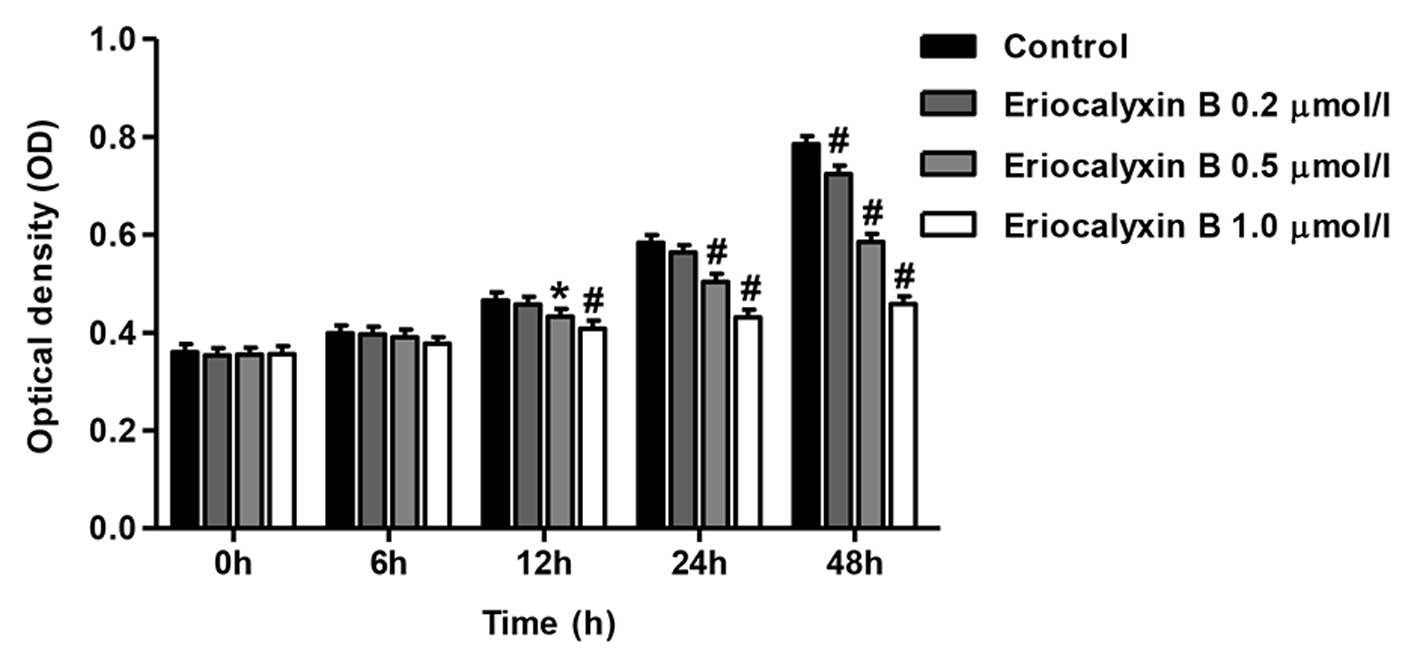
Eriocalyxin B inhibits the proliferation
of SW1116 cells
SW1116 cells were serum-starved overnight and
subsequently cultured with or without various concentrations of
Eriocalyxin B for 0, 6, 12, 24 and 48 h. Eriocalyxin B inhibited
the proliferation of SW1116 cells in a time- and dose-dependent
manner. Following initial experimentation, Eriocalyxin B at dose of
1 µmol/l significantly inhibited cell proliferation,
compared with Eriocalyxin B at dose of 0.2 µmol/l, and
significant differences in cell proliferation occurred between 0.2,
0.5 and 1 µmol/l concentrations at 48 h (P<0.05 and
P<0.01; Fig. 1).
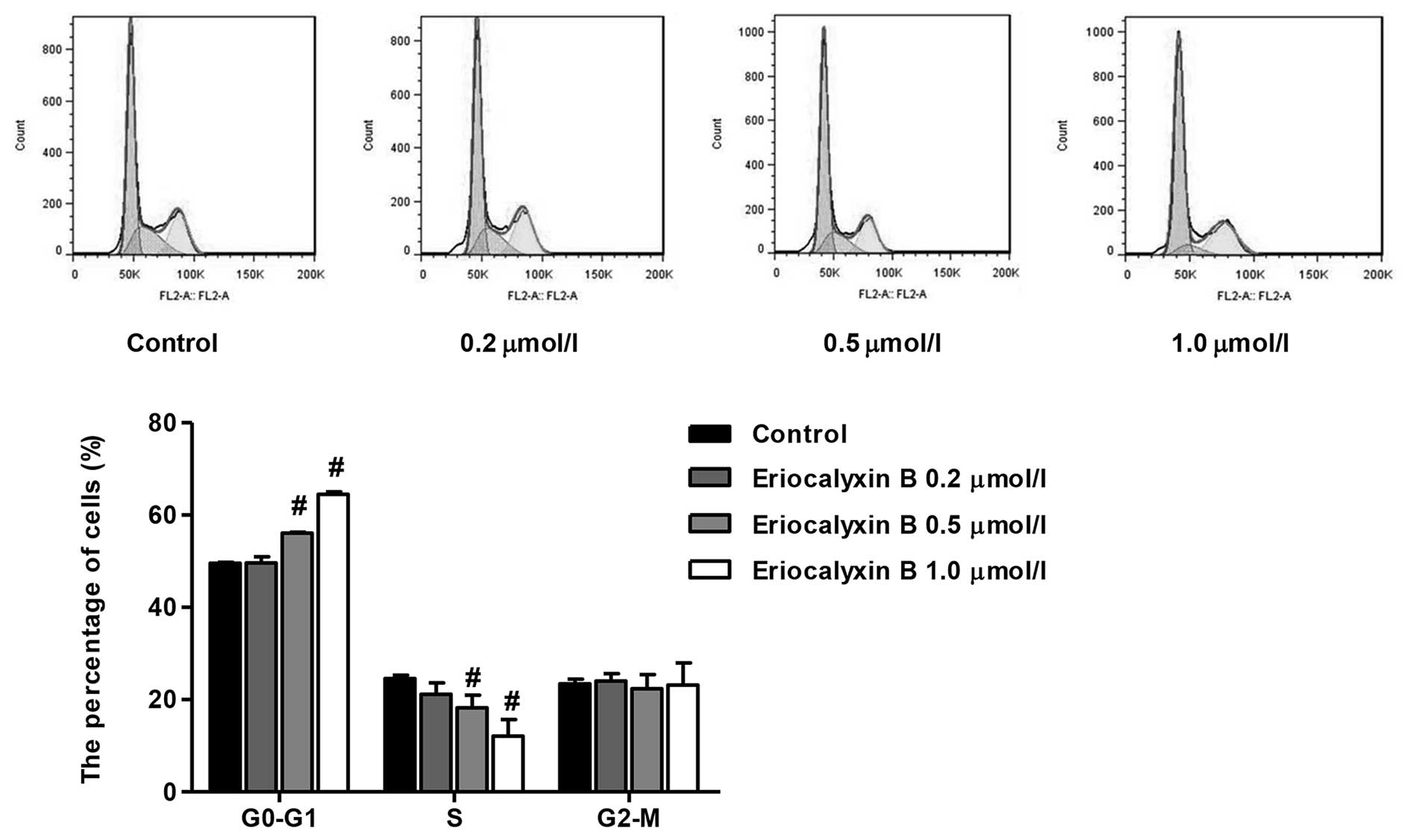
Eriocalyxin B arrests the cell cycle of
SW1116 cells
The cell cycle distribution of SW1116 cells was
analyzed by flow cytometry following 48 h exposure to Eriocalyxin B
at various concentrations (0.2, 0.5 and 1 µmol/l). The
number of cells in the G0–G1 phase following
Eriocalyxin B treatment increased to 64.49% following 48 h of
incubation. The number of cells in the S phase decreased to 12.13%
following 48 h of incubation. In the control group, the number of
cells in the G0–G1 and S phase were 49.57 and
24.58%, respectively (Fig. 2). No
significant difference was observed in the number of cells in the
G2-M phase.
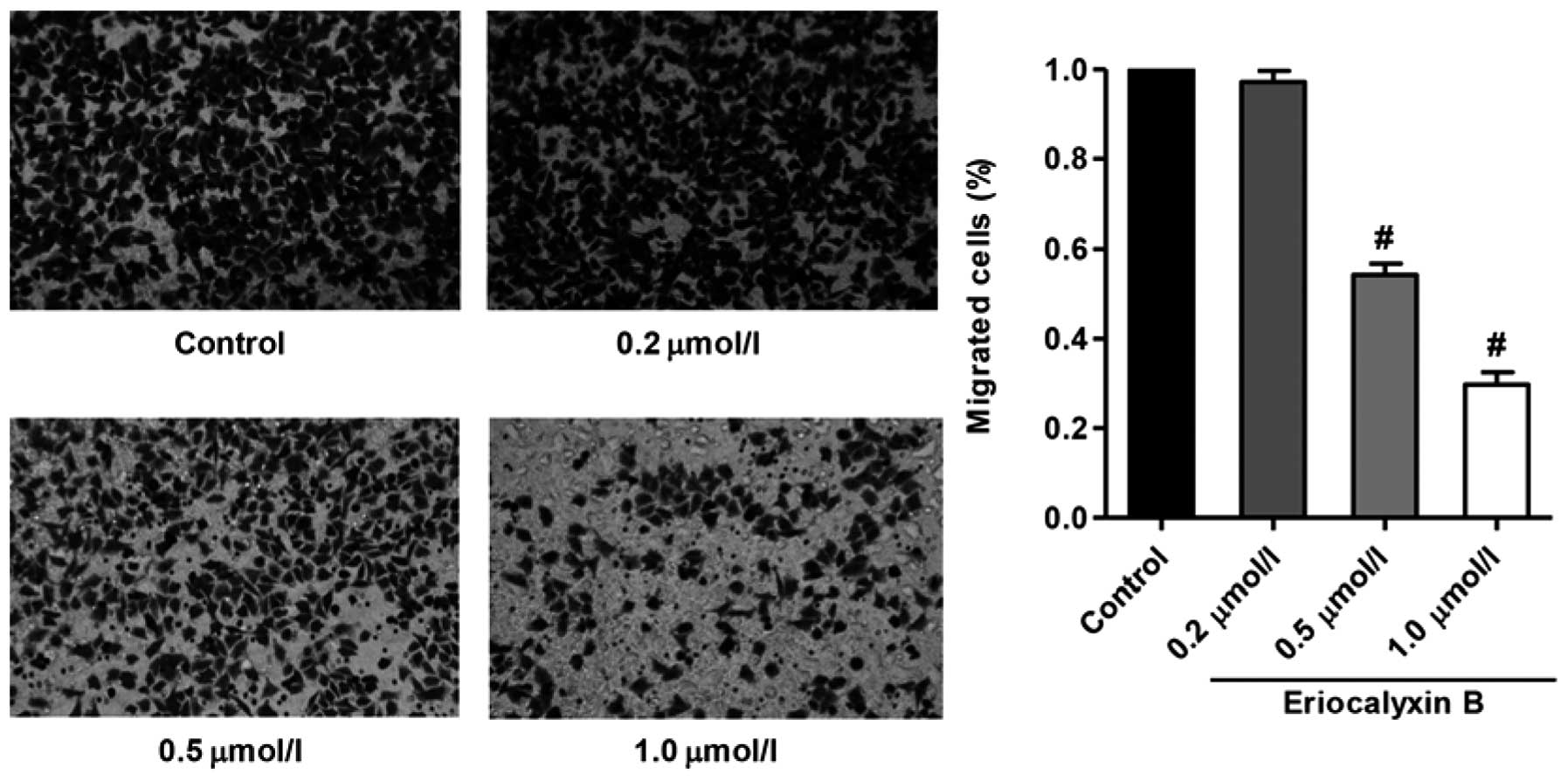
Eriocalyxin B inhibits the migration of
SW1116 cells
The migratory inhibition of SW1116 cells by
Eriocalyxin B was analyzed using a Transwell assay, the results of
which are presented in Fig. 3.
Eriocalyxin B significantly inhibited SW1116 cell migration;
however, this effect was not observed at 0.2 µmol/l
Eriocalyxin B (P<0.01).
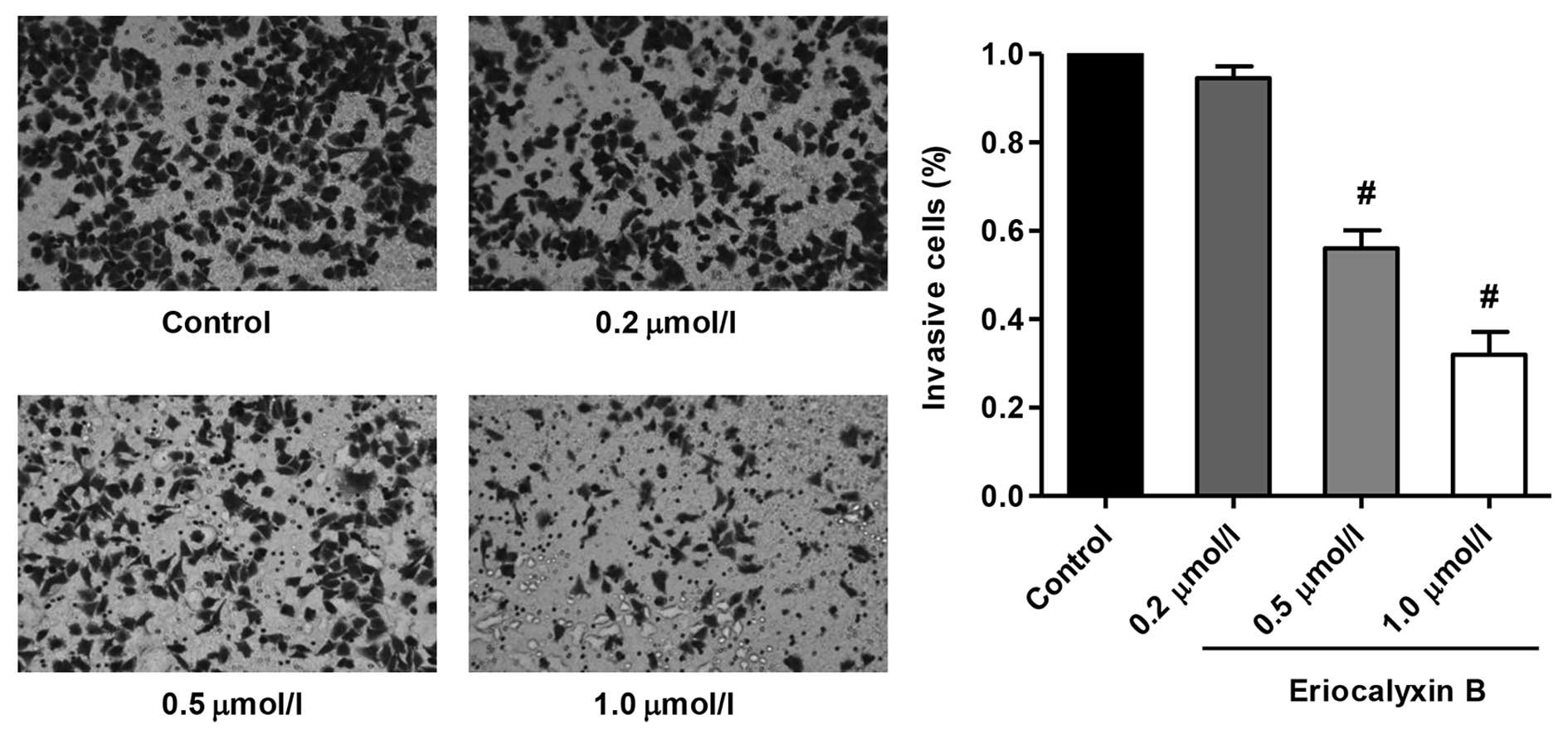
Eriocalyxin B inhibits the invasion of
SW1116 cells
The invasion inhibition of the SW1116 cells by
Eriocalyxin B was measured using a Matrigel-based Transwell assay,
and the results are presented in Fig.
4. Eriocalyxin B significantly inhibited SW1116 cell invasion
(P<0.01), however the effect of Eriocalyxin B at 0.2
µmol/l was not observed. These results clearly suggest that
Eriocalyxin B targets SW1116 cells by exhibiting antimigratory and
anti-invasive effects.
Eriocalyxin B effects the expression
levels of SW1116 cell proteins
The effects of Eriocalyxin B on the JAK2/STAT3
signaling pathway and tumorigenesis-associated proteins in the
SW1116 cells following treatment with Eriocalyxin B were determined
by western blotting. Eriocalyxin B inhibited the expression levels
of p-JAK2 and p-STAT3 in SW1116 cells (P<0.05 and P<0.01;
Fig. 5A), however the expression
levels of JAK2 and STAT3 remained unaltered by Eriocalyxin B
treatment. The expression levels of MMP9, MMP2, PCNA, VEGF and
VEGFR2 were significantly decreased, as compared with the
corresponding control groups (P<0.05 and P<0.01; Fig. 5B). These effects may result in the
inhibition of proliferation, migration, invasion and angiogenesis
in SW1116 cells following exposure to 1 µmol/l Eriocalyxin
B.
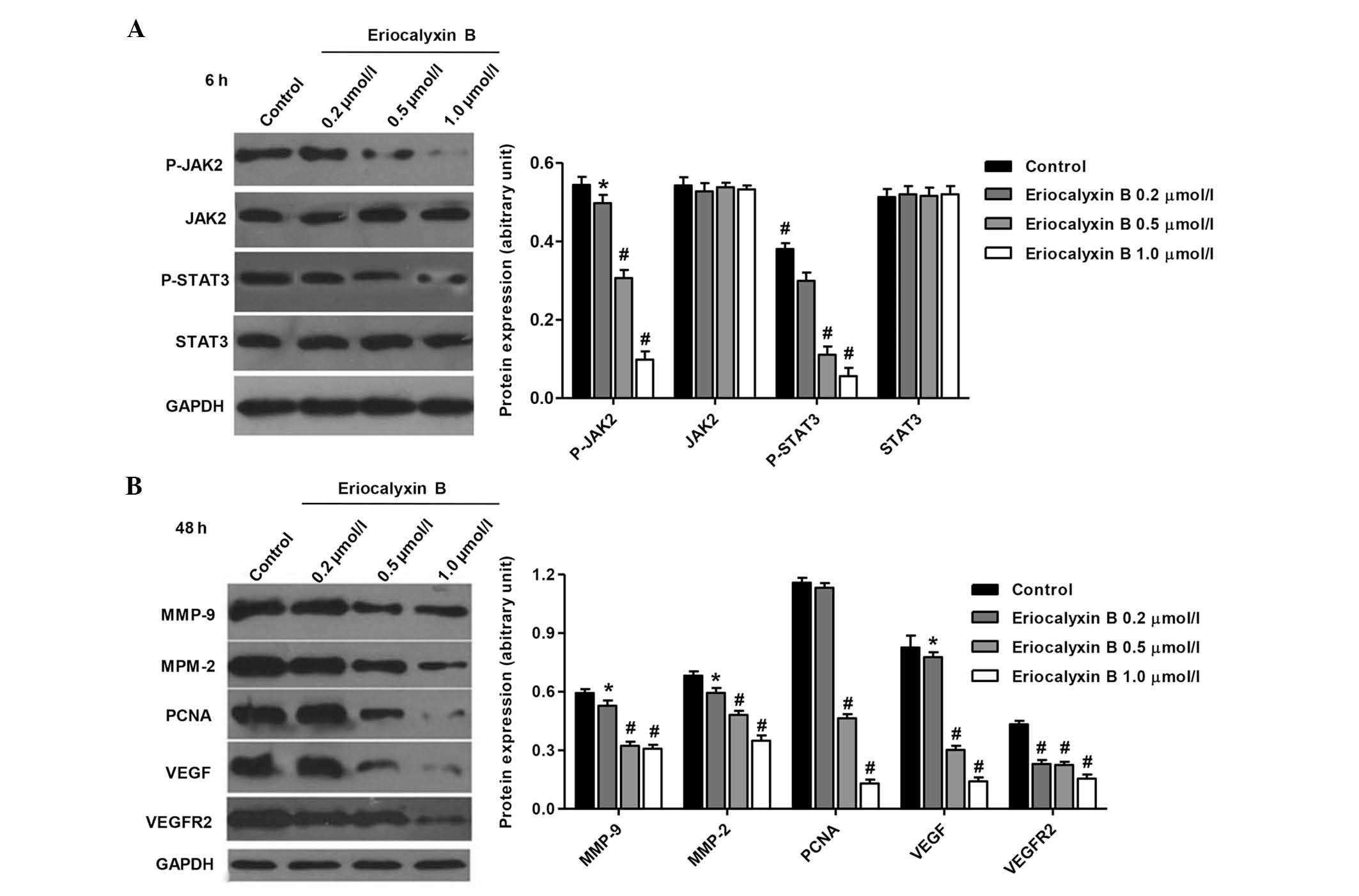 | Figure 5Effects of Eriocalyxin B treatment on
the protein expression levels of p-JAK2, p-STAT3, MMP-2, MMP-9,
PCNA, VEGF and VEGFR2. (A) The SW1116 cells were treated with
various concentrations of Eriocalyxin B for 6 h and (B) 48 h. The
protein expression levels were quantified by western blotting.
Equal quantities of total cellular protein (20 µg) were
separated by 10–15% SDS-PAGE and GAPDH was used as a loading
control. Each bar represents the mean ± standard deviation of three
independent experiments. *P<0.05 and
#P<0.01, compared with control cells. p,
phosphorylated; JAK2, Janus kinase 2; STAT3, signal transducer and
activator of transcription 3; MMP, matrix metalloproteinase; PCNA,
proliferating cell nuclear antigen; VEGF, vascular endothelial
growth factor 2; VEGFR2, vascular endothelial growth factor
receptor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Human colon cancer is the fourth most common type of
cancer in men and, the third most common in women worldwide,
accounting for ~8% of all cancer-associated mortality (19,20).
To date, to the best of our knowledge, no study has explored the
effects of Eriocalyxin B on the development and progression of
human colon cancer. Several studies have reported that modulation
of constitutive JAK2/STAT3 activity via genetic and pharmacological
approaches, demonstrated the role of abnormal activity of
JAK2/STAT3 in malignant transformation and tumor progression, and
thus suggested that JAK2/STAT3 may serve as a novel cancer drug
target (21,22).
Eriocalyxin B suppressed cell proliferation,
invasion, metastasis, and angiogenesis through a mechanism that has
yet to be fully elucidated (23–25).
Numerous genes regulated by signal transducers and transcription
activators were involved in these processes, therefore it was
hypothesized that Eriocalyxin B functions in human colon cancer by
modulating JAK2/STAT3 activation. The present study demonstrated
that Eriocalyxin B inhibited SW1116 cell proliferation by
inhibiting the cell cycle at the G0–G1 phase
and decreasing the number of cells in the S phase. In addition, no
significant differences were observed in the number of cells in the
G2-M phases. Furthermore, the protein expression levels
of PCNA were significantly downregulated following SW1116 cell
treatment with various concentrations of Eriocalyxin B for 48 h.
PCNA is overexpressed in numerous types of tumor regulated by
STAT3, and is required for cells to proliferate (26).
The findings of the present study demonstrated that
Eriocalyxin B inhibited SW1116 cell migratory and invasive
abilities, predominantly via the downregulation of gene product
expression, specifically of genes involved in cell migration,
invasion and angiogenesis, such as MMP-2, MMP-9, VEGF and VEGFR2,
which are regulated by STAT3. MMP-2 and MMP-9 have important roles
in human colon cancer progression, tumor migration, invasion and
angiogenesis (27). A previous
study reported that the upregulation of MMP-2 and MMP-9 expression
is associated with poor survival prognosis in patients with human
colon cancer, which resulted from degradation of the extracellular
matrix, and induction of cell metastasis (28). Angiogenesis is critical for cancer
growth and progression. VEGF is a potent angiogenic growth factor
in human colon cancer, and inhibition of VEGF resulted in
inhibition of angiogenesis and cancer growth (29). VEGFR2 activation is closely
associated with tumor- and vessel-mediated processes in human colon
cancer, and is responsible for tumor survival, as well as tumor
growth and metastasis (30).
To further investigate the mechanism underlying
Eriocalyxin B-induced JAK2/STAT3 signaling inhibition in SW1116
cells, the present study further examined the proteins upstream of
STAT3. JAK2 is considered to be associated with STAT3 activation.
Phosphorylation of JAK2 was suppressed by treatment with
Eriocalyxin B in the SW1116 cells. These results suggested that
Eriocalyxin B exerts its inhibitory effects on STAT3 activation by
inhibiting the activation of JAK2. Activation of the STAT3
signaling pathway has been observed in numerous patients with
cancer (31). Upregulated levels
of p-STAT3 expression is associated with poor survival rates in
patients with colon (32), breast
(33) and gastric cancer (34). A previous study reported a
correlation between STAT3 pathway activation and high
clinicopathological grade with advanced stage in a variety of
cancer types (35). These results
suggest that the JAK2/STAT3 signaling pathway may function as a
therapeutic target and a prognostic marker in patients with colon
cancer (36).
In conclusion, to the best of our knowledge, prior
to the present study no research had been conducted on the role of
Eriocalyxin B in SW1116 human colon cancer cells, nor its
JAK2/STAT3 signaling protein targets. The present study therefore
reports for the first time the anticancer mechanism underlying the
effects of Eriocalyxin B in SW1116 cell tumorigenesis and colon
cancer progression. It was demonstrated that this predominantly
occurred by inhibiting the activation of the JAK2/STAT3 signaling
pathway. Therefore, the JAK2/STAT3 signaling pathway has a
significant role in human colon cancer oncogenesis, and may serve
as a potential therapeutic target for human colon cancer
treatment.
Acknowledgments
The present study was supported by the Doctoral
Innovation Fund Projects from Shanghai Jiao Tong University School
of Medicine (no. BXJ201437).
References
|
1
|
Hedvat M, Huszar D, Herrmann A, Gozgit JM,
Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et
al: The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: Clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koppikar P, Lui VW, Man D, Xi S, Chai RL,
Nelson E, Tobey AB and Grandis JR: Constitutive activation of
signal transducer and activator of transcription 5 contributes to
tumor growth, epithelial-mesenchymal transition and resistance to
epidermal growth factor receptor targeting. Clin Cancer Res.
14:7682–7690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park KW, Kundu J, Chae IG, Kim DH, Yu MH,
Kundu JK and Chun KS: Carnosol induces apoptosis through generation
of ROS and inactivation of STAT3 signaling in human colon cancer
HCT116 cells. Int J Oncol. 44:1309–1315. 2014.PubMed/NCBI
|
|
5
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012.PubMed/NCBI
|
|
6
|
Lee J, Kim JC, Lee SE, Quinley C, Kim H,
Herdman S, Corr M and Raz E: Signal transducer and activator of
transcription 3 (STAT3) protein suppresses adenoma-to-carcinoma
transition in Apcmin/+ mice via regulation of Snail-1 (SNAI)
protein stability. J Biol Chem. 287:18182–18189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Musteanu M, Blaas L, Mair M, Schlederer M,
Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L,
et al: Stat3 is a negative regulator of intestinal tumor
progression in Apc min mice. Gastroenterology. 138:1003–1011. 2010.
View Article : Google Scholar
|
|
8
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
9
|
Marisa L, de Reyniès A, Duval A, Selves J,
Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D,
Ayadi M, et al: Gene expression classification of colon cancer into
molecular subtypes: Characterization, validation, and prognostic
value. PloS Med. 10:e10014532013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang R, Wang H, Deng L, Hou J, Shi R, Yao
M, Gao Y, Yao A, Wang X, Yu L and Sun B: IL-22 is related to
development of human colon cancer by activation of STAT3. BMC
Cancer. 13:592013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikezoe T, Chen SS, Tong XJ, Heber D,
Taguchi H and Koeffler HP: Oridonin induces growth inhibition and
apoptosis of a variety of human cancer cells. Int J Oncol.
23:1187–1193. 2003.PubMed/NCBI
|
|
12
|
Wang L, Zhao WL, Yan JS, Liu P, Sun HP,
Zhou GB, Weng ZY, Wu WL, Weng XQ and Sun XJ: Eriocalyxin B induces
apoptosis of t(8;21) leukemia cells through NF-kappaB and MAPK
signaling pathways and triggers degradation of AML1-ETO oncoprotein
in a caspase-3-dependent manner. Cell Death Differ. 14:306–317.
2007. View Article : Google Scholar
|
|
13
|
Leizer AL, Alvero AB, Fu HH, Holmberg JC,
Cheng YC, Silasi DA, Rutherford T and Mor G: Regulation of
inflammation by the NF-κB pathway in ovarian cancer stem cells. Am
J Reprod Immunol. 65:438–447. 2011. View Article : Google Scholar
|
|
14
|
Li L, Yue GG, Lau CB, Sun H, Fung KP,
Leung PC, Han Q and Leung PS: Eriocalyxin B induces apoptosis and
cell cycle arrest in pancreatic adenocarcinoma cells through
caspase- and p53-dependent pathways. Toxicol Appl Pharmacol.
262:80–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YW, Jiang XX, Chen QS, Shi WY, Wang
L, Sun HD, Shen ZX, Chen Z, Chen SJ and Zhao WL: Eriocalyxin B
induces apoptosis in lymphoma cells through multiple cellular
signaling pathways. Exp Hematol. 38:191–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin SY, Lai WW, Chou CC, Kuo HM, Li TM,
Chung JG and Yang JH: Sodium ascorbate inhibits growth via the
induction of cell cycle arrest and apoptosis in human malignant
melanoma A375.S2 cells. Melanoma Res. 16:509–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen HW, Yu SL, Chen JJ, Li HN, Lin YC,
Yao PL, Chou HY, Chien CT, Chen WJ, Lee YT and Yang PC:
Anti-invasive gene expression profile of curcumin in lung
adenocarcinoma based on a high throughput microarray analysis. Mol
Pharmacol. 65:99–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moghimi-Dehkordi B and Safaee A: An
overview of colorectal cancer survival rates and prognosis in Asia.
World J Gastrointest Oncol. 4:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar
|
|
22
|
Rivat C, Rodrigues S, Bruyneel E, Piétu G,
Robert A, Redeuilh G, Bracke M, Gespach C and Attoub S: Implication
of STAT3 signaling in human colonic cancer cells during intestinal
trefoil factor 3 (TFF3)- and vascular endothelial growth
factor-mediated cellular invasion and tumor growth. Cancer Res.
65:195–202. 2005.PubMed/NCBI
|
|
23
|
Lu Y, Chen B, Song JH, Zhen T, Wang BY, Li
X, Liu P, Yang X, Zhang QL, Xi XD, et al: Eriocalyxin B ameliorates
experimental autoimmune encephalomyelitis by suppressing Th1 and
Th17 cells. Proc Natl Acad Sci USA. 110:2258–2263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leung CH, Grill SP, Lam W, Gao W, Sun HD
and Cheng YC: Eriocalyxin B inhibits nuclear factor-kappaB
activation by interfering with the binding of both p65 and p50 to
the response element in a noncompetitive manner. Mol Pharmacol.
70:1946–1955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coupland VH, Lagergren J, Lüchtenborg M,
Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N and Møller H:
Hospital volume, proportion resected and mortality from oesophageal
and gastric cancer: A population-based study in England, 2004–2008.
Gut. 62:961–966. 2013. View Article : Google Scholar
|
|
26
|
Camargo MC, Kim WH, Chiaravalli AM, Kim
KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R,
Meneses-Gonzalez F, et al: Improved survival of gastric cancer with
tumour Epstein-Barr virus positivity: An international pooled
analysis. Gut. 63:236–243. 2014.
|
|
27
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langers A, Verspaget H, Hawinkels L,
Kubben FJ, van Duijn W, van der Reijden JJ, Hardwick JC, Hommes DW
and Sier CF: MMP-2 and MMP-9 in normal mucosa are independently
associated with outcome of colorectal cancer patients. Brit J
Cancer. 106:1495–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahluwalia A, Jones MK, Matysiak-Budnik T
and Tarnawski AS: VEGF and colon cancer growth beyond angiogenesis:
Does VEGF directly mediate colon cancer growth via a non-angiogenic
mechanism? Curr Pharm Design. 20:1041–1044. 2014. View Article : Google Scholar
|
|
30
|
Jayasinghe C, Simiantonaki N, Habedank S
and Kirkpatrick CJ: The relevance of cell type-and tumor
zone-specific VEGFR-2 activation in locally advanced colon cancer.
J Exp Clin Cancer Res. 34:422015. View Article : Google Scholar
|
|
31
|
Kim JE, Patel M, Ruzevick J, Jackson CM
and Lim M: STAT3 activation in glioblastoma: Biochemical and
therapeutic implications. Cancers (Basel). 6:376–395. 2014.
View Article : Google Scholar
|
|
32
|
Cross-Knorr S, Lu S, Perez K, Guevara S,
Brilliant K, Pisano C, Quesenberry PJ, Resnick MB and Chatterjee D:
RKIP phosphorylation and STAT3 activation is inhibited by
oxaliplatin and camptothecin and are associated with poor prognosis
in stage II colon cancer patients. BMC Cancer. 13:4632013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dave B, Landis MD, Tweardy D, Chang JC,
Dobrolecki LE, Wu MF, Zhang X, Westbrook TF, Hilsenbeck SG, Liu D
and Lewis MT: Selective small molecule Stat3 inhibitor reduces
breast cancer tumor-initiating cells and improves recurrence free
survival in a human-xenograft model. PloS One. 7:e302072012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong H, Du W, Wang JL, Wang YC, Tang JT,
Hong J and Fang JY: Constitutive activation of STAT3 is predictive
of poor prognosis in human gastric cancer. J Mol Med (Berl).
90:1037–1046. 2012. View Article : Google Scholar
|
|
35
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fletcher S, Turkson J and Gunning PT:
Molecular approaches towards the inhibition of the signal
transducer and activator of transcription 3 (Stat3) protein.
ChemMedChem. 3:1159–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|